Abstract
Background
Acetaminophen (APAP) hepatotoxicity is currently the most frequent cause of acute liver failure in the US and many European countries. Although intracellular signaling mechanisms are critical for hepatocellular injury, a contribution of inflammatory cells, especially neutrophils, has been suggested. However, conflicting results were obtained when using immunological intervention strategies.
Aims
The role of neutrophils was investigated using a CD18-deficient mouse model.
Results
Treatment of C57Bl/6 wild type mice with 300 mg/kg APAP resulted in severe liver cell necrosis at 12 and 24 h. This injury was accompanied by formation of cytokines and chemokines and accumulation of neutrophils in the liver. However, there was no difference in the inflammatory response or liver injury in CD18-deficient mice compared to wild type animals. In contrast to treatment with endotoxin, no upregulation of CD11b or priming for reactive oxygen was observed on neutrophils isolated from the peripheral blood or the liver after APAP administration. Furthermore, animals treated with endotoxin 3 h after APAP experienced an exaggerated inflammatory response as indicated by substantially higher cytokine and chemokine formation and twice the number of neutrophils in the liver. However, liver injury in the two-hit model was the same as with APAP alone.
Conclusions
Our data do not support the hypothesis that neutrophils contribute to APAP hepatotoxicity or that a neutrophil-mediated injury phase could be provoked by a second, pro-inflammatory hit. Thus, APAP-induced liver injury in mice is dominated by intracellular mechanisms of cell death rather than by neutrophilic inflammation.
Keywords: drug hepatotoxicity, acetaminophen, innate immunity, neutrophils, integrins, oxidant stress, inflammation
INTRODUCTION
Acetaminophen (APAP) overdose is currently the most frequent cause of acute liver failure in the US and many other countries (1,2). The mechanisms of cell injury induced by APAP have been extensively studied in vivo and in primary cultured hepatocytes during the last several decades. The main intracellular events critical for hepatotoxicity include the formation of a reactive metabolite, which causes glutathione depletion and covalent protein modifications, mitochondrial dysfunction and oxidant stress, peroxynitrite formation, and nuclear DNA fragmentation by mitochondrial-derived endonuclease G and apoptosis-inducing factor (3–5). Ultimately these events, especially the oxidant stress and peroxynitrite formation in the mitochondria (6), trigger the opening of the membrane permeability transition pore and the collapse of the mitochondrial membrane potential leading to necrotic cell death (7,8).
In addition to the intracellular signaling events, a potential contribution of inflammatory cells to the overall injury after APAP overdose came into focus during the last decade (9,10). During an inflammatory response, neutrophils can accumulate in sinusoids, extravasate and kill stressed hepatocytes mainly through reactive oxygen formation (11). It is well documented that liver injury can be aggravated by neutrophils during ischemia-reperfusion injury (12), endotoxemia (13), alcoholic hepatitis (14,15), and obstructive cholestasis (16) as well as during drug- and chemical-induced hepatotoxicity by α-naphthylisothiocyanate (17,18), halothane (19) and concanavalin A (20). However, the role of neutrophils in acetaminophen hepatotoxicity is controversially discussed (21). Evidence for (22–24) and against (25–27) a contribution of neutrophils to the overall injury has been presented.
More recent reports show that danger-associated molecular patterns (DAMPs) released from necrotic hepatocytes after APAP overdose can activate toll-like receptors on Kupffer cells and induce the formation of inflammatory mediators through the inflammasome (28–30). The implication is again that inflammatory cytokines such as interleukin-1β promote a neutrophil-mediated aggravation of the initial injury (28–30). A problem with the previous studies evaluating a potential effect of neutrophils on APAP hepatotoxicity was the use of immunological agents, i.e. neutropenia-inducing antibodies, which resulted in conflicting results (22, 23, 26, 27). To avoid these issues, we applied a genetic approach by using mice deficient in the β-chain (CD18) of β2 integrins (CD11/CD18). β2 integrins on neutrophils are critical for their adhesion to receptors on endothelial cells and hepatocytes as well as the adherence-dependent generation of reactive oxygen species (31). Consequently, neutrophil accumulation during peritonitis was drastically reduced in CD18-deficient mice (32). In addition, we showed previously in these mice impaired extravasation of neutrophils and reduced reactive oxygen formation in the liver resulting in less inflammatory cell injury during obstructive cholestasis (16). Based on these data, we tested the hypothesis that if neutrophils play a relevant role in the progression of APAP hepatotoxicity, CD18-deficient mice should be at least partially protected.
MATERIALS AND METHODS
Animals
Male CD18-deficient mice (B6.129S7-Itgb2tm1Bay/J; Stock number: 002128), which are backcrossed to a C57BL/6J background and their age-matched control littermates (C57BL/6J) with an average weight of 18 to 20 g were purchased from Jackson Laboratory (Bar Harbor, Maine). All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food (# 8604 Teklad Rodent, Harlan, Indianapolis, IN) and water. The experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise.
Experimental design
Mice were intraperitoneally injected with 300 mg/kg APAP (dissolved in warm saline) after overnight fasting. The animals were killed 12 or 24 h after APAP treatment, blood was withdrawn from the vena cava into a heparinized syringe for measurement of alanine aminotransferase (ALT) activities (Kinetic Test Kit 68-B, Biotron Diagnostics, Inc., Hernet, CA) and cytokine levels. The liver was removed and was rinsed in saline; liver sections were fixed in 10% phosphate buffered saline for histological analyses. The remaining liver was snap-frozen in liquid nitrogen and stored at −80 °C. In additional experiments, groups of animals were first treated with 300 mg/kg APAP and 3 h later either with saline (10 ml/kg) or 100 µg/kg Salmonella abortus equi endotoxin (ET) (i.p.). As positive controls for neutrophil-mediated liver injury, a few animals were subjected to common bile duct ligation (BDL) for 2 days as described in detail (16).
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 µm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for blinded evaluation of the areas of necrosis by the pathologist. The percent of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared to the entire cross section. Additional sections were stained for neutrophils. Endogenous peroxidase was suppressed with peroxidase suppressor (Thermo, Waltham, MA). Tissue was blocked with serum free blocker (Dako, Carpinteria, CA). Trypsin antigen retrieval was performed (Abcam, Cambridge, MA). Anti-mouse neutrophil allotypic marker antibody (AbD Serotec, Raleigh, NC) was incubated for 1 hour at room temperature. Secondary antibody coupled with biotin was then added (Vector, Burlingame, CA) followed by streptavadin-HRP conjugate (Vector). DAB substrate was used (Dako) and then counterstained with hematoxylin (Sigma). Positively stained cells consistent with neutrophil morphology were quantified in randomly selected high power fields (HPF, ×400).
mRNA expression
Quantification of mRNA expression of several cytokines and chemokines was performed by real-time PCR (RT-PCR) analysis as previously described (33). cDNA was generated by reverse transcription of total RNA by M-MLV reverse transcriptase in the presence of random primers (Invitrogen, Carlsbad, CA). Forward and reverse primers for the genes were designed using Primer Express software (Applied Biosystems, Foster City, CA). After normalization of cDNA concentrations, SYBR green PCR Master Mix (Applied Biosystems) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the ABI 7900 instrument (Applied Biosystems). All genes evaluated were first normalized to β-actin and then expressed as a fold increase relative to control which was arbitrarily set as 1.0. Calculations are made by assuming one cycle is equivalent to a two-fold difference in copy number which is the 2^(-ddCt) formula.
Plasma cytokine measurements
Quantification of cytokines was performed in plasma by the Bio-Plex bead-based multiplex assay following the kit instructions (Bio-Rad Laboratories, Hercules, CA) and analyzed on the Bio-Plex 200 instrument (Bio-Rad).
Isolation of hepatic non-parenchymal cells
The procedure was adapted from a method decribed by Watanabe et al. (34). Under isoflurane anesthesia mice were exsanguinated from the caudal vena cava into heparinized tubes and the blood was placed on ice; the liver was then perfused through the portal vein with 1.0 mL ice-cold Dulbecco’s PBS. The liver was immediately excised, placed in ice-cold PBS and minced with scissors. The tissue was then pressed through a 200-gauge stainless steel mesh into a 50mL conical tube. The cell suspension was centrifuged at 50 × g for 2 minutes to remove hepatocytes and large debris. The supernatant containing non-parenchymal cells was then centrifuged at 350 × g for 5 minutes and cells were washed twice in a 15 mL conical bottom tube. Viable, nucleated cells were counted by trypan blue exclusion and brought to a uniform cell density.
Flow Cytometric Analysis of CD11b Expression and Reactive Oxygen Formation
CD11b/Gr-1 staining (35,36)
5µg Fc receptor (FcR) blocking antibody (BioLegend, San Diego, CA) diluted in 100µL 0.1% BSA in PBS was added to 100µL non-parenchymal cell suspension for 20 minutes on ice. To 50µL whole blood or 200µL FcR-blocked hepatic non-parenchymal cells, saturating concentrations of PE-Cy5-labeled-anti-Gr-1 (BioLegend) and PE-labeled-anti-CD11b (BioLegend) diluted in 0.1% BSA in PBS were added. Tubes were incubated in the dark, on ice for 30 minutes. After washing with 0.1% BSA, red blood cells were lysed using RBC lysing solution (0.155 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Pellets were washed three times with 0.1% BSA then fixed with 2.5% buffered formalin. Samples were measured on the FACSCalibur (BD, Franklin Lakes, NJ). We gated on Gr-1high cells, which is also the only Ly6G-positive cell population (36). Cells, which are Gr-1high were identified as neutrophils by cell morphology (36). Other leukocytes are either Gr-1intermediate (mainly monocytes and eosinophils) or Gr-1negative (monocytes and lymphocytes) (36). The data were analyzed using the BD FACSDiva 6.0 software.
Reactive Oxygen Production (37,38)
To 50µL whole blood or 100µL hepatic non-parenchymal cells, 10 µM diphenyleneiodonium or vehicle (0.1% DMSO) was added to each tube for 20 minutes. 2 µM phorbol 12-myristate 13-acetate (PMA) or saline was added and incubated for 10 minutes at 37°C. 4 µM dihydrorhodamine-123 was added and incubated for 10 minutes at 37°C. Ice-cold 0.1% BSA in PBS was added and pellets were washed. The non-parenchymal cell suspension was blocked with FcR blocking antibody (BioLegend) as previously described and cells were stained with saturating concentrations of PE-Cy5-labeled-anti-Gr-1 (BioLegend) diluted in 0.1% BSA. Tubes were incubated in the dark, on ice for 30 minutes. After washing with 0.1% BSA, red blood cells were lysed using RBC lysing solution. Pellets were washed three times with 0.1% BSA then fixed with 2.5% buffered formalin. Samples were measured on the FACSCalibur (BD) and the data were analyzed using the BD FACSDiva 6.0 software.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. If the data were not normally distributed, we used the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test. P < 0.05 was considered significant.
RESULTS
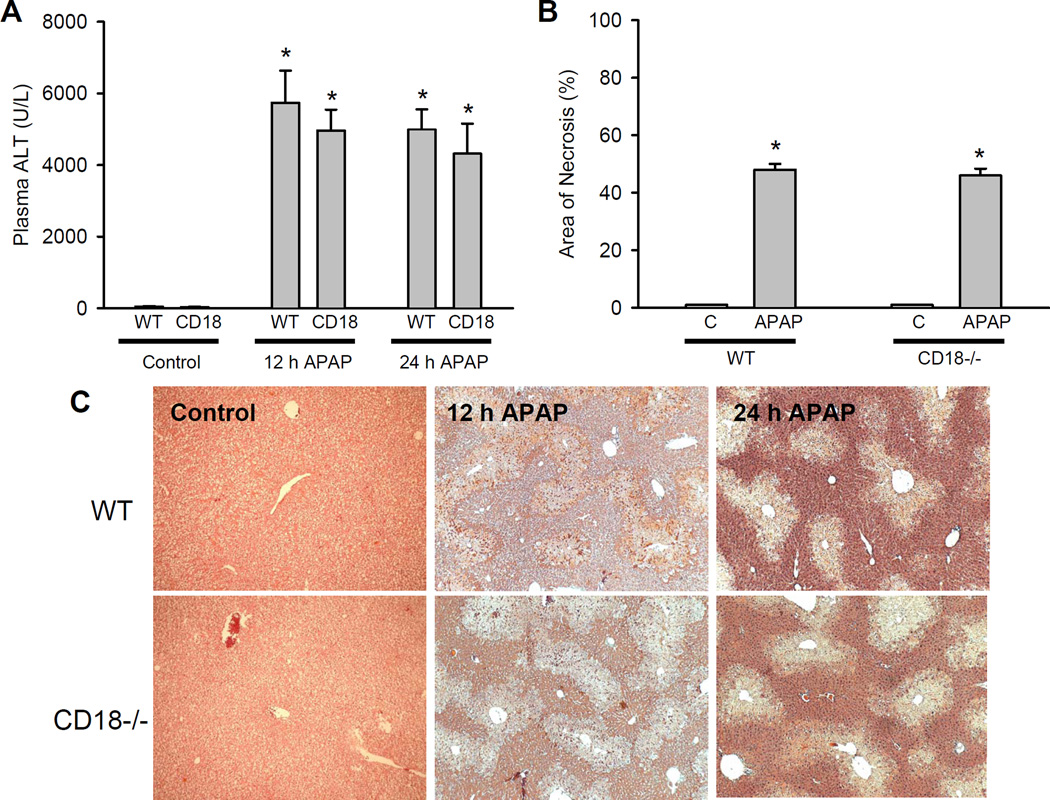
Administration of 300 mg/kg APAP to C57Bl/6 mice resulted in severe liver injury at 12 and 24 h as indicated by the highly elevated plasma ALT activities (Figure 1A) and the extensive centrilobular necrosis (Figure 1B, C). Blinded quantitation of cell death revealed that approximately 50% of all hepatocytes were necrotic in wild type animals at 24 h (Figure 1B). Hepatic glutathione (GSH+GSSG), which is depleted by >90% during the first hour after APAP (39), was only partially recovered at 12 h but reached baseline values at 24 h (Figure 2A). APAP induced a significant oxidant stress as indicated by the increase of hepatic GSSG values and the increase of the GSSG-to-GSH ratio (Figure 2B,C). Immunohistochemical staining for neutrophils showed extensive accumulation of these leukocytes in the liver at 12 h and a substantial further increase at 24 h (Figure 3A). However, despite the fairly high numbers of neutrophils around the area of necrosis after APAP (Figure 3B), animals subjected to bile duct ligation, where neutrophils play a critical role in liver injury (16,40), showed a substantially higher accumulation of neutrophils around the bile infarcts (necrotic foci) (Figure 3B). Overall, the APAP data are consistent with previous findings regarding the degree of injury, oxidant stress and inflammation in this model (27,39). Treatment of CD18-deficient mice with APAP resulted in similar liver injury as compared to wild type animals as judged by both plasma ALT activities and histology (Figure 1) and showed similar oxidant stress (Figure 2) and hepatic neutrophil accumulation (Figure 3).
Figure 1.
Acetaminophen-induced liver injury in C57Bl/6 wild type and CD18-deficient mice. Animals were treated with 300 mg/kg APAP and plasma ALT was measured at 12 h and at 24 h (A) and the area of necrosis was quantified at 24 h (B). Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls). C. Representative H&E-stained liver sections of animals treated for 12 h and 24 h with 300 mg/kg APAP (×50 for all panels).
Figure 2.
Hepatic content of total GSH (GSH + GSSG) (Panel A) and GSSG (Panel B) were measured in untreated controls (C) and in C57Bl/6 wild type and CD18-deficient mice treated with 300 mg/kg APAP for 12 h or 24 h. In addition, the GSSG-to-GSH ratio (Panel C) was calculated from each animal. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to respective control)
Figure 3.
Hepatic neutrophil accumulation after APAP treatment in C57Bl/6 wild type and CD18-deficient mice. A. Neutrophil numbers were quantified in 15 randomly selected high power fields (HPF; ×400). Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls). B. Comparison of neutrophil accumulation in an area of necrosis 12 h after 300 mg/kg APAP or 2 days after common bile duct ligation (BDL). (×200). Red arrows highlight selected neutrophils in sinusoids and on the periphery of necrosis in the APAP-treated liver.
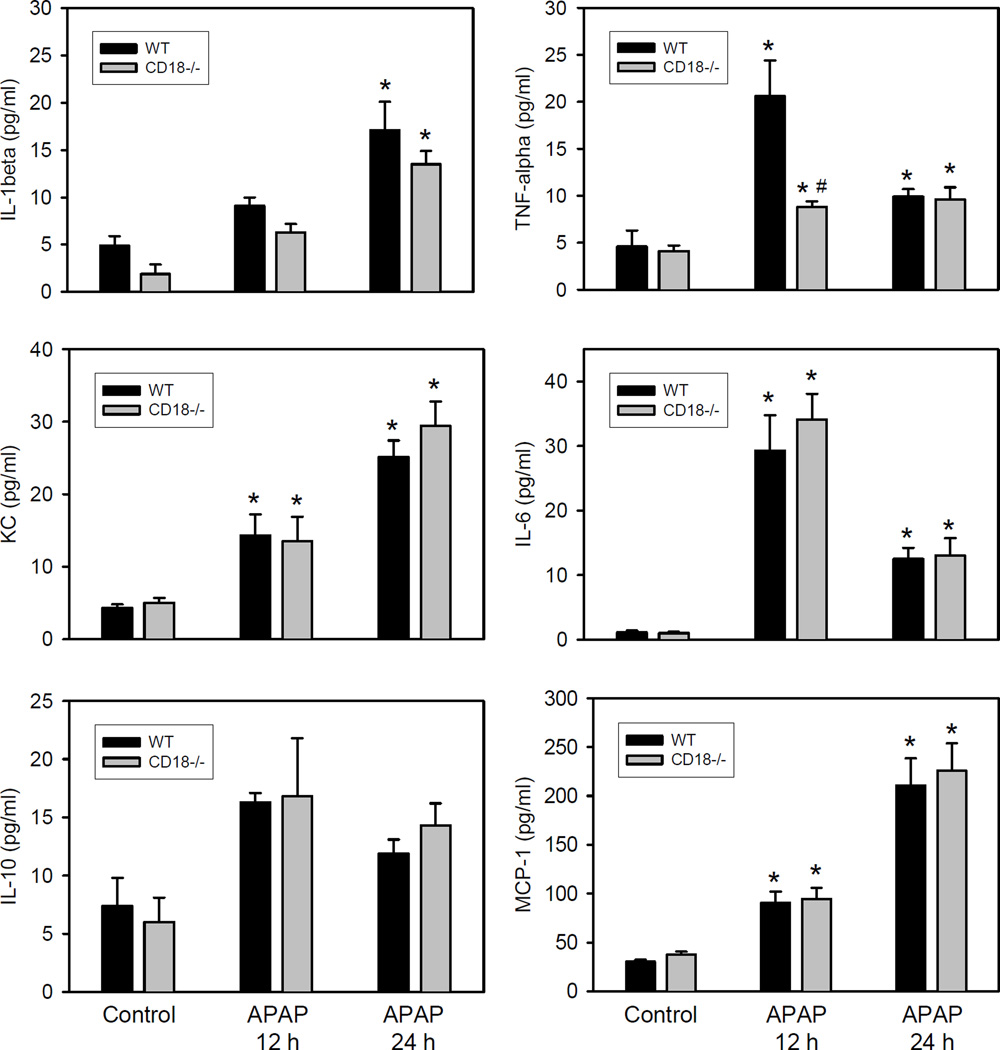
To evaluate pro-inflammatory mediator formation, the hepatic mRNA of selected cytokines and chemokines were measured (Table 1). A variable but significant increase above untreated controls was found for all cytokines/chemokines at 12 h and at 24 h after APAP. Consistent with these findings, plasma protein levels of most of these cytokines and chemokines were significantly increased at both time points (Figure 4). However, the increase was generally moderate and limited to levels between 2-fold to less than 10-fold above baselines. Most importantly, no significant difference in mRNA or serum protein levels of any cytokine or chemokine between wild type and CD18-deficient animals was observed (except the 12 h plasma TNF-α levels). These data support the conclusion that a neutrophilic inflammatory response occurs early after the APAP-induced liver injury. However, animals with a deficiency in CD18 develop the identical degree of injury after APAP overdose suggesting that neutrophils do not play a critical role in the pathophysiology.
Table 1.
Hepatic Cytokine mRNA Induction by Acetaminophen in Wild Type and CD18-deficient Mice
| Real Time PCR (fold change) | ||||
|---|---|---|---|---|
| Cytokine mRNA | WT 12 h | CD18−/− 12 h | WT 24 h | CD18−/− 24 h |
| TNF-α | 8.8 ± 4.2 | 9.9 ± 3.9 | 12.6 ± 1.7 | 18.2 ± 4.8 |
| IL-1β | 6.4 ± 2.7 | 5.6 ± 2.5 | 4.9 ± 1.1 | 6.9 ± 2.0 |
| IL-10 | 35.3 ± 19.1 | 17.0 ± 3.8 | 7.1 ± 1.8 | 8.5 ± 1.6 |
| KC | 10.6 ± 6.6 | 6.7 ± 1.4 | 3.8 ± 1.1 | 3.1 ± 1.2 |
| MIP-2 | 238 ± 126 | 257 ± 71 | 14.0 ± 2.5 | 11.1 ± 3.2 |
Hepatic cytokine/chemokine mRNA levels were measured 12 and 24 h after administration of 300 mg/kg acetaminophen (APAP) in C57Bl/6 wild type animals and in CD18-deficient mice (CD18−/−). mRNA levels are expressed as the cytokine mRNA-to-β-actin mRNA ratio. The values of untreated controls were set as 1 and the fold change of the APAP-treated animals was calculated. Data represent means ± SE of n = 6–7 animals per group. All values are significantly different from untreated controls but there is no significant difference between any data from wild type and CD18-deficient animals.
Figure 4.
Plasma cytokine and chemokine levels after acetaminophen overdose in C57Bl/6 wild type and CD18-deficient mice. Animals were treated with 300 mg/kg APAP and plasma cytokine/chemokine levels were measured at 12 h and at 24 h after APAP using the Bioplex system. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls). #P<0.05 (compared to wild type animals).
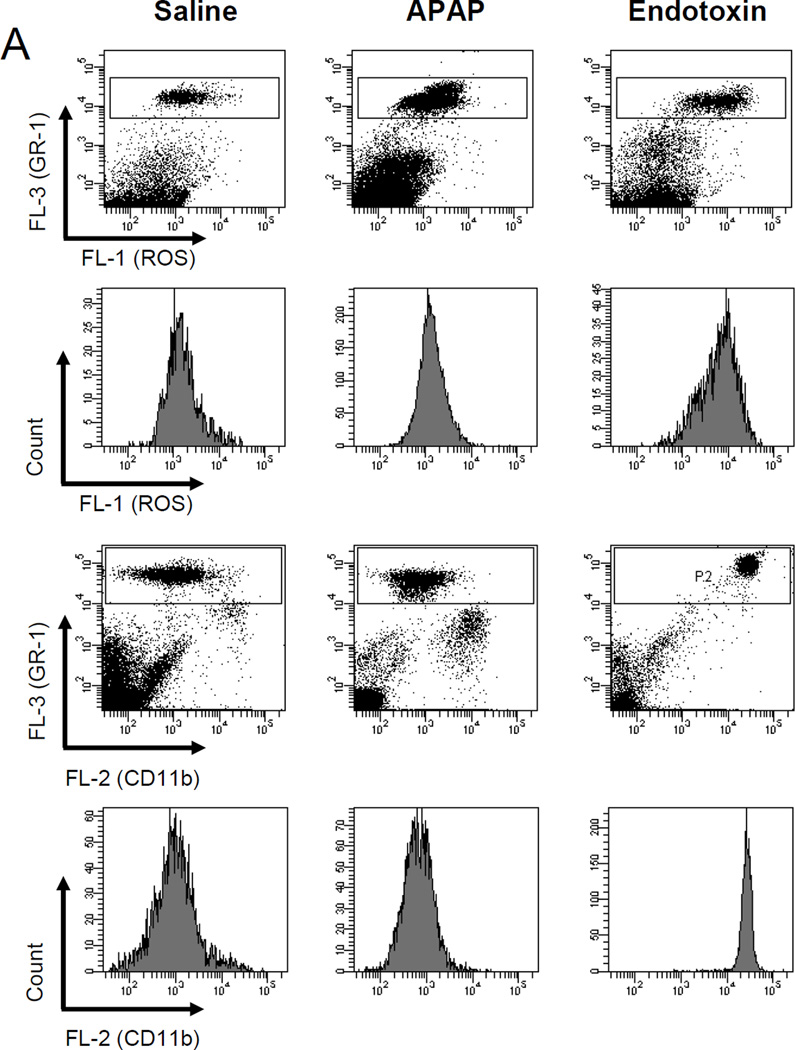
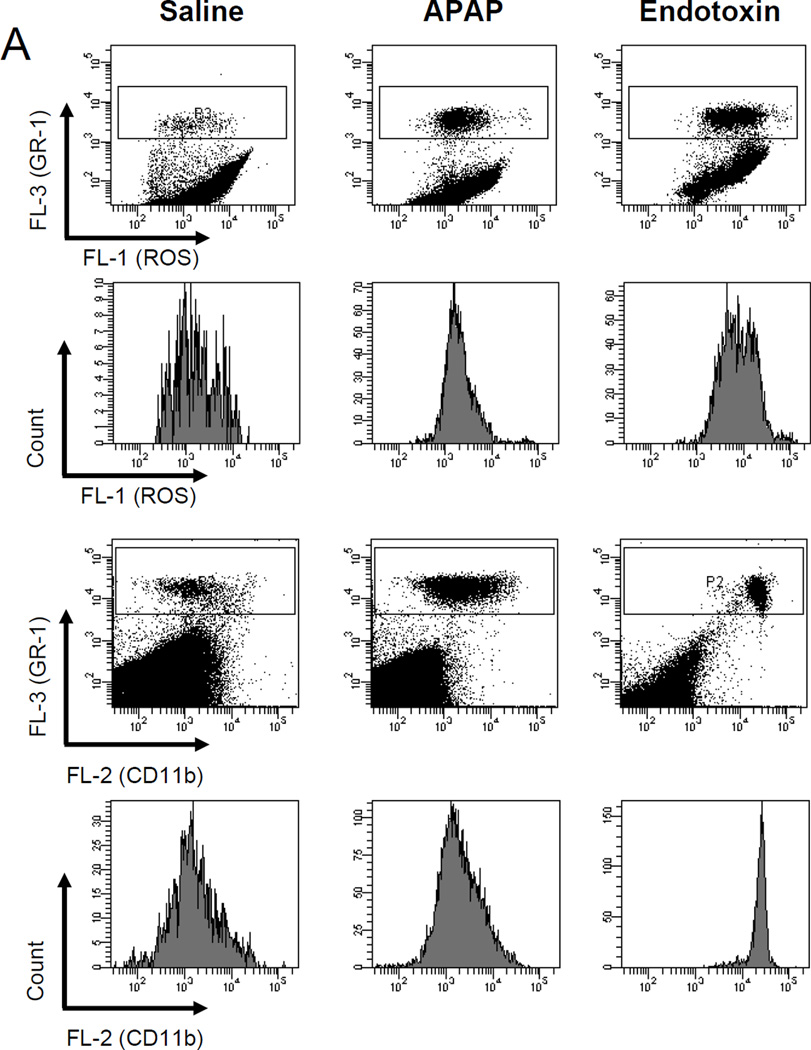
In order to further characterize a potential neutrophil priming or activation after APAP, the surface expression of Mac-1 (CD11b/CD18) and the capacity for the formation of reactive oxygen species (ROS) was determined in peripheral neutrophils (Figure 5) and in liver neutrophils (Figure 6) at 6 h after 300 mg/kg APAP. Upregulation of Mac-1 and priming for reactive oxygen formation of neutrophils is a hallmark of all models where neutrophils actively contribute to liver injury (16,35,41). Despite significant injury at 6 h (ALT: 2840 ± 300 U/L; n=5), ROS formation and the expression of Mac-1 on peripheral neutrophils (Figure 5) and hepatic neutrophils (Figure 6) from APAP-treated animals were similar compared to cells obtained from untreated controls. As a positive control experiment, animals were treated with 100 µg/kg of endotoxin. Peripheral blood and hepatic neutrophils were evaluated at 1.5 h. The well-known cytokine response after endotoxin caused a 2.6-fold (2.0-fold) increase in ROS formation and a 25-fold (7.5-fold) increase in Mac-1 expression on peripheral (hepatic) neutrophils (Figure 5 and 6). The quantitatively slightly lower activation of the hepatic neutrophils compared to the peripheral neutrophils may be due to the increased stress of isolating the cells from the liver. However, most importantly, the data obtained from peripheral neutrophils closely reflect the activation status of liver neutrophils. Measurements of blood and liver neutrophils in CD18-deficient mice showed similar ROS formation as obtained in wild type animals (data not shown). The reason for this is the use of PMA as stimulant. PMA directly activates protein kinase C intracellularly and does not depend on surface receptors. As expected (16), CD11b expression was substantially lower in CD18-deficient samples compared to wild type (data not shown).
Figure 5.
Reactive oxygen species (ROS) priming and CD11b expression on peripheral neutrophils. C57Bl/6 mice (n=4 per group) were treated with 20 ml saline/kg for 6 hours, 100 µg endotoxin/kg for 90 minutes or 300 mg APAP/kg for six hours. A. To determine reactive oxygen species (ROS) priming, mice were treated with APAP or endotoxin in vivo then whole blood was stimulated ex vivo with PMA. Upon PMA-induced ROS production DHR-123 is converted to R-123 and quantified in neutrophils by flow cytometry. In addition, immediately after in vivo stimulation with APAP or endotoxin whole blood was stained for CD11b surface expression and neutrophils were analyzed by flow cytometry. The Gr-1high cell population consists of neutrophils. Representative ROS or CD11b histograms or mean fluorescence intensities (B) for saline, the positive control endotoxin, and APAP are shown. *P<0.05 (compared to saline controls)
Figure 6.
Reactive oxygen species (ROS) priming and CD11b expression on liver accumulated neutrophils. C57Bl/6 mice (n=4 per group) were treated with 20 ml saline/kg for 6 hours, 100 µg endotoxin/kg for 90 minutes or 300 mg APAP/kg for six hours. A. To determine reactive oxygen species (ROS) priming, mice were treated with APAP or endotoxin in vivo, non-parenchyma cells were isolated and then stimulated ex vivo with PMA. Upon PMA-induced ROS production DHR-123 is converted to R-123 and quantified in neutrophils by flow cytometry. In addition, immediately after in vivo stimulation with APAP or endotoxin, the isolated non-parenchymal cells were stained for CD11b surface expression and neutrophils were analyzed by flow cytometry. The Gr-1high cell population consists of neutrophils. Representative ROS or CD11b histograms or mean fluorescence intensities (B) for saline, the positive control endotoxin, and APAP are shown. *P<0.05 (compared to saline control)
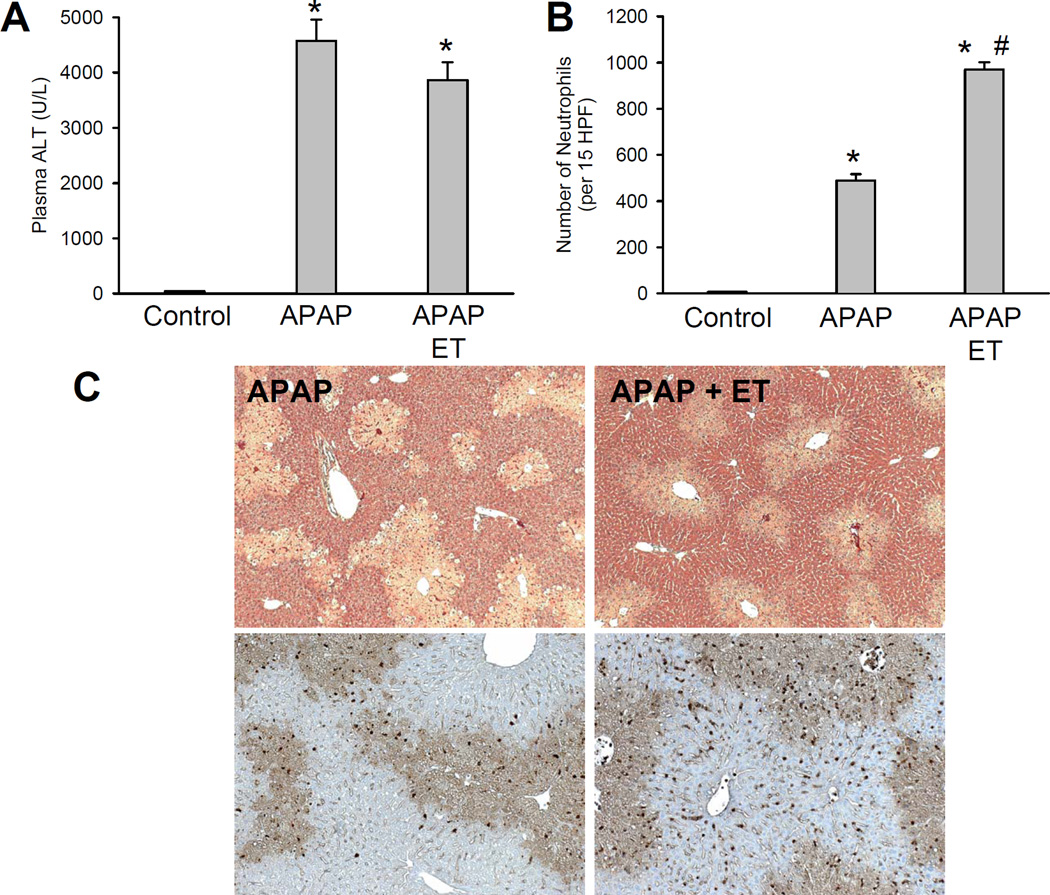
Because endotoxin caused a substantial activation of neutrophils, it was tested if treatment with endotoxin 3 h after APAP enhances neutrophil recruitment into the liver and potentially aggravates APAP-induced liver injury during the 24 h observation period. However, liver injury after the sequential administration of APAP and ET was the same as with APAP alone (Figure 7A,C) despite the doubling of the number of neutrophils in the liver (Figure 7B). Although the peaks of cytokine/chemokine formation after endotoxin occur well before the 24 h time point (42), there was still a more than 5-fold elevation of all mRNA levels and a 2- to 80-fold increase of plasma protein levels detectable at this late time (Table 2). These data demonstrate that the additional treatment with endotoxin drastically enhanced the pro-inflammatory mediator formation and further promoted hepatic neutrophil accumulation. However, despite this substantial boost of the inflammatory response, liver injury was not different from injury with APAP alone (Figure 7).
Figure 7.
Effect of endotoxin treatment on acetaminophen-induced liver injury. Animals were first treated with 300 mg/kg APAP and 3 h later either with saline (10 ml/kg) or 100 µg/kg endotoxin (ET) (i.p.). Plasma ALT activities (A) and the number of liver accumulating neutrophils (B) were measured at 24 h. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls). #P<0.05 (compared to APAP alone). C. Representative H&E-stained liver sections (×50) and neutrophil staining (×100) of animals treated for 24 h with 300 mg/kg APAP alone or in combination with endotoxin.
Table 2.
Plasma Cytokine Levels and Hepatic Cytokine mRNA Induction by Acetaminophen and Endotoxin
| Plasma Protein Levels (pg/ml) |
Real Time PCR (fold change) | |||
|---|---|---|---|---|
| Cytokine | APAP 24 h | APAP 24 h ET |
APAP 24 h | APAP 24 h ET |
| TNF-α | 10 ± 1 | 23 ± 6* | 4 ± 1 | 20 ± 6* |
| IL-1β | 17 ± 3 | 60 ± 10* | 3 ± 1 | 18 ± 7* |
| IL-6 | 13 ± 2 | 1035 ± 292* | 1 ± 0 | 21 ± 6* |
| IL-10 | 12 ± 2 | 456 ± 90* | 6 ± 2 | 152 ± 20* |
| KC | 25 ± 2 | 324 ± 133* | 10 ± 4 | 52 ± 11* |
Plasma cytokine/chemokine concentrations and hepatic mRNA levels were measured 24 h after administration of 300 mg/kg acetaminophen (APAP). Some animals were additionally treated with 100 µg/kg endotoxin (ET) at 3 h after APAP. mRNA levels are expressed as the cytokine mRNA-to-β-actin mRNA ratio. The values of untreated controls were set as 1 and the fold change of the APAP-treated animals was calculated. Data represent means ± SE of n = 4–5 animals per group.
P<0.05 (compared to APAP alone)
DISCUSSION
The main objective of this investigation was to test in a model with genetic deficiency of neutrophil function if these phagocytes are sufficiently activated and receive the appropriate signals during APAP overdose to further aggravate liver injury. This approach was necessary because previous attempts to evaluate the role of neutrophils in APAP hepatotoxicity yielded conflicting results due to the use of immunological intervention strategies, i.e. neutropenia-inducing antibodies (23,24,27). The problem with this approach is that pretreatment with such an antibody results in the accumulation of antibody-tagged neutrophils in sinusoids. During the 24 h pretreatment time used in experiments that showed a protection against APAP toxicity (23,24), Kupffer cells remove these inactivated neutrophils by phagocytosis, which causes the activation of Kupffer cells (43). Cytokines produced by these phagocytes act on hepatocytes and induce a number of genes, e.g., metallothionein, heme oxygenase-1 and others, some of which have been shown to protect against APAP overdose (44). Although these findings cannot rule out that the protective effect with pretreatment of a neutropenia-inducing antibody was caused by the elimination of neutrophils, there are at least serious concerns that the induction of hepatoprotective genes (preconditioning effect) was at least in part if not completely responsible for the reduced injury after APAP. Our current experiments with CD18-deficient mice, which showed no protection against APAP hepatotoxicity, strongly support the hypothesis that neutrophils are not involved in the injury process in mice. In addition, the fact that endotoxin, which dramatically exaggerated the inflammatory response, did not affect the overall injury further supports this conclusion.
Members of β2 integrin family, especially CD11a/CD18 and CD11b/CD18, are essential for firm adhesion of neutrophils to endothelial cells via binding to intercellular adhesion molecule-1 (ICAM-1), for their extravasation and for the adhesion to potential target cells, where a long-lasting, adhesion-dependent oxidant stress can be triggered (31). The neutrophil-dependent oxidant stress is responsible for target cell killing (45). In contrast to this general mechanism of neutrophil adhesion, which applies mainly to postcapillary venules in a variety of vascular beds, in the liver, the pathophysiologically most relevant accumulation of neutrophils occurs in sinusoids (46). Because of the low shear stress in sinusoids, selectins, CD18 or ICAM-1 are not necessary for hepatic neutrophil recruitment (47,48). If the sinusoidal endothelium is intact, blocking these adhesion molecules with an antibody or deficiency of CD18 or ICAM-1 does not prevent neutrophil accumulation in sinusoids (14,48,49). An effect on neutrophil numbers in the liver can only be seen after longer time period as documented in CD18- and ICAM-1-deficient animals 3 days after bile duct ligation (16,40). This reflects the reduced inflammatory response due to the reduced tissue injury rather than a direct inhibition of neutrophil adhesion (16,40). However, blocking CD18 inhibits the extravasation of neutrophils and prevents the neutrophil-induced oxidant stress and injury (13,16,40,49). Even if the endothelium is severely damaged and does not provide a relevant barrier for neutrophils, e.g. during hepatic ischemia-reperfusion injury, CD18 antibodies are still highly effective due to the attenuation of the adhesion-dependent oxidant stress (13,41). In all models where neutrophils aggravate liver injury it was consistently shown that a neutrophil component was evident during the first 24 h (12,13,17,50). Taken together, if neutrophils contributed to the APAP hepatotoxicity, a beneficial effect should have been observed in CD18-deficient mice during the first 24 h after APAP administration. However, despite the mild inflammatory response as judged by cytokine and chemokine formation and the accumulation of neutrophils in the liver, there was no evidence that any of these effects are reduced in CD18-deficient animals. Because neutrophil-induced cell killing in the liver also requires reactive oxygen (11), these data are consistent with previous reports showing that inhibitors of NADPH oxidase (27) or genetic deficiency in the function of this enzyme (51) do not protect against APAP-mediated liver injury. The lack of priming for reactive oxygen formation in peripheral and in liver accumulated neutrophils is also in agreement with this finding. Nevertheless, the fact that neutrophil accumulation is moderate (Figure 3), occurs mainly after the onset of severe cell injury (25,27) and can be reduced by antibodies against DAMPs, e.g. high mobility group box proteins (30,52), suggest this is a classical scenario of a sterile inflammatory response.
Because it appears that the modest activation of neutrophils during APAP hepatotoxicity is insufficient to trigger an active attack of these phagocytes, a more aggressive inflammatory response was induced by treatment with endotoxin several hours after APAP. This schedule was chosen to mimic a realistic scenario of massive cytokine formation and neutrophil activation at the time of APAP-induced injury. Previously, we could show that administration of a low dose of endotoxin after hepatic ischemia-reperfusion drastically aggravated the inflammatory response including neutrophil activation and the neutrophil-mediated injury (53,54). In this combination model of two insults, even the delayed treatment with an antibody against CD18 was highly effective in reducing reperfusion injury (54). Although the enhanced cytokine and chemokine formation after endotoxin recruited substantially more activated neutrophils into the liver after APAP overdose, no increased injury was detected. These results may be surprising, however, they are consistent with our mechanistic understanding of neutrophil-mediated liver injury (11,15,55). Various cytokines (TNF-α, IL-1), chemokines (IL-8, MIP-2, KC), activated complement factors (C5a) and other proinflammatory mediators can individually and in combination activate neutrophils to various degrees and induce their accumulation in sinusoids (35,46,49). This process does not require CD18 (48), hence CD18-deficient mice did not show less sinusoidal neutrophil accumulation (Figure 3 and 7). In addition, primed neutrophils in sinusoids do not randomly cause liver injury (46). For neutrophils to actually participate in killing of larger cells such as hepatocytes, neutrophils need to extravasate and adhere to the target. This process requires CD18 and ICAM-1 (13,16,40,49). In addition, it requires a distress signal from the parenchyma that indicates to neutrophils located in the sinusoid a cell might be dying and needs to be removed (15,56). The attack on the target involves an adherence-dependent oxidant stress (CD18-dependent) and killing by reactive oxygen including hypochlorous acid (13,16,40,41,56). Whether or not such an attack actually aggravates liver injury depends on the initial insult. If the stress is mild and non-lethal, the stressed cell can trigger a neutrophil attack, which will kill the cell and therefore aggravate the injury. If the stress is already lethal by itself, e.g. APAP toxicity in hepatocytes, the accumulation and attack of neutrophils will have no impact on the overall injury. Thus, levels of cytokines and other pro-inflammatory mediators determine the degree of neutrophil activation and accumulation in sinusoids; the distress signal from injured cells determines the degree of extravasation and attack on hepatocytes. However, whether this makes a difference in the extent of liver damage is determined by the original stress or insult. Based on our data we can conclude that the intracellular signaling mechanisms induced by APAP in hepatocytes determine whether or not a cell will die and the neutrophilic inflammatory response has no impact on the injury. However, this does not exclude a critical role of neutrophils and macrophages in the removal of cell debris and preparation for regeneration.
In summary, our data documented severe liver injury after APAP overdose, which was accompanied by pro-inflammatory mediator formation and moderate hepatic neutrophil accumulation. However, animals with a deficiency of CD18, which has been shown to be critical for neutrophil-induced liver injury in a number of liver disease models, were not protected against APAP hepatotoxicity. In addition, stimulated cytokine formation and enhanced hepatic recruitment of activated neutrophils did not aggravate APAP-induced liver injury. Together these data do not support the hypothesis that neutrophils contribute to APAP hepatotoxicity or that a neutrophil-mediated injury phase could be provoked by a second, pro-inflammatory hit. Thus, APAP-induced liver injury in mice is dominated by intracellular mechanisms of cell death rather than by neutrophilic inflammation.
ACKNOWLEDGMENT
This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916 to H.J., and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. C.D. Williams was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
REFERENCES
- 1.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 2.Larson AM, Polson J, Fontana RJ, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 3.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 6.Cover C, Mansouri A, Knight TR, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 7.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 8.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2005;1:389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- 10.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 13.Jaeschke H, Farhood A, Smith CW. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am J Physiol. 1991;261:G1051–G1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- 14.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein-2 production and intercellular adhesion molecule-1 expression in the liver. Hepatology. 1997;25:335–342. doi: 10.1002/hep.510250214. [DOI] [PubMed] [Google Scholar]
- 15.Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicology Mechanisms and Methods. 2007;17:431–440. doi: 10.1080/00952990701407702. [DOI] [PubMed] [Google Scholar]
- 16.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 17.Dahm LJ, Schultze AE, Roth RA. An antibody to neutrophils attenuates alpha-naphthylisothiocyanate-induced liver injury. J Pharmacol Exp Ther. 1991;256:412–420. [PubMed] [Google Scholar]
- 18.Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–G363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- 19.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 20.Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699–701. doi: 10.1002/hep.22556. [DOI] [PubMed] [Google Scholar]
- 22.Smith GS, Nadig DE, Kokoska ER, et al. Role of neutrophils in hepatotoxicity induced by oral acetaminophen administration in rats. J Surg Res. 1998;80:252–258. doi: 10.1006/jsre.1998.5441. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 24.Ishida Y, Kondo T, Kimura A, et al. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- 25.Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 26.Bauer I, Vollmar B, Jaeschke H, et al. Transcriptional activation of heme oxygenase-1 and its functional significance in acetaminophen-induced hepatitis and hepatocellular injury in the rat. J Hepatol. 2000;33:395–406. doi: 10.1016/s0168-8278(00)80275-5. [DOI] [PubMed] [Google Scholar]
- 27.Cover C, Liu J, Farhood A, et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Chen CJ, Kono H, Golenbock D, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 29.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CW. Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharmacol. 1993;71:76–87. doi: 10.1139/y93-012. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RW, Ballantyne CM, Smith CW, et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–1578. [PubMed] [Google Scholar]
- 33.Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol Sci. 2008;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe H, Ohtsuka K, Kimura M, et al. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 35.Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- 36.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 37.Emmendörffer A, Hecht M, Lohmann-Matthes M, Roesler J. A fast and easy method to determine the production of reactive oxygen intermediates by human and murine phagocytes using dihydrorhodamine 123. J Immunol Methods. 1990;131:269–275. doi: 10.1016/0022-1759(90)90198-5. [DOI] [PubMed] [Google Scholar]
- 38.Smith J, Weidemann M. Further characterization of the neutrophil oxidative burst by flow cytometry. J Immunol Methods. 1993;162:261–268. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- 39.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001 Aug;62(2):212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 40.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschke H, Farhood A, Bautista AP, et al. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–923. [PubMed] [Google Scholar]
- 42.Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2005;288:G880–G886. doi: 10.1152/ajpgi.00317.2004. [DOI] [PubMed] [Google Scholar]
- 43.Bautista AP, Spolarics Z, Jaeschke H, Smith CW, Spitzer JJ. Antineutrophil monoclonal antibody (1F12) alters superoxide anion release by neutrophils and Kupffer cells. J Leukoc Biol. 1994;55:328–335. doi: 10.1002/jlb.55.3.328. [DOI] [PubMed] [Google Scholar]
- 44.Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective (Letter) Hepatology. 2007;45:1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- 45.Entman ML, Youker K, Shoji T, et al. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- 47.Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaeschke H, Farhood A, Fisher MA, Smith CW. Sequestration of neutrophils in the hepatic vasculature during endotoxemia is independent of beta 2 integrins and intercellular adhesion molecule-1. Shock. 1996;6:351–356. doi: 10.1097/00024382-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Essani NA, Fisher MA, Farhood A, et al. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995;21:1632–1639. [PubMed] [Google Scholar]
- 50.Dold S, Laschke MW, Lavasani S, et al. Simvastatin protects against cholestasis-induced liver injury. Br J Pharmacol. 2009;156:466–474. doi: 10.1111/j.1476-5381.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- 52.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 53.Liu P, Vonderfecht SL, Fisher MA, McGuire GM, Jaeschke H. Priming of phagocytes for reactive oxygen production during hepatic ischemia-reperfusion potentiates the susceptibility for endotoxin-induced liver injury. Circ Shock. 1994;43:9–17. [PubMed] [Google Scholar]
- 54.Liu P, McGuire GM, Fisher MA, et al. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- 55.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 56.Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]