Abstract
We enrolled 22 patients with gas-forming pyogenic liver abscess in a study to assess the mechanism of gas formation. Klebsiella pneumoniae was cultured from specimens from all patients. Gas and pus samples from abscesses revealed four major components: nitrogen, oxygen, carbon dioxide, and hydrogen; this implicates mixed acid fermentation of glucose as the mechanism of gas formation.
Pyogenic liver abscess is uncommon, accounting for 8 to 25 cases per 100,000 hospital admissions (14, 18). Over the past 2 decades, its case fatality rate was around 11.5 to 40% (6, 10, 18, 22). Gas-forming pyogenic liver abscess (GFPLA), which accounts for 7 to 24% of pyogenic liver abscess (5, 11, 12, 19, 21, 22), has a high fatality rate in spite of aggressive management (27.7 to 37.1%) (5, 17, 22). Klebsiella pneumoniae is the most common pathogen of pyogenic liver abscesses in Taiwan (2, 3, 5, 22), especially in GFPLA (5) and in patients with diabetic mellitus (DM) (2). To our knowledge, the pathogenesis of gas formation in K. pneumoniae liver abscess has not been elucidated (22). To reveal the possible mechanism of gas formation, we analyzed the gas composition in liver abscesses of diabetic patients. Clinical manifestations of patients were also studied.
We enrolled all patients with GFPLA, diagnosed at the emergency department of a university hospital from July 1988 to June 1992. Samples of gas and pus were aspirated under echo-guidance during the course of percutaneous drainage (13) and collected in a plastic syringe sealed with a rubber plug. A portion of the pus was sent to the microbiology laboratory, and another portion was used to determine the pH value. Gas samples were aspirated from the sealed syringe with a Hamilton gas-tight syringe and analyzed using an HP 5880A gas chromatography-thermal conductivity detector, which provides automated analyses of fixed gases and light hydrocarbons, and an HP 5970 gas chromatography-mass spectrometer detector, which detects unknown components in gas samples. Sulfur compounds were analyzed by a Varian 3400 gas chromatography-flame photometric detector. The percentage of a particular gas in a sample was calculated by the following formula: % component gas of sample gas = (area under the curve of sample gas/area under the curve of standard gas) × % standard gas (13).
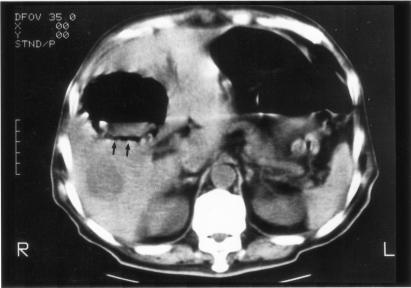
Among the 69 patients with pyogenic liver abscess identified during the study period, 22 (32%) had GFPLA, and their ages ranged from 38 to 79 years (mean = 58.7 years) (Table 1). Underlying diseases could be identified in all patients, and 21 (95%) had DM. Pathogens were isolated from pus in all except two patients (91%) who received medical treatment only. K. pneumoniae bacteremia was noted in 17 patients (77%), and K. pneumoniae was isolated from both blood and pus in 15 (68%). The size of abscesses ranged from 1.5 to 11 cm (mean = 6.5 cm), and abnormal gas accumulation in the liver (Fig. 1) was noted in chest radiographs of eight patients (36%). Both abdominal sonography and computerized tomography (Fig. 2) had a 100% detection rate of gas accumulation. Nineteen patients (86.4%) received both medical treatment and percutaneous aspiration or drainage, and seven died, yielding a case fatality rate of 32%.
TABLE 1.
Summary of clinical characteristics of 22 patients with gas-forming liver abscess caused by K. pneumoniae
| Patient no. | Age (yr) | Gender | Underlying disease(s) | Blood culture | Pus culture | Abscess length (cm) | Treatment(s)a | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 | Male | Diabetes mellitus | K. pneumoniae, Enterobacter cloacae, Streptococcus agalactiae | 4.0 | A | Death | |
| 2 | 62 | Female | Diabetes mellitus; hyperthyroidism | K. pneumoniae | K. pneumoniae | 9.0 | A, PCD | Death |
| 3 | 66 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 5.0 | A, PCD | Death |
| 4 | 50 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 5.0 | A, PCD | Death |
| 5 | 38 | Male | Diabetes mellitus, alcoholic liver disease | K. pneumoniae | 8.0 | A, PCD | Survival | |
| 6 | 45 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 5.0 | A, PCD | Survival |
| 7 | 44 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 6.0 | A, PCD | Survival |
| 8 | 54 | Female | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 7.0 | A, PCD | Survival |
| 9 | 62 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae, Bacteroides fragilis | 8.0 | A, S | Survival |
| 10 | 67 | Male | Diabetes mellitus | K. pneumoniae | K. pneumoniae | 5.5 | A, PCD | Survival |
| 11 | 48 | Male | Diabetes mellitus | K. pneumoniae, Stenotrophomonas maltophilia | 2.0 | A, PCD | Survival | |
| 12 | 71 | Female | DM | K. pneumoniae | 10.0 | A, PCD | Survival | |
| 13 | 63 | Male | DM | K. pneumoniae | 4.0 | A, PCD | Survival | |
| 14 | 68 | Female | DM | K. pneumoniae | K. pneumoniae | 4.6 | A, PCD | Survival |
| 15 | 55 | Female | DM | K. pneumoniae | K. pneumoniae | 10.0 | A, PCD | Survival |
| 16 | 77 | Male | Gall bladder stones | K. pneumoniae | K. pneumoniae | 4.0 | A, PCD | Survival |
| 17 | 50 | Female | DM | K. pneumoniae | 7.0 | A, PCD | Survival | |
| 18 | 60 | Female | DM | K. pneumoniae | 11.0 | A | Death | |
| 19 | 61 | Male | DM, hyperthyroidism | K. pneumoniae | K. pneumoniae | 8.0 | A, PCD | Death |
| 20 | 59 | Male | DM | K. pneumoniae | K. pneumoniae, Proteus mirabilis, Enterococcus sp. | 5.0 | A, PCD | Survival |
| 21 | 56 | Female | DM | K. pneumoniae | K. pneumoniae | 10.0 | A, PCD | Survival |
| 22 | 56 | Male | DM | K. pneumoniae | K. pneumoniae | 5.5 | A, PCD | Death |
Abbreviations: A, antimicrobial therapy; PCD, percutaneous catheter drainage; S, surgery.
FIG. 1.
Chest radiograph showing gas with alveolar pattern in the liver (arrows).
FIG. 2.
Nonenhanced computerized tomograph of the abdomen confirming a huge gas-containing abscess with air-fluid level in the right lobe of the liver parenchyma (arrows).
Gas samples were successfully collected from five patients, and the composition was nitrogen (N2; 65.8 to 78.1%), oxygen (O2; 1.2 to 7.3%), carbon dioxide (CO2; 5.4 to 14.8%), and hydrogen (H2; 9.0 to 18.3%) (Table 2). N2 predominated in all samples, and none contained oxygen-containing hydrocarbon or sulfur-containing compounds.
TABLE 2.
Analyses of gas samples from five patients with gas-forming liver abscess due to K. pneumoniae
| Gas sample | Patient no. | % of gas sample
|
pH | |||
|---|---|---|---|---|---|---|
| N2 | O2 | H2 | CO2 | |||
| 1 | 10 | 78.1 | 5.4 | 14.8 | 5.4 | 6.00 |
| 2 | 12 | 67.3 | 4.4 | 18.3 | 10.0 | 5.89 |
| 3 | 13 | 76.3 | 7.3 | 9.0 | 7.0 | 5.67 |
| 4 | 15 | 66.9 | 6.0 | 18.0 | 9.1 | 6.02 |
| 5 | 21 | 65.8 | 1.2 | 18.1 | 14.8 | 5.52 |
GFPLA is uncommon in western countries (11, 16, 19) but is often reported in Taiwan (5, 17, 22). We observed a higher proportion (32%) than previous studies (5, 11, 19, 22), probably because the enrollment of patients was through an emergency department. Our case fatality rate (32%) was compatible with those reported in two studies (30.4 and 37.1%) of GFPLA (17, 22), which is much higher than those in unspecified liver abscess (3, 4, 9, 18).
Bacteria usually obtain their energy through the fermentation of glucose, which involves a variety of pathways, including lactic fermentation, alcoholic fermentation, mixed acid (formic acid) fermentation, butyric fermentation, butanediol fermentation, and propionic fermentation (8, 15). The formate produced in the mixed acid fermentation by members of the Enterobacteriaceae is relatively stable at an alkaline pH. However, fermentation often leads to accumulation of acids, and when pH reaches 6 or less, gas-forming microorganisms, such as Escherichia coli, will produce formic hydrogenlyase, which converts formic acid to CO2 and H2 (8). Thus, production of H2 is the hallmark of mixed acid fermentation because none of the other five pathways produces H2 as an end product (Table 3). Therefore, our results may imply that mixed acid fermentation was operative and that K. pneumoniae (like E. coli) might also form formic hydrogenlyase, which produces acid and gas.
TABLE 3.
Summary of the fermentative pathways of bacteria, the major end products, and the organism type carrying out the fermentationa
| Fermentative pathway | Major end product(s) | Organism type or genus |
|---|---|---|
| Alcohol fermentation | Ethanol, CO2 | Many yeasts, bacterium Zymomonas |
| Mixed acid fermentation | Formate, CO2, H2 | Most of the Enterobacteriaceae |
| Butanediol (acetoin) fermentation | 2,3-Butylene glycol, diacetyl, CO2 | Enterobacter, Klebsiella, Serratia, Aeromonas, some Bacillus spp. |
| Propionic fermentation | Propionate, CO2 | Propionibacterium |
| Butyric-butylic fermentation | Butanol, isopropanol, CO2 | Clostridium |
| Lactic fermentation | Lactate | Streptococcus, Lactobacillus, many enterobacteria |
Based on data from references 8 and 15.
Gas-forming infection depends on rapid catabolism and impaired transport of the end products at inflammatory sites (23). The high level of glucose in tissue and compromised immunity in diabetic patients provide microbes with a microenvironment in favor of vigorous metabolism and growth (13, 20). Local tissue damage caused by gas-forming bacteria and compounded by the diabetic microangiopathy would perhaps markedly retard the transport of catabolic end products away from the lesion and thereby result in gas accumulation. Considering results of our study and other studies (1, 4, 7, 13, 23), we hypothesized that the gas formation involves increased production of gas, impaired transportation of gas, and equilibrium between the gas in local tissues and that in abscesses. Because N2 exists in all body fluids and tissues in high concentrations, it is not surprising that N2 levels were high in gas samples taken from abscesses.
In terms of specific pathogens, E. coli and K. pneumoniae are by far the most common isolates in pyogenic liver abscess (2, 3, 4, 5, 22), and GFPLA has a high percentage of K. pneumoniae (5, 22). In our study, K. pneumoniae infection in patients with DM was the cornerstone for the development of GFPLA. In patients with DM, the high level of blood glucose may provide gas-forming microorganisms with a more favorable environment for forming gas via mixed acid fermentation of glucose.
In conclusion, results from our study tend to implicate mixed acid fermentation of glucose as the pathway by which GFPLA develops. In order to reduce fatality, adequate antibiotic, good control of blood sugar to stop the rapid catabolism, and, most importantly, an adequate drainage to improve the tissue perfusion to facilitate the gas transport are desirable.
Acknowledgments
We thank Chi-Yu Chen for her assistance in gas analyses and Ji-Je Young for helping with data collection.
REFERENCES
- 1.Chen, K. W., J. J. Huang, M. H. Wu, X. Z. Lin, C. Y. Chen, and M. K. Ruaan. 1994. Gas in hepatic veins: a rare and critical presentation of emphysematous pyelonephritis. J. Urol. 151:125-126. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, D. L., Y. C. Liu, M. Y. Yen, C. Y. Liu, F. W. Shi, and L. S. Wang. 1989. Causal bacteria of pyogenic liver abscess. J. Formosan Med. Assoc. 88:1008-1011. [PubMed] [Google Scholar]
- 3.Cheng, D. L., Y. C. Liu, M. Y. Yen, C. Y. Liu, F. W. Shi, and L. S. Wang. 1990. Pyogenic liver abscess: clinical manifestations and value of percutaneous catheter drainage treatment. J. Formosan Med. Assoc. 89:571-576. [PubMed] [Google Scholar]
- 4.Chiu, C. T., D. Y. Liu, C. S. Wu, C. S. Chang-Chien, I. S. Sheen, and Y. F. Liaw. 1987. A clinical study on pyogenic liver abscess. J. Formosan Med. Assoc. 86:405-412. [PubMed] [Google Scholar]
- 5.Chou, F. F., S. M. Sheen-Chen, Y. S. Chen, and T. Y. Lee. 1995. The comparison of clinical course and results of treatment between gas-forming and non-gas-forming pyogenic liver abscess. Arch. Surg. 130:401-405. [DOI] [PubMed] [Google Scholar]
- 6.Conter, R. L., H. A. Pitt, R. K. Tompkins, and W. P. Longmire, Jr. 1986. Differentiation of pyogenic from amebic hepatic abscesses. Surg. Gynecol. Obstet. 162:114-120. [PubMed] [Google Scholar]
- 7.Cook, D. J., M. R. Achong, and J. Dobranowski. 1989. Emphysematous pyelonephritis. Complicated urinary tract infection in diabetes. Diabetes Care 12:229-232. [DOI] [PubMed] [Google Scholar]
- 8.Davis, B. D. 1990. Nutrition; energy; membrane transport; chemotaxis, p. 65-88. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 4th ed. J. B. Lippincott Company, Philadelphia, Pa.
- 9.Farges, O., T. Leese, and H. Bismuth. 1988. Pyogenic liver abscess: an improvement in prognosis. Br. J. Surg. 75:862-865. [DOI] [PubMed] [Google Scholar]
- 10.Gyorffy, E. J., C. F. Frey, J. Silva, Jr., and J. McGahan. 1987. Pyogenic liver abscess. Diagnostic and therapeutic strategies. Ann. Surg. 206:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halvorsen, R. A., Jr., W. L. Foster, Jr., R. H. Wilkinson, Jr., P. M. Silverman, and W. H. Thompson. 1988. Hepatic abscess: sensitivity of imaging tests and clinical findings. Gastrointest. Radiol. 13:135-141. [DOI] [PubMed] [Google Scholar]
- 12.Hochbergs, P., L. Forsberg, E. Hederstrom, and R. Andersson. 1990. Diagnosis and percutaneous treatment of pyogenic hepatic abscesses. Acta Radiol. 31:351-353. [PubMed] [Google Scholar]
- 13.Huang, J. J., K. W. Chen, and M. K. Ruaan. 1991. Mixed acid fermentation of glucose as a mechanism of emphysematous urinary tract infection. J. Urol. 146:148-151. [DOI] [PubMed] [Google Scholar]
- 14.Johannsen, E. C., C. D. Sifri, and L. C. Madoff. 2000. Pyogenic liver abscesses. Infect. Dis. Clin. N. Am. 14:547-563. [DOI] [PubMed] [Google Scholar]
- 15.Jurtshuk, P., Jr. 1991. Bacterial metabolism, p. 69-89. In S. Baron and P. M. Jennings (ed.), Medical microbiology, 3rd ed. Churchill Livingstone Inc., New York, N.Y.
- 16.Kressel, H. Y., and R. A. Filly. 1978. Ultrasonographic appearance of gas-containing abscesses in the abdomen. Am. J. Roentgenol. 130:71-73. [DOI] [PubMed] [Google Scholar]
- 17.Lee, T. Y., Y. L. Wan, and C. C. Tsai. 1994. Gas-containing liver abscess: radiological findings and clinical significance. Abdom. Imaging 19:47-52. [DOI] [PubMed] [Google Scholar]
- 18.Pineiro-Carrero, V. M., and J. M. Andres. 1989. Morbidity and mortality in children with pyogenic liver abscess. Am. J. Dis. Child. 143:1424-1427. [DOI] [PubMed] [Google Scholar]
- 19.Pitt, H. A. 1990. Surgical management of hepatic abscesses. World J. Surg. 14:498-504. [DOI] [PubMed] [Google Scholar]
- 20.Rayfield, E. J., M. J. Ault, G. T. Keusch, M. J. Brothers, C. Nechemias, and H. Smith. 1982. Infection and diabetes: the case for glucose control. Am. J. Med. 72:439-450. [DOI] [PubMed] [Google Scholar]
- 21.Rubinson, H. A., M. B. Isikoff, and M. C. Hill. 1980. Diagnostic imaging of hepatic abscesses: a retrospective analysis. Am. J. Roentgenol. 135:735-745. [DOI] [PubMed] [Google Scholar]
- 22.Yang, C. C., C. Y. Chen, X. Z. Lin, T. T. Chang, J. S. Shin, and C. Y. Lin. 1993. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am. J. Gastroenterol. 88:1911-1915. [PubMed] [Google Scholar]
- 23.Yang, W. H., and N. C. Shen. 1990. Gas-forming infection of the urinary tract: an investigation of fermentation as a mechanism. J. Urol. 143:960-964. [DOI] [PubMed] [Google Scholar]