Abstract
The currently used method for immunological detection of tuberculosis infection, the tuberculin skin test, has low specificity. Antigens specific for Mycobacterium tuberculosis to replace purified protein derivative are therefore urgently needed. We have performed a rigorous assessment of the diagnostic potential of four recently identified antigens (Rv2653, Rv2654, Rv3873, and Rv3878) from genomic regions that are lacking from the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine strains as well as from the most common nontuberculous mycobacteria. The fine specificity of potential epitopes in these molecules was evaluated by sensitive testing of the T-cell responses of peripheral blood mononuclear cells derived from M. bovis BCG-vaccinated healthy individuals to synthesized overlapping peptides. Three of the four molecules contained regions with significant specificity problems (Rv2653, Rv3873, and Rv3878). We selected and combined the specific peptide stretches from the four proteins not recognized by M. bovis BCG-vaccinated individuals. These peptide stretches were tested with peripheral blood mononuclear cells obtained from patients with microscopy- or culture-confirmed tuberculosis and from healthy M. bovis BCG-vaccinated controls. The combination of the most promising stretches from this analysis showed a sensitivity level (57%) comparable to the level found with the two well-known M. tuberculosis-specific proteins ESAT-6 and CFP-10 (75 and 66%, respectively). The combination of ESAT-6, CFP-10, and the novel specific peptide stretches gave an overall sensitivity of 84% at a specificity of 97%. In a validation experiment with new experimental groups, the sensitivities obtained were 57% for the combination of peptides and 90% for the combination of the peptides, ESAT-6, and CFP-10. This combination gave a specificity of 95%.
Tuberculosis is a major cause of morbidity and mortality throughout the world. It is estimated that nearly 1% of the world population is newly infected each year and that approximately one-third of the world population is latently infected with Mycobacterium tuberculosis.
On average, immunocompetent individuals infected with M. tuberculosis have a lifetime risk of developing active tuberculosis of 10%, but this risk increases to a 10% yearly risk in persons coinfected with human immunodeficiency virus. If left untreated, a patient with active pulmonary tuberculosis will transmit the infection to 10 to 15 contacts each year (World Health Organization, tuberculosis fact sheet no. 104, http://www.who.int/mediacentre/factsheets/who104/en/, 2000). Therefore, novel tools to detect and subsequently treat infected individuals before the disease progresses to active contagious tuberculosis are an international research priority (10).
In addition to chest radiographs to detect pulmonary tuberculosis, the current diagnostic assays for the detection of infection with M. tuberculosis include culture, microscopy and PCR of relevant patient material, and the tuberculin skin test. The first three methods are based on identification of the bacteria and hence depend on a certain bacterial load and access to the infection site. This makes them inapplicable for detection of extrapulmonary tuberculosis or early diagnosis of the preclinical latent stages of infection.
The standard tuberculin skin test represents a delayed-type hypersensitivity reaction based on immunological recognition of mycobacterial antigens in exposed individuals and is a simple and inexpensive assay. It employs intradermal injection of purified protein derivative (PPD), which is a crude and poorly defined mixture of mycobacterial antigens, many of which are shared with proteins from the vaccine strain Mycobacterium bovis bacillus Calmette-Guérin (M. bovis BCG) and from nontuberculous environmental mycobacteria (4, 20). The broad cross-reactivity of PPD results in poor specificity of the tuberculin skin test, leading to a situation in which M. bovis BCG vaccination and exposure to nontuberculous mycobacteria give a test result that can be similar to that seen in individuals infected with M. tuberculosis (15, 17, 21, 25). To overcome the problem of sensitization to nontuberculous mycobacteria, comparative skin testing with PPD derived from two sources, M. tuberculosis and Mycobacterium avium, has been used. A blind study showed that M. avium sensitin-dominant skin tests can discriminate M. avium infection from tuberculosis with high specificity in humans (42) and furthermore that infection with nontuberculous mycobacteria is responsible for the majority of PPD reactions of 5 to 14 mm in the annual tuberculin skin test performed on U.S.-born health care workers and medical students (41). The limited specificity of a test based on PPD also characterizes in vitro assays, as was demonstrated by early attempts to develop serodiagnostic tests based on PPD (12) and more recently from the whole blood QuantiFERON-TB test, in which PPD is used to induce the secretion of gamma interferon (IFN-γ) from sensitized T cells in whole blood (9).
The sequenced genomes of not only M. tuberculosis but also M. bovis BCG and M. avium provide a blueprint for the rational design of the next generation of specific immunodiagnostic reagents (http://www.pasteur.fr/english.html) and allowed the identification of the regions representing genomic deletions in the M. bovis BCG vaccine strain compared to the virulent M. tuberculosis strain (7). Proteins from these regions, in particular the subset of proteins which are also lacking in M. avium and other nontuberculous mycobacteria, represent an excellent source of candidate antigens for a highly specific tuberculosis diagnostic test. A number of antigens from these regions have recently been characterized and have undergone initial evaluation as potential diagnostic reagents for human or cattle tuberculosis (3). The antigens characterized in most detail are ESAT-6 and CFP-10, which have already shown great potential for tuberculosis diagnosis (5, 17, 40), but a number of more recently identified candidate molecules have also been reported. In the present study, we evaluated four of these recently characterized antigens, Rv3873 and Rv3878, encoded in region of deletion 1, in addition to Rv2653 and Rv2654, encoded in region of deletion 11 (1a, 1c, 32).
We report that although these molecules are encoded in the deleted regions, M. bovis BCG-vaccinated individuals recognized epitopes in three of the four molecules. By excluding these cross-reactive parts of the molecules, we demonstrate that it is possible to compose a highly specific cocktail of peptides for sensitive and specific tuberculosis diagnosis. The potential of combining this cocktail with ESAT-6 and CFP-10 is addressed.
MATERIALS AND METHODS
Recombinant proteins and synthetic peptides.
Recombinant CFP-10 and ESAT-6 were produced as previously described (8, 33). The overlapping peptides (18- or 20-mers) from the four proteins Rv2653, Rv2654, Rv3873, and Rv3878 were synthesized by standard solid-phase methods at Schafer-N, Copenhagen, Denmark. The peptides were purified by reverse-phase high-pressure liquid chromatography. Purified peptides were lyophilized and stored dry until reconstitution in phosphate-buffered saline. The sequences of the peptides synthesized are shown in Fig. 1.
FIG. 1.
Amino acid sequences of the four diagnostic antigens. Synthetic 18- to 20-mer peptides spanning the proteins are indicated by horizontal double lines. Peptide numbers are used throughout the article.
Subjects. (i) Derivation panel.
The healthy M. bovis BCG-vaccinated subjects (controls, n = 29) were all Danish-born adults between 25 and 67 years of age (mean age, 40 years; 8 males and 21 females) who had received M. bovis BCG vaccination during their childhood 20 to 62 years before venipuncture. They were recruited through advertisement. All controls answered a questionnaire to reveal potential exposure to tuberculosis: travel history, contact with tuberculosis patients, or occupational exposure to M. tuberculosis, and all controls with possible exposure were excluded. Vaccination status was determined through the questionnaire. Thirty-four healthy M. bovis BCG-vaccinated individuals were recruited, and 6 were excluded based on information revealed in the questionnaire.
Peripheral blood mononuclear cells (PBMCs) from 60 tuberculosis patients 14 to 72 years old (mean age, 39 years; 40 males and 20 females) with microscopy- or culture-proven infection were used. Patients were recruited from hospitals in The Netherlands and Denmark. Blood samples were drawn from tuberculosis patients shortly after diagnosis and treatment initiation; five of the patients had their blood drawn later, i.e., 2 years after diagnosis (n = 1), 6 years after diagnosis (n = 2), and 6 months after diagnosis (n = 2) (range, 0 to 6.6 years; median, 0.12 years). About a third of the patients were of Caucasian Danish or Dutch origin, and two-thirds were immigrants born in North Africa, Central and South Africa, Southeast Asia, Central Asia, Southwest Asia, South America, and eastern Europe. Thirty-six of the patients had pulmonary tuberculosis, two patients had milliary tuberculosis, and the remaining patients had extrapulmonary tuberculosis, e.g., of the lymph nodes (neck, axillae, and groin), pericardium, spine, thoracic spine, pleura, mammae, joints, or peritoneum.
Results for one of the patients were excluded based on a low response to phytohemagglutinin (PHA) (<150 pg/ml), suggestive of a low survival rate of the PBMCs after freezing and thawing of the cells. Three of the patients had in vitro anergy (low response to PPD).
(ii) Validation panel.
For validation of the findings, PBMCs from 43 individuals were used, 22 M. bovis BCG-vaccinated controls and 21 individuals with M. tuberculosis infection, comprising 13 individuals with latent tuberculosis and 8 patients with active tuberculosis.
The latently infected individuals (10 males and 3 females; mean age, 17 years) were Danish-born high school students with close contact to a tuberculosis patient with sputum microscopy- and culture-positive pulmonary tuberculosis. They were M. bovis BCG nonvaccinated, had a strongly positive tuberculin skin test (>15-mm induration), and therefore received prophylactic treatment according to the tuberculin skin test results. They were recruited from a local tuberculosis outbreak.
The patients with active tuberculosis (six males and two females aged 17 to 46 years; mean, 37 years) were recruited from a Danish hospital, and all had culture- or microscopy-positive tuberculosis, and their diagnosis was confirmed within 1 month before or after venipuncture. One patient was Danish born, and seven were immigrants from Pakistan, North Africa, Central and South Africa, and Greenland. Seven patients had pulmonary tuberculosis, and one patient had extrapulmonary tuberculosis (psoas abscess). One patient was also human immunodeficiency virus positive, and one patient received immunosuppressive treatment.
The control group in the validation panel (n = 22; 12 males and 10 females; mean age, 45 years) comprised healthy Danish adults; they had a low risk of exposure to M. tuberculosis and were M. bovis BCG vaccinated in childhood.
All participating individuals gave their informed written consent before blood sampling. The study was approved by the Local Ethical Committee for Copenhagen and Frederiksberg (RH 01-282/96 and KF 01-369/98) and by the Institutional Review Board of the Leiden University Medical Center (protocol P136/97).
Lymphocyte preparations and cell culture.
PBMCs were freshly isolated by gradient centrifugation of heparinized blood on Lymphoprep (Nycomed, Oslo, Norway) and stored in liquid nitrogen until use. The viability and number of cells were determined by Nigrosin staining. Cell cultures were established in triplicate cultures of 1.25 × 105 PBMCs in 100-μl microtiter plates (Nunc, Roskilde, Denmark) and stimulated with 5 μg of PPD, 5 μg of recombinant CFP-10, 5 μg of recombinant ESAT-6 and 10 μg of the individual peptides per ml. Peptide mixtures were used at the concentration that was found to be optimal for the individual mixtures upon testing in preliminary experiments. The optimal concentration was 10 μg/ml for each individual peptide in the mixture except for peptide mixture Rv3978 A and B, which had optimal performance at 5 μg/ml for each individual peptide in the mixture. For all analyses, the standard error was generally below 30% of the mean.
Cell cultures without antigen were included as negative controls, and PHA (2 μg/ml) was used as a mitogenic positive control (results not shown). Cell cultures were incubated for 5 days at 37°C in a humidified (5% CO2, 95% air) incubator. Cell-free supernatants were harvested and kept frozen at −20°C until use.
Cytokine analysis.
IFN-γ was detected with a standard sandwich enzyme-linked immunosorbent assay technique with a commercially available pair of monoclonal antibodies (Endogen) and used according to the manufacturer's instruction. Recombinant IFN-γ (Endogen) was used as a standard. Cytokine levels are given as picograms of protein per milliliter of supernatant. The detection limit of the assay was 20 pg/ml. For all individuals values from the unstimulated control well, 0 to 400 pg/ml (median, 2.0; 95% confidence intervals [CI], 9.9 to 33.6), were subtracted from stimulation wells if detectable.
Cell culture and cytokine analysis for the selection of specific peptide stretches and validation of the diagnostic performance were performed by trained laboratory assistants and conducted in a random blind fashion, so that the clinical status of the tested subjects was unknown.
Statistical methods.
Comparisons of proportions were made with a two-sided chi-square test. Calculations of diagnostic performance were made with SISA statistical analysis (http://home.clara.net/sisa/diagnos.htm). The 95% CI are given throughout the text.
RESULTS
Fine specificity of T-cell responses to diagnostic antigens.
The main purpose of the present study was to identify specific molecules or parts of molecules for a future diagnostic peptide cocktail. The four antigens Rv2653, Rv2654, Rv3873, and Rv3878 are encoded in the regions of the M. tuberculosis genome deleted in M. bovis BCG and lacking in most nontuberculous mycobacteria. These antigens have all previously been described to be well recognized by tuberculosis patients, but some recognition in healthy controls was also observed (1a, 1c, 32). Therefore, the fine specificity of these antigens was evaluated in detail by studying the responses in healthy M. bovis BCG-vaccinated individuals with panels of overlapping peptides covering the entire sequences of these proteins (Fig. 1) and stimulating PBMCs in vitro (Fig. 2A).
FIG. 2.
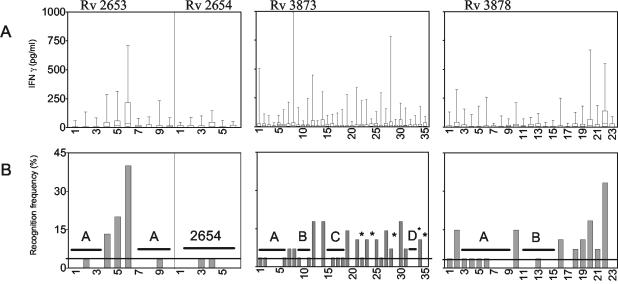
Recognition of the single peptides in M. bovis BCG-vaccinated individuals. (A) Level of recognition of single peptides spanning the four proteins in M. bovis BCG-vaccinated individuals. Synthetic peptides spanning the whole proteins were tested by measuring IFN-γ release after stimulation of PBMCs from healthy M. bovis BCG-vaccinated individuals (n = 28). Boxes represent the 25th to 75th percentiles, and bars show the maximum response. (B) Recognition frequencies of single peptides in M. bovis BCG-vaccinated individuals. Recognition frequencies (individuals with responses of >100 pg/ml to the individual peptides) of the single peptides in M. bovis BCG-vaccinated individuals are shown. Horizontal bars indicate the regions selected and pooled as diagnostic cocktails. Asterisks indicate the single peptides included in the Rv3873D cocktail.
In general, most individual peptides induced low IFN-γ responses, with maximum responses being below 300 pg/ml in the M. bovis BCG-vaccinated individuals. However, some peptides did induce IFN-γ release above this background in several individuals, in particular, those for Rv3873, for which recognition in controls was seen scattered throughout the molecule, and peptides 1, 8, 12, and 28 were recognized, with a high level of IFN-γ release in controls. For Rv3878, peptides 2, 20, and 22 from the C-terminal end of the molecule were recognized by controls, while for Rv2653 a strong recognition of the central part of the molecule (especially peptide 6) was seen. On the other hand, the peptides covering the Rv2654 molecule were not recognized by any of the M. bovis BCG-vaccinated individuals.
In order to get an overview of the regions within Rv3873, Rv3878, and Rv2653 with nonspecific activity in M. bovis BCG-vaccinated individuals, the IFN-γ responses shown in Fig. 2A were converted into recognition frequencies (percentage of individuals with responses of >100 pg/ml to the individual peptides) (Fig. 2B). On the basis of these data, the stretches of the molecules without specificity problems were selected by excluding peptides recognized (>100 pg/ml) by more than one individual out of the groups of 28 individuals (corresponding to more than 4%). Additionally, peptides that gave rise to recognition with an IFN-γ release of over 500 pg/ml in any control subject were excluded. For the Rv3873 protein, three major regions were identified that were not recognized by cells from M. bovis BCG-vaccinated individuals. These regions, designated A, B, and C, contained peptides 2 to 6 (Rv3873A), 9 to 11 (Rv3873B), and 15 to 18 (Rv3873C). Furthermore, to explore the specific peptides in the C-terminal part of the molecule, a number of specific single peptides (peptides 22, 24, 29, 32, 33, and 35) were combined into one cocktail, designated Rv3873D.
In protein Rv3878, two specific regions were selected, peptides 3 to 9 (Rv3878A) and peptides 11 to 15 (Rv3878B).
The recognition frequency for single peptides spanning the Rv2653 protein showed that this protein contains a cross-reactive stretch of approximately 36 amino acids (peptides 4, 5, and 6) in the central part of the protein. The two specific regions (peptides 1 to 3 and peptides 7 to 10) were chosen, combined, and designated Rv2653A. The Rv2654 molecule did not contain nonspecific segments and was therefore used as a cocktail of all six peptides covering the sequence.
Diagnostic potential of peptide stretches.
To evaluate the diagnostic potential of the selected specific regions from the four proteins, the peptide mixtures were tested in in vitro assays on a panel of PBMCs from tuberculosis patients with culture- and microscopy-proven tuberculosis and in parallel in M. bovis BCG-vaccinated healthy controls. These control PBMCs were derived from the same individuals as used for the selection of specific peptide stretches. They had a second venipuncture, and PBMCs from one additional individual were included. The eight different peptide mixtures were compared with the well-known M. tuberculosis-specific proteins CFP-10 and ESAT-6 in order to determine not only the diagnostic potential of each of the peptide cocktails, but also the additive value of the new proteins combined with ESAT-6 and CFP-10.
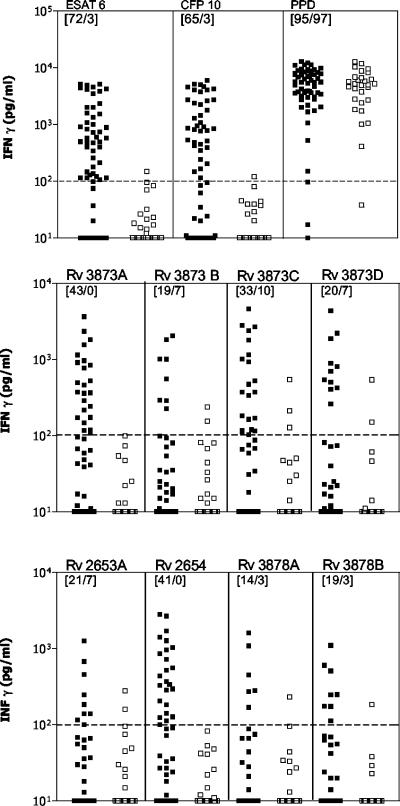
In confirmation of earlier results (5, 9, 30, 40), both ESAT-6 and CFP-10 were recognized by a large proportion of the patients (75 and 66%, respectively) and induced the release of high levels of IFN-γ in this group, while only low levels were induced in M. bovis BCG-vaccinated controls (Fig. 3). The mean IFN-γ release for ESAT-6 for all patients was 1,130 pg/ml, versus 25 pg/ml for controls. CFP-10 also showed an excellent discrimination between patients and controls, with a mean IFN-γ release of 1,249 pg/ml for patients and 23 pg/ml for controls. PPD was recognized by all except one individual and induced high levels of IFN-γ but, in contrast to the two specific proteins, did not discriminate between patients and controls.
FIG. 3.
T-cell responses to ESAT-6, CFP-10, PPD, and the peptide cocktails. The proteins and peptide mixtures were tested in a panel of PBMCs from tuberculosis patients and M. bovis BCG-vaccinated healthy controls. Horizontal lines indicate the cutoff value of 100 pg/ml used for the initial selection of peptides. Solid squares represent patient responses to Rv2653A, Rv3873B, Rv3878A, and Rv3878B (n = 44) or ESAT-6, CFP-10, PPD, Rv3873A, Rv3873C, Rv3873D, and Rv2654 (n = 59), and open squares represent the 29 M. bovis BCG-vaccinated controls tested. For each antigen-peptide cocktail, recognition (>100 pg/ml) is given as the percentage of positive patients among M. bovis BCG-vaccinated controls and is indicated in the upper right-hand corner.
The peptide cocktails were each recognized by cells from between 14 and 43% of tuberculosis patients with responses above the established cutoff (100 pg/ml). However, for some of the cocktails, the discrimination between M. bovis BCG-vaccinated and tuberculosis patients was not as clear-cut as would have been expected based on the results from the individual peptides. One example was Rv3873C, which despite being recognized by 33% of the patients was also recognized by 10% of the M. bovis BCG-vaccinated individuals. Four of the peptide mixtures (Rv2654, Rv3873A, Rv3878A, and Rv3878B), on the other hand, were recognized in a highly specific fashion, with no more than one responder out of the 29 M. bovis BCG-vaccinated individuals. Of these specific peptide cocktails, Rv2654 and Rv3873A were the most frequently recognized, with 41 and 43% of the patients responding above the cutoff level. Low levels of IFN-γ were found in the control wells for both the M. bovis BCG-vaccinated group (median, 2.0 pg/ml; 95% CI, 9.9 to 33.6 pg/ml) and the tuberculosis patients (median, 3.0 pg/ml; 95% CI, 5.5 to 34.0 pg/ml). For the PHA wells, no significant difference was found for the patients and M. bovis BCG-vaccinated individuals (median, 10,530 and 9,533 pg/ml, respectively).
Combining peptide cocktails for optimal diagnostic performance.
The diagnostic performance of the most promising peptide cocktails (Rv2654, Rv3873A, and Rv3878B) was evaluated, and the sensitivity was calculated based on the cutoff values established by analysis of receiver-operating characteristic curves at a specificity level of 97% in the derivation panel of subjects (Table 1).
TABLE 1.
Diagnostic performance of the novel peptide mixtures and of ESAT-6 and CFP-10 alone and in combination for the derivation panel
| Antigen(s) | Recognitiona (no. responding/no. tested) | Sensitivity,b % (95% CI) | Specificity,c %(95% CI) |
|---|---|---|---|
| Rv2654 | 28/59 | 47 (35-60) | 97 (90-103) |
| Rv3873A | 27/59 | 46 (33-57) | 97 (90-103) |
| Rv3878B | 14/44 | 32 (18-46) | 97 (90-103) |
| Rv2654 + Rv3873A + Rv3878B | 25/44 | 57 (42-72) | 97 (90-103) |
| ESAT-6 | 44/59 | 75 (64-86) | 97 (90-103) |
| CFP-10 | 39/59 | 66 (54-78) | 97 (90-103) |
| ESAT-6 + CFP-10 | 48/59 | 81 (71-91) | 97 (90-103) |
| ESAT-6 + CFP-10 + Rv2654 + Rv3873A + Rv3878B | 37/44 | 84 (73-95) | 97 (90-103) |
Number of patients responding/number tested. The cutoff was determined by receiver-operating characteristic curve analysis; there was ≥97% specificity for the single proteins and peptide cocktails: ESAT-6, 94 pg/ml; CFP-10, 80 pg/ml; Rv2654, 53 pg/ml; Rv3873A, 73 pg/ml; and Rv3878B, 38 pg/ml.
Percentage of responding patients out of all patients tested.
Percentage of true-negative controls out of all control individuals tested.
The two epitope cocktails Rv2654 and Rv3873A gave a sensitivity of approximately 50%, while Rv3878B had a sensitivity of 32%. To maximize the sensitivity of a future diagnostic reagent, we investigated whether it would be advantageous to combine the selected peptide cocktails. By combining Rv2654, Rv3873A, and Rv3878B peptides a sensitivity of 57% (95% CI, 42 to 72%) was obtained (Table 1). This sensitivity level is not significantly different from the sensitivity obtained with ESAT-6 or CFP-10 (75 and 66%, respectively) when tested in a two-sided chi-square test in which the peptide mixture (57%) was compared to ESAT-6 (75%; P = 0.0898) and the peptide mixture (57%) was compared to CFP-10 (66%; P = 0.4714). Furthermore, there was a trend of an additive but not statistically significant (P = 0.8958) value of the combination of ESAT-6, CFP-10, and the novel specific peptide cocktails, resulting in a combined sensitivity of 84% (95% CI, 73 to 95%) compared to the 81% (95% CI, 71 to 91%) sensitivity of the combination of ESAT-6 and CFP-10 alone (Table 1).
For the sensitivities, it is noteworthy that three of the patients had in vitro anergy and were negative to all antigens tested, including PPD. Two of these patients received immunosuppressive treatment, and one was severely ill with extensive disease.
Validation of the findings.
To validate the findings, a new panel of 21 M. tuberculosis-infected individuals (13 with latent tuberculosis and 8 with active tuberculosis) and control subjects were investigated. Sensitivity and specificity were calculated with the five antigens and the five cutoff values established in the derivation panel of subjects (Table 2). The results from the group of latently infected individuals and the group of individuals with active tuberculosis were very similar, with no significant differences, and are reported together below.
TABLE 2.
Diagnostic performance of the novel peptide mixtures and of ESAT-6 and CFP-10 alone and in combinations for the validation panela
| Antigen(s) | Recognition (no. responding/no. tested) | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Rv2654 | 9/21 | 42 (22-64) | 100 (100-100) |
| Rv3873A | 8/21 | 38 (17-59) | 95 (87-104) |
| Rv3878B | 12/21 | 57 (36-78) | 100 (100-100) |
| Rv2654 + Rv3873A + Rv3878B | 12/21 | 57 (36-78) | 95 (87-104) |
| ESAT-6 | 18/21 | 86 (71-101) | 100 (100-100) |
| CFP-10 | 15/21 | 71 (52-91) | 100 (100-100) |
| ESAT-6 + CFP-10 | 18/21 | 86 (71-101) | 100 (100-100) |
| ESAT-6 + CFP-10 + Rv2654 + Rv3873A + Rv3878B | 19/21 | 90 (78-103) | 95 (87-104) |
See Table 1, footnotes a, b, and c.
The combination of the three new peptide stretches gave a sensitivity of 57% (95% CI, 36 to 78%) and the specificity was 95% (95% CI, 87 to 104%). The combination of ESAT-6 and CFP-10 gave a sensitivity for all infected of 86% (95% CI, 71 to 101%) and a specificity of 100% (95% CI, 100 to 100%). The combination of all five antigens gave a sensitivity of 90% (95% CI, 78 to 103%) for all infected and a specificity of 95% (95% CI, 87 to 104%).
DISCUSSION
A next-generation PPD in the form of selected antigens specific to M. tuberculosis has been on the research agenda for many years. In the 1970s, attempts were made to extract M. tuberculosis-specific antigens by absorption with cross-reacting antibodies (39). Since then, most attempts have been based on affinity purification with monoclonal antibodies (44, 45) or conventional biochemical purification (31). These efforts have more recently resulted in the discovery of ESAT-6 (2, 38) and CFP-10 (8, 13), which in a number of studies from different laboratories have demonstrated a great potential for immune-based tuberculosis diagnosis (5, 9, 24, 40). However, although they are highly specific for tuberculosis infection, the underlying problem has been the insufficient sensitivity often obtained with single antigens, and therefore attempts have been made to discover more antigens with diagnostic potential (11, 26).
In the present study we conducted a postgenomic antigen discovery program focused on antigens recently identified in the regions of the M. tuberculosis genome that are not present in the M. bovis BCG genome (7, 19, 27). We demonstrate that it is possible to combine carefully selected specific peptides and antigens as ingredients in a sensitive and highly specific diagnostic cell-mediated immunity-based test. Future studies will reveal whether these ingredients can be mixed into a single reagent. We evaluated four recently characterized antigens with reported diagnostic potential: Rv3873 (32) (a protein belonging to the proline-rich family of proteins, the PPE family) and Rv3878 (1c), both encoded in the RD1 region, and the two RD11 region proteins Rv2653 and Rv2654 (1a).
An unexpected finding in the present investigation was that although an antigen in itself may be absent from M. bovis BCG and other strains of mycobacteria, it may still contain stretches with T-cell epitopes that are homologous to the products of genes outside the deleted regions or may even contain limited regions homologous to genes outside the mycobacterial genus. This is in agreement with recent attempts to develop tuberculosis diagnostic reagents for cattle. In a recent study by Cockle et al. (11), this cross-reactivity was observed for several antigens, e.g., 17% of M. bovis BCG-vaccinated cattle recognized the Rv3873 protein. Rv3873 was also found in the present study to contain many epitopes recognized by M. bovis BCG-vaccinated individuals. This protein belongs to the large PPE family, which contains members residing both inside and outside the RD regions, and new data (32) indicate that one of the main epitopes recognized by M. bovis BCG-vaccinated individuals in fact represents a highly conserved epitope shared among the different PPE family members.
For the other proteins in this study, no homology to other mycobacterial proteins was found in database searches, and therefore there is no obvious explanation for the observed “cross-reactivity” (1a, 1c). In the present study, instead of abandoning the nonspecific molecules as diagnostic reagents, we dissected the proteins by testing overlapping peptides spanning their sequence and divided them into specific and nonspecific moieties. A peptide-based diagnostic approach has also been attempted for leprosy, but although high specificity was demonstrated, the selected specific peptides gave a relatively low sensitivity (14). We made the same observation in the present study; selecting only the regions of the protein without cross-reactive epitopes decreased the sensitivity. However, by combining peptides from different molecules we obtained a combined sensitivity at the same level as the reference reagents ESAT and CFP-10 without jeopardizing specificity.
Combining tuberculosis-specific antigens to improve diagnostic performance does not represent a new idea. In one study, tuberculosis-infected guinea pigs were skin tested with MPT64 and ESAT-6. All infected animals responded to the combination of the two antigens, but a number of nonresponders were found when the antigens were employed separately (16). Furthermore, combinations of ESAT-6 and CFP-10 have been used in IFN-γ-based assays, resulting in increased sensitivity compared to the sensitivity provided by the individual antigens without jeopardizing specificity (40). Very recent data confirm the strong potential of this combination for detection of latent M. tuberculosis infection (17).
The data in the present study demonstrate that it may be possible to improve the diagnostic performance of ESAT-6 and CFP-10 even further by combining these two antigens with the new highly selected peptides. The additive effect seen was not statistically significant with the 44 individuals in the derivation panel of patients, but the trend was also seen in the validation set of M. tuberculosis-infected individuals. This trend could very well be relevant and have advantages if tested on a broader scale. Recent data from a study in cattle indicated that combining antigens widens the repertoire of responsive individuals and is due not only to the genetic restriction of responses to individual epitopes but interestingly also to a very pronounced variation in T-cell responses to the individual antigens over the course of infection (1b). By analogy with the present study, Rv3878 was found in that study to be a candidate molecule, which supplemented ESAT-6 and CFP-10 for diagnosis of cattle tuberculosis, and interestingly this antigen was recognized at time points when the animals were nonresponsive to the other antigens. This factor may be of particular importance, as ESAT-6 and CFP-10 both belong to the ESAT-6 gene family (37) and reside within one operon. Therefore, both antigens may be expressed and available for immune recognition in the same phase of infection.
Evidence for such a sequential appearance of antigen responses has recently been provided by Gennaro and colleagues, both by monitoring gene expression (35) and most recently by the sequential appearance of antibody responses (36). These findings suggest that even though the ESAT-6 family members are unusually rich in T-cell epitopes and would potentially be recognized by genetically heterogeneous populations (6, 23, 34), a multiantigen cocktail for tuberculosis diagnosis would have the potential advantage of enabling the detection of patients in different phases of infection. The present study was based on cells from tuberculosis patients as well as individuals with latent tuberculosis infection, indicating that the new specific peptides have potential for the diagnosis of both active and latent tuberculosis.
In the present study, cell-mediated immune responses were monitored by using in vitro IFN-γ assays with PBMCs isolated from whole blood and frozen until use. This is a very convenient research method but not a practical diagnostic assay for clinical application. Currently, two different methods are under development for cell-mediated immunity-based tuberculosis diagnosis measuring IFN-γ, the ELISPOT and the QuantiFERON-TB whole blood test. The QuantiFERON technology is currently based on PPD and has been demonstrated to correlate nicely with the tuberculin skin test in its ability to detect latent tuberculosis (28, 29). However, being based on PPD, QuantiFERON-TB has a low specificity (9). Attempts to adapt this methodology to include specific antigens have provided promising data (22), and very recently the second-generation QuantiFERON-TB test, the so-called QuantiFERON-TB Gold assay, which uses ESAT-6 and CFP-10, has been released to the market for clinical application. The use of ESAT-6 and CFP-10 for ELISPOT-based diagnosis of latent tuberculosis, the so-called CLINISPOT-TB assay, has recently attracted a lot of interest. In a contact-tracing study, it was found that although the ELISPOT results showed an overall concordance of 89% compared with the tuberculin skin test, recognition of ESAT-6 and CFP-10 correlated significantly more closely to the level of exposure to the index case than did the tuberculin skin test (17).
Both the QuantiFERON-TB whole-blood assay and the ELISPOT assay are based on fresh blood and may be difficult to implement in the Third World for practical and logistical reasons. In this setting, an improved skin test in which PPD is replaced by a cocktail of specific antigens would be an attractive alternative to the classic tuberculin skin test. ESAT-6 and CFP-10 have previously been demonstrated to induce strong skin test responses in guinea pigs (16, 26, 40), and a recent demonstration of the potential of ESAT-6 as a skin test reagent for the diagnosis of bovine tuberculosis (33) looks promising for its potential in a human skin test as well. Rv2654 has also proven to be able to induce skin test responses in M. tuberculosis-infected guinea pigs (1a). This antigen is therefore an obvious diagnostic candidate to be combined with CFP-10 and ESAT-6.
We have now demonstrated that it is possible to combine carefully selected specific peptides and antigens as ingredients in a highly sensitive and specific cell-mediated immunity-based diagnostic test. The next-generation skin test or blood-based diagnostic test for tuberculosis infection must discriminate between individuals sensitized not only by M. bovis BCG and nontuberculous mycobacteria but also by any new vaccine developed for tuberculosis in the future. It is therefore important that a new diagnostic reagent be carefully aligned with the efforts to develop a new vaccine in coming years.
Acknowledgments
We thank Vita Elleby Skov, Thomas Okkels Thomasen, and Birgitte Smedegaard for dedicated assistance in T-cell assays and Jette Pedersen, Kathryn Wattam, and Vivi Andersen for excellent technical assistance. We also thank Tom H. M. Ottenhoff for continuous practical and mental support and constructive discussions. Axel Kok-Jensen from the Department of Pulmonary Disease, Gentofte Hospital, and Kirsten Stax Jacobsen, Department of Pulmonary Disease, Viborg Hospital, Denmark, and Richard van Altena, Tineke van Ooijen, and Atie de Boer at the tuberculosis clinic Beatrixoord, Haaren (Gr), The Netherlands, are gratefully acknowledged for recruitment, blood sampling, and providing clinical data for the patients in this study. Timothy Mark Doherty is gratefully acknowledged for critical reading of the manuscript.
This work was supported by the Commission of the European Community, The Netherlands Organisation for Scientific Research, and the Netherlands, Leprosy Foundation.
REFERENCES
- 1a.Aagaard C, I. Brock, A. Olsen, T. H. Ottenhoff, K. Weldingh, and P. Andersen. 2004. Mapping of the immune reactivity of Rv2653 and Rv2654; two novel low molecular mass antigens found specifically in the Mycobacterium tuberculosis complex. J. Infect. Dis. 189:812-819. [DOI] [PubMed]
- 1b.Aagaard, C., M. Govaerts, L. M. Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to the identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed]
- 1c.Agger, E. M., I. Brock, L. M. Okkels, S. M. Arend, C. S. Aagaard, K. N. Weldingh, and P. Andersen. 2003. Human T-cell responses to the RD1-encoded protein TB27.4 (Rv3878) from Mycobacterium tuberculosis. Immunology 110:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 3.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, Å., and B. P. Bengaard. 1994. Proteins and antigens of Mycobacterium tuberculosis. ASM Press, Washington, D.C.
- 5.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 6.Arend, S. M., A. Geluk, K. E. van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 8.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 9.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 10.Centers for Disease Control. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Morb. Mortal. Wkly. Rep. 49:1-51. [PubMed] [Google Scholar]
- 11.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, T. M. 1989. Rapid diagnosis of tuberculosis: laboratory techniques applicable in developing countries. Rev. Infect. Dis. 11(Suppl. 2):S471-S478. [DOI] [PubMed] [Google Scholar]
- 13.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dockrell, H. M., S. Brahmbhatt, B. D. Robertson, S. Britton, U. Fruth, N. Gebre, M. Hunegnaw, R. Hussain, R. Manandhar, L. Murillo, M. C. Pessolani, P. Roche, J. L. Salgado, E. Sampaio, F. Shahid, J. E. Thole, and D. B. Young. 2000. A postgenomic approach to identification of Mycobacterium leprae-specific peptides as T-cell reagents. Infect. Immun. 68:5846-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, L. B., F. A. Acquaviva, V. T. Livesay, F. W. Cross, and C. E. Palmer. 1969. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am. Rev. Respir. Dis. 99(Suppl.):1-132. [PubMed] [Google Scholar]
- 16.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 18.Felten, M. K., and C. A. van-der-Merwe. 1989. Random variation in tuberculin sensitivity in schoolchildren. Serial skin testing before and after preventive treatment for tuberculosis. Am. Rev. Respir. Dis. 140:1001-1006. [DOI] [PubMed] [Google Scholar]
- 19.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 20.Harboe, M. 1981. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am. Rev. Respir. Dis. 124:80-87. [DOI] [PubMed] [Google Scholar]
- 21.Huebner, R. E., M. F. Schein, and J. B. Bass. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 24.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 25.Lind, A., L. O. Larsson, M. W. Bentzon, M. Magnusson, J. Olofson, I. Sjogren, I. L. Strannegard, and B. E. Skoogh. 1991. Sensitivity to sensitins and tuberculin in Swedish children. I. A study of schoolchildren in an urban area. Tubercle 72:29-36. [DOI] [PubMed] [Google Scholar]
- 26.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazurek, G. H., P. A. LoBue, C. L. Daley, J. Bernardo, A. A. Lardizabal, W. R. Bishai, M. F. Iademarco, and J. S. Rothel. 2001. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 286:1740-1747. [DOI] [PubMed] [Google Scholar]
- 29.Mazurek, G. H., and M. E. Villarino. 2003. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:15-18. [PubMed] [Google Scholar]
- 30.Munk, M. E., S. M. Arend, I. Brock, T. H. Ottenhoff, and P. Andersen. 2001. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J. Infect. Dis. 183:175-176. [DOI] [PubMed] [Google Scholar]
- 31.Nagai, S., H. G. Wiker, M. Harboe, and M. Kinomoto. 1991. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59:372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okkels, L. M., I. Brock, F. Follmann, E. M. Agger, S. M. Arend, T. H. Ottenhoff, F. Oftung, I. Rosenkrands, and P. Andersen. 2003. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71:6116-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock, J. M., J. McNair, H. Bassett, J. P. Cassidy, E. Costello, H. Aggerbeck, I. Rosenkrands, and P. Andersen. 2003. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J. Clin. Microbiol. 41:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, C. F. von-Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 35.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva, V. M., G. Kanaujia, M. L. Gennaro, and D. Menzies. 2003. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int. J. Tuberc. Lung Dis. 7:478-484. [PubMed] [Google Scholar]
- 37.Skjot, R. L., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turcotte, R. 1975. The participation of common and species-“specific” antigens of mycobacteria in the tuberculin skin reaction. Can. J. Microbiol. 21:774-783. [DOI] [PubMed] [Google Scholar]
- 40.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Reyn, C. F., C. R. Horsburgh, K. N. Olivier, P. F. Barnes, R. Waddell, C. Warren, S. Tvaroha, A. S. Jaeger, A. D. Lein, L. N. Alexander, D. J. Weber, and A. N. Tosteson. 2001. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int. J. Tuberc. Lung Dis. 5:1122-1128. [PubMed] [Google Scholar]
- 42.von Reyn, C. F., D. E. Williams, C. R. Horsburgh, Jr., A. S. Jaeger, B. J. Marsh, K. Haslov, and M. Magnusson. 1998. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative to discriminate pulmonary disease due to M. avium complex from pulmonary disease due to Mycobacterium tuberculosis. J. Infect. Dis. 177:730-736. [DOI] [PubMed] [Google Scholar]
- 43.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worsaae, A., L. Ljungqvist, K. Haslov, I. Heron, and J. Bennedsen. 1987. Allergenic and blastogenic reactivity of three antigens from Mycobacterium tuberculosis in sensitized guinea pigs. Infect. Immun. 55:2922-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worsaae, A., L. Ljungqvist, and I. Heron. 1988. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J. Clin. Microbiol. 26:2608-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]