Abstract
In this study, we aimed to evaluate the effects of exenatide (EXE) treatment on exocrine pancreas of nonhuman primates. To this end, 52 baboons (Papio hamadryas) underwent partial pancreatectomy, followed by continuous infusion of EXE or saline (SAL) for 14 weeks. Histological analysis, immunohistochemistry, Computer Assisted Stereology Toolbox morphometry, and immunofluorescence staining were performed at baseline and after treatment. The EXE treatment did not induce pancreatitis, parenchymal or periductal inflammatory cell accumulation, ductal hyperplasia, or dysplastic lesions/pancreatic intraepithelial neoplasia. At study end, Ki-67–positive (proliferating) acinar cell number did not change, compared with baseline, in either group. Ki-67–positive ductal cells increased after EXE treatment (P = 0.04). However, the change in Ki-67–positive ductal cell number did not differ significantly between the EXE and SAL groups (P = 0.13). M-30–positive (apoptotic) acinar and ductal cell number did not change after SAL or EXE treatment. No changes in ductal density and volume were observed after EXE or SAL. Interestingly, by triple-immunofluorescence staining, we detected c-kit (a marker of cell transdifferentiation) positive ductal cells co-expressing insulin in ducts only in the EXE group at study end, suggesting that EXE may promote the differentiation of ductal cells toward a β-cell phenotype. In conclusion, 14 weeks of EXE treatment did not exert any negative effect on exocrine pancreas, by inducing either pancreatic inflammation or hyperplasia/dysplasia in nonhuman primates.
Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-IV (DPP-IV) inhibitors represent new classes of therapeutic agents for type 2 diabetes mellitus treatment acting by augmenting β-cell function while decreasing food intake and body weight.1, 2, 3, 4, 5, 6, 7
Isolated cases of pancreatitis have been reported in diabetic patients treated with GLP-1 receptor agonists and DPP-IV inhibitors, prompting the US Food and Drug Administration to issue alerts on possible adverse effects.8 Acute pancreatitis is a severe clinical condition characterized by pancreatic pathological changes and increased serum amylase and lipase levels. Obstructive gallstone disease, alcohol abuse, hypertriglyceridemia, obesity, and type 2 diabetes mellitus are the most common risk factors for pancreatitis and pancreatic cancer.9, 10, 11
Although pancreatitis and pancreatic cancer have been suggested to be more frequent in diabetic patients treated with GLP-1–based therapies, the methodologically heterogeneous literature available does not support a firm conclusion on whether GLP-1 receptor agonists or DPP-IV inhibitors are directly implicated in pancreatitis or pancreatic cancer.8, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Animal studies designed to examine the effect of GLP-1–based therapies on exocrine pancreas have also yielded conflicting results.26, 27, 28, 29, 30, 31
In this study, we directly evaluated whether the GLP-1 receptor agonist exenatide (EXE) can promote inflammation/pancreatitis and hyperplasia/dysplasia in exocrine pancreas of baboons. Baboons have interesting genetic and physiological similarity to humans, develop similar pathological features, and represent a valuable model to study human diseases, such as insulin resistance, obesity, and type 2 diabetes mellitus.32, 33, 34, 35, 36, 37, 38
Materials and Methods
Study Population and Analytical Determinations
Fifty-two baboons (Papio hamadryas; 38 females and 14 males) were selected and studied at the Southwest National Primate Research Center at the Texas Biomedical Research Institute (San Antonio, TX). Baboons underwent a partial resection (biopsy) of the pancreatic tail, followed by 14-week treatment with either continuous i.v. EXE (Amylin, San Diego, CA; Eli Lilly & Co, Indianapolis, IN) (0.014 μg/kg body weight hour; n = 26, 19 females and 7 males) or saline (SAL; n = 26, 19 females and 7 males). EXE or SAL i.v. infusion was performed using heparin-coated polyurethane 7 French catheters that were placed in the internal jugular veins under general anesthesia. These catheters were routed s.c. to the interscapular region of the back, where the catheters exited the skin into a molded plastic box (backpack) attached outside of a special jacket and were connected to an infusion pump placed above the cage.39 After catheter placement, baboons were housed in single indoor metal cages with enrichment games and ad libitum access to water and food, as previously described.36 All animal care and procedures were approved by the Texas Biomedical Research Institute Animal Care and the Use Committee as well as the University of Texas Health Science Center at San Antonio (San Antonio, TX), Institutional Animal Care and Use Committee (protocols 1126PC and 1175PC). Anthropometric and biochemical data were collected at baseline, before the partial pancreatectomy/pancreatic biopsy, and after 14 weeks of SAL or EXE infusion. Serum levels of EXE were analyzed by AC2993 IEMA Assay (Amylin) before and after treatment, as previously described.4
An additional cohort of 16 baboons, which had never been exposed to any pharmacological treatment or surgical procedures, was used as an additional control group. The unmanipulated control animals were housed in two 6-acre outdoor corrals. At necropsy, the pancreas of these baboons was collected and the tail portion was divided from head/body.
Surgery
Under general anesthesia, isofluorane 1.5% v/v, the pancreas was first dissected along the inferior edge, exposing the posterior avascular plane. The superior pancreas was then dissected off the splenic artery and vein. The distal tail of the pancreas was dissected away from the hilum of the spleen, preserving the spleen. Ten percent to thirty percent of the pancreas was removed. No drains were placed. Absorbable interrupted sutures were used to close the fascia, and nylon sutures were used to close the skin. During the same anesthetic procedure, heparin-coated polyurethane 7 French catheters were placed in the internal jugular veins.
After the surgery, i.v. parenteral nutrition with 5% glucose solution, potassium, and amino acids was provided during a recovery period of 4 days, along with antibiotics and analgesics. The perioperative/postoperative mortality was 0%. Solid food was again introduced after the recovery period, and baboons were housed inside individual cages while on tether for treatment.
At the conclusion of the study, all baboons were humanely euthanized by i.v. administration of an overdose of sodium pentobarbital, 48 hours after cessation of the EXE or SAL infusion, and necropsy was performed to collect the head/body of the pancreas, as previously described.37, 40
Pathological Data
Pancreas samples removed at baseline (tail) and at necropsy (head-body) were fixed in 10% neutral-buffered formalin, embedded in paraffin wax, cut into sections (5 μm thick), and stained with hematoxylin and eosin (H&E). The same tissue collection and processing protocol was followed for pancreas samples (tail and head-body) obtained from unmanipulated control group. Two board-certified veterinary pathologists (M.O. and E.J.D.), who were blinded to treatment groups, evaluated pancreas slides to identify the following: i) lesions consistent with acute or chronic pancreatitis (necrosis hemorrhage, suppurative infiltrates, or fibrosis), ii) ductal hyperplasia, and iii) ductal dysplasia.
Focal parenchymal or periductal mononuclear inflammatory cell accumulation (MICA)40 was evaluated in both groups, and cells were also characterized using the monoclonal anti-CD68 (clone KP1; Dako, Carpinteria, CA), anti-CD20 (clone L26; Dako), and anti-CD3 (clone PS1; BioGenex, San Ramon, CA) antibodies, which identify macrophages, B lymphocytes, and T lymphocytes, respectively. Severity scores for MICA were as follows: 0 indicates none; 1 indicates minimal (<10 cells per focus); 2 indicates mild (<50 cells per focus or a single focus of up to 100 cells); and 3 indicates moderate (>50 cells per focus in more than one location).
Ductal hyperplasia was defined as the presence of columnar epithelium in small or midsized pancreatic ducts, in the absence of any significant architectural and cytological atypia, and was scored as absent (0) or present (1). Columnar epithelium seen in larger ducts was considered normal.
Acinar and ductal cell proliferation was evaluated by Ki-67 immunostaining, using the monoclonal MIB-1 antibody (Dako). Acinar and ductal cell apoptosis was evaluated using the monoclonal antibody M-30 (Roche, Penzberg, Germany), which recognizes the caspase-cleaved formalin-resistant epitope of cytokeratin 18. The number of proliferating or apoptotic cells was evaluated by counting the positive cells in a total of 1500 acinar and 150 ductal cells per slide.
Electron Microscopy
For ultrastructural analysis, pancreatic samples from SAL and EXE groups were fixed for 2 hours at 4°C in 2% paraformaldehyde and 2% glutaraldehyde in 0.05 mol/L (pH 7.3) cacodylate buffer (Karnovsky fixative), post-fixed in 1% osmium tetroxide, and embedded in Epon-Araldite (Sigma-Aldrich, St. Louis, MO). Thin sections were counterstained with uranyl acetate and lead citrate. All samples were examined with a Philips Morgagni electron microscope (FEI Company, Eindhoven, The Netherlands).33
Morphometric Analysis
Morphometric analysis was performed using the Computer Assisted Stereology Toolbox (CAST) 2.0 system (Visiopharm, Ballerup, Denmark) coupled with an Olympus BX61 microscope (Olympus Corporation of the Americas, Center Valley, PA). Duct morphometry was evaluated on H&E-stained sections by using the stereology fundamentals previously described.33, 34, 41, 42 Each field had an area of 142,600 μm2 and was randomly selected from the whole H&E section using the CAST meander sampling. On average, we analyzed 142 fields per slide; in each field, point counting of total pancreatic tissue and ducts was performed at the magnification of ×40; 64 points were inserted on each field, and an average of 9090 points per slide was used. The quantification of the relative duct volume/% pancreas was calculated by using the following formula: where DP indicates points that hit pancreatic ducts; and TP, total pancreas points. We also quantified the following: i) total number of ducts, ii) total duct area and volume, iii) ducts’ numerical density (number of ducts/mm2), iv) total number of duct cells, v) average duct cell size (μm2), vi) duct cells’ numerical density (duct cells/1000 μm2), and vii) total duct mass (mg). All these measurements were done before and after treatment, with either SAL or EXE.
Immunofluorescence Staining
Pancreas sections (5 μm thick) were fixed and immunostained as previously described.33, 42 Nuclei were visualized by staining with DAPI (Sigma-Aldrich). The following primary antibodies were used: anti-insulin guinea pig monoclonal (Dako), anti–c-kit rabbit polyclonal antibody (Cell Signaling, Beverly, MA), and MIB-1 mouse monoclonal antibody to detect Ki-67 (Dako).
Microscopy analysis was performed using a Zeiss (Oberkochen, Germany) Axiovert 200 inverted fluorescence microscope equipped with a Retiga SRV charge-coupled device camera (QImaging, Surrey, BC, Canada). Optical sections covering the whole tissue section were acquired in 0.4-μm z-axis steps with the Zeiss Axiovert widefield fluorescence microscope and deblurred using the Nearest Neighbor algorithm (Image ProPlus 6.2 3D Analyser; Media Cybernetics, Rockville, MD).
Statistical Analysis
Anthropometric and biochemical parameters are expressed as means ± SEM or median (interquartile range) for variables with a skewed distribution. Differences between groups were assessed by unpaired Student's t-test or by Mann-Whitney test. Paired Student's t-test and Wilcoxon test were used to compare changes between baseline and end of study (EOS) in each group. Comparisons between pretreatment and post-treatment scores for SAL and EXE groups were performed with two-sided Fisher's exact test. To compare the changes (basal-EOS) in the MICA and hyperplasia scores between EXE and SAL groups, we used the two-sample Wilcoxon rank test. P ≤ 0.05 was considered statistically significant. The changes in MICA and hyperplasia score, Ki-67, and M-30–positive cell number were obtained by subtracting pretreatment from post-treatment data. Statistical analyses were performed with SPSS program for Windows version 17 (SPSS Inc., Chicago, IL). With a sample size of 52, two-sided testing, and a type 1 error of 0.05, the study had power of 90% to detect a minimum difference of 20% in periductal inflammation and ductal hyperplasia between groups, considering that surgery may increase these parameters by 15%; as well as power of 95% to detect a minimum difference in the number of acinar and ductal cells in proliferation (5 and 0.3 cells, respectively).
Results
EXE Treatment Is Not Associated with Increased Serum Amylase Levels
The anthropometric and biochemical characteristics at baseline and at the study end were not significantly different between EXE and SAL group (Table 1). At the EOS, serum EXE levels in the EXE group were significantly higher (758 ± 162 pg/mL) than in the SAL group (0.0 pg/mL). Food consumption assessed during EXE and SAL infusion was not significantly different between both groups (EXE versus SAL, 55.2 ± 4.03 versus 53.6 ± 2.6 Kcal/kg per day; P = 0.43), and no anorexia, vomiting, or behavior changes were observed in EXE-treated animals as well as in SAL-treated baboons.
Table 1.
Anthropometric and Biochemical Characteristics of Study Population at Baseline and Study End
| Clinical parameters | SAL BAS | EXE BAS | SAL EOS | EXE EOS |
P value |
|||
|---|---|---|---|---|---|---|---|---|
| SAL BAS vs EXE BAS | SAL BAS vs SAL EOS | EXE BAS vs EXE EOS | SAL EOS vs EXE EOS | |||||
| Age (years) | 10.9 ± 4.7 | 10.8 ± 6.9 | 0.45 | |||||
| Sex (M:F ratio) | 7:19 | 7:19 | 0.89 | |||||
| BMI (kg/m2) | 22.5 ± 8.3 | 23.3 ± 7.6 | 21.4 ± 8.8 | 22.1 ± 6.5 | 0.89 | 0.003 | 0.001 | 0.90 |
| Waist (m) | 0.48 ± 0.1 | 0.5 ± 0.2 | 0.47 ± 0.1 | 0.47 ± 0.1 | 0.35 | 0.76 | 0.003 | 0.87 |
| FPG (mmol/L) | 4.46 ± 0.12 | 4.56 ± 0.21 | 4.45 ± 0.13 | 4.53 ± 0.22 | 0.69 | 0.57 | 0.17 | 0.91 |
| HbA1c [% (mmol/mol)] | 4.1 ± 0.4 (21.3 ± 4.4) | 4.3 ± 0.3 (23.5 ± 3.3) | 4.3 ± 0.8 (23.5 ± 8.7) | 4.3 ± 0.6 (23.5 ± 6.6) | 0.08 | 0.06 | 0.30 | 0.98 |
| Albumin (g/L) | 39.1 ± 1.0 | 39.1 ± 1.1 | 36.0 ± 1.0 | 36.1 ± 1.1 | 0.75 | 0.07 | 0.08 | 0.87 |
| ALT (μkat/L) | 0.57 ± 0.20 | 0.49 ± 0.23 | 0.53 ± 0.32 | 0.46 ± 0.23 | 0.68 | 0.87 | 0.065 | 0.12 |
| Amylase (μkat/L) | 2.27 ± 0.48 | 2.52 ± 1.14 | 2.24 ± 0.92 | 2.34 ± 0.99 | 0.32 | 0.80 | 0.90 | 0.78 |
| Creatinine (μmol/L) | 90.2 ± 3.5 | 92.8 ± 3.5 | 85.8 ± 3.5 | 84.9 ± 3.5 | 0.65 | 0.044 | 0.047 | 0.81 |
| Tot. Chol (mmol/L) | 2.43 ± 0.11 | 2.62 ± 0.12 | 2.67 ± 0.12 | 2.80 ± 0.13 | 0.35 | 0.13 | 0.15 | 0.47 |
| HDL (mmol/L) | 1.06 ± 0.04 | 1.14 ± 0.05 | 1.09 ± 0.06 | 1.17 ± 0.08 | 0.2 | 0.60 | 0.80 | 0.45 |
| LDL (mmol/L) | 1.14 ± 0.06 | 1.19 ± 0.07 | 1.24 ± 0.08 | 1.27 ± 0.08 | 0.55 | 0.02 | 0.24 | 0.76 |
| Trigly (mmol/L) | 0.52 ± 0.24 | 0.51 ± 0.29 | 0.61 ± 0.34 | 0.69 ± 0.34 | 0.96 | 0.037 | 0.043 | 0.75 |
ALT, alanine transaminase; BAS, baseline; BMI, body mass index; EOS, end of study; EXE, exenatide; F, female; M, male; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SAL, saline; Tot. Chol, total cholesterol; Trigly, triglycerides.
We observed a significant decrease in body mass index at study end compared with baseline in SAL (21.4 ± 8.8 versus 22.5 ± 8.3 kg/m2; P = 0.003) and EXE (22.1 ± 6.5 versus 23.3 ± 7.6 kg/m2; P = 0.001) groups (Table 1). EXE-treated baboons also showed a slight decrease in waist circumference (0.47 ± 0.1 versus 0.5 ± 0.2 m; P = 0.003). Serum low-density lipoprotein increased modestly in the SAL group and remained unchanged in the EXE group. Interestingly, serum amylase levels did not change significantly after SAL and EXE treatment in comparison to baseline [SAL, 2.24 ± 0.92 versus 2.27 ± 0.48 μkat/L (P = 0.80); EXE, 2.34 ± 0.99 versus 2.52 ± 1.14 μkat/L (P = 0.90)] (Table 1).
EXE Treatment Does Not Induce Pancreatic Inflammation and Ductal Cell Hyperplasia
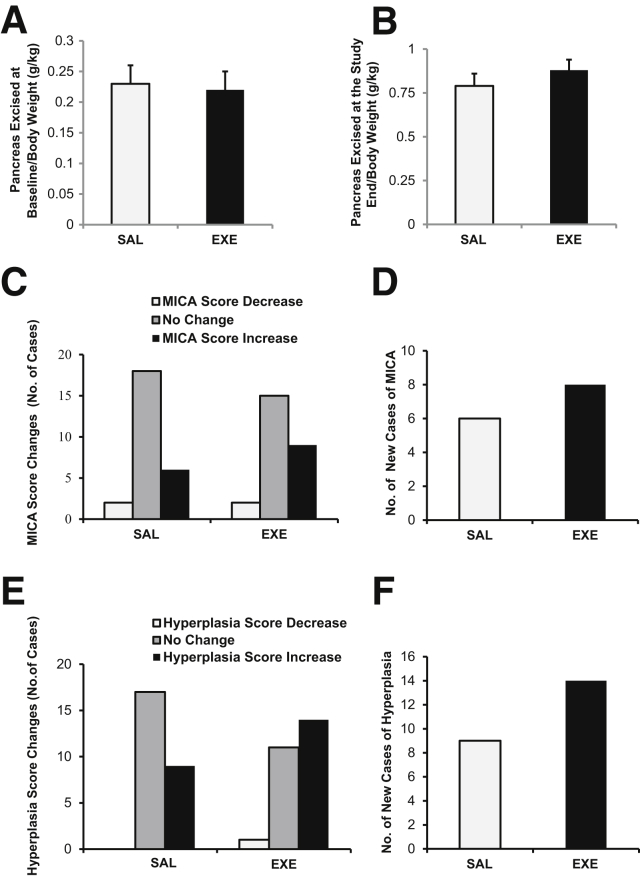
The weight of the pancreatic portion excised at baseline was similar in the SAL and EXE groups (4.59 ± 0.49 g versus 4.44 ± 0.46 g; P = 0.82), as was the weight of the pancreas collected at study end (16.52 ± 1.16 g versus 17.59 ± 0.99 g; P = 0.49) and total pancreatic weight (22.04 ± 0.86 g versus 22.12 ± 1.05 g; P = 0.5). In addition, no differences between groups were observed when pancreas weight at baseline and at study end was normalized for body weight (P = 0.9 and P = 0.34, respectively) (Figure 1, A and B).
Figure 1.
Pancreatic tissue weight and mononuclear inflammatory cell accumulation (MICA) and ductal hyperplasia score changes in saline (SAL) and exenatide (EXE) groups. A: Weight of pancreas (g) excised at baseline/body weight (kg) at baseline. B: Weight of pancreas (g) excised at study end/body weight (kg) at the EOS. C: Number of baboons with increase, decrease, and no change in MICA score after EXE or SAL treatment. D: Number of animals with pancreas MICA newly identified after SAL (white bar) and EXE treatment (black bar). E: Number of baboons with increase, decrease, and no change in ductal hyperplasia score after EXE or SAL treatment. F: Number of animals with pancreatic hyperplasia newly identified after SAL (white bar) and EXE treatment (black bar). Number of sections for each baboon = 4 (H&E staining; 2 sections at baseline and 2 sections at the end of the study). None of the comparisons were statistically significant.
There was no histological evidence of lesions consistent with pancreatitis (necrosis, hemorrhage, suppurative infiltrates, or fibrosis) either at baseline or after treatment with SAL or EXE (Table 2). Some samples in both groups exhibited minimal or mild focal parenchymal/periductal MICA. CD3 immunostaining demonstrated that MICA was mainly composed of T lymphocytes (Supplemental Figures S1 and S2). At baseline, in the EXE group, 17 (65.4%), 7 (26.9%), and 2 (7.7%) of baboons had pancreatic MICA scores of 0, 1, and 2, respectively, whereas in the SAL group, 22 (84.6%), 4 (15.4%), and 0 baboons had pancreatic MICA scores of 0, 1, and 2, respectively (P = 0.24) (Table 2).
Table 2.
Pancreatitis, MICA, PanIN, and Hyperplasia Scores at Baseline and at the End of the Study
| Variable | SAL BAS, n = 26 | EXE BAS, n = 26 | SAL EOS, n = 26 | EXE EOS, n = 26 |
P value |
|||
|---|---|---|---|---|---|---|---|---|
| SAL BAS vs EXE BAS | SAL BAS vs SAL EOS | EXE BAS vs EXE EOS | SAL EOS vs EXE EOS | |||||
| Pancreatitis | 0 | 0 | 0 | 0 | ||||
| MICA score | ||||||||
| 0 | 22 (84.6) | 17 (65.4) | 18 (69.2) | 10 (38.5) | 0.24 | 0.25 | 0.17 | 0.098 |
| 1 | 4 (15.4) | 7 (26.9) | 6 (23.1) | 12 (46.2) | ||||
| 2 | 0 | 2 (7.7) | 2 (7.7) | 4 (15.4) | ||||
| 3 | 0 | 0 | 0 | 0 | ||||
| PanIN | 0 | 0 | 0 | 0 | ||||
| Hyperplasia score | ||||||||
| 0 | 23 (88.5) | 22 (84.6) | 14 (53.8) | 9 (34.6) | 1.0 | 0.006 | 0.003 | 0.26 |
| 1 | 3 (11.5) | 4 (15.4) | 12 (46.2) | 17 (65.4) | ||||
Data are given as number (percentage) unless otherwise indicated. All comparisons between EXE and SAL group were performed by Fisher's exact test.
BAS, baseline; EOS, end of the study; EXE, exenatide; MICA, mononuclear inflammatory cells accumulations; PanIN, pancreatic intraepithelial neoplasia; SAL, saline.
At study end, 18 (69.2%), 6 (23.1%), and 2 (7.7%) of SAL group baboons had pancreatic MICA scores of 0, 1, and 2, respectively. These scores were slightly, but not significantly, increased in comparison to baseline and likely represent a surgery effect (P = 0.25) (Table 2).
Also at study end, 10 (38.5%), 12 (46.2%), and 4 (15.4%) of EXE-treated baboons had pancreatic MICA scores of 0, 1, and 2, respectively. As observed in SAL group, MICA scores after EXE treatment were slightly, but not significantly, increased (P = 0.17) (Table 2). No significant difference was observed in MICA scores at the EOS between SAL- and EXE-treated animals (P = 0.089) (Table 2), and the change (basal-EOS) in MICA score between the two study groups was not statistically different (Z = −0.546, P = 0.585).
At study end, MICA score was increased in nine EXE- and six SAL-treated baboons. There was a decrease of MICA score in two baboons in both groups and no change in 15 EXE- and 18 SAL-treated baboons (P = 0.50) (Figure 1C).
We analyzed the 17 EXE- and 22 SAL-treated baboons without exocrine pancreas MICA at baseline (score, 0). At study end, newly identified MICA was observed in eight EXE- and in six SAL-treated baboons, demonstrating that EXE did not induce a significant increase in new pancreatic MICA compared with SAL (P = 0.31) (Table 3 and Figure 1D).
Table 3.
Presence or Absence of MICA and Hyperplasia at Baseline and after Treatment in EXE and SAL Groups
| Variables | EXE, n = 26 | SAL, n = 26 | P value |
|---|---|---|---|
| No. of cases of newly identified MICA at study end | 8 | 6 | 0.31 |
| No. of samples without MICA before and after treatment | 9 | 16 | 0.31 |
| No. of samples with MICA at baseline | 9 | 4 | 0.49 |
| No. of cases of new identified hyperplasia at study end | 14 | 9 | 0.21 |
| No. of samples without hyperplasia before and after treatment | 8 | 14 | 0.21 |
| No. of samples with hyperplasia at baseline | 4 | 3 | 1.0 |
All comparisons between EXE and SAL group were performed by Fisher's exact test.
EXE, exenatide; MICA, mononuclear inflammatory cell accumulation; SAL, saline.
The relative risk of an increase in MICA score and the onset of MICA in the EXE group was 1.5 (95% CI, 0.6–3.5; P = 0.5) and 1.7 (95% CI, 0.7–4; P = 0.3) compared with SAL group. Collectively, these data suggest that EXE treatment did not significantly increase the risk of developing or aggravating the extent of pre-existent MICA.
The presence of ductal cell hyperplasia and dysplasia in EXE and SAL groups was investigated by histological analysis (Supplemental Figure S3). Notably, no dysplastic lesions, pancreatic intraepithelial neoplasia (PanIN), or lesions resembling pancreatic cancer were observed in any pancreatic specimen examined at baseline or after treatment in either animal group43, 44 (Table 2).
No difference in ductal hyperplasia score was observed at baseline between the EXE and SAL groups (P = 1.0) (Table 2). At study end, the number of samples with ductal hyperplasia was increased in the SAL [12 (46.2%) versus 3 (11.5%); P = 0.006] and EXE [17 (65.4%) versus 4 (15.4%); P = 0.003] groups in comparison to baseline (Table 2), suggesting a significant effect of the surgery on ductal cell proliferation as a physiological attempt of the remnant pancreas to compensate for the partial pancreatic resection. Hyperplasia score at the EOS was not significantly different between SAL and EXE group (P = 0.26) (Table 2), and no significant difference was observed between both groups in the change of hyperplasia score from baseline to the study end (Z = −0.943, P = 0.346).
The hyperplasia score increased after EXE or SAL treatment in 14 and 9 baboons, respectively (P = 0.21), and did not change in 11 EXE- and 17 SAL-treated baboons (Table 3 and Figure 1, E and F). At study end, the hyperplasia score was decreased (from 1 to 0) in one baboon from the EXE group. The relative risk of a hyperplasia score increase was 1.6 (95% CI, 0.86–3.0; P = 0.2) in EXE-treated baboons compared with SAL. Therefore, EXE did not significantly increase the risk of ductal hyperplasia.
We also evaluated sex-specific changes in MICA and hyperplasia score and observed that EXE treatment did not significantly affect MICA or hyperplasia score in males or females (Supplemental Table S1).
In addition, we analyzed pancreatic tail and head/body portions collected from a separate cohort of 16 baboons (8 males and 8 females) that were not treated with SAL or EXE. Notably, we observed different stages of MICA and ductal hyperplasia in some of the pancreatic tail and head/body samples and no significant differences in MICA and hyperplasia scores between the EXE, SAL, and control groups, suggesting that MICA and ductal hyperplasia are physiologically present in baboon exocrine pancreas (Supplemental Table S2).
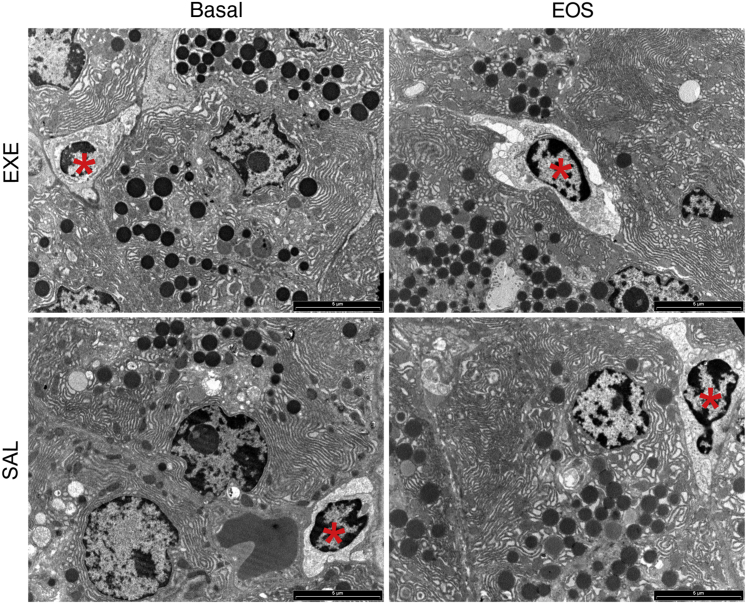
Electron microscopic analysis confirmed the presence of a few lymphocytes among acinar cells at baseline and at the study end in both SAL and EXE groups. Acinar cells were morphologically normal appearing, showing regular nuclei, abundant endoplasmic reticulum, and numerous zymogen granules at both baseline and after either EXE or SAL treatment (Figure 2).
Figure 2.
Exocrine pancreas electron microscopy. Electron microscopic analysis confirms the presence of lymphocytes (asterisks) among exocrine acinar cells in both saline (SAL) and exenatide (EXE) groups, at baseline and at the study end. Acinar cells are morphologically normal appearing with regular nuclei, abundant endoplasmic reticulum, and zymogen granules, before and after EXE treatment as well as before and after SAL treatment.
EXE Does Not Significantly Affect Baboon Pancreas Acinar and Ductal Cell Proliferation
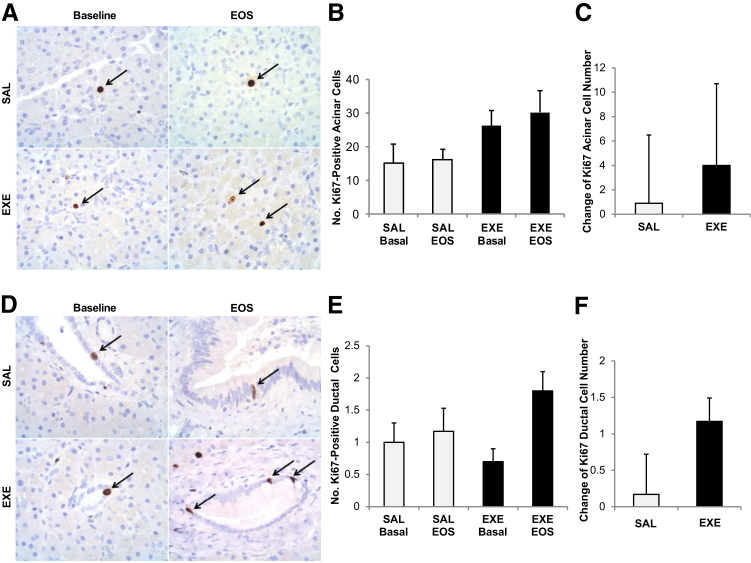
The number of Ki-67–positive acinar cells did not differ between the EXE and SAL group at baseline (P = 0.14) and at the study end (P = 0.07). Interestingly, it also did not change significantly between baseline and EOS in the SAL group (P = 0.87) and in the EXE group as well (P = 0.56) (Figure 3, A and B). Moreover, the change in Ki-67–positive acinar cell number from baseline to study end was not different between SAL and EXE group (P = 0.72) (Figure 3C).
Figure 3.
Exocrine pancreatic cell proliferation evaluation by Ki-67 immunostaining. A: Acinar cells positive for Ki-67 immunostaining (arrows). B: Mean number of Ki-67–positive acinar cells in aline (SAL, white bars) and exenatide (EXE, black bars) groups at baseline and at the end of the study (EOS) (B). C: Change in Ki-67–positive acinar cell number between baseline and EOS in both groups. D: Ductal cells positive for Ki-67 immunostaining (arrows). E: Mean number of Ki-67–positive ductal cells in SAL (white bars) and EXE (black bars) groups at baseline and at EOS. F: Change in the number of Ki-67–positive ductal cells between baseline and EOS in both groups. Number of sections with Ki-67 staining for each baboon = 4 (2 sections at baseline and 2 sections at the EOS). None of the comparisons were statistically significant.
The number of Ki-67–positive ductal cells at baseline and at the study end was similar in the two groups (P = 0.40 and P = 0.17, respectively). No significant difference was observed in the SAL group between study end and baseline (P = 0.77) (Figure 3, D and E). In contrast, the number of Ki-67–positive ductal cells was significantly higher at study end than at baseline in EXE-treated animals (P = 0.04) (Figure 3, D and E). However, the change of Ki-67–positive ductal cell number from baseline to the EOS was not significantly greater in the EXE in comparison to SAL group (P = 0.13) (Figure 3F).
EXE Treatment Does Not Affect Baboon Pancreas Acinar and Ductal Cell Apoptosis
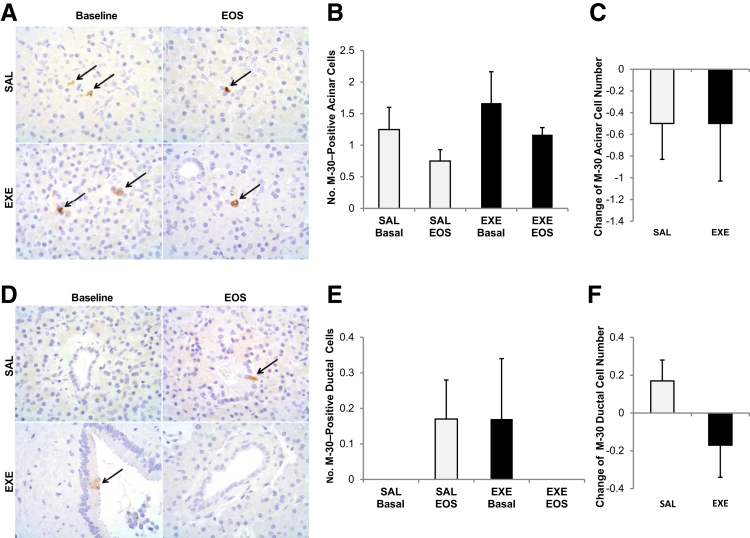
The number of M-30–positive acinar cells was not significantly different between groups at baseline (P = 0.5) and at the study end (P = 0.08) and did not change significantly after SAL (P = 0.17) or EXE (P = 0.37) treatment (Figure 4, A and B).
Figure 4.
Exocrine pancreatic cell apoptosis evaluation by M-30 immunostaining. A: Acinar cells positive for M-30 immunostaining (arrows). B: Mean number of M-30–positive acinar cells in saline (SAL, white bars) and exenatide (EXE, black bars) groups at baseline and at end of the study (EOS) (B). C: Change in M-30–positive acinar cell number. D: Ductal cells positive for M-30 immunostaining (arrows). E: Mean number of M-30–positive ductal cells in SAL (white bars) and EXE (black bars) groups at baseline and at EOS. F: Change in the number of M-30–positive ductal cells. Number of sections with M-30 immunostaining for each baboon = 4 (2 sections at baseline and 2 sections at the EOS). None of the comparisons were statistically significant.
Accordingly, the change in M-30–positive acinar cell number from baseline did not differ significantly between SAL- and EXE-treated baboons; P = 1.0) (Figure 4C).
The number of M-30–positive ductal cells was also not significantly different between SAL and EXE group at baseline (P = 0.33) and at the study end (P = 0.1), and did not change significantly after SAL (P = 0.17) or EXE (P = 0.34) treatment (Figure 4, D and E). In addition, the change in M-30–positive ductal cell number at study end did not differ significantly between groups (P = 0.11) (Figure 4F).
Quantitative Duct Analysis by CAST
When comparing the quantitative effects of SAL and EXE on duct morphometry, there were no statistical differences between groups before and after treatment as well as between the delta-changes between groups in duct numerical density (P = 0.71 and P = 0.83, respectively) (Supplemental Figure S4, A and B), relative duct volume/% pancreas (P = 0.53 and P = 0.61, respectively) (Supplemental Figure S4, C and D), duct cell size (P = 0.32 and P = 0.98, respectively) (Supplemental Figure S4, E and F), duct cell numerical density (P = 0.47 and P = 0.51, respectively) (Supplemental Figure S4, G and H), and total duct mass (P = 0.78 and P = 0.82, respectively) (Supplemental Figure S4, I and J). These data are consistent and reinforce the semiquantitative/qualitative data on ductal cell hyperplasia.
EXE Treatment Promotes the Differentiation of Ductal Cells Toward a β-Cell Phenotype
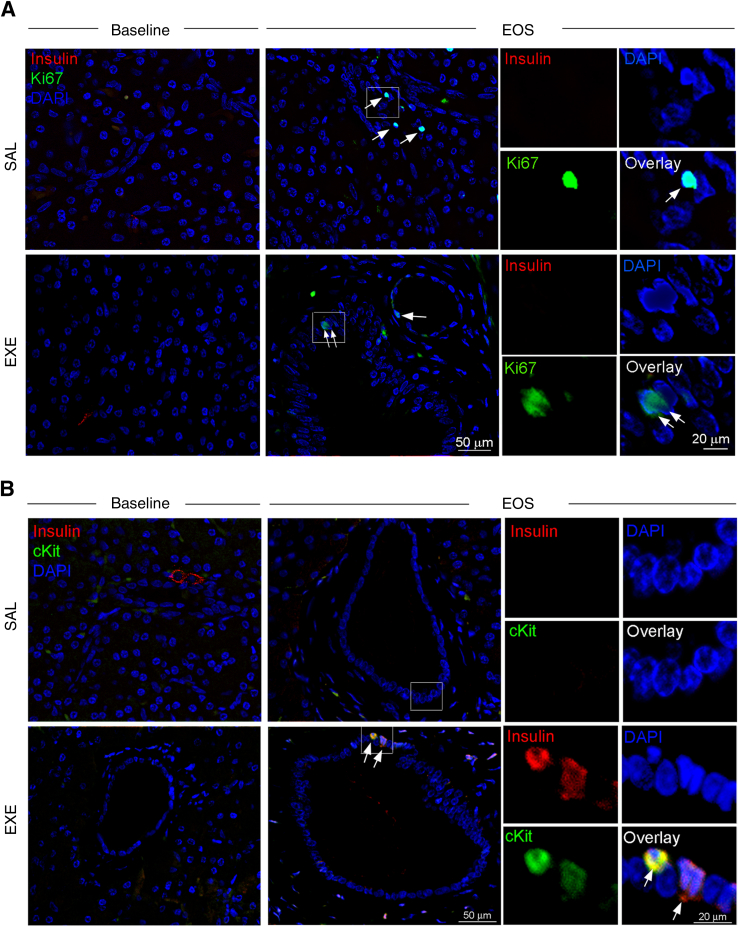
It has been reported that EXE promotes the proliferation and differentiation of β-cells from ductal progenitor cells in rodents.1, 3, 45 We, therefore, used triple-immunofluorescence staining to evaluate the presence of insulin-positive cells in association with markers of cell proliferation (Ki-67) or cell transdifferentiation (c-kit) in pancreatic ducts. Proliferating Ki-67–positive nuclei in duct structures were rare in both the EXE and the SAL groups, and costained occasionally with insulin (Figure 5A and Supplemental Figure S5A). Interestingly, only in the EXE group at study end, we detected some c-kit–positive cells co-expressing insulin in large duct structures (Figure 5B and Supplemental Figure S5B), thus suggesting that EXE may promote transdifferentiation of ductal cells to insulin-producing cells.
Figure 5.
Detection of insulin-expressing cells in pancreatic ducts by immunofluorescence staining. A: Representative images of pancreas sections triple stained with insulin (red), Ki-67 (green), and DAPI (blue), at baseline and at study end, in saline (SAL) and exenatide (EXE) groups. After pancreatectomy (EOS), Ki-67–positive nuclei (arrows) were found in duct structures of either the SAL or EXE group. B: Representative images of pancreas sections triple stained with insulin (red), c-kit (a marker of differentiation) (green), and DAPI (blue) at baseline and at study end in SAL and EXE groups. Rare cells double labeled with c-kit and insulin (yellow staining) were identified in duct structures only at study end in the EXE group (arrows). Examples of c-kit–positive ductal cells co-expressing insulin are also shown at higher magnification. The boxed areas in white in the SAL- and EXE-treated samples are magnified in the single channel and overlay photos on the right. Original magnification, ×2.5.
Discussion
The clinical literature on the association between pancreatitis, pancreatic cancer, DPP-IV inhibitors, and GLP-1 receptor agonists is controversial and does not allow firm conclusions.8, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Animal studies have also provided contradictory data in relation to inflammation, proliferation, and metaplasia in exocrine pancreas. More important, in none of these studies, there was a direct comparison between pretreatment and post-treatment pancreas in the same animals.26, 27, 28, 29, 30, 31 The Food and Drug Administration and the European Medicines Evaluation Agency have recently re-analyzed all studies on GLP-1 receptor agonist and DPP-IV inhibitors and concluded that there is no significantly increased risk of either pancreatitis or dysplastic lesion/pancreatic cancer in rodents, nonhuman primates, and humans, but suggested continuous monitoring of these drugs.46
Herein, we examined the effects of EXE treatment on exocrine pancreas of nonhuman primates, performing a direct comparison between samples taken before and at the end of treatment.
Interestingly, 14-week EXE continuous infusion was not associated with an increase in amylase levels or pancreatic mass, which are indirect indicators of pancreas inflammation/pancreatitis.
Although serum EXE levels in EXE-treated baboons were fourfold higher than those achieved in diabetic-treated patients,4 there was no evidence of acute or chronic pancreatitis (necrosis, hemorrhage, suppurative infiltrates, or fibrosis). Focal parenchymal or periductal MICA, which has been described previously in nonhuman primates,37, 40 was observed in SAL and EXE groups and in control baboons, and was not significantly increased by EXE treatment.
We did not observe any evidence of PanIN, either before or after treatment with either SAL or EXE.43, 44 The ductal hyperplasia score was increased by fourfold at the study end compared with baseline in both groups; consistent with a surgical effect, no significant differences between SAL and EXE group were observed. Moreover, ductal hyperplasia was observed also in pancreas of control baboons with a frequency not significantly different from SAL and EXE group. Although a trend toward an increase in hyperplasia and MICA score was observed in EXE-treated animals, the difference between SAL and EXE groups was not significant. Moreover, the presence of mononuclear inflammatory cells in pancreatic parenchyma was not associated with an impaired structural integrity of exocrine pancreas cells, as assessed by electron microscopy. In addition, no histological lesions suggestive of pancreatitis or ductal dysplasia/PanIN were observed, and we only detected focal inflammatory cell accumulation (T cells) and simple hyperplasia in the absence of any architectural and cytological atypia, which represent subtle histological changes likewise detectable in control group (ie, in physiological conditions).
The number of M-30–positive acinar and ductal cells observed after EXE treatment by immunohistochemistry was not significantly different from baseline, demonstrating that EXE does not affect exocrine pancreatic cell apoptosis. The number of Ki-67–positive acinar cells did not change significantly after treatment in the EXE group. Ki-67–positive ductal cell number after EXE treatment was slightly increased compared with baseline; however, the change of Ki-67–positive ductal cell number at study end from baseline was not significantly different between EXE and SAL group. To analyze in greater depth the effect of EXE on pancreatic ducts, duct morphometric analyses were also performed by CAST.33, 34, 41, 42 No significant changes in ducts' numerical density, relative duct volume/% pancreas, ductal cell size, ductal cell numerical density, or total duct mass were observed at the EOS in comparison to baseline in EXE- and SAL-treated baboons.
In consideration of previous data reporting the effect of GLP-1 in promoting the proliferation and transdifferentiation of β-cells from ducts, we evaluated whether EXE treatment was associated with a differentiation of ductal cells toward a β-cell phenotype.1, 3, 45, 47, 48 Interestingly, only in EXE group at study end, some c-kit (a marker of transdifferentiation) positive cells co-expressing insulin were detected in large duct structures, suggesting that EXE may promote the differentiation of ductal progenitor cells to insulin-producing cells. However, the evaluation of EXE effects on β-cell mass and secretion was not the goal of this study, and additional studies are required to confirm and evaluate in-depth GLP-1 receptor agonist actions on β-cell neogenesis from ductal progenitor cells in nonhuman primates and in diabetic patients.
Baboons represent an interesting nonhuman primate model to study human diseases because of their genetic and pathophysiological similarity to humans.32, 33, 34, 35, 36, 37, 38, 40, 42 Because the incidence of pancreatitis in baboons is similar to humans, these findings may have an impact on clinical practice.9, 37 The strengths of the present study are its relatively large sample size considering that it was conducted in large nonhuman primates, a direct comparison between pancreatic samples taken before and at the end of the treatment in the same animals, a semiquantitative histological evaluation of pancreatic inflammation and hyperplasia performed by two veterinary pathologists who were blinded to treatment groups, a quantitative analysis of exocrine pancreatic cell turnover, and a quantitative analysis of pancreatic ductal mass by CAST. Although some toxicologists prefer to avoid the blinded examination,49 we elected to evaluate pancreatic slides in blind to avoid biases in underestimation or overestimation of any negative effect of EXE treatment.
This study has some limitations, including a medium duration dosing time (3.5 months) and the lack of a recovery group, which may limit us to detecting small effects of EXE on exocrine pancreas. However, the high serum EXE levels in treated animals could be sufficient to detect some potential negative effects of the drug. The comparison of different portions of pancreas may represent a limitation of the study; however, although islet of Langerhans distribution is different between tail and body/head, no differences in exocrine tissue histological features exist between tail and body/head.
In conclusion, we did not observe a significant association between EXE treatment and pancreatic inflammation and/or ductal hyperplasia/dysplasia in nonhuman primates. Our data also provide preliminary evidence suggesting that EXE may promote the differentiation of pancreatic ductal cell toward a β-cell phenotype in baboons and possibly in humans affected by type 2 diabetes mellitus. However, further studies of longer duration are needed to confirm the long-term safety of EXE treatment and the capability of GLP-1 receptor agonists to promote the transdifferentiation from ductal cells to β-cells, in nonhuman primates.
Acknowledgments
We thank Renee Escalona, Tony Perez, and Jesse Martinez for their anatomical pathology support; Drs. Patricia Frost and Cassondra A. Bauer for clinical support; Vicky Mattern for clinical chemistry; and Prof. James H. Ware (Harvard School of Public Health, Boston, MA) for critically reviewing the statistical analyses and the manuscript.
T.V.F. analyzed the data and wrote and edited the manuscript; M.O. and E.J.D. performed semiquantitative histological analysis; G.A. and G.H. performed pancreatic surgeries; S.L.R., A.M., G.F., C.C., and F.S. performed quantitative immunocytochemistry; C.P. and E.S.D.C. performed immunofluorescence staining and image analyses; F.C. and A.P. collected the data; R.G.M. contributed to data analysis; A.A., A.D., P.F., A.G.C., and M.S. contributed to discussion; R.A.D. revised the manuscript; and F.F. designed the study, analyzed and reviewed all data, and wrote, edited, and revised the manuscript. F.F. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported in part by NIH grant RO1 DK080148 (F.F.), Amylin/Lilly (R.A.D. and F.F.), and the National Center for Research Resources, NIH, Southwest National Primate Research Center grant P51 RR013986, which are currently supported by the Office of Research Infrastructure Programs grant P51 OD011133. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs, NIH, grants C06 RR015456 and C06 RR014578. T.V.F. was supported in part by a fellowship from Fondazione per la Ricerca Diabetologica (Fo.Ri.SID), Italy.
T.V.F. and M.O. contributed equally to this work.
Disclosures: None declared.
Portions of this work were presented at the Gordon Research Conference at Mount Holyoke College in South Hadley, MA, July 21-26, 2013.
Current address of F.F., Faculdade de Ciências Médicas, Obesity and Comorbidities Research Center, Universidade Estadual de Campinas, São Paulo, Brazil.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2014.09.009.
Supplemental Data
Histological analysis of pancreatic mononuclear inflammatory cell accumulation (MICA). Pancreatic MICA score on H&E staining: 0 (absence of MICA) in saline (SAL; A) and exenatide group (EXE; B) groups; 1 (minimal MICA) in SAL (C) and EXE (D) groups; 2 (mild MICA) in SAL (E) and EXE (F) groups.
Exocrine pancreas CD3 immunostaining. CD3 immunostaining in exocrine pancreas with MICA score 1 (A) and 2 (B) demonstrates the presence of T lymphocytes in the exocrine pancreas.
Histological analysis of exocrine pancreas hyperplasia. H&E staining ductal hyperplasia score 0 (absence of hyperplasia) in saline (SAL; A) and exenatide group (EXE; B) groups, ductal hyperplasia score 1 (presence of hyperplasia) in SAL (C) and EXE (D) groups.
Morphometric analysis by cast of the pancreatic ducts in the study groups before and after treatment. Duct numerical density (A), delta of duct numerical density (B), relative duct volume/percentage pancreas (C), delta of relative duct volume/percentage pancreas (D), duct cell size (E), delta of duct cell size (F), duct cell numerical density (G), delta of duct cell numerical density (H), duct mass (I), delta of duct mass (J) in saline (SAL, white bars) and exenatide (EXE, black bars) group at baseline and at end of the study (EOS). Number of sections with H&E staining for each baboon = 4 (2 sections at baseline and 2 sections at the EOS). None of the comparisons were statistically significant.
Detection of insulin-expressing cells, in duct structures by immunofluorescence staining. A: Representative images of pancreas sections triple stained with insulin (red), Ki-67 (green), and DAPI (blue), at baseline and at study end, in saline (SAL) and exenatide (EXE) groups. At the end of study, Ki-67–positive nuclei (arrows) were found in large duct structures of either the SAL or EXE groups and occasionally costaining with insulin was detected. B: Representative images of pancreas sections triple stained with insulin (red), c-kit (a marker of transdifferentiation) (green), and DAPI (blue), at baseline and at study end, in SAL and EXE groups. Rare cells double labeled with c-kit and insulin (yellow staining) were identified in duct structures only at study end, in the EXE group (arrows). Examples of c-kit–positive ductal cells co-expressing insulin are also shown at higher magnification. The boxed areas in white in the SAL- and EXE-treated samples are magnified in the single channel and overlay photos on the right. Original magnification, ×2.5.
References
- 1.Xu G., Stoffers D.A., Habener J.F., Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 2.Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U., Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 3.Hui H., Wright C., Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785–796. doi: 10.2337/diabetes.50.4.785. [DOI] [PubMed] [Google Scholar]
- 4.Fineman M.S., Bicsak T.A., Shen L.Z., Taylor K., Gaines E., Varns A., Kim D., Baron A.D. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 5.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein B.J., Feinglos M.N., Lunceford J.K., Johnson J., Williams-Herman D.E., Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 7.Meier J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 8.Meier J.J., Nauck M.A. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia. 2014;57:1320–1324. doi: 10.1007/s00125-014-3231-y. [DOI] [PubMed] [Google Scholar]
- 9.Forsmark C.E., Billie J. AGA institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 10.Girman C.J., Kou T.D., Cai B., Alexander C.M., O'Neill E.A., Williams-Herman D.E., Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12:766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 11.Ben Q., Xu M., Ning X., Liu J., Hong S., Huang W., Zhang H., Li Z. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Nauck M.A. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126–2132. doi: 10.2337/dc12-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monami M., Dicembrini I., Nardini C., Fiordelli I., Mannucci E. Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabetes Res Clin Pract. 2014;103:269–275. doi: 10.1016/j.diabres.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Monami M., Dicembrini I., Mannucci E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:48–56. doi: 10.1111/dom.12176. [DOI] [PubMed] [Google Scholar]
- 15.Wenten M., Gaebler J.A., Hussein M., Pelletier E.M., Smith D.B., Girase P., Noel R.A., Braun D.K., Bloomgren G.L. Relative risk of acute pancreatitis in initiators of exenatide twice daily compared with other anti-diabetic medication: a follow-up study. Diabet Med. 2012;29:1412–1418. doi: 10.1111/j.1464-5491.2012.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg R., Chen W., Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349–2354. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drucker D.J., Sherman S.I., Bergenstal R.M., Buse J.B. The safety of incretin-based therapies: review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 18.Elashoff M., Matveyenko A.V., Gier B., Elashoff R., Butler P.C. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macconell L., Brown C., Gurney K., Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes. 2012;5:29–41. doi: 10.2147/DMSO.S28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White W.B., Cannon C.P., Heller S.R., Nissen S.E., Bergenstal R.M., Bakris G.L., Perez A.T., Fleck P.R., Mehta C.R., Kupfer S., Wilson C., Cushman W.C., Zannad F., EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 21.Scirica B.M., Bhatt D.L., Braunwald E., Steg P.G., Davidson J., Hirshberg B., Ohman P., Frederich R., Wiviott S.D., Hoffman E.B., Cavender M.A., Udell J.A., Desai N.R., Mosenzon O., McGuire D.K., Ray K.K., Leiter L.A., Raz I., SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 22.Drucker D.J., Sherman S.I., Gorelick F.S., Bergenstal R.M., Sherwin R.S., Buse J.B. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428–433. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler A.E., Campbell-Thompson M., Gurlo T., Dawson D.W., Atkinson M., Butler P.C. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harja E., Lord J., Skyler J.S. An analysis of characteristics of subjects examined for incretin effects on pancreatic pathology. Diabetes Technol Ther. 2013;15:609–618. doi: 10.1089/dia.2013.0177. [DOI] [PubMed] [Google Scholar]
- 25.Bonner-Weir S., In't Veld P.A., Weir G.C. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab. 2014;16:661–666. doi: 10.1111/dom.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachnani J.S., Bulchandani D.G., Nookala A., Herndon B., Molteni A., Pandya P., Taylor R., Quinn T., Weide L., Alba L.M. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2010;53:153–159. doi: 10.1007/s00125-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 27.Matveyenko A.V., Dry S., Cox H.I., Moshtaghian A., Gurlo T., Galasso R., Butler A.E., Butler P.C. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes. 2009;58:1604–1615. doi: 10.2337/db09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gier B., Matveyenko A.V., Kirakossian D., Dawson D., Dry S.M., Butler P.C. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras (G12D) mouse model. Diabetes. 2012;61:1250–1262. doi: 10.2337/db11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatarkiewicz K., Smith P.A., Sablan E.J., Polizzi C.J., Aumann D.E., Villescaz C., Hargrove D.M., Gedulin B.R., Lu M.G., Adams L., Whisenant T., Roy D., Parkes D.G. Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am J Physiol Endocrinol Metab. 2010;299:E1076–E1086. doi: 10.1152/ajpendo.00479.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyborg N.C., Mølck A.M., Madsen L.W., Knudsen L.B. The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes. 2012;61:1243–1249. doi: 10.2337/db11-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrang N., Jelsing J., Simonsen L., Jensen A.E., Thorup I., Søeborg H., Knudsen L.B. The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab. 2012;303:E253–E264. doi: 10.1152/ajpendo.00182.2012. [DOI] [PubMed] [Google Scholar]
- 32.Chavez A.O., Lopez-Alvarenga J.C., Tejero M.E., Triplitt C., Bastarrachea R.A., Sriwijitkamol A., Tantiwong P., Voruganti V.S., Musi N., Comuzzie A.G., DeFronzo R.A., Folli F. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 33.Guardado-Mendoza R., Davalli A.M., Chavez A.O., Hubbard G.B., Dick E.J., Majluf-Cruz A., Tene-Perez C.E., Goldschmidt L., Hart J., Perego C., Comuzzie A.G., Tejero M.E., Finzi G., Placidi C., La Rosa S., Capella C., Halff G., Gastaldelli A., DeFronzo R.A., Folli F. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guardado-Mendoza R., Jimenez-Ceja L., Majluf-Cruz A., Kamath S., Fiorentino T.V., Casiraghi F., Velazquez A.O., DeFronzo R.A., Dick E., Davalli A., Folli F. Impact of obesity severity and duration on pancreatic α- and β-cells dynamics in normoglycemic non-human primates. Int J Obes (Lond) 2013;37:1071–1078. doi: 10.1038/ijo.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamath S., Chavez A.O., Gastaldelli A., Casiraghi F., Halff G.A., Abrahamian G.A., Davalli A.M., Bastarrachea R.A., Comuzzie A.G., Guardado-Mendoza R., Jimenez-Ceja L.M., Mattern V., Paez A.M., Ricotti A., Tejero M.E., Higgins P.B., Rodriguez-Sanchez I.P., Tripathy D., DeFronzo R.A., Dick E.J., Jr., Cline G.W., Folli F. Coordinated defects in hepatic long chain fatty acid metabolism and triglyceride accumulation contribute to insulin resistance in non-human primates. PLoS One. 2011;6:e27617. doi: 10.1371/journal.pone.0027617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casiraghi F., Lertwattanarak R., Luzi L., Chavez A.O., Davalli A.M., Naegelin T., Comuzzie A.G., Frost P., Musi N., Folli F. Energy expenditure evaluation in humans and non-human primates by SenseWear Armband: validation of energy expenditure evaluation by SenseWear Armband by direct comparison with indirect calorimetry. PLoS One. 2013;8:e73651. doi: 10.1371/journal.pone.0073651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bommineni Y.R., Dick E.J., Jr., Malapati A.R., Owston M.A., Hubbard G.B. Natural pathology of the Baboon (Papio spp.) J Med Primatol. 2011;40:142–155. doi: 10.1111/j.1600-0684.2010.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guardado-Mendoza R., Dick E.J., Jr., Jimenez-Ceja L.M., Davalli A., Chavez A.O., Folli F., Hubbard G.B. Spontaneous pathology of the baboon endocrine system. J Med Primatol. 2009;38:383–389. doi: 10.1111/j.1600-0684.2009.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coelho A.M., Jr., Carey K.D. A social tethering system for nonhuman primates used in laboratory research. Lab Anim Sci. 1990;40:388–394. [PubMed] [Google Scholar]
- 40.McClure H.M., Chandler F.W. A survey of pancreatic lesions in nonhuman primates. Vet Pathol Suppl. 1982;7:193–209. [PubMed] [Google Scholar]
- 41.Mandarim-de-Lacerda C.A. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75:469–486. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- 42.Quinn A.R., Blanco C.L., Perego C., Finzi G., La Rosa S., Capella C., Guardado-Mendoza R., Casiraghi F., Gastaldelli A., Johnson M., Dick E.J., Jr., Folli F. The ontogeny of the endocrine pancreas in the fetal/newborn baboon. J Endocrinol. 2012;214:289–299. doi: 10.1530/JOE-12-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hruban R.H., Adsay N.V., Albores-Saavedra J., Compton C., Garrett E.S., Goodman S.N., Kern S.E., Klimstra D.S., Klöppel G., Longnecker D.S., Lüttges J., Offerhaus G.J. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Hruban R.H., Takaori K., Klimstra D.S., Adsay N.V., Albores-Saavedra J., Biankin A.V., Biankin S.A., Compton C., Fukushima N., Furukawa T., Goggins M., Kato Y., Klöppel G., Longnecker D.S., Lüttges J., Maitra A., Offerhaus G.J., Shimizu M., Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 45.Bonner-Weir S., Inada A., Yatoh S., Li W.C., Aye T., Toschi E., Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 46.Egan A.G., Blind E., Dunder K., de Graeff P.A., Hummer B.T., Bourcier T., Rosebraugh C. Pancreatic safety of incretin-based drugs: FDA and EMA assessment. N Engl J Med. 2014;370:794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- 47.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 48.Mezza T., Muscogiuri G., Sorice G.P., Clemente G., Hu J., Pontecorvi A., Holst J.J., Giaccari A., Kulkarni R.N. Insulin resistance alters islet morphology in non-diabetic humans. Diabetes. 2014;63:994–1007. doi: 10.2337/db13-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holland T., Holland C. Unbiased histological examinations in toxicological experiments (or, the informed leading the blinded examination) Toxicol Pathol. 2011;39:711–714. doi: 10.1177/0192623311406288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological analysis of pancreatic mononuclear inflammatory cell accumulation (MICA). Pancreatic MICA score on H&E staining: 0 (absence of MICA) in saline (SAL; A) and exenatide group (EXE; B) groups; 1 (minimal MICA) in SAL (C) and EXE (D) groups; 2 (mild MICA) in SAL (E) and EXE (F) groups.
Exocrine pancreas CD3 immunostaining. CD3 immunostaining in exocrine pancreas with MICA score 1 (A) and 2 (B) demonstrates the presence of T lymphocytes in the exocrine pancreas.
Histological analysis of exocrine pancreas hyperplasia. H&E staining ductal hyperplasia score 0 (absence of hyperplasia) in saline (SAL; A) and exenatide group (EXE; B) groups, ductal hyperplasia score 1 (presence of hyperplasia) in SAL (C) and EXE (D) groups.
Morphometric analysis by cast of the pancreatic ducts in the study groups before and after treatment. Duct numerical density (A), delta of duct numerical density (B), relative duct volume/percentage pancreas (C), delta of relative duct volume/percentage pancreas (D), duct cell size (E), delta of duct cell size (F), duct cell numerical density (G), delta of duct cell numerical density (H), duct mass (I), delta of duct mass (J) in saline (SAL, white bars) and exenatide (EXE, black bars) group at baseline and at end of the study (EOS). Number of sections with H&E staining for each baboon = 4 (2 sections at baseline and 2 sections at the EOS). None of the comparisons were statistically significant.
Detection of insulin-expressing cells, in duct structures by immunofluorescence staining. A: Representative images of pancreas sections triple stained with insulin (red), Ki-67 (green), and DAPI (blue), at baseline and at study end, in saline (SAL) and exenatide (EXE) groups. At the end of study, Ki-67–positive nuclei (arrows) were found in large duct structures of either the SAL or EXE groups and occasionally costaining with insulin was detected. B: Representative images of pancreas sections triple stained with insulin (red), c-kit (a marker of transdifferentiation) (green), and DAPI (blue), at baseline and at study end, in SAL and EXE groups. Rare cells double labeled with c-kit and insulin (yellow staining) were identified in duct structures only at study end, in the EXE group (arrows). Examples of c-kit–positive ductal cells co-expressing insulin are also shown at higher magnification. The boxed areas in white in the SAL- and EXE-treated samples are magnified in the single channel and overlay photos on the right. Original magnification, ×2.5.