Abstract
Experimental alcohol-induced liver injury is exacerbated by a high polyunsaturated fat diet rich in linoleic acid. We postulated that bioactive oxidized linoleic acid metabolites (OXLAMs) play a critical role in the development/progression of alcohol-mediated hepatic inflammation and injury. OXLAMs are endogenous ligands for transient receptor potential vanilloid 1 (TRPV1). Herein, we evaluated the role of signaling through TRPV1 in an experimental animal model of alcoholic liver disease (ALD). Chronic binge alcohol administration increased plasma OXLAM levels, specifically 9- and 13-hydroxy-octadecadienoic acids. This effect was associated with up-regulation of hepatic TRPV1. Exposure of hepatocytes to these OXLAMs in vitro resulted in activation of TRPV1 signal transduction with increased intracellular Ca2+ levels. Genetic depletion of TRPV1 did not blunt hepatic steatosis caused by ethanol, but prevented hepatic injury. TRPV1 deficiency protected from hepatocyte death and prevented the increase in proinflammatory cytokine and chemokine expression, including tumor necrosis factor-α, IL-6, macrophage inflammatory protein-2, and monocyte chemotactic protein 1. TRPV1 depletion markedly blunted ethanol-mediated induction of plasminogen activator inhibitor-1, an important alcohol-induced hepatic inflammation mediator, via fibrin accumulation. This study indicates, for the first time, that TRPV1 receptor pathway may be involved in hepatic inflammatory response in an experimental animal model of ALD. TRPV1-OXLAM interactions appear to play a significant role in hepatic inflammation/injury, further supporting an important role for dietary lipids in ALD.
Alcohol consumption remains one of the most common and important causes of liver disease in the United States and worldwide. Alcoholic liver disease (ALD) ranges from steatosis and steatohepatitis to advanced injury, such as fibrosis, cirrhosis, and hepatocellular carcinoma. It has been estimated that 15% to 30% of heavy drinkers develop advanced ALD.1–3 Despite the significant progress made on ALD pathogenesis, the specific mechanism(s) responsible for ALD development and progression remain poorly understood. Due, in part, to this incomplete understanding of the mechanisms by which alcohol damages the liver, there is still no Food and Drug Administration–approved therapy for this common and often devastating disease. Understanding the molecular mechanisms involved in the pathogenesis of alcohol-induced liver injury may, therefore, lead to the development of new therapeutic options and/or preventive interventions.
Dietary fat is an important determinant of ALD development and progression.4–7 Recent publications have shown that experimental and clinical alcohol-induced liver steatosis and injury were associated with elevated oxidized linoleic acid metabolites (OXLAMs), specifically 9- and 13-hydroxy-octadecadienoic acids (9- and 13-HODEs).8,9 It has been reported that 9- and 13-HODEs are natural endogenous ligands for the transient receptor potential vanilloid 1 (TRPV1).10,11 The TRPV1 receptor is a ligand-gated nonselective cation channel with high permeability for Ca2+,12 which is expressed in many cells and tissues, including liver.13–22 The TRPV1 is a polymodal molecular detector of multiple stimuli responding to a large variety of physical (eg, noxious heat), and chemical (eg, H+ ions) stimuli. In addition to HODEs, several exogenous and endogenous TRPV1 agonists have been identified, including capsaicin,12 cannabinoids,23 retinoids,24 and metabolites of arachidonic acid.25
Accumulating evidence suggests an important role of TRPV1 in several diseases and pathological conditions, including chronic pain,26 neurogenic inflammation,27 diabetes,28,29 metabolic syndrome and obesity,19,30 and liver diseases.13,31,32 To the best of our knowledge, there are no data assessing the role of TRPV1 in ALD. The present study evaluates the role of TRPV1 in the development of ethanol-induced liver steatosis, inflammation, and injury using an experimental animal model of ALD. Our findings collectively indicate that the genetic deficiency of TRPV1 protects against alcohol-induced liver inflammation and injury but not steatosis. Our data point toward a role for TRPV1-OXLAM receptor-ligand interactions as a potentially relevant pathway contributing to alcohol-mediated steatohepatitis.
Materials and Methods
Animal Model of ALD
Animals were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and the study protocol was approved by the University of Louisville (Louisville, KY) Institutional Animal Care and Use Committee. Eight-week-old male TRPV1 knockout mice (B6.129X1-TRPV1tm1Jul/J, 11th backcross generation) and their genetically unaltered wild-type (WT; C57Bl6/J) counterparts were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals were fed Lieber-DeCarli control (isocaloric maltose-dextrin) or ethanol (5% w/v) liquid diets ad libitum for 10 days plus a single binge ethanol administration (5 g/kg, body weight, 20% ethanol) by gavage, whereas mice in control groups were gavaged with isocaloric dextrin maltose.33 Both diets were prepared fresh daily. In the control group diet, the levels of protein, carbohydrate, and fat were held constant at 17%, 43%, and 40% of total energy, respectively. In the alcohol diet, ethanol (35% of total calories) was substituted for carbohydrate energy. The diet was enriched in corn oil containing a high amount of polyunsaturated linoleic fatty acid, and purchased from Research Diet (New Brunswick, NJ). At the conclusion of the experiment, the mice were anesthetized; and blood and tissue samples were obtained. Plasma was stored at −80°C. Portions of liver tissue were frozen immediately in liquid nitrogen, whereas others were fixed in 10% neutral-buffered formalin or embedded in frozen specimen medium (Tissue-Tek OCT compound; Sakura Finetek, Torrance, CA).
Blood and Liver Biochemical Analysis
Plasma alanine transaminase (ALT) and aspartate transaminase (AST) activity, cholesterol, triglycerides (TGs), glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very LDL (VLDL) were determined by Lipid Panel Plus using the Piccolo Xpress chemistry analyzer (Abaxis, Union City, CA). Blood alcohol levels were measured using nicotinamide adenine dinucleotide-alcohol dehydrogenase (NAD-ADH) Reagent Multiple Test (Sigma, St. Louis, MO), according to the manufacturer's instructions. Plasma endotoxin levels were measured with the Limulus Amoebocyte Lysate kit (Lonza, Walkersville, MD). For the determination of hepatic lipid levels, hepatic lipids were extracted with an aqueous extract from chloroform and methanol. Hepatic TGs were measured, as previously described,34 using TG reagent (Thermo Fisher Scientific Inc., Middletown, VA). Liver cholesterol was assayed using reagents from Sigma.
Liver Histological Examination and Staining
For histological analysis, liver sections were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections (5 μm thick) were prepared and stained with hematoxylin and eosin. Oil-Red-O staining was performed to evaluate hepatic fat accumulation. Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay using the ApopTag Peroxidase In Situ Apoptosis Detection kit (Millipore, Billerica, MA), according to the manufacturer's instructions. Neutrophil accumulation in the livers was assessed by chloroacetate esterase (CAE) staining using a commercially available kit (Sigma), according to the manufacturer's instructions. Immunofluorescence detection of hepatic fibrin deposition was performed in frozen tissue, as previously described.35
Hepatic Caspase-3 Activity Assessment
Caspase-3 activity was determined using 200 μg whole liver protein with the caspase-3 colorimetric kit (Abcam, Cambridge, MA), according to the manufacturer's instructions.
RNA Isolation and Real-Time RT-PCR Assay
Total liver RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Reverse transcription was performed with qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD) and quantitative real-time RT-PCR (RT-qPCR) with Perfecta SYBR Green FastMix (Quanta Biosciences) using an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). The reverse and forward specific primers are presented in Table 1. Primers were designed using Primer3 software version 4.0.0 (http://bioinfo.ut.ee/primer3-0.4.0/primer3).36 All primer pairs were validated by demonstrating high-amplification efficiency, consistent single-peak dissociation patterns, and the presence of single products of the expected size on agarose gels. The relative gene expression was normalized with 18s rRNA as the internal control, and calculated using the 2−ΔΔCT method.
Table 1.
Primer Sequences for the Targeted Mouse Gene RT-qPCR Assay
| Primer set | Forward sequence | Reverse sequence |
|---|---|---|
| 18s | 5′-CTCAACACGGGAAACCTCAC-3′ | 5′-CGCTCCACCAACTAAGAACG-3′ |
| TRPV1 | 5′-TGGACAGCTACAGTGAGATACTTTTC-3′ | 5′-CCATGGAAGCCACATACTCC-3′ |
| IL-6 | 5′-TGGAAATGAGAAAAGAGTTGTGC-3′ | 5′-CCAGTTTGGTAGCATCCATCA-3′ |
| TNF-α | 5′-GTGATCGGTCCCCAAAGG-3′ | 5′-GGTGGTTTGCTACGACGTG-3′ |
| MIP-2 | 5′-GCGCCCAGACAGAAGTCATA-3′ | 5′-TCCAGGTCAGTTAGCCTTGC-3′ |
| MCP-1 | 5′-GGCTCAGCCAGATGCAGT-3′ | 5′-TGAGCTTGGTGACAAAAACTACAG-3′ |
| PAI-1 | 5′-TCAATGACTGGGTGGAAAGG-3′ | 5′-AGGCGTGTCAGCTCGTCTAC-3′ |
| IL-1b | 5′-TTCATCTTTGAAGAAGAGCCCAT-3′ | 5′-TCGGAGCCTGTAGTGCAGTT-3′ |
| IL-1a | 5′-CAAGCAACGGGAAGATTCTG-3′ | 5′-CTGATCTGGGTTGGATGGTC-3′ |
| LCN2 | 5′-ATGTCACCTCCATCCTGGTC-3′ | 5′-ACCTGAGGATACCTGTGCAT-3′ |
Western Blot Analysis
Western blot analysis was performed to evaluate the phospho–extracellular signal-regulated kinase (ERK) 1/2 (p42/44) mitogen-activated protein kinase (MAPK) and nuclear phospho–NF-κB p65 protein levels using commercially available primary antibody from Cell Signaling (Danvers, MA). Equal amounts of proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane. Immunoreactive signals were visualized using enhanced chemiluminescence light detection reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Band intensities were quantified using ImageJ software version 1.49j (NIH, Bethesda, MD). The protein content was normalized to total NF-κB p65 (for pNF-κB p65) and β-actin [for pERK 1/2 (p42/44) MAPK]. The results were expressed as the ratio of protein of interest/NF-κB p65 or β-actin.
Plasma OXLAM Measurement
Lipid extraction from plasma and quantification of 9- and 13-HODEs were performed as previously described.37,38 Briefly, plasma samples with antioxidant solution, internal standards [synthetic 9(s)-HODE-d4; 13(s)-HODE-d4], and potassium hydroxide were added to glass test tubes, and overlaid with argon. After hydrolysis under argon atmosphere, the released fatty acids were extracted twice into the hexane layer by liquid/liquid extraction. The combined hexane extracts were dried under nitrogen gas and resuspended in 85% methanol/water. Reconstituted lipid extracts were analyzed by high-performance liquid chromatography. Quantification of oxidized fatty acids was done on a triple quadrupole mass spectrometer (model API 365; Applied Biosystems, Foster City, CA) with Ionics EP 10þ upgrade (Ionics Mass Spectrometry, Concord, ON, Canada) using stable isotope dilution methods and multiple reaction monitoring with characteristic parent-to-daughter ion transitions.
Cell Culture and Treatments
HepG2, a human hepatoma cell line obtained from ATCC (Manassas, VA), was used for in vitro experiments. Cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C in a humidified 5% CO2, 95% air atmosphere. Cells were plated in 96-well plates at the density of 25,000 cells per well. Cells were treated with 9-HODE (10 μmol/L), 13-HODE (10 μmol/L), and capsaicin (10 μmol/L) for 24 hours. The HODE concentration was chosen on the basis of the observation that serum OXLAM levels were up to 1000 nmol/L in our experimental model of ALD, and the knowledge that HODEs can be found in human blood in the low μmol/L range.39 HODEs were purchased from Cayman Chemical Company (Ann Arbor, MI), capsaicin from Sigma, and the dyes for Cellomics assays from Invitrogen. By using the cell viability MTT assay, we have confirmed that concentrations of up to 25 μmol/L of HODEs cause minimal cell death in HepG2 cells (data not shown). After treatment, cells were incubated for 1 hour in growth media containing the following dyes: i) Hoechst (for nuclear fluorescence), ii) Fluo-4 (for free calcium), and iii) TOTO-3 (for cell membrane permeability). Cellomics analysis was performed using a Thermo Scientific Array Scan VTI HCS Reader (Thermo Fisher Scientific Inc., Waltham, MA), as described by the manufacturer. Cellomics Array Scan 60 software version 7.6.2.1-1.00x (Thermo Fisher Scientific Inc.) was used to determine fluorescence intensities of the dyes. Well averages, as well as individual cell data, were recorded and analyzed.
Statistical Analysis
The data were expressed as means ± SEM. A Student's t-test (two tailed) was performed to evaluate significant differences between alcohol- and pair-fed animals. Two-way analysis of variance, followed by the Tukey's multiple-comparison test, was used to evaluate significant differences between experimental groups (WT–pair fed, WT-ethanol, TRPV1−/−–pair fed, and TRPV1−/−-ethanol). P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc., La Jolla, CA).
Results
Chronic Binge Ethanol Administration Increases Circulating OXLAM Levels and Induces Hepatic TRPV1 Expression
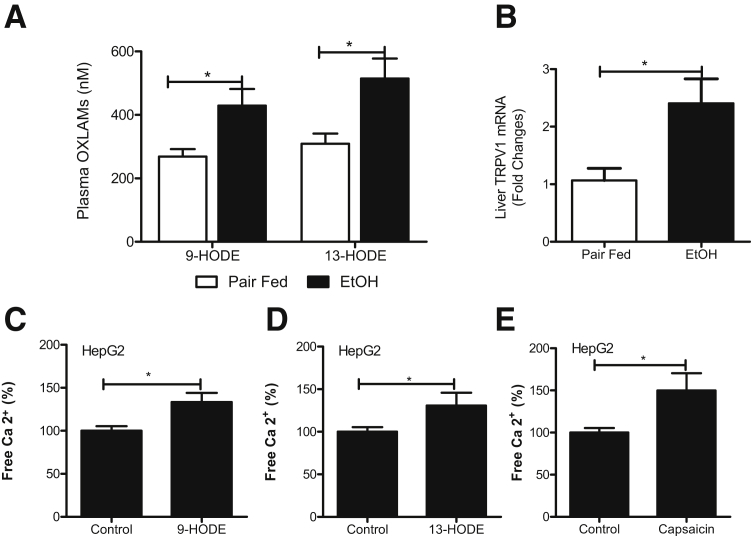
Recent clinical and experimental studies have demonstrated that alcohol-induced liver inflammation and injury are associated with elevated levels of bioactive OXLAMs.8,9 Indeed, chronic binge ethanol administration significantly elevated plasma OXLAM levels, specifically 9- and 13-HODEs, compared with their pair-fed controls (Figure 1A). A similar trend was found for 9- and 13-oxo-octadecadenoic acids (oxoODEs) (data not shown). OXLAMs, specifically 9- and 13-HODEs, have been reported as endogenous activators/agonists of TRPV1,10,11 a ligand-gated nonselective cation channel with high permeability for Ca2+.12 On the basis of these observations, we examined TRPV1 expression in the livers of control pair- and ethanol-fed animals. We observed that ethanol exposure increased hepatic TRPV1 mRNA expression in parallel with the increase in circulating OXLAMs in ethanol but not in control pair-fed animals (Figure 1B). Next, we performed in vitro studies using HepG2 cells as a prototype for liver hepatocytes to determine whether OXLAMs were able to activate TRPV1 and thereby increase intracellular Ca2+. We found that both 9- and 13-HODE exposure increased intracellular Ca2+ levels (Figure 1, C and D), analogous to capsaicin, a classic TRPV1 agonist (Figure 1E).
Figure 1.
Chronic binge ethanol (EtOH) administration increases circulating OXLAM levels associated with TRPV1 up-regulation. A: Plasma 9- and 13-HODE levels. B: Hepatic TRPV1 mRNA up-regulation in response to chronic binge alcohol exposure. The relative mRNA expression was measured by RT-qPCR. Gene expression was normalized to 18s rRNA as an internal control. C–E: Intracellular Ca2+ levels in HepG2 cells determined by Cellomics. Cells were treated with 10 μmol/L 9-HODE, 10 μmol/L 13-HODE, and 10 μmol/L capsaicin for 24 hours. All stimulations were performed in triplicate. Results are presented as means ± SEM (Student's t-test). n = 5 to 9 animals per group (A); n = 5 to 6 animals per group (B); n = 3 animals per group (C–E). ∗P < 0.05.
Metabolic Characteristics of TRPV1−/− Mice in Response to Chronic Binge Ethanol Feeding
To determine whether increased expression of TRPV1 plays a role in alcohol-induced liver injury, and to examine the potential role of OXLAM/TRPV1 interactions, we next evaluated the effects of TRPV1 deletion in an experimental animal model of ALD. Both WT and TRPV1−/− animals tolerated the experimental protocol, and no mortality was observed. The effects of genotype and ethanol on body weight and clinical chemistry variables are presented in Table 2. Food consumption was similar in WT and TRPV1−/− mice fed an alcohol-containing diet, and there were no significant differences in body weight between the experimental groups. Ethanol exposure significantly increased liver/body weight ratios, which were not affected by mouse strain. At the end of ethanol feeding, 9 hours after a single ethanol gavage, elevated blood alcohol levels were observed in WT compared with TRPV1−/− animals. No significant differences were detected between the experimental groups in plasma lipopolysaccharide (LPS) levels, a marker of gut permeability and blood endotoxemia. Plasma glucose, TG, HDL, LDL, and VLDL levels were not substantially altered by ethanol exposure in both WT and TRPV1−/− animals. Plasma cholesterol was significantly increased in WT + ethanol compared with pair-fed mice; this effect of ethanol was not observed in TRPV1−/− animals.
Table 2.
Metabolic Characteristics of WT and TRPV1-Deficient Mice in an Experimental Animal Model of Chronic Binge Alcohol-Induced Liver Injury
| Characteristic | WT pair fed | WT-EtOH | TRPV1−/− pair fed | TRPV1−/− EtOH |
|---|---|---|---|---|
| Weight | ||||
| Initial BW (g) | 24.3 ± 0.45 | 23.8 ± 0.33 | 26.3 ± 0.75 | 26.9 ± 0.55 |
| Final BW (g) | 23.7 ± 0.31 | 23.8 ± 0.24 | 27.8 ± 1.23 | 26.2 ± 0.37 |
| Liver/BW (%) | 34 ± 0.001 | 45 ± 0.001∗ | 32 ± 0.001 | 44 ± 0.001∗ |
| Food consumption (g per day per mouse) | 9.4 ± 0.13 | 9.4 ± 0.17 | 9.8 ± 0.26 | 9.6 ± 0.20 |
| Blood Biochemical Characteristics | ||||
| LPS (EU/mL) | 0.15 ± 0.01 | 0.12 ± 0.02 | 0.15 ± 0.01 | 0.14 ± 0.02 |
| Blood alcohol levels (mg/dL) | ND | 238.1 + 10.4 | ND | 163.9 ± 20.6† |
| Glucose (mg/dL) | 165.6 ± 29.2 | 160.7 ± 6.1 | 149.3 ± 19.6 | 131.1 ± 12.5 |
| TGs (mg/dL) | 42.0 ± 3.3 | 42.8 ± 4.1 | 35.5 ± 1.2 | 46.5 ± 2.4 |
| Cholesterol (mg/dL) | 76.0 ± 4.4 | 92.5 ± 2.9∗ | 71.0 ± 18.4 | 89.43 ± 2.8 |
| HDL (mg/dL) | 55.8 ± 1.7 | 63.5 ± 3.3 | 65.3 ± 10.0 | 58.8 ± 2.2 |
| LDL (mg/dL) | 12.0 ± 3.1 | 20.0 ± 1.5 | 15.0 ± 0.01 | 21.4 ± 2.8 |
| VLDL (mg/dL) | 8.6 ± 0.6 | 8.6 ± 0.9 | 7.25 ± 0.2 | 9.3 ± 0.4 |
Values are expressed as means ± SEM (n = 5 to 9 animals per group).
BW, body weight; EtOH, ethanol; ND, not determined.
P < 0.05 for pair-fed versus EtOH-fed mice.
P < 0.05 for WT-EtOH versus TRPV1−/− EtOH.
Deficiency of the TRPV1 Ameliorates Chronic Binge Ethanol-Induced Liver Injury with No Effects on Hepatic Steatosis
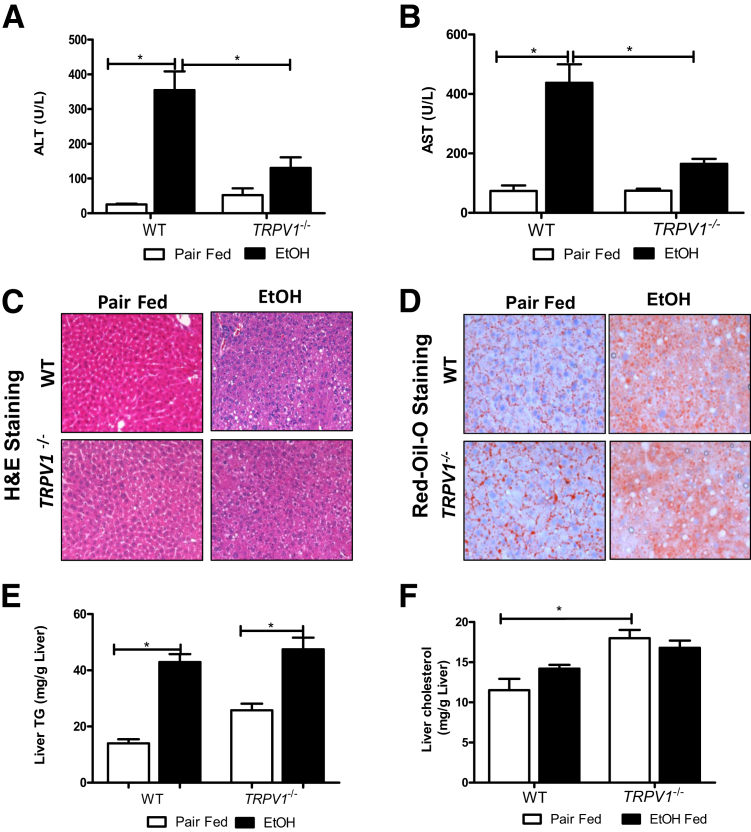
TRPV1 deficiency significantly attenuated chronic binge ethanol-induced liver injury. Thus, compared with WT, TRPV1−/− mice had significantly reduced plasma ALT and AST levels (Figure 2, A and B). Histological examination of the liver samples and analysis of Oil-Red-O staining revealed that alcohol exposure similarly increased hepatic fat deposition in both TRPV1−/− and WT mice (Figure 2, C and D); this effect was confirmed by hepatic TG measurement (Figure 2E). Although increases in hepatic cholesterol levels were observed in pair-fed TRPV1−/− mice compared with pair-fed WT animals, there were no differences in liver cholesterol in response to ethanol exposure in both TRPV1−/− and WT animals (Figure 2F). Hence, the absence of TRPV1 protected against chronic binge ethanol-induced hepatic injury, despite not blunting steatosis caused by ethanol exposure.
Figure 2.
Disruption of TRPV1 gene attenuates chronic binge ethanol (EtOH)–induced liver injury. A: Plasma ALT levels. B: Plasma AST levels. There is a significant increase in ALT and AST levels in response to ethanol in WT, but not TRPV1−/−, animals. C: Representative image of hepatic hematoxylin and eosin (H&E) staining. D: Representative image of Oil-Red-O staining. E: Liver TG levels. F: Liver cholesterol levels. A, B, E, and F: Values are means ± SEM. n = 5 to 9 animals per group (A, B, E, and F). ∗P < 0.05 (two-way analysis of variance, followed by the Tukey's multiple-comparison test). Original magnification: ×200 (C); ×400 (D).
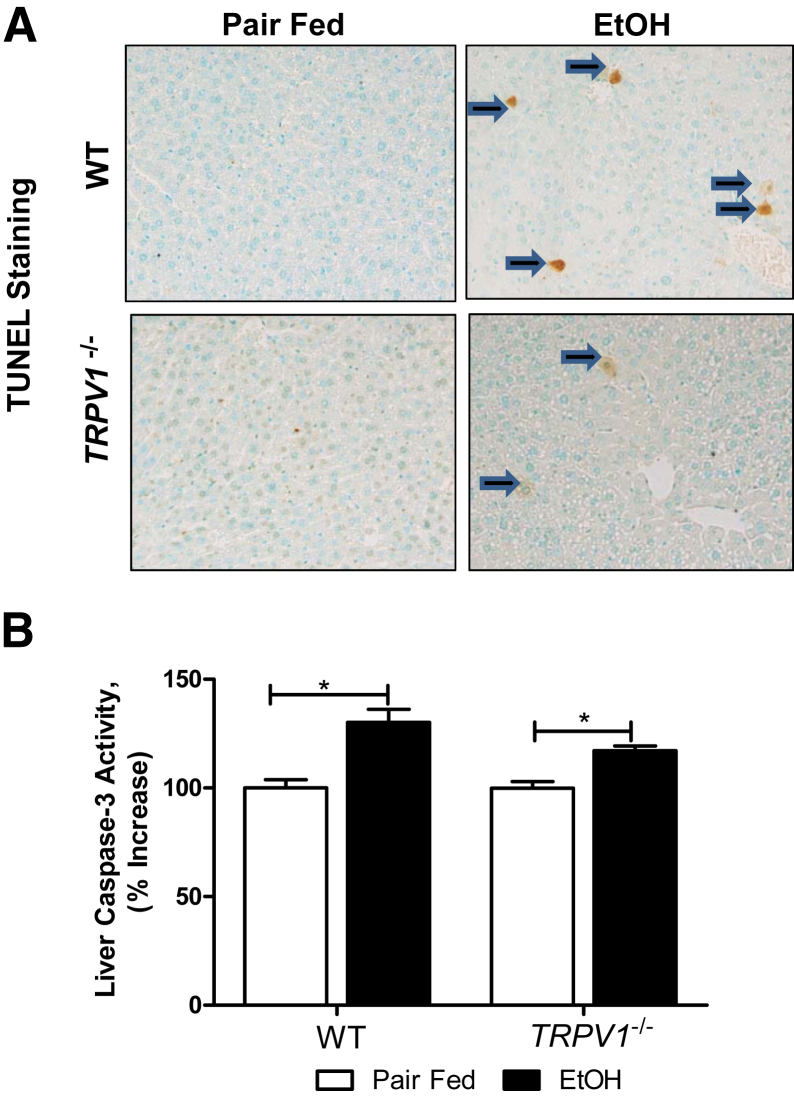
To investigate the possible mechanism(s) involved in the TRPV1 deficiency protection against chronic binge ethanol-induced liver injury, we next examined whether TRPV1 depletion may attenuate ethanol-induced hepatic apoptotic cell death. Ethanol exposure to WT mice increased positive staining for TUNEL in hepatocytes (Figure 3A); markedly fewer TUNEL-positive cells were observed in the livers of TRPV1−/− animals. In addition, we found that the alcohol-mediated increase in hepatic cleaved caspase-3 activity, an established marker of apoptosis, was attenuated in TRPV1−/− compared with WT mice (Figure 3B).
Figure 3.
TRPV1 deficiency prevents chronic binge ethanol (EtOH)–induced hepatic apoptosis. A: Representative images of TUNEL staining. Arrows indicate TUNEL-positive hepatocytes. B: Hepatic caspase-3 activity. Values are means ± SEM. n = 5 to 8 animals per group. ∗P < 0.05 (two-way analysis of variance, followed by the Tukey's multiple-comparison test). Original magnification, ×200 (A).
TRPV1−/− Mice Are Resistant to Chronic Binge Ethanol-Induced Hepatic Inflammation
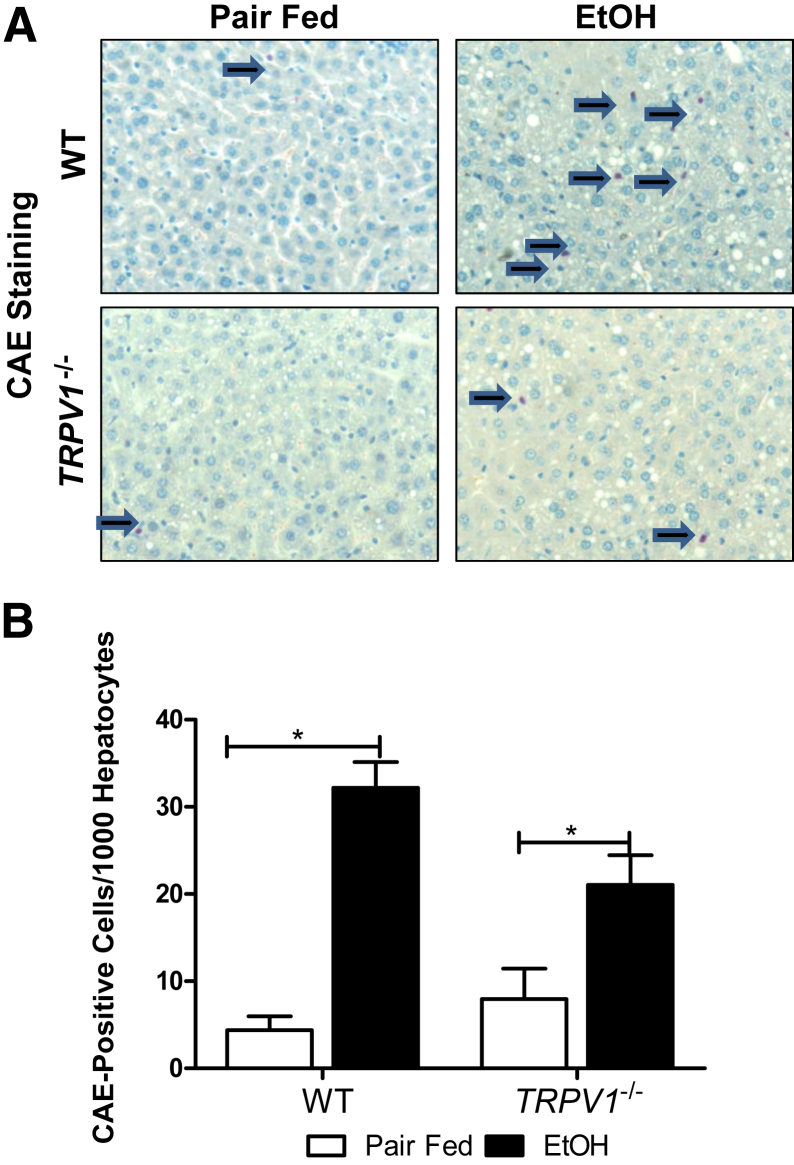
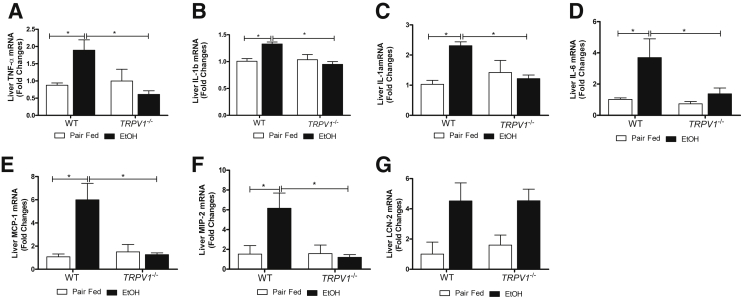
To further investigate the mechanism(s) underlying hepatoprotective effects of TRPV1 deficiency on alcohol-induced liver injury, we examined the ethanol-mediated hepatic proinflammatory response. As expected,40 ethanol exposure under these conditions caused a robust inflammatory response and increased the number of recruited neutrophils in WT animals; TRPV1 deficiency significantly blunted this increase caused by ethanol exposure (Figure 4, A and B). Next, we examined expression of proinflammatory cytokines and chemokines known to play important roles in alcohol-induced liver injury. Analysis of mRNA revealed that hepatic levels of proinflammatory cytokines TNF-α, IL-1β, IL-1α, and IL-6 (Figure 5, A–D) and chemokines monocyte chemotactic protein (MCP)-1 and macrophage inflammatory protein (MIP)-2 (Figure 5, E and F) were significantly induced by ethanol in WT, but not in TRPV1−/−, animals. Given that lipocalin 2 (LCN2) has previously been characterized as an adipokine/cytokine playing a role in modulation of inflammation,41 we determined hepatic LCN2 mRNA levels. A similarly elevated LCN2 expression was observed in both WT and TRPV1−/− mice in response to ethanol feeding, not reaching statistical significance because of the high intragroup variability (Figure 5G).
Figure 4.
Disruption of TRPV1 gene attenuates chronic binge ethanol (EtOH)–induced hepatic neutrophil infiltration. A: Representative images depict CAE staining. Arrows indicate CAE-positive neutrophils. B: Quantification of CAE staining performed by counting CAE-positive neutrophils per 1000 hepatocytes. Values are means + SEM. n = 3 to 8 animals per group. ∗P < 0.05 (two-way analysis of variance, followed by the Tukey's multiple-comparison test). Original magnification, ×400 (A).
Figure 5.
Absence of TRPV1 prevents against chronic binge ethanol (EtOH)–induced hepatic inflammation. RT-qPCR analysis of mRNA levels of hepatic proinflammatory cytokines and chemokines: TNF-α (A), IL-1b (B), IL-1a (C), IL-6 (D), MCP-1 (E), MIP-2 (F), and LCN2 (G). RT-qPCR data were normalized to 18s rRNA as an internal control. Values are means ± SEM (n = 5 to 6 animals per group). ∗P < 0.05 (two-way analysis of variance, followed by the Tukey's multiple-comparison test).
TRPV1 Deficiency Decreases Hepatic PAI-1 Up-Regulation and Fibrin Deposition Caused by Chronic Binge Ethanol Treatment
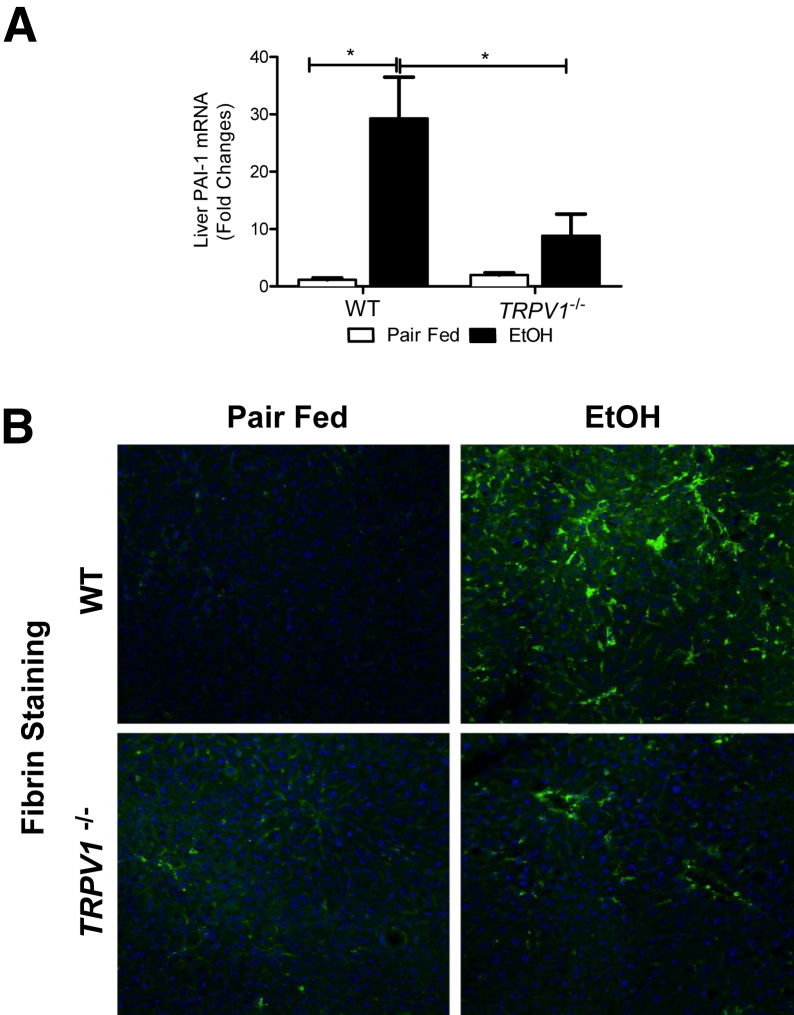
Our data indicated that TRPV1 deficiency conferred protection against alcohol-induced inflammation, despite not affecting steatosis. Previous work has demonstrated that the inhibition of hepatic fibrin degradation by plasminogen activator inhibitor-1 (PAI-1) also plays a selective role in hepatic inflammation caused by ethanol.42 The role of PAI-1 and fibrin deposition in the current study was, therefore, determined. Indeed, PAI-1 mRNA levels were markedly up-regulated in response to ethanol in both WT and TRPV1−/− mice; the increase in WT mice was greater than fourfold than that in TRPV1−/− mice (Figure 6A). Furthermore, we observed that this substantial PAI-1 up-regulation in the livers of WT mice fed ethanol was associated with a marked increase in fibrin deposition in sinusoidal spaces of the liver lobule (assessed by immunofluorescence staining), and this effect was blunted by TRPV1 deficiency (Figure 6B).
Figure 6.
TRPV1−/− mice exhibit significantly decreased hepatic PAI-1 expression and fibrin deposition. A: Hepatic PAI-1 mRNA. PAI-1 levels were measured by RT-qPCR, and normalized to 18s rRNA as an internal control. B: Representative confocal images depict immunofluorescence detection of hepatic fibrin (green) against a Hoechst counterstain (blue). A: Values are means ± SEM. n = 5 to 6 animals per group (A). ∗P < 0.05 (two-way analysis of variance, followed by the Tukey’s multiple-comparison test). Original magnification, ×400 (B). EtOH, ethanol.
TRPV1 Depletion Prevents Ethanol-Induced Activation of Hepatic NF-κB and ERK 1/2 MAPK Signaling Pathways
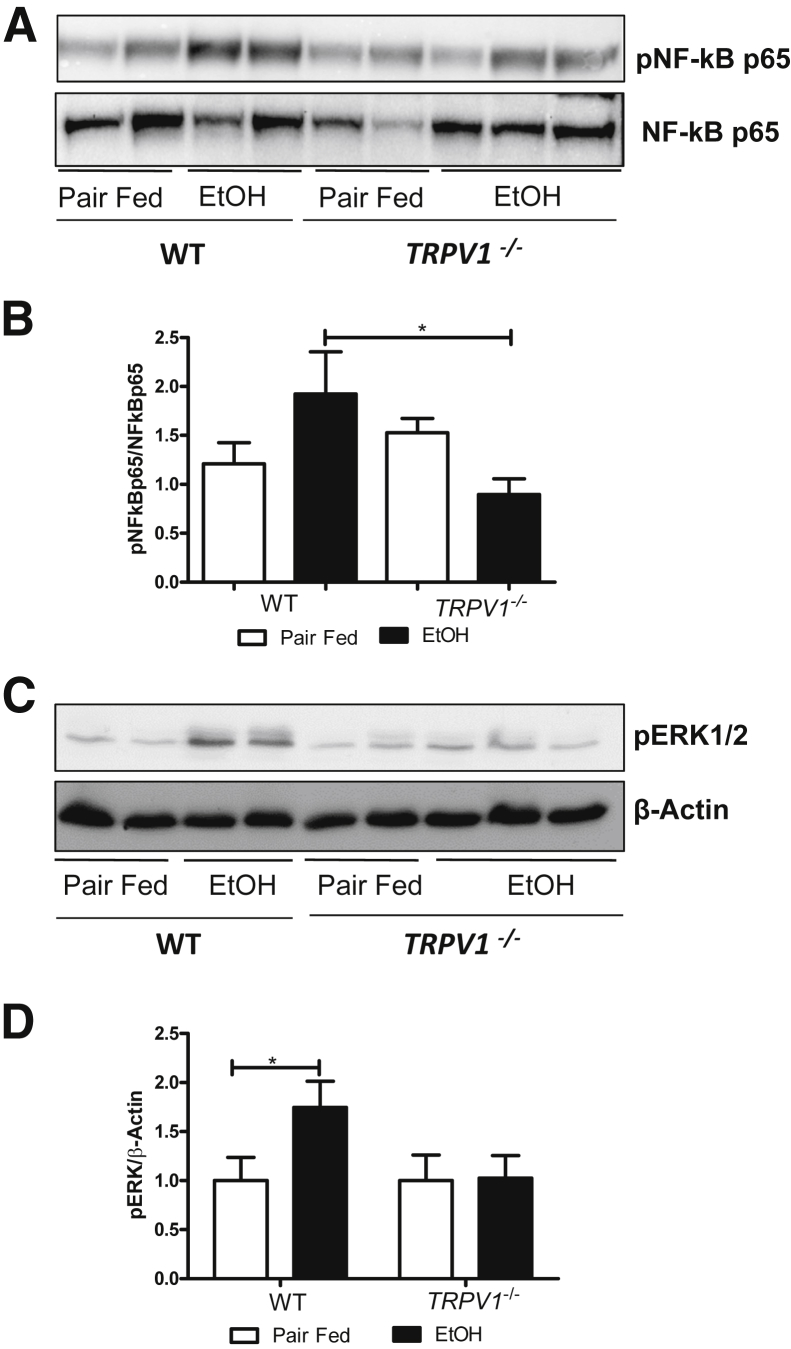
We next evaluated which signaling cascades might be involved in the ethanol-mediated hepatic proinflammatory response and the protective effects of TRPV1 deletion. The NF-κB signaling pathway regulates the expression of many cytokines and is known to play an important role in the proinflammatory response in ALD. Activation of the hepatic NF-κB signaling pathway, confirmed by an increase in nuclear pNF-κB p65, was observed in WT, but not TRPV1-deficient, animals in response to ethanol treatment (Figure 7, A and B). We also observed that chronic binge ethanol administration activated pERK 1/2 MAPK signaling, and TRPV1 deficiency completely abolished ethanol-induced pERK 1/2 activation (Figure 7, C and D). Activation of other MAPKs, including p38 MAPK and c-Jun N-terminal kinase, was not found in either WT or TRPV1−/− ethanol-fed mice (data not shown).
Figure 7.
Effects of ethanol (EtOH) and TRPV1 deficiency on phospho–NF-κB p65 and phospho-ERK 1/2. Representative images of Western blot and quantitative densitometric analyses for nuclear pNF-κB p65 (A and B) and whole liver lysate pERK 1/2 (C and D). The intensity of protein bands was quantified by densitometry using the ImageJ software. Values are means ± SEM. n = 4 to 6 animals per group. ∗P < 0.05 (two-way analysis of variance, followed by the Tukey's multiple-comparison test).
Discussion
Dietary fat and alcohol both play important roles in the pathogenesis of ALD. Our group and others have demonstrated that dietary fat enriched in linoleic acid (LA) exacerbated ethanol-induced hepatic steatosis, inflammation, and injury.4–7 Moreover, it has been reported that dietary LA is required for the development of experimental ALD.43 However, the mechanisms by which the combination of LA and alcohol promotes liver injury are not fully understood. Our current data and recently published reports8,9 demonstrate that ethanol-induced liver inflammation and injury are associated with elevated plasma levels of bioactive OXLAMs in experimental and clinical ALD. OXLAMs, specifically 9- and 13-HODEs, have recently been implicated as natural endogenous ligands for the TRPV1 receptor,10,11 a ligand-gated channel with high permeability for Ca2+.12 Given these data, it was hypothesized that the TRPV1 receptor is activated during ALD and may contribute to liver injury. Although the contribution and detailed mechanism(s) of OXLAMs/TRPV1-mediated hepatic pathological features remain to be established, a critical role of OXLAM/TRPV1 interactions has been previously identified in other pathological conditions. For example, it has been reported that activation of TRPV1 by OXLAMs in the spinal cord contributed to inflammatory hyperalgesia.11 Inhibition of TRPV1 reduced 13-S-HODE–mediated mitochondria dysfunction and bronchial epithelial injury in vitro and severe airway obstruction, and increased proinflammatory cytokines in vivo.44 In our study, ethanol administration up-regulated hepatic TRPV1 mRNA in parallel with the elevated levels of circulating OXLAMs. We also showed that 9- and 13-HODEs increased intracellular Ca2+ (as a marker of TRPV1 activation) in vitro, in HepG2 cells, suggesting that these OXLAMs may serve as endogenous ligands for hepatic TRPV1 in vivo. Evidence suggests that ethanol may also sensitize TRPV1 to endogenous agonists/activators. For example, it lowered the threshold for TRPV1 heat activation in primary sensory nerves.45
Ethanol-mediated increases in circulating OXLAMs and TRPV1 levels were associated with hepatic steatosis, inflammation, and injury. More important, we found that TRPV1 deficiency protected against chronic binge alcohol-induced hepatic inflammation and injury, as assessed by decreased plasma ALT levels, decreased ethanol-induced hepatocyte cell death via apoptosis, as indicated by TUNEL staining, and decreased ethanol-induced caspase-3 activity. In light of previous reports of TRPV1-mediated Ca2+-dependent apoptosis in several cell types (eg, primary cortical neurons46 and retinal ganglion cells47), we propose that TRPV1 activation promotes apoptosis in hepatocytes via a Ca2+-mediated mechanism.
TRPV1 deficiency had no impact on the degree of alcohol-induced hepatic steatosis, indicating that the most prominent role of TRPV1 is in progression to steatohepatitis. Control TRPV1−/− mice fed only a high-fat diet (no ethanol was added) did accumulate slightly more hepatic fat compared with WT animals, indicating that TRPV1 may play a limited role in hepatic lipid metabolism. Notably, recently published reports showed that TRPV1 activation by dietary capsaicin, an exogenous TRPV1 agonist, prevented development of high-fat diet–induced fatty liver in mice via up-regulation of hepatic uncoupling protein 2, a mitochondria membrane transporter involved in fatty acid oxidation,13 and peroxisome proliferator-activated receptor-δ–dependent autophagy enhancement.31
A critical issue in the development of ALD is the progression from the simple steatosis to the inflamed state, steatohepatitis, and to fibrosis. However, the exact mechanisms driving/underlying hepatic inflammation during the transition from steatosis to more advanced stages of ALD are not well defined. Our data suggest that OXLAM/TRPV1 interactions may contribute to this progression. Indeed, in our study, we observed that on the background of the equal alcohol-mediated hepatic fat accumulation, TRPV1−/− mice had decreased susceptibility to hepatic inflammation compared with WT animals. TRPV1 deficiency prevented alcohol-mediated hepatic inflammation and consequent liver injury by significantly reducing TNF-α expression, one of the main cytokines involved in hepatocyte injury, as well as other hepatic proinflammatory cytokines, including IL-1β, IL-1α, and IL-6. Ethanol-induced hepatic MCP-1 and MIP-2 mRNA levels, chemokines involved in hepatic inflammatory cell infiltration, were also decreased in parallel with reduced hepatic neutrophil infiltration in TRPV1−/− compared with WT mice. One of the possible mechanism(s) underlying hepatic inflammation during the progression from steatosis to steatohepatitis in our model might relate to the nature of TRPV1 as a channel with high permeability for Ca2+. As a second messenger, intracellular Ca2+ is essential for many cellular responses, including proinflammatory responses. In this regard, a recently published study demonstrated a Ca2+- and protein kinase C–dependent signaling pathway for NF-κB activation, increased inducible nitric oxide synthase expression, and TNF-α production in LPS-stimulated rat peritoneal macrophages.48 Our observation that TRPV1 deficiency attenuated NF-κB pathway activation suggests that TRPV1 contributes to the hepatic NF-κB activation via a Ca2+-dependent mechanism; however, further studies are needed to support this concept. Increases in intracellular Ca2+ have recently been suggested as a critical factor of NLRP3 inflammasome activation with the consequent increase in IL-1β release,49–51 an important proinflammatory response in ALD.52 Intracellular Ca2+ also plays an important role in inflammasome-independent, calcium-sensitive, cysteine protease calpain-mediated processing of pro–IL-1α and production of IL-1α.53 Moreover, calcium-channel blockers have been shown to have a hepatoprotective effect in animal models with alcohol-induced liver injury.54
Resistance of TRPV1−/− mice to ethanol-induced hepatic PAI-1 up-regulation and fibrin accumulation observed in our study may also contribute to the protective effects of TRPV1 deficiency against alcohol-induced liver inflammation. Preventing PAI-1 induction completely protected against chronic alcohol-induced inflammation.55 Furthermore, the enhanced LPS-induced inflammatory liver injury caused by ethanol pre-exposure was shown to be mediated, at least in part, by fibrin accumulation in livers, mediated by an inhibition of fibrinolysis by PAI-1.56 The detailed molecular mechanisms linking TRPV1 receptor to the alcohol-induced PAI-1 up-regulation are not well understood. One of the possible mechanism(s) might be an ethanol/OXLAM/TRPV1-mediated increase in intracellular Ca2+. Indeed, intracellular Ca2+, as an important intracellular messenger, plays a significant role in the up-regulation of PAI-1 gene expression in several cell lines (eg, human lymphoma-derived histocytic cell line,57 human dermal fibroblasts,58 and human hepatocellular carcinoma HepG2 cells59). It has been shown in HepG2 cells that Ca2+ stimulated the expression of PAI-1 via hypoxia-inducible factor-1α transcription, which is, in turn, induced by elevation of cytosolic Ca2+ via the ERK signaling pathway.59 These in vitro observations are consistent with our in vivo findings demonstrating that ethanol-induced activation of hepatic ERK is in parallel with the liver PAI-1 up-regulation in WT, but not in TRPV1-deficient, animals. In addition, linoleic acid itself may enhance secretion of PAI-1,60 possibly via a direct effect of linoleic acid on fatty acid–responsive regulatory elements present on the PAI-1 gene.61
The results from the current study are in agreement with the concept that TRPV1 activation is proinflammatory; this has been demonstrated in numerous nonhepatic cell types62–64 and animal models under different proinflammatory conditions.65 It has been also shown that TRPV1 deficiency decreased high-fat diet–induced IL-1β and IL-6 release.30 However, anti-inflammatory and protective effects of TRPV1 have also been reported both in vitro and in vivo in certain experimental paradigms.32,66–68 Thus, TRPV1 may exhibit both proinflammatory and anti-inflammatory properties that most likely depend on the nature of TRPV1 activation, downstream signaling pathways involved in the response, type of cells, diseases, and conditions.
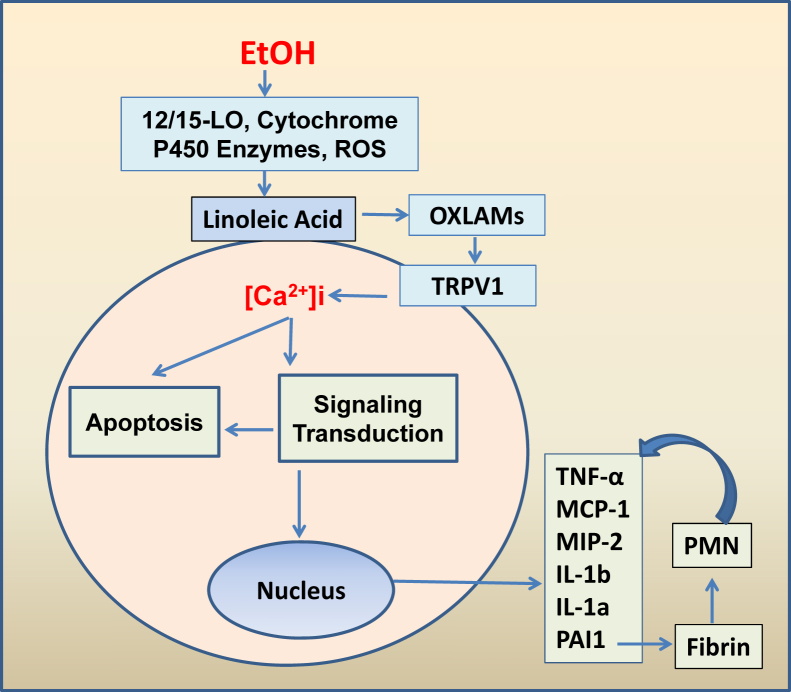
In conclusion, these data demonstrate that TRPV1 deficiency protected against experimental ALD through the modulation/reduction of ethanol-induced proinflammatory responses. Compared with WT, TRPV1−/− mice displayed less ethanol-induced liver inflammation/injury but similar levels of hepatic steatosis. These data provide new insights into ALD pathogenesis, and suggest the involvement of OXLAM/TRPV1 interactions in the alcohol-induced inflammation and injury (Figure 8).
Figure 8.
The proposed model of the OXLAM/TRPV1 contribution to the alcohol-mediated hepatic inflammation and injury. Ethanol (EtOH) activates metabolic pathways of linoleic acid oxidation and increase in OXLAM production, which, in turn, may activate TRPV1 with the consequent elevation in intracellular Ca2+. Intracellular Ca2+ is essential for many cellular responses, including apoptosis and proinflammatory responses. Furthermore, the increase in PAI-1 among other proinflammatory cytokines associated with elevated fibrin deposition may mediate PMN accumulation and feedback induction of proinflammatory cytokines. 12/15-LO, 12/15-lipoxygenase; PMN, polymorphonuclear leukocyte; ROS, reactive oxygen species.
Acknowledgments
We thank Jingwen Zhang for assistance with tissue staining, Dr. David Barker for qPCR primer design, and Marion McClain for proofreading the manuscript.
Footnotes
Supported by NIH grants R21 AA020849-01A1 (I.A.K.), DK082451 (A.E.F.), P01 AA017103 (C.J.M.), R01 AA023681 (C.J.M.), R01 AA018016 (C.J.M.), R37 AA010762 (C.J.M.), R01 AA018869 (C.J.M.), and U01 AA022489 (A.E.F., C.J.M.), the Department of Veterans Affairs grant BX000350 (C.J.M.), and the Intramural Program of the National Institute on Alcohol Abuse and Alcoholism (C.E.R.).
Disclosures: None declared.
References
- 1.Kim W.R., Brown R.S., Jr., Terrault N.A., El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Hassan M.M., Hwang L.Y., Hatten C.J., Swaim M., Li D., Abbruzzese J.L., Beasley P., Patt Y.Z. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 3.Kung H.C., Hoyert D.L., Xu J., Murphy S.L. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 4.Ronis M.J., Korourian S., Zipperman M., Hakkak R., Badger T.M. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 5.Nanji A.A. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Kirpich I.A., Feng W., Wang Y., Liu Y., Barker D.F., Barve S.S., McClain C.J. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirpich I.A., Feng W., Wang Y., Liu Y., Beier J.I., Arteel G.E., Falkner K.C., Barve S.S., McClain C.J. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol. Alcohol. 2013;47:257–264. doi: 10.1016/j.alcohol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raszeja-Wyszomirska J., Safranow K., Milkiewicz M., Milkiewicz P., Szynkowska A., Stachowska E. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat. 2012;99:51–56. doi: 10.1016/j.prostaglandins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Latchoumycandane C., McMullen M.R., Pratt B.T., Zhang R., Papouchado B.G., Nagy L.E., Feldstein A.E., McIntyre T.M. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patwardhan A.M., Akopian A.N., Ruparel N.B., Diogenes A., Weintraub S.T., Uhlson C., Murphy R.C., Hargreaves K.M. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patwardhan A.M., Scotland P.E., Akopian A.N., Hargreaves K.M. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Chen J., Ni Y., Feng X., Zhao Z., Wang P., Sun J., Yu H., Yan Z., Liu D., Nilius B., Zhu Z. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflugers Arch. 2012;463:727–732. doi: 10.1007/s00424-012-1078-y. [DOI] [PubMed] [Google Scholar]
- 14.Miao X., Liu G., Xu X., Xie C., Sun F., Yang Y., Zhang T., Hua S., Fan W., Li Q., Huang S., Wang Q., Liu G., Zhong D. High expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2008;186:25–32. doi: 10.1016/j.cancergencyto.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Rychkov G.Y., Barritt G.J. Expression and function of TRP channels in liver cells. Adv Exp Med Biol. 2011;704:667–686. doi: 10.1007/978-94-007-0265-3_35. [DOI] [PubMed] [Google Scholar]
- 16.Vriens J., Janssens A., Prenen J., Nilius B., Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium. 2004;36:19–28. doi: 10.1016/j.ceca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Li X.H., McGrath K.C., Tran V.H., Li Y.M., Mandadi S., Duke C.C., Heather A.K., Roufogalis B.D. Identification of a calcium signalling pathway of S-[6]-gingerol in HuH-7 cells. Evid Based Complement Alternat Med. 2013;2013:951758. doi: 10.1155/2013/951758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L.L., Yan Liu D., Ma L.Q., Luo Z.D., Cao T.B., Zhong J., Yan Z.C., Wang L.J., Zhao Z.G., Zhu S.J., Schrader M., Thilo F., Zhu Z.M., Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 20.Akiba Y., Kato S., Katsube K., Nakamura M., Takeuchi K., Ishii H., Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- 21.Heiner I., Eisfeld J., Halaszovich C.R., Wehage E., Jungling E., Zitt C., Luckhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders C.I., Kunde D.A., Crawford A., Geraghty D.P. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol Immunol. 2007;44:1429–1435. doi: 10.1016/j.molimm.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Smart D., Gunthorpe M.J., Jerman J.C., Nasir S., Gray J., Muir A.I., Chambers J.K., Randall A.D., Davis J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin S., Luo J., Qian A., Du J., Yang Q., Zhou S., Yu W., Du G., Clark R.B., Walters E.T., Carlton S.M., Hu H. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest. 2013;123:3941–3951. doi: 10.1172/JCI66413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang S.W., Cho H., Kwak J., Lee S.Y., Kang C.J., Jung J., Cho S., Min K.H., Suh Y.G., Kim D., Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 27.Richardson J.D., Vasko M.R. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 28.Gram D.X., Hansen A.J., Wilken M., Elm T., Svendsen O., Carr R.D., Ahren B., Brand C.L. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. 2005;153:963–969. doi: 10.1530/eje.1.02046. [DOI] [PubMed] [Google Scholar]
- 29.Gram D.X., Ahren B., Nagy I., Olsen U.B., Brand C.L., Sundler F., Tabanera R., Svendsen O., Carr R.D., Santha P., Wierup N., Hansen A.J. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 30.Marshall N.J., Liang L., Bodkin J., Dessapt-Baradez C., Nandi M., Collot-Teixeira S., Smillie S.J., Lalgi K., Fernandes E.S., Gnudi L., Brain S.D. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–252. doi: 10.1161/HYPERTENSIONAHA.112.201434. [DOI] [PubMed] [Google Scholar]
- 31.Li Q., Li L., Wang F., Chen J., Zhao Y., Wang P., Nilius B., Liu D., Zhu Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor delta activation. Pflugers Arch. 2013;465:1303–1316. doi: 10.1007/s00424-013-1274-4. [DOI] [PubMed] [Google Scholar]
- 32.Avraham Y., Zolotarev O., Grigoriadis N.C., Poutahidis T., Magen I., Vorobiav L., Zimmer A., Ilan Y., Mechoulam R., Berry E.M. Cannabinoids and capsaicin improve liver function following thioacetamide-induced acute injury in mice. Am J Gastroenterol. 2008;103:3047–3056. doi: 10.1111/j.1572-0241.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- 33.Bertola A., Mathews S., Ki S.H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirpich I.A., Gobejishvili L.N., Bon Homme M., Waigel S., Cave M., Arteel G., Barve S.S., McClain C.J., Deaciuc I.V. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J Nutr Biochem. 2011;22:38–45. doi: 10.1016/j.jnutbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beier J.I., Guo L., von Montfort C., Kaiser J.P., Joshi-Barve S., Arteel G.E. New role of resistin in lipopolysaccharide-induced liver damage in mice. J Pharmacol Exp Ther. 2008;325:801–808. doi: 10.1124/jpet.108.136721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Untergrasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3–new capabilities and interfaces. Nucleic Acids Research. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldstein A.E., Lopez R., Tamimi T.A., Yerian L., Chung Y.M., Berk M., Zhang R., McIntyre T.M., Hazen S.L. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zein C.O., Lopez R., Fu X., Kirwan J.P., Yerian L.M., McCullough A.J., Hazen S.L., Feldstein A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–1299. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willker W., Leibfritz D. Lipid oxidation in blood plasma of patients with neurological disorders. Brain Res Bull. 2000;53:437–443. doi: 10.1016/s0361-9230(00)00375-0. [DOI] [PubMed] [Google Scholar]
- 40.Bertola A., Park O., Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D.A., Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–1426. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beier J.I., Arteel G.E. Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism. Exp Biol Med (Maywood) 2012;237:1–9. doi: 10.1258/ebm.2011.011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanji A.A., French S.W. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44:223–227. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- 44.Mabalirajan U., Rehman R., Ahmad T., Kumar S., Singh S., Leishangthem G.D., Aich J., Kumar M., Khanna K., Singh V.P., Dinda A.K., Biswal S., Agrawal A., Ghosh B. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep. 2013;3:1349. doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trevisani M., Smart D., Gunthorpe M.J., Tognetto M., Barbieri M., Campi B., Amadesi S., Gray J., Jerman J.C., Brough S.J., Owen D., Smith G.D., Randall A.D., Harrison S., Bianchi A., Davis J.B., Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 46.Song J., Lee J.H., Lee S.H., Park K.A., Lee W.T., Lee J.E. TRPV1 activation in primary cortical neurons induces calcium-dependent programmed cell death. Exp Neurobiol. 2013;22:51–57. doi: 10.5607/en.2013.22.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sappington R.M., Sidorova T., Long D.J., Calkins D.J. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci. 2009;50:717–728. doi: 10.1167/iovs.08-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Yang W., Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem. 2006;281:31337–31347. doi: 10.1074/jbc.M602739200. [DOI] [PubMed] [Google Scholar]
- 49.Triantafilou K., Hughes T.R., Triantafilou M., Morgan B.P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 50.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrasek J., Bala S., Csak T., Lippai D., Kodys K., Menashy V., Barrieau M., Min S.Y., Kurt-Jones E.A., Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freigang S., Ampenberger F., Weiss A., Kanneganti T.D., Iwakura Y., Hersberger M., Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 54.Iimuro Y., Ikejima K., Rose M.L., Bradford B.U., Thurman R.G. Nimodipine, a dihydropyridine-type calcium channel blocker, prevents alcoholic hepatitis caused by chronic intragastric ethanol exposure in the rat. Hepatology. 1996;24:391–397. doi: 10.1002/hep.510240217. [DOI] [PubMed] [Google Scholar]
- 55.Bergheim I., Guo L., Davis M.A., Lambert J.C., Beier J.I., Duveau I., Luyendyk J.P., Roth R.A., Arteel G.E. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beier J.I., Luyendyk J.P., Guo L., von Montfort C., Staunton D.E., Arteel G.E. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peiretti F., Fossat C., Anfosso F., Alessi M.C., Henry M., Juhan-Vague I., Nalbone G. Increase in cytosolic calcium upregulates the synthesis of type 1 plasminogen activator inhibitor in the human histiocytic cell line U937. Blood. 1996;87:162–173. [PubMed] [Google Scholar]
- 58.Kye K.C., Chae E.K., Piao Y.J., Park S., Park J.K., Kim C.D., Lee J.H., Suhr K.B. Signaling events during induction of plasminogen activator inhibitor-1 expression by sphingosylphosphorylcholine in cultured human dermal fibroblasts. J Invest Dermatol. 2004;122:1365–1371. doi: 10.1111/j.0022-202X.2004.22615.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q., Moller U., Flugel D., Kietzmann T. Induction of plasminogen activator inhibitor I gene expression by intracellular calcium via hypoxia-inducible factor-1. Blood. 2004;104:3993–4001. doi: 10.1182/blood-2004-03-1017. [DOI] [PubMed] [Google Scholar]
- 60.Banfi C., Rise P., Mussoni L., Galli C., Tremoli E. Linoleic acid enhances the secretion of plasminogen activator inhibitor type 1 by HepG2 cells. J Lipid Res. 1997;38:860–869. [PubMed] [Google Scholar]
- 61.Kariko K., Rosenbaum H., Kuo A., Zurier R.B., Barnathan E.S. Stimulatory effect of unsaturated fatty acids on the level of plasminogen activator inhibitor-1 mRNA in cultured human endothelial cells. FEBS Lett. 1995;361:118–122. doi: 10.1016/0014-5793(95)00170-e. [DOI] [PubMed] [Google Scholar]
- 62.Zhang F., Yang H., Wang Z., Mergler S., Liu H., Kawakita T., Tachado S.D., Pan Z., Capo-Aponte J.E., Pleyer U., Koziel H., Kao W.W., Reinach P.S. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213:730–739. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]
- 63.Sappington R.M., Calkins D.J. Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest Ophthalmol Vis Sci. 2008;49:3004–3017. doi: 10.1167/iovs.07-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma J., Altomare A., Guarino M., Cicala M., Rieder F., Fiocchi C., Li D., Cao W., Behar J., Biancani P., Harnett K.M. HCl-induced and ATP-dependent upregulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012;303:G635–G645. doi: 10.1152/ajpgi.00097.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alawi K., Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181–195. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J.F., Ching L.C., Kou Y.R., Lin S.J., Wei J., Shyue S.K., Lee T.S. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-alpha-induced inflammation in macrophages: role of liver X receptor alpha. Mediators Inflamm. 2013;2013:925171. doi: 10.1155/2013/925171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark N., Keeble J., Fernandes E.S., Starr A., Liang L., Sugden D., de Winter P., Brain S.D. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21:3747–3755. doi: 10.1096/fj.06-7460com. [DOI] [PubMed] [Google Scholar]
- 68.Hegde V.L., Nagarkatti P.S., Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. doi: 10.1371/journal.pone.0018281. [DOI] [PMC free article] [PubMed] [Google Scholar]