Abstract
Using a group-specific PCR assay, we investigated the presence of enterohepatic Helicobacter species in gut specimens from patients with inflammatory bowel disease. Enterohepatic Helicobacter species were detected in 12% (3 of 25) of the patients with Crohn's disease, in 17% (3 of 18) of the ulcerative colitis samples, and in 4% (1 of 23) of the controls.
Members of the family Helicobacteraceae are able to colonize various ecological niches in the gastrointestinal tract (19). With respect to their preferential site of colonization, Helicobacter species are divided into two subgroups. The better-known gastric Helicobacter species, which preferably colonize the host's stomach, represent only one-third of the known species of Helicobacteraceae. The remaining two-thirds of Helicobacter species are referred to as enterohepatic because they predominantly colonize the intestine and the hepatobiliary system (19). Recently, enterohepatic Helicobacter species have been discovered in inflammatory bowel disease (IBD) of rodents (9, 14, 16), carnivores (7, 8), and primates (10, 18). Infection experiments, moreover, demonstrated that enterohepatic Helicobacter species can trigger IBD in susceptible animals (4, 5, 13, 15). In order to investigate if enterohepatic Helicobacter species are also present in human IBD, we performed this prospective evaluation.
A series of 115 consecutive patients who underwent colonoscopy were screened for eligibility to participate in this study. Within this series, all patients with clinically established diagnosis of Crohn's disease (n = 25) or ulcerative colitis (n = 18) as well as a control group of patients defined by the absence of macroscopic or microscopic abnormalities (n = 23) were selected. The 49 remaining patients had other pathologies such as neoplasia, diverticulosis, or human immunodeficiency virus-associated enteritis and were excluded from further analysis. Of the three patient groups examined, the patients with Crohn's disease were significantly younger (average age, 41.0 ± 11.7 years) than the ulcerative colitis patients (average age, 51.7 ± 10.3 years). The patients without morphological changes were significantly older (average age, 65.1 ± 9.1 years) than both IBD groups.
Biopsy specimens from the terminal ileum and the colon of each patient were analyzed by a group-specific PCR assay with primers C97 (5′-GCTATGACGGGTATCC-3′) and H2 (5′-TCGCAATGAGTATTCCTCTT-3′) as previously described (2, 3). In order to identify the Helicobacter species, amplification products were sequenced as described before (3).
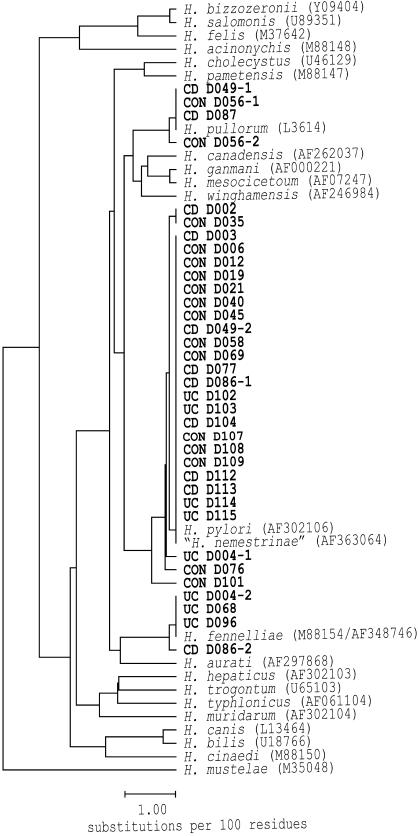
Nine of 25 (36%) of the Crohn's disease patients and 7 of 18 (39%) of the ulcerative colitis samples were positive for Helicobacteraceae, compared to 15 of 23 (65%) in the control group. DNA sequencing of the complete amplification product in these 31 positive samples revealed single Helicobacter species in 28 patients; two patients in the Crohn's disease group and one patient in the ulcerative colitis group were coinfected with two different Helicobacter species. All individual 16S rRNA gene sequences could be assigned to a known Helicobacter species, as documented in Fig. 1. H. pylori was identified in 27 patients, H. fennelliae in four patients, and H. pullorum in three patients. In two patients in the Crohn's disease group, H. pullorum was detected. An additional Crohn's disease patient in whom Yersinia enterocolitica was also detected in stool cultures was positive for H. fennelliae. In three patients with ulcerative colitis, H. fennelliae was identified. One patient in the control group was H. pullorum positive.
FIG. 1.
Phylogenetic tree based on the evolutionary distance of the detected 16S rRNA sequences and Helicobacter reference strains. The scale bar is equal to 1 substitution per 100 residues, as determined by measuring the lengths of the horizontal lines connecting two species. Subjects are represented by bold letters that represent the diagnosis of Crohn's disease (CD), ulcerative colitis (UC), or control status (CON) and the patient code. A number after a dash indicates that several sequences were obtained from a single patient. The code in brackets after the names of the reference strains indicates the GenBank accession number of the corresponding 16S rRNA sequence.
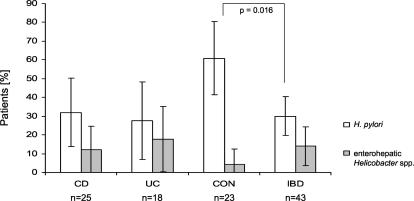
Altogether, enterohepatic Helicobacter species were detected in 12% (3 of 25) of Crohn's disease cases, in 17% (3 of 18) of ulcerative colitis cases, and in 4% (1 of 23) of the controls. In 32% (8 of 25) of the patients with Crohn's disease, in 28% (5 of 18) of the ulcerative colitis patients, and in 61% (14 of 23) of the controls, H. pylori DNA was detected in the gut mucosa. Figure 2 shows the proportion of patients with enterohepatic Helicobacter species and H. pylori in the individual patient groups. H. pylori was significantly more frequent in controls than in patients with IBD (P = 0.02). In contrast, enterohepatic Helicobacter species were more frequent in IBD, but this difference was not statistically significant.
FIG. 2.
Prevalence of H. pylori and enterohepatic Helicobacter species in Crohn's disease (CD) patients, ulcerative colitis (UC) patients, controls (CON), and both types of IBD patients combined (IBD). The error bars indicate the 95% confidence interval.
The prevalence of enterohepatic Helicobacter species of 12 and 17% in Crohn's disease and ulcerative colitis patients, respectively, indicates that a significant portion of patients with IBD carry these potentially harmful bacteria. Interestingly, in patients with ulcerative colitis, only H. fennelliae was identified. Recently, H. fennelliae was also detected in homosexual men with proctitis and proctocolitis (21). These patients typically present symptoms that are similar to those in ulcerative colitis: rectal pain, tenesmus, and diarrhea. As in ulcerative colitis, the symptoms are limited to the large bowel. In patients with Crohn's disease, H. pullorum was the dominant enteric Helicobacter species. Recently, H. pullorum was isolated from patients with gastroenteritis, in whom, as in Crohn's disease, the complete gastrointestinal tract is affected (20).
Another interesting aspect is the production of cytolethal distensing toxin by H. pullorum but not H. fennelliae. Cytolethal distensing toxin is an important pathogenicity factor that is also produced by Campylobacter species, pathogenic Escherichia coli strains, and Shigella spp. It causes cellular distension, cytoskeletal abnormalities, G2/M cell cycle arrest, and cytolethality (23, 24). The expression of cytolethal distensing toxin by enterohepatic Helicobacter species could potentially affect the development of IBD. One could speculate that the production of cytolethal distensing toxin by H. pullorum in patients with Crohn's disease may play a role in the involvement of deeper layers of the bowel which is typical of Crohn's disease. In consequence, it seems logical that H. fennelliae, which lacks this toxin, is linked to ulcerative colitis, in which inflammation is usually limited to the mucosa.
During the review of our manuscript, another study that investigated the presence of Helicobacter species in human IBD in the United Kingdom was published (1). In that study, neither H. pylori nor other Helicobacter species could be detected in 30 patients with IBD. In an area where the prevalence of H. pylori is approximately 40 to 50%, one would have expected several H. pylori-positive patients. Therefore, the inability to detect H. pylori in that study is surprising and suggests a methodological problem or another bias. Our finding that the frequency of H. pylori is significantly lower in patients with IBD compared to controls is consistent with other studies that reported similar results with different methods (6, 11, 12, 17, 22). Since it has been reported that the low prevalence of H. pylori in patients with IBD is not due to therapeutic effects, infection with H. pylori might directly reduce the relative risk of IBD (17, 22). Possible mechanisms could be immunomodulatory effects, direct interactions with the intestinal mucosa, or mechanisms that prevent the colonization of the host by enterohepatic Helicobacter species.
We emphasize that this study was designed as a pilot study and was not intended to prove a causative pathogenic role of enterohepatic Helicobacter species in human IBD. Based on our results, we conclude that these bacteria exist in at least a subgroup of patients with IBD. This is an important novel finding because intestinal bacteria are known to play an important role in human IBD and enterohepatic Helicobacter species are able to induce IBD in susceptible animals. It will now be important to investigate whether enteric Helicobacter species play a causative role in human IBD.
Acknowledgments
We thank Marion Holley and Ursula Stolz for technical assistance and all colleagues of the endoscopy unit for their cooperation.
Ulrich Bohr was supported by an NBL-3 grant from the Bundesministerium für Bildung und Forschung.
REFERENCES
- 1.Bell, S. J., S. A. Chisholm, R. J. Owen, S. P. Borriello, and M. A. Kamm. 2003. Evaluation of Helicobacter species in inflammatory bowel disease. Aliment. Pharmacol. Ther. 18:481-486. [DOI] [PubMed] [Google Scholar]
- 2.Bohr, U. R. M., A. Primus, A. Zagoura, B. Glasbrenner, T. Wex, and P. Malfertheiner. 2002. A group-specific PCR assay for the detection of Helicobacteraceae in human gut. Helicobacter 7:378-383. [DOI] [PubMed] [Google Scholar]
- 3.Bohr, U. R. M., I. Segal, A. Primus, T. Wex, H. Hassan, R. Ally, and P. Malfertheiner. 2003. Detection of a putative novel Wolinella species in patients with squamous cell carcinoma of the esophagus. Helicobacter 8:608-612. [DOI] [PubMed] [Google Scholar]
- 4.Burich, A., R. Hershberg, K. Waggie, W. Zeng, T. Brabb, G. Westrich, J. L. Viney, and L. Maggio-Price. 2001. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281:764-778. [DOI] [PubMed] [Google Scholar]
- 5.Chin, E. Y., C. A. Dangler, J. G. Fox, and D. B. Schauer. 2000. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alphabeta mutant mice. Comp. Med. 50:586-594. [PubMed] [Google Scholar]
- 6.El-Omar, E., I. Penman, G. Cruikshank, S. Dover, S. Banerjee, C. Williams, and K. E. McColl. 1994. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut 35:1385-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley, J. E., S. L. Marks, L. Munson, A. Melli, F. E. Dewhirst, S. Yu, Z. Shen, and J. G. Fox. 1999. Isolation of Helicobacter canis from a colony of Bengal cats with endemic diarrhea. J. Clin. Microbiol. 37:3271-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley, J. E., J. V. Solnick, J. M. Lapointe, S. Jang, and N. C. Pedersen. 1998. Identification of a novel enteric Helicobacter species in a kitten with severe diarrhea. J. Clin. Microbiol. 36:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foltz, C. J., J. G. Fox, R. Cahill, J. C. Murphy, L. Yan, B. Shames, and D. B. Schauer. 1998. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter 3:69-78. [DOI] [PubMed] [Google Scholar]
- 10.Fox, J. G., L. Handt, S. Xu, Z. Shen, F. E. Dewhirst, B. J. Paster, C. A. Dangler, K. Lodge, S. Motzel, and H. Klein. 2001. Novel Helicobacter species isolated from rhesus monkeys with chronic idiopathic colitis. J. Med. Microbiol. 50:421-429. [DOI] [PubMed] [Google Scholar]
- 11.Guslandi, M., L. Fanti, and P. A. Testoni. 2002. Helicobacter pylori seroprevalence in Crohn's disease: lack of influence by pharmacological treatment. Hepatogastroenterology 49:1296-1297. [PubMed] [Google Scholar]
- 12.Halme, L., H. Rautelin, M. Leidenius, and T. U. Kosunen. 1996. Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J. Clin. Pathol. 49:65-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, H. Q., N. Kushnir, M. C. Thurnheer, N. A. Bos, and J. J. Cebra. 2002. Monoassociation of SCID mice with Helicobacter muridarum, but not four other enterics, provokes IBD upon receipt of T cells. Gastroenterology 122:1346-1354. [DOI] [PubMed] [Google Scholar]
- 14.Lee, A., M. W. Phillips, J. L. O'Rourke, B. J. Paster, F. E. Dewhirst, G. J. Fraser, J. G. Fox, L. I. Sly, P. J. Romaniuk, T. J. Trust, and S. Kouprach. 1992. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int. J. Syst. Bacteriol. 42:27-36. [DOI] [PubMed] [Google Scholar]
- 15.Maggio-Price, L., D. Shows, K. Waggie, A. Burich, W. Zeng, S. Escobar, P. Morrissey, and J. L. Viney. 2002. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a-/-) mice. Am. J. Pathol. 160:739-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendes, E. N., D. M. Queiroz, F. E. Dewhirst, B. J. Paster, S. B. Moura, and J. G. Fox. 1996. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int. J. Syst. Bacteriol. 46:916-921. [DOI] [PubMed] [Google Scholar]
- 17.Pearce, C. B., H. D. Duncan, L. Timmis, J. R. Green. 2000. Assessment of the prevalence of infection with Helicobacter pylori in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 12:439-443. [DOI] [PubMed] [Google Scholar]
- 18.Saunders, K. E., Z. Shen, F. E. Dewhirst, B. J. Paster, C. A. Dangler, and J. G. Fox. 1999. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J. Clin. Microbiol. 37:146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solnick, J. V., and D. B. Schauer. 2001. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin. Microbiol. Rev. 14:59-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley, J., D. Linton, A. P. Burnens, F. E. Dewhirst, S. L. On, A. Porter, R. J. Owen, and M. Costas. 1994. Helicobacter pullorum sp. nov.—genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology 140:3441-3449. [DOI] [PubMed] [Google Scholar]
- 21.Totten, P. A., C. L. Fennell, F. C. Tenover, J. M. Wezenberg, P. L. Perine, W. E. Stamm, and K. K. Holmes. 1985. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J. Infect. Dis. 151:131-139. [DOI] [PubMed] [Google Scholar]
- 22.Vare, P. O., B. Heikius, J. A. Silvennoinen, R. Karttunen, S. E. Niemela, J. K. Lehtola, and T. N. Karttunen. 2001. Seroprevalence of Helicobacter pylori infection in inflammatory bowel disease: is Helicobacter pylori infection a protective factor? Scand. J. Gastroenterol. 36:1295-1300. [DOI] [PubMed] [Google Scholar]
- 23.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]
- 24.Young, V. B., K. A. Knox, and D. B. Schauer. 2000. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 68:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]