Abstract
The anti-oxidant lipoic acid (LA) potently suppresses clinical and pathologic disease in the animal model of multiple sclerosis, experimental autoimmune encephalomyelitis, by inhibiting the migration of pathogenic T cells to the spinal cord. The mechanism by which this occurs is largely unknown. In this report we demonstrate that LA induces increases in cyclic AMP, a known immunosuppressant, in human T cells. The increase in cAMP is associated with increased adenylyl cyclase activity and is partially blocked by prostanoid receptor antagonists. We present evidence that LA also stimulates cAMP production in natural killer (NK) cells. This novel mechanism of action is highly relevant to the immunomodulatory effects of LA and provides further support for the study of LA as a therapeutic agent for multiple sclerosis and other autoimmune diseases.
Keywords: lipoic acid, cAMP, lymphocyte, NK cell
Introduction
Lipoic acid (LA) is an anti-oxidant that is highly effective in treating the animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE). Several laboratories have demonstrated that LA can completely suppress clinical and pathologic EAE by preventing migration of pathogenic T cells and monocytes into the spinal cord [1, 2], [3]. Impaired migration is associated with decreased expression of the cellular adhesion markers ICAM-1 and VCAM-1 on neuronal endothelial cells [4]. LA has also been shown to decrease migration of human T cells in vitro [5]. Importantly, a double blind placebo controlled trial of LA in MS subjects suggested that LA could decrease two biomarkers related to T cell migration, matrix metalloproteinase-9 (MMP-9) and sICAM-1 [6]. However the mechanism by which LA elicits these effects is still unclear.
The small molecule second messenger cyclic AMP (cAMP) has been studied as a potent inhibitor of the immune response and T cell activation for many years. Agents that increase intracellular concentrations of cAMP modulate the immune system in a variety of ways including inhibiting T lymphocyte activation and cell migration [7, 8]. Intracellular levels of cAMP are elevated by modulation of adenylyl cyclases (AC), through conversion of ATP to cAMP, and phosphodiesterases (PDE), via degradation of cAMP into AMP and Pi. Consequently, cAMP levels can be increased through stimulation of ACs or inhibition of PDEs. Prostaglandins stimulate G-protein coupled receptors and stimulatory G proteins (Gαs) to activate AC and increase cAMP [9].
Here we demonstrate for the first time that a brief treatment of human primary enriched T cells, purified human T cells, or purified human NK cells; with LA results in a concentration-dependent increase in cAMP levels. The increase in cAMP is dependent on adenylate cyclase activity and is partially blocked using antagonists of the prostaglandin E receptors, but requires components in addition to the prostanoid E receptors.
Materials and Methods
Cell culture
Human peripheral blood mononuclear cells (PBMC) were obtained from source leukocytes (buffy coat) from the Portland Red Cross (approval #VACARR) within three hours of being drawn from the donor. Enriched leukocytes were subjected to ficoll purification (Amersham). The interface was collected and washed with RPMI 1640 (Life Technologies, Gaithersburg, MD). For T-cell enriched PBMCs, cells from the interface were subjected to two rounds of adherence to plastic, one for 1 hr in RPMI the second overnight in RPMI 1%FCS both at 37°C, 5% CO2. Non-adherent cells were collected. The T-cell enriched population contains >90% CD3 positive cells, <3% CD19 positive cells and <1% CD14 positive cells, as determined by flow cytometry. Cells were cryo-frozen in RPMI 1640, 25% FCS, 12%DMSO, (Nalgene cryofreezing container) and stored in liquid nitrogen. Cells were thawed into RPMI 1640 and allowed to rest at 37°C, 5% CO2 for one hour prior to use.
Monocyte-derived dendritic cells were prepared according to the method of Romani et al. [10]. Purified T or NK cells were obtained by negative selection using Dynal T cell negative isolation kit version II or Dynal NK cell negative selection kit (Invitrogen, Inc.). Purified T cells are 97% CD3+, <2% CD56+, <1% CD14+, <0.1% CD19+ as determined by flow cytometry. Purified NK cells are 96% CD56+, <1% CD3+, <2 % CD19+as determined by flow cytometry.
HEK293 EBNA were maintained in Dulbecco’s modified eagles medium high glucose with 10% FCS.
Cyclic AMP assays
Except where indicated otherwise, two million cells in 500 μl RPMI 1640 were used in cAMP assays. After treatment, cells were lysed with 0.1M HCl and the cAMP ELISA was performed as per manufacturer’s protocol (Assay Designs, Ann Arbor, MI). LA was a gift from Integrative Technologies, Inc. (Wilsonville, OR). Dihydrolipoic acid (DHLA) and dimethyl lipoic acid (DMLA) were synthesized and generously provided by Dr. Rolf Winter at the Portland VA Medical Center (Portland, OR). Lipoamide (LPM) (DL 6,8-thioctic acid amide), was purchased from Sigma (St. Louis, MO). LA, DHLA, DMLA, and LPM were prepared fresh weekly as described [1]. Cells treated with LA, DHLA, DMLA, LPM, were incubated at the indicated concentrations for one minute, centrifuged for one minute at 13000 × g, and lysed in 0.1M HCl. Cells treated with forskolin (Sigma-Aldrich) were incubated for 10 minutes at 37°C. The AC inhibitor 2’5’-dideoxy-adenosine (Biomol, Plymouth Meeting PA) was pre-incubated with cells for 25 minutes at 37°C before treating with either forskolin or LA. Cells treated with prostaglandin E2 (PGE2; Sigma-Aldrich) or prostaglandin D2 (PGD2; Cayman Chemicals Ann Arbor, MI) were incubated for 1.5 minutes, centrifuged and lysed. BWA868C (Cayman Chemicals), AH23848 (Sigma-Aldrich), or AH6809 (Sigma-Aldrich) were added to cells 30 minutes before incubation with LA or the prostanoids.
PDE Assay
1.5 × 106 PBMC were lysed in 120 μl lysis buffer (5mM Tris pH6.8, 5 mM EGTA, 250 mM Sucrose, 0.1% Triton X-100 and protease inhibitor cocktail (Sigma-Aldrich)) and kept on ice. LA (200 μM) or isobutylmethylxanthine (IBMX) (1 mM) were added to the lysates and PDE activity was assayed. 30 μl of lysate was incubated with a master mix of 10 μl of PDE buffer A [100 mM MOPS pH 7.5, 4 mM EGTA, 1 mg/ml bovine serum albumin (BSA)], 10 μl of PDE buffer B (100 mM MOPS pH 7.5 75 mM MgAc, 100,000 c.p.m. of [3H] cAMP (Dupont, NEN)), and 1 μl of 1 mM cAMP in a total volume of 250 μl. The reaction was terminated by boiling and the addition of 10 μl of snake venom (2.5 mg/ml). The samples were then transferred to an ion exchange column and [3H] nucleoside was eluted into scintillation vials. Scintillation fluid was added to the eluted samples, mixed and counted in a scintillation counter.
Receptor transfections
HEK293 EBNA cells (2 ×105) were seeded into a 12 well plate and allowed to grow overnight. Cells were transfected with 5 μg pcDNA3 EP2 or pcDNA3 EP4 using the standard Lipofectamine 2000 protocol (Invitrogen). After 24 hours cells were treated with PGE2 (10 μM) or LA (100μg/ml) for one hour in media containing 100μM IBMX. The media was aspirated and 0.1M HCl was added, after 10 minutes the lysed cells were scraped out of the well and transferred with the HCl to an eppendorf tube, boiled for 5 minutes, centrifuged for 1 minute at 13,000 × g and the supernatant was used in a cAMP assay.
Results and Discussion
Lipoic acid increases cAMP levels in human T cell enriched PBMC
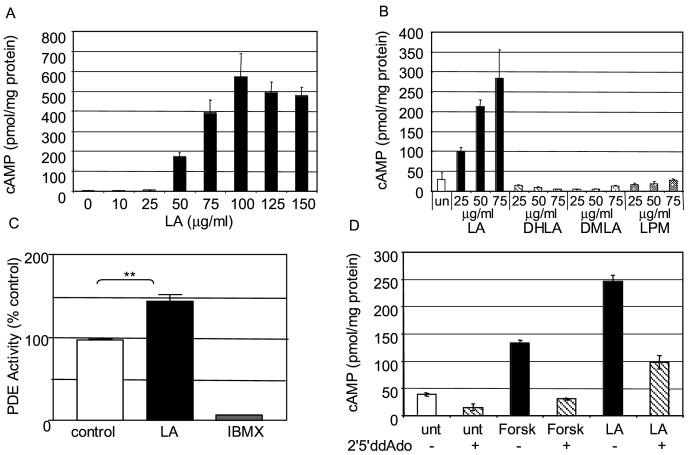
LA prevents the migration of inflammatory T cells to the spinal cords of mice, and inhibits human T cell migration across a fibronectin barrier in vitro [5]. To determine if the anti-inflammatory effects of LA are related to cAMP levels, we treated human T cell enriched PBMC with LA and measured cAMP levels. A one-minute treatment of human T cell enriched PBMC with physiological concentrations of LA results in a concentration-dependent increase in cAMP levels (Figure 1A). The half maximal LA concentration and the maximal cAMP levels varied between donors from 5-50 μg/ml LA (data not shown) and 300-1500 pmol/mg protein cAMP, respectively, but consistently resulted in 5-8 fold increases in cAMP (n=13). Thus, subsequent experiments were performed on T cell-enriched PBMC that had been calibrated by LA dose response and treated with levels of LA that would elicit maximal increases in cAMP. Derivatives of LA, including its reduced form DHLA, as well as DMLA and LPM had no effect on cAMP levels (Figure 1B).
Figure 1.
LA increases cAMP levels in T-cell enriched PBMC. A) Two milllion T-cell enriched PBMCs were incubated with the indicated concentrations of LA for one minute. The cells were lysed and cAMP levels were quantitated by ELISA. B) LA derivatives DHLA, DMLA and LPM do not increase cAMP levels. PBMC were incubated with the indicated concentrations of LA, DHLA, DMLA, or LPM for one minute. Cells were lysed and cAMP levels were quantitated by ELISA. C) PDE Activity Assay. Two milllion PBMC were lysed, LA (200 μM) or IBMX (1 mM) was added to the lysate, and PDE activity was assayed. D) Adenylyl Cyclase assay. Two milllion PBMC were incubated with 250 μM 2’5’dideoxy-adenosine for 30 minutes at 37°C before incubation with forskolin (25 μM, 30 minutes) or LA (10 μg/ml, one minute). Each graph is one representative experiment of at least three in which cAMP values were determined in triplicate (error bars=SEM, (C) student paired t-test p=0.007) and replicate experiments were conducted with different donors to eliminate donor specific artifacts.
The concentration of cAMP within a cell is controlled by AC, which catalyzes the production of cAMP, and PDE which catalyses the degradation of cAMP. To ascertain if the increase in cAMP levels was the result of inhibited PDE activity, we treated PBMC with LA and measured PDE activity. PBMC were lysed, LA (200 μM) or IBMX (1 mM) was added to the lysate and PDE activity was quantitated. As illustrated in Figure 1C, LA did not decrease PDE activity but rather increased it.
To determine if LA increased cAMP levels through activation of AC, PBMC were treated with LA in the presence and absence of the cell-permeable inhibitor of AC, 2’5’ dideoxyadenosine (2’5’dd-ADO). As a control, cells were incubated with forskolin in the presence and absence of 2’5’dd-ADO. As illustrated in Figure 1D, incubation of the cells with LA or forskolin increased cAMP levels, but when cells were incubated with LA or forskolin in the presence of the AC inhibitor the increase in cAMP was reduced. These data suggest that LA increases cAMP levels via activation of AC.
Involvement of EP receptors
AC is stimulated through interaction with G-proteins and G-protein coupled receptors, and prostaglandins are known to induce rapid increases in cAMP. We therefore investigated the possibility that LA is activating prostanoid receptors. To examine the involvement of these G-protein coupled receptors, cells were exposed to LA in the presence of prostanoid receptor antagonists.
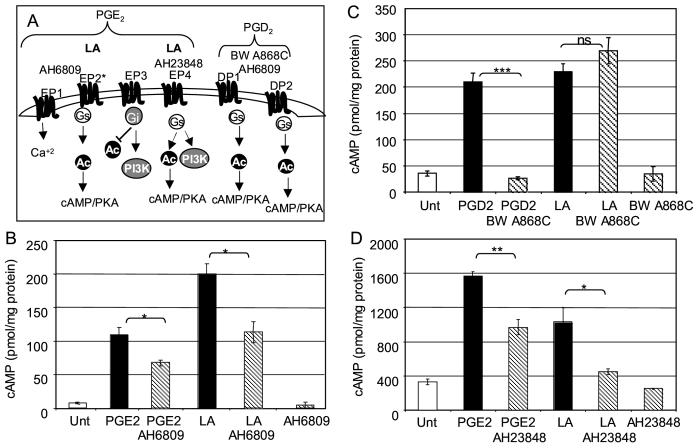
Prostaglandins can elicit a wide variety of effects on T cells through the activation of multiple signaling pathways [11]. Relevant to cAMP signaling are the roles of PGE2 and PGD2. PGE2 stimulates four prostanoid receptors EP1, EP2, EP3, and EP4. EP2, EP4 and certain splice variants of EP3 can result in increased cAMP levels. The EP1 receptor is coupled to changes in calcium while other splice variants of the EP3 receptor are coupled to the inhibitory G protein (Gi) resulting in decreased cAMP levels. The PGD2 receptors DP1 and DP2 are also coupled to Gαs and activation results in increased cAMP levels, see cartoon Figure 2A.
Figure 2.
EP receptor antagonists, but not DP receptor antagonists, inhibit LA mediated elevation of cAMP. A) Cartoon illustrating EP and DP receptor pathways, sites of antagonist action, and potential sites of LA action. B) The EP/DP antagonist AH6809 interferes with LA induction of cAMP. Two milllion PBMCs were incubated with AH6809 (10 μM for 30 min pretreatment), PGE2 (10 μM for 1.5 minutes), or LA (50 μg/ml, one minute). C) The DP specific antagonist BWA868C does not interfere with LA induction of cAMP. Two milllion PBMCs were incubated with BWA868C (1 μM for 30 min pretreatment), PDE2 (300 nM for 1.5 minutes), or LA (50 μg/ml, one minute). D) The EP4 antagonist AH23848 interferes with LA induction of cAMP. Two milllion PBMCs were incubated with AH23848 (30 μM for 30 min pretreatment), PGE2 (10 μM for 1.5 minutes), or LA (50 μg/ml, one minute). In each experiment, after incubation the cells were lysed and cAMP levels were quantitated by ELISA. Each graph is one representative experiment of at least three in which cAMP values were determined in triplicate (error bars=SEM, student unpaired T test; *, p=0.01-0.05; **, p=0.001-0.01; ***, p<0.001). Replicate experiments were conducted with different donors to eliminate donor specific artifacts.
A specific antagonist to the EP2 receptor is not commercially available. AH6809 has the greatest specificity for DP receptors but also inhibits EP1 and EP2 receptors [12, 13]. Treatment of PBMC with PGE2 or LA increased cAMP levels, however, treatment of PBMC with AH6809 before incubation with PGE2 or LA blocked the increase in cAMP levels by 30% and 40%, respectively. Incubation of the cells with AH6809 alone had no effect on cAMP levels (Figure 2B).
Evidence that LA increases cAMP levels through interactions with the EP2 prostanoid receptor, but not the DP prostanoid receptor is presented in Figure 2C. Pretreatment with the DP specific antagonist BWA868C fully inhibited increases in cAMP levels elicited by PGD2, BWA868C had no effect on increases in cAMP levels induced by LA. Incubation of the cells with BWA868C alone had no effect on cAMP levels.
Data to support LA activation of the EP4 receptor in addition to the EP2 receptor, is shown in Figure 2D. Treatment of PBMC with PGE2 or LA for one minute again results in increased cAMP levels. Pretreatment of PBMC with the EP4 specific antagonist, AH23848, for 30 minutes followed by incubation with PGE2 or LA inhibits the increase in cAMP levels by 39% and 57%, respectively. Incubation of the cells with AH23848 alone does not affect cAMP levels.
EP receptor over expression studies
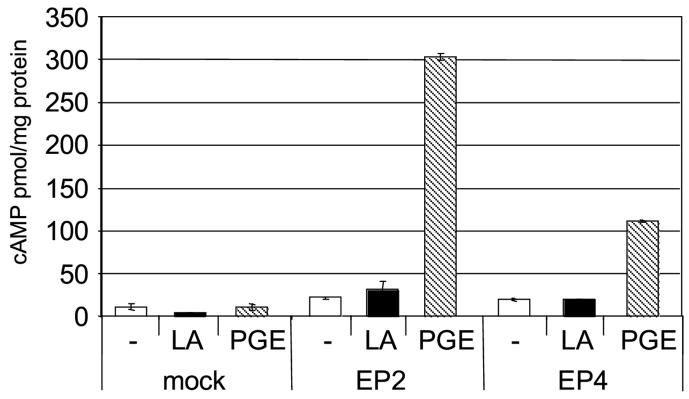
The above data suggest that LA is inducing cAMP though EP receptors. To test this directly we transfected HEK 293 EBNA cells with EP2 or EP4 receptor constructs and assayed the ability of LA or PGE2 to stimulate cAMP production (Figure 3). Our data confirm experiments published by Regan and colleagues, that untransfected HEK 293 EBNA cells do not respond to PGE2, and PGE2 treatment after transfection with the EP2 receptor results in more cAMP than PGE2 treatment after transfection with the EP4 receptor[14]. However, treatment with LA did not stimulate cAMP production from either the EP2 or EP4 receptor transfected cells. Similar results were obtained from receptor transfections in Hela cells confirming this is not a cell dependent phenomenon (data not shown). These data suggest that components in addition to the EP2 or EP4 receptor are required for LA mediated stimulation of cAMP production.
Figure 3.
EP2 and EP4 receptor transfection studies. Hek293 EBNA cells were transiently transfected with pcDNA3 (mock) or pcDNA3 containing either EP2 or EP4 receptor DNA and incubated without (-) or with 100 μg/ml LA (solid bars) or 10 μM PGE2 (hatched bars) for one hour in media containing 100 μM IBMX. Cells were lysed in 0.1M HCl and cAMP levels were determined. Shown is one representative experiment of five in which transfections were conducted in triplicate. Error bars are S.E.M.
Effects of LA on other immune cell types
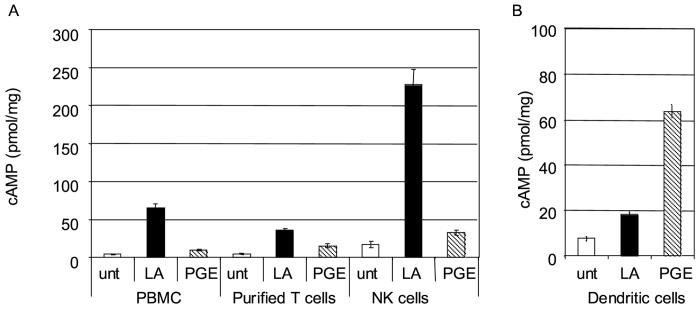
To look at different populations of primary immune cells we purified T cells and NK cells by negative selection, and cultured dendritic cells using GM-CSF and IL-4 treatment of adherent PBMC. We treated 1 × 106 purified T cells, 2 × 105 NK cells or 2 × 105 dendritic cells with LA or PGE2. We found that NK cells produced the most cAMP in response to LA, purified T cells produced significant amounts of cAMP, but less than NK cells, while dendritic cells produced the least cAMP (Figure 4).
Figure 4.
LA stimulates cAMP from NK cells. A) T cells or NK cells were purified by negative selection. 1×106 PBMCs or purified T cells, or 2×105 NK cells in RPMI 1640 were incubated for one minute with 100 μg/ml LA or 100 nM PGE2. B, 2×105 dendritic cells in RPMI 1640 were incubated for five minutes with 100 μg/ml LA or 100 μM PGE2. Cells were lysed in 0.1M HCl and assayed for cAMP.
Recently NK cells have been shown to mediate the immunomodulatory effects of IL-2 receptor-targeted therapy in MS [15]. NK cells expressing CD11chigh are associated with patients experiencing an MS relapse, and that NK cell stimulatory cytokines such as IL-15 upregulate CD11c expression on NK cells [16]. CD11c has also been shown to be involved in adhesion to stimulated endothelium [17]. We have demonstrated that cAMP induction in T cells results in inhibition of IL-15 [18]. Thus we might hypothesize that LA mediated stimulation of cAMP in NK cells could inhibit IL-15 mediated induction of CD11c, altering the ability of NK cells to regulate T cell migration and the course of MS.
In summary, we have demonstrated for the first time that LA, but not DHLA, can increase cAMP levels in T cells and NK cells by stimulating AC activity. This novel mechanism of action may explain some of the dramatic effects LA has on EAE and provides further rationale for studying LA as a treatment for MS and other autoimmune diseases.
Acknowledgments
This research was supported by Department of Veterans Affairs Biomedical Laboratory Research & Development Service (DWC and DNB), by NIH grant P50AT00066-01 (DNB), and the Nancy Davis Center Without Walls (DNB).
Abbreviations
- AC
Adenylyl Cyclase
- cAMP
cyclic AMP
- DHLA
dihydrolipoic acid
- DMLA
dimethyl lipoic acid
- IBMX
isobutylmethylxanthine
- LA
Lipoic Acid
- LPM
lipoamide (DL 6,8-thioctic acid amide)
- NK
natural killer cell
- PDE
phosphodiesterase
- PGE2
Prostaglandin E2
- PGD2
Prostaglandin D2
- PKA
Protein Kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;131:104–114. doi: 10.1016/s0165-5728(02)00269-2. [DOI] [PubMed] [Google Scholar]
- [2].Morini M, Roccatagliata L, Dell′Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–153. doi: 10.1016/j.jneuroim.2003.11.021. [DOI] [PubMed] [Google Scholar]
- [3].Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol SM, Hendrikx EM, Dopp ED, Dijkstra CD, Drukarch B, de Vries HE. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006;177:2630–2637. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- [4].Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [5].Marracci GH, McKeon GP, Marquardt WE, Winter RW, Riscoe MK, Bourdette DN. Alpha lipoic acid inhibits human T-cell migration: implications for multiple sclerosis. J Neurosci Res. 2004;78:362–370. doi: 10.1002/jnr.20255. [DOI] [PubMed] [Google Scholar]
- [6].Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11:159–165. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- [7].Kuklina EM, Shirshev SV. Role of cAMP-dependent signal transduction in the control of T lymphocyte activation. Biochemistry (Mosc) 2000;65:629–639. [PubMed] [Google Scholar]
- [8].Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [9].Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- [10].Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- [12].Keery RJ, Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br J Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- [14].Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- [15].Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aranami T, Miyake S, Yamamura T. Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J Immunol. 2006;177:5659–5667. doi: 10.4049/jimmunol.177.8.5659. [DOI] [PubMed] [Google Scholar]
- [17].Stacker SA, Springer TA. Leukocyte integrin P150,95 (CD11c/CD18) functions as an adhesion molecule binding to a counter-receptor on stimulated endothelium. J Immunol. 1991;146:648–655. [PubMed] [Google Scholar]
- [18].Kasyapa CS, Stentz CL, Davey MP, Carr DW. Regulation of IL-15-stimulated TNF-alpha production by rolipram. J Immunol. 1999;163:2836–2843. [PubMed] [Google Scholar]