Abstract
Deregulated Hedgehog (HH)/GLI signaling plays an etiologic role in the initiation, progression and maintenance of many cancers. Small molecule targeting of HH signaling by inhibiting the essential pathway effector Smoothened (SMO) has proven exceptionally efficient for the treatment of advanced and metastatic basal cell carcinoma. That said, severe side effects, limited response rates, SMO-independent GLI signaling and rapid development of drug resistance limit the therapeutic success of SMO antagonists, urgently calling for the identification of alternative and additional strategies repressing oncogenic HH signaling.
In this perspective article we highlight recent findings showing that the Toll-like receptor-7/8 (TLR7/8) agonist imiquimod (IMQ), an immune modulator approved for the treatment of basal cell carcinoma, can also act as a potent cell autonomous inhibitor of oncogenic HH signaling. Surprisingly, IMQ reduces HH signal strength independent of TLR signaling, via adenosine receptor (ADORA)/Adenylate cyclase (AC)/Protein kinase A (PKA) activation. We here highlight the molecular mechanisms of IMQ-mediated repression of HH/GLI and discuss the possible benefits as well as challenges of using ADORA agonists for the treatment of HH-associated cancer.
Keywords: Hedgehog signaling, Imiquimod, GLI proteins, basal cell carcinoma, Adenosine receptors, ADORA, Protein Kinase A, PKA
INTRODUCTION
HH/GLI signaling is crucial for proper embryonic development and in adults for tissue maintenance and regeneration by regulating stem cell activation and self-renewal. In line with the requirement of exquisite regulation of signal strength and duration, deregulated HH/GLI signaling can have fatal consequences causing developmental anomalies and cancer (for extensive reviews see [1-11]).
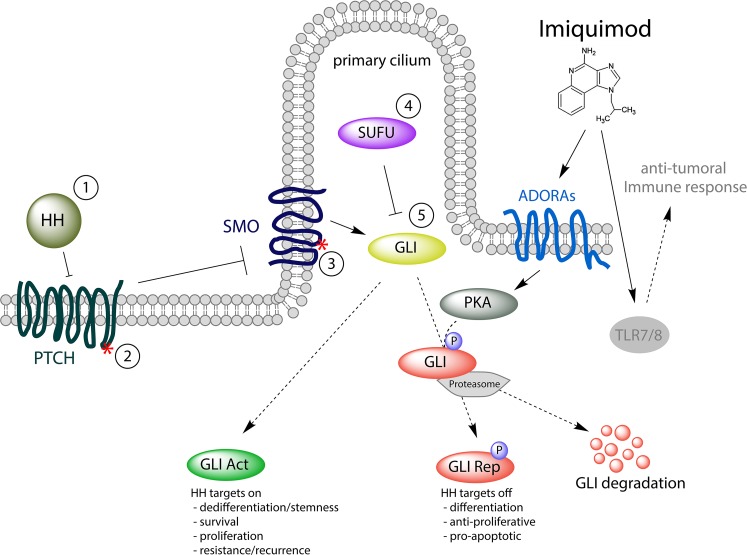
HH pathway activation is initiated by binding of HH ligand to Patched (PTCH), a twelve transmembrane domain protein that blocks Smoothened (SMO) in the absence of HH. Ligand binding inhibits the repressive function of PTCH, allowing SMO to enter the primary cilium, an antenna-like organelle essential for HH signal coordination and transduction [12-17]. Ciliary activated SMO subsequently activates GLI2/3 transcription factors, which represent the downstream effectors of canonical HH signaling (Figure 1). HH/GLI target genes include feedback signaling proteins (e.g. PTCH, HHIP and GLI1) and genes involved in proliferation/cell cycle regulation, differentiation, self-renewal/stemness, metastasis and survival (for reviews see [3, 6, 18-20]). Suppressor of Fused (SUFU), a key negative regulator of mammalian HH signaling, directly binds to GLIs, thereby preventing their activation and nuclear localization [21]. In addition, proteolytic processing yielding C-terminally truncated GLI repressor forms and proteasome-dependent degradation of GLI proteins constitute critical negative regulatory mechanisms. In this context, phosphorylation of GLIs by protein kinase A (PKA) is a key repressive step in HH/GLI signaling that promotes GLI repressor formation and GLI destabilization [22-36].
Figure 1. IMQ represses HH/GLI signaling via ADORA/PKA activation.
Classical Hedgehog (HH) signaling is activated by secreted HH protein binding to its receptor PTCH (1). In cancer, loss of function mutations in PTCH (2), activating mutations in SMO (3), genetic loss of SUFU (4) or GLI1/2 amplification/overexpression (5) result in aberrant HH signaling and an increased GLI activator (GLI Act) to GLI repressor (GLI Rep) ratio, inducing HH target gene expression (e.g. GLI1, HHIP) and oncogenic transformation. Imiquimod (IMQ) activates Protein Kinase A (PKA) by engaging adenosine receptors (ADORAs), leading to GLI phosphorylation and functional inactivation via proteasome-mediated GLI repressor formation and/or GLI degradation. We propose that IMQ can block HH signaling in all pathway-activating events illustrated, even in settings where SMO inhibitors may no longer be effective (i.e. in settings 4 and 5, where GLI activation occurs in a SMO-independent manner, including GLI activation by other oncogenic pathways, for details see main text).
Of note, GLI transcription factors can be activated also in a non-canonical SMO independent manner, thereby reversing the therapeutic effect of SMO inhibitors used for targeted inhibition of oncogenic HH signaling [37-52].
The oncogenic role of HH signaling in cancer was first discovered in patients suffering from nevoid basal cell carcinoma syndrome (NBCCS) caused by genetic loss of PTCH function. NBCCS patients are prone to developing multiple basal cell carcinomas (BCC) in response to ligand independent constitutive activation of the HH pathway [53-56]. Clinical trials with the first FDA approved HH pathway inhibitor vismodegib (Erivedge), a selective SMO inhibitor, showed that targeting HH in BCC patients dramatically reduces tumor burden and prevents growth of new lesions [57-61]. However, more than 50% of patients receiving vismodegib discontinued drug treatment due to severe side effects including muscle cramps, nausea, hair, taste and weight loss [58, 59]. The efficacy of SMO inhibitors can be further limited by rapid development of drug resistance via mutations in SMO, genetic alterations downstream of SMO (e.g. loss of SUFU or gain of GLI copy number) or by the activation of compensatory pathways such as PI3K/AKT [50, 62-65].
The immune modulator imiquimod (IMQ, applied as 5% cream formulation referred to as Aldara) represents another FDA approved drug successfully applied for the treatment of superficial BCC, when surgery is less favorable [66-70].
IMQ is a synthetic nucleoside analogue of the imidazoquinoline family [71]. Its anti-tumor activity is multifactorial and not completely understood. IMQ is known to bind to and activate Toll-like receptors 7/8 (TLR7/8) thus stimulating TLR-MYD88 signaling. The resulting inflammatory reaction and antitumor response involves plasmacytoid dendritic and cytotoxic CD8+ cells attacking the tumor [72-74]. A direct effect of IMQ on oncogenic HH/GLI signaling in BCC has not been reported until recently.
In a screen for modifiers of HH/GLI signaling that comprised several TLR agonists including IMQ, our group noticed that IMQ has a direct repressive effect on GLI activity in mouse embryonic fibroblasts (H. Esterbauer, personal communication and unpublished data). In light of the well-documented therapeutic effect on BCC, this led us to hypothesize that IMQ may directly repress oncogenic HH/GLI signaling independent of its immune modulating function.
In the study by Wolff et al. [75], we tested for a putative direct effect of IMQ on HH signaling and found that IMQ directly blocks HH pathway activation in cultured murine BCC cells as evidenced by the repression of HH target genes including Gli1. Surprisingly, BCC cells do not express detectable levels of the cognate IMQ receptors TLR7/8, neither did genetic inhibition of the essential TLR effector MYD88 affect the repressive activity of IMQ on HH/GLI signaling. This suggested a non-classical, TLR-MYD88 independent effect of IMQ on HH/GLI signaling.
Two previous studies were key to interpret these unexpected and puzzling findings. Schön et al. have shown that IMQ can affect adenylate cyclase (AC) and protein kinase A (PKA) activity via binding to adenosine receptors (ADORAs) independent of TLR7/8 [76]. Equally important, a study analyzing hematopoietic progenitors in flies has identified adenosine/ADORA signaling as a negative regulator of Hh signaling via activation of PKA and repression of the fly GLI homologue Cubitus interruptus [77].
In line with these data, we observed that treatment of BCC cells or human GLI expressing keratinocytes with IMQ induced PKA-mediated GLI phosphorylation, thereby reducing the level of GLI activator and oncogenic HH signal strength, respectively (Figure 1).
The study by Wolff et al. therefore identified ADORAs as possible targets for inhibition of HH signaling in BCC, and it is tempting to speculate that other small molecule ADORA agonists currently in clinical or preclinical evaluation may also hold promise for anti-cancer therapy by repressing HH/GLI signaling. In this context it will also be important to address whether ADORA agonists can overcome current limitations of SMO inhibitors for the treatment of acquired or de novo SMO inhibitor resistant malignancies including cancers with SMO-independent GLI activation [9, 46, 48, 50, 63-65].
OPEN QUESTIONS AND FUTURE CHALLENGES
Although the surprising finding of a direct repressive role of IMQ on HH/GLI via stimulation of ADORA/AC/ PKA signaling revealed a new mode of action of a well-known drug, several questions remain to be addressed before any of these findings may be translated into clinical applications:
(1) Which ADORA subtype is responsible for the cell autonomous effect of IMQ?
In Wolff et al. [75] we investigated the expression of ADORA subtypes in human BCCs and demonstrated that ADORA2A and ADORA3 are overexpressed in BCCs compared to normal skin, whereas expression of ADORA1 and ADORA2B was comparable to normal skin samples [75]. By applying selective ADORA2A agonists and antagonists [78, 79] we showed that ADORA2A has a key role in mediating the HH-repressive effects of IMQ. However, quantification of GLI2 phosphorylation showed stronger phosphorylation by IMQ than by ADORA2A agonists. This may be explained by an additional receptor-independent activation of PKA by IMQ or by an additive effect resulting from activation of multiple ADORA subtypes by IMQ [76]. Subtype specific knockdown of each of the four ADORAs alone or in combination will therefore be important to understand the individual contribution of the respective ADORA family members to HH signal repression.
(2) Do distinct cancer entities engage different ADORA subtypes in the modulation of HH/GLI signaling?
A number of HH-associated cancer entities such as cancers of the skin, breast, lung and prostate express high levels of different ADORA subtypes [9, 80]. While the study by Wolff et al. suggests that ADORA2A may be the main negative regulator of HH signaling in BCC, it is well possible that other ADORA subtypes negatively or even positively modulate HH signaling in cancer entities other than BCC. The regulatory complexity is likely to be very high given the fact that ADORA signaling can also act as inducer of extracellular signal-regulated kinase (ERK) 1/2 activity [81, 82]. As ERK1/2 activation is a potent stimulus and modifier of GLI activity [39, 41, 44, 45, 83], ADORA signaling may also positively affect HH/ GLI signal strength in a context dependent manner. The use of IMQ as potential therapeutic for HH/GLI associated cancers therefore needs to be carefully evaluated for each tumor entity.
(3) Where does IMQ interact with HH/GLI signaling?
Detailed epistatic mapping of the repressive mechanism of IMQ on HH/GLI signaling will be key to stratify patients with HH-associated cancer into putative responders and non-responders. The data by Wolff et al. support a model where IMQ interferes with HH signaling downstream of SMO (Figure 1), suggesting that ADORA agonists may prove beneficial also for SMO-inhibitor resistant and SMO-independent cancer entities. This would also be an indication that combination treatments with vismodegib and ADORA agonists may improve therapeutic efficacy and possibly also prevent or at least delay the development of drug resistance. For this, additional experiments need to be performed to evaluate the efficacy of IMQ in cancer cells expressing drug-resistant variants of SMO or lacking SUFU, the key negative regulator of GLI.
The identification of IMQ as inhibitor of HH/GLI signaling has strong potential to broaden the spectrum of applications for this drug or derivatives thereof. However, addressing the open questions raised in this article is critical and there is still a significant way to go before optimized ADORA agonists can be clinically evaluated as potential drugs for the treatment of HH/GLI dependent cancers.
Acknowledgments
The authors are grateful to their lab members, in particular to Christina Sternberg and Pedro Del Burgo Martinez for discussions and critical reading of the manuscript. Work of the authors was funded by the Austrian Science Fund FWF (Projects W1213 to F.A. and A-M.F., P20652 and P25629 to F.A.) and the priority program Biosciences and Health of the Paris-Lodron University of Salzburg. The authors apologize to all colleagues whose work has not been cited or discussed due to space constraints.
REFERENCES
- 1.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–3457. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 3.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 4.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwenhuis E, Hui CC. Hedgehog signaling and congenital malformations. Clin Genet. 2005;67:193–208. doi: 10.1111/j.1399-0004.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 9.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–7851. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]
- 11.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 12.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 13.Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol. 2007;9:1005–1009. doi: 10.1038/ncb435. [DOI] [PubMed] [Google Scholar]
- 14.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr., Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varjosalo M, Taipale J. Hedgehog signaling. J Cell Sci. (Pt 1) 2007;120(Pt 1):3–6. doi: 10.1242/jcs.03309. [DOI] [PubMed] [Google Scholar]
- 19.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 20.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 21.Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 22.Huntzicker EG, Estay IS, Zhen H, Lokteva LA, Jackson PK, Oro AE. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20:276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 24.Di Marcotullio L, Greco A, Mazza D, Canettieri G, Pietrosanti L, Infante P, Coni S, Moretti M, De Smaele E, Ferretti E, Screpanti I, Gulino A. Numb activates the E3 ligase Itch to control Gli1 function through a novel degradation signal. Oncogene. 2011;30:65–76. doi: 10.1038/onc.2010.394. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Wang B, Jiang J. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes Dev. 1999;13:2828–2837. doi: 10.1101/gad.13.21.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 27.Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2009;326:177–189. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126:3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- 30.Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80:563–572. doi: 10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- 32.Lepage T, Cohen SM, Diaz-Benjumea FJ, Parkhurst SM. Signal transduction by cAMP-dependent protein kinase A in Drosophila limb patterning. Nature. 1995;373:711–715. doi: 10.1038/373711a0. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- 34.Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R. Gli protein activity is controlled by multisite phosphorylation in vertebrate Hedgehog signaling. Cell reports. 2014;6:168–181. doi: 10.1016/j.celrep.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks SA, Kalderon D. Regulation of mammalian Gli proteins by Costal 2 and PKA in Drosophila reveals Hedgehog pathway conservation. Development. 2011;138:2533–2542. doi: 10.1242/dev.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotani T. Protein kinase A activity and Hedgehog signaling pathway. Vitamins and hormones. 2012;88:273–291. doi: 10.1016/B978-0-12-394622-5.00012-2. [DOI] [PubMed] [Google Scholar]
- 37.Aberger F, Ruiz IAA. Context-dependent signal integration by the GLI code: The oncogenic load, pathways, modifiers and implications for cancer therapy. Seminars in cell & developmental biology. 2014;33C:93–104. doi: 10.1016/j.semcdb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stecca B, Ruiz IAA. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riobo NA, Haines GM, Emerson CP., Jr Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 2006;66:839–845. doi: 10.1158/0008-5472.CAN-05-2539. [DOI] [PubMed] [Google Scholar]
- 42.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauth M, Bergstrom A, Toftgard R. Phorbol esters inhibit the Hedgehog signalling pathway downstream of Suppressor of Fused, but upstream of Gli. Oncogene. 2007;26:5163–5168. doi: 10.1038/sj.onc.1210321. [DOI] [PubMed] [Google Scholar]
- 44.Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G, Philpott MP, Aberger F. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283–6298. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M, Aberger F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 48.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, Endo Y, Rubin JS, Toretsky J, Uren A. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelczar P, Zibat A, van Dop WA, Heijmans J, Bleckmann A, Gruber W, Nitzki F, Uhmann A, Guijarro MV, Hernando E, Dittmann K, Wienands J, Dressel R, Wojnowski L, Binder C, Taguchi T, et al. Inactivation of Patched1 in mice leads to development of gastrointestinal stromal-like tumors that express Pdgfralpha but not kit. Gastroenterology. 2013;144:134–144 e136. doi: 10.1053/j.gastro.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494:484–488. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillman RT, Feng BY, Ni J, Woo WM, Milenkovic L, Hayden Gephart MG, Teruel MN, Oro AE, Chen JK, Scott MP. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333–2346. doi: 10.1101/gad.173054.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X, Lee DF, Hsu JM, Huo L, Labaff AM, Liu D, Huang TH, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr., Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 55.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 56.Gailani MR, Stahle-Backdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, Unden AB, Dean M, Brash DE, Bale AE, Toftgard R. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 57.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr., de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. The New England journal of medicine. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 58.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, Epstein EH., Jr Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. The New England journal of medicine. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Low JA, Mackey HM, Yauch RL, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. The New England journal of medicine. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nature reviews Drug discovery. 2012;11:437–438. doi: 10.1038/nrd3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA, Zerivitz KL, Low JA, Von Hoff DD. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England journal of medicine. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, Bazan JF, Kan Z, Seshagiri S, Hann CL, Gould SE, Low JA, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, Hsiao K, Yuan J, Green J, Ospina B, Yu Q, Ostrom L, Fordjour P, Anderson DL, Monahan JE, Kelleher JF, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Jr., Beachy PA, Rudin CM. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marks R, Gebauer K, Shumack S, Amies M, Bryden J, Fox TL, Owens ML, Australasian Multicentre Trial G. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. Journal of the American Academy of Dermatology. 2001;44:807–813. doi: 10.1067/mjd.2001.113689. [DOI] [PubMed] [Google Scholar]
- 67.Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. Journal of the American Academy of Dermatology. 2004;50:722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 68.Geisse JK, Rich P, Pandya A, Gross K, Andres K, Ginkel A, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: a double-blind, randomized, vehicle-controlled study. Journal of the American Academy of Dermatology. 2002;47:390–398. doi: 10.1067/mjd.2002.126215. [DOI] [PubMed] [Google Scholar]
- 69.Lacarrubba F, Potenza MC, Gurgone S, Micali G. Successful treatment and management of large superficial basal cell carcinomas with topical imiquimod 5% cream: a case series and review. The Journal of dermatological treatment. 2011;22:353–358. doi: 10.3109/09546634.2010.548503. [DOI] [PubMed] [Google Scholar]
- 70.Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: an overview. The British journal of dermatology. 2003;149(Suppl 66):53–56. doi: 10.1046/j.0366-077x.2003.05626.x. [DOI] [PubMed] [Google Scholar]
- 71.Perry CM, Lamb HM. Topical imiquimod: a review of its use in genital warts. Drugs. 1999;58:375–390. doi: 10.2165/00003495-199958020-00017. [DOI] [PubMed] [Google Scholar]
- 72.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. The Journal of clinical investigation. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holcmann M, Drobits B, Sibilia M. How imiquimod licenses plasmacytoid dendritic cells to kill tumors. Oncoimmunology. 2012;1:1661–1663. doi: 10.4161/onci.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schon MP, Schon M. Imiquimod: mode of action. The British journal of dermatology. 2007;157(Suppl 2):8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 75.Wolff F, Loipetzberger A, Gruber W, Esterbauer H, Aberger F, Frischauf AM. Imiquimod directly inhibits Hedgehog signalling by stimulating adenosine receptor/protein kinase A-mediated GLI phosphorylation. Oncogene. 2013;32:5574–5581. doi: 10.1038/onc.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7 - and TLR8- independent fashion. The Journal of investigative dermatology. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 77.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell - and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hutchison AJ, Webb RL, Oei HH, Ghai GR, Zimmerman MB, Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. The Journal of pharmacology and experimental therapeutics. 1989;251:47–55. [PubMed] [Google Scholar]
- 79.Todde S, Moresco RM, Simonelli P, Baraldi PG, Cacciari B, Spalluto G, Varani K, Monopoli A, Matarrese M, Carpinelli A, Magni F, Kienle MG, Fazio F. Design, radiosynthesis, and biodistribution of a new potent and selective ligand for in vivo imaging of the adenosine A(2A) receptor system using positron emission tomography. Journal of medicinal chemistry. 2000;43:4359–4362. doi: 10.1021/jm0009843. [DOI] [PubMed] [Google Scholar]
- 80.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 81.Schulte G, Fredholm BB. Human adenosine A, A(2A), A(2B), and A receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular- regulated kinase 1/2. Molecular pharmacology. 2000;58:477–482. [PubMed] [Google Scholar]
- 82.Graham S, Combes P, Crumiere M, Klotz KN, Dickenson JM. Regulation of p42/p44 mitogen-activated protein kinase by the human adenosine A3 receptor in transfected CHO cells. European journal of pharmacology. 2001;420:19–26. doi: 10.1016/s0014-2999(01)00976-1. [DOI] [PubMed] [Google Scholar]
- 83.Whisenant TC, Ho DT, Benz RW, Rogers JS, Kaake RM, Gordon EA, Huang L, Baldi P, Bardwell L. Computational prediction and experimental verification of new MAP kinase docking sites and substrates including Gli transcription factors. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]