Abstract
Objective
We compared metoclopramide 20 mg IV, combined with diphenhydramine 25 mg IV, to ketorolac 30 mg IV in adults with tension-type headache and all non-migraine, non-cluster recurrent headaches.
Methods
In this ED-based randomized, double-blind study, we enrolled adults with non-migraine, non-cluster recurrent headaches. Patients with tension-type headache were a subgroup of special interest. Our primary outcome was a comparison of the improvement in pain score between baseline and one hour later, assessed on a 0 to 10 verbal scale. We defined a between-group difference of 2.0 as the minimum clinically significant difference. Secondary endpoints included: 1) need for rescue medication in the ED; 2) achieving headache freedom in the ED and sustaining it for 24 hours; and 3) patient’s desire to receive the same medication again.
Results
We included 120 patients in the analysis. The metoclopramide/diphenhydramine arm improved by a median of 5 (IQR 3,7) scale units while the ketorolac arm improved by a median of 3 (IQR 2,6) (95%CI for difference: 0, 3). Metoclopramide + diphenhydramine were superior to ketorolac for all three secondary outcomes: the number needed to treat for not requiring ED rescue medication was 3 (95%CI: 2, 6), for sustained headache freedom 6 (95%CI: 3, 20), and for wish to receive the same medication again 7 (95%CI: 4, 65). Tension-type headache subgroup results were similar.
Conclusions
For adults who presented to an ED with tension-type headache or with non-migraine, non-cluster recurrent headache, IV metoclopramide + diphenhydramine provided more headache relief than IV ketorolac.
Introduction
Non-steroidal anti-inflammatory drugs are commonly used to treat tension-type headache.1. Several studies have also demonstrated efficacy of parenteral dopaminergic antagonists such as chlorpromazine2 and metoclopramide3 for these headaches. Comparative efficacy studies of the dopamine antagonists versus the non-steroidals have yet to be performed. One aim of this study was to compare the efficacy in tension-type headache of intravenous metoclopramide, a safe and well tolerated dopamine receptor antagonist, to that of intravenous ketorolac, a parenteral non-steroidal anti-inflammatory drug.
Patients who present to an ED for treatment of an acute exacerbation of a recurrent headache disorder at times cannot be given a formal headache diagnosis because of bland or conflicting headache features, prolonged headache duration, or a history of only infrequent recurrence of headache4. These difficult to classify headaches will either continue to recur, and ultimately meet criteria for one of the named headache disorders, such as tension-type, migraine, or cluster, or resolve and thus not require classification. In clinical practice, when these headaches present to our ED acutely, we treat them as presumptive tension-type headache with non-steroidal anti-inflammatory drugs or as presumptive migraine, with dopamine antagonists.
In this study, we lumped non-migraine, non-cluster recurrent headaches together with tension-type headache because this reflects a clinical reality: once clinicians exclude a pathological underlying cause of headache from the differential diagnosis, and when the headache lacks the requisite features to support the diagnosis of migraine or cluster, subtleties in headache nosology are of only marginal practical use to emergency clinicians. This approach has ample precedent in emergency medicine headache research, in which researchers often aggregate all benign headaches 5–7. It may also reflect a reality of headache nociception known as the “convergence hypothesis,” which posits that various distinct primary headaches are manifestations of the same underlying neuropathophysiology.8
In this study we tested two distinct hypotheses:
Hypothesis 1: In a population of patients with an exacerbation of a recurrent headache meeting neither migraine nor cluster headache criteria, 20 mg of intravenous metoclopramide combined with 25 mg of intravenous diphenhydramine will produce greater relief of headache 60 minutes after medication administration than will 30 mg of intravenous ketorolac.
Hypothesis 2: Within the subset of patients meeting International Headache Society criteria for tension-type headache, 20 mg of intravenous metoclopramide combined with 25 mg of intravenous diphenhydramine will also produce greater relief of headache 60 minutes after medication administration than will 30 mg of intravenous ketorolac.
Methods
Study design and setting
This was a randomized, double blind trial, comparing two parenteral treatments among patients presenting to our ED with 1) non-migraine, non-cluster recurrent headache and 2) tension-type headache. The Montefiore Medical Center IRB approved this protocol. We registered it at http://clinicaltrials.gov (NCT01011673).
This study was performed in the ED of Montefiore Medical Center, an urban teaching hospital, with over 100,000 adult visits annually. Salaried, trained, fluently bilingual (English and Spanish) research associates staff the ED 24 hours per day, seven days per week.
Selection of Participants
Research associates screened adult patients younger than 65 who presented to our ED with headache. Those who had a recurrent episode of a headache experienced at least once before were eligible for participation, provided they did not meet migraine or cluster headache criteria as defined by the International Headache Society’s (IHS) International Classification of Headache Disorders, 2nd edition.9 We excluded patients if the attending physician was suspicious of a serious secondary cause of headache, for temperature ≥100.4 degrees, a new objective neurologic abnormality, allergy, active gastritis or peptic ulcer disease, history of upper gastrointestinal bleeding, organ transplant, use of a monoamine oxidase inhibitor, pregnancy, lactation, or previous enrollment. We asked patients a series of close-ended questions about their current headache and their headache history, which allowed us to define the sub-group who met criteria for tension-type headache (ICHD 2.1, ICHD 2.2 , ICHD 2.3)9(Figure 1).
Figure 1.
Tension-type headache criteria. From the International Headache Society’s International Classification of Headache Disorders, 2nd edition. Tension-type headaches can be further subdivided into infrequent episodic, frequent episodic, or chronic
Interventions
The research pharmacist performed randomization in blocks of six using an online random number generator. The pharmacist filled medication vials and placed these vials into sequentially numbered research bags. Research associates then allocated the research bags to patients in order. Only the pharmacist, whose records were maintained in a location distant from the ED and unavailable to the investigators, knew the assignment. Every research bag in the metoclopramide/diphenhydramine arm held two vials, one containing 20 mg of metoclopramide and one containing 25 mg of diphenhydramine. Every bag in the ketorolac arm also held two vials, one containing ketorolac 30 mg and one containing normal saline placebo. The contents of these vials were clear and indistinguishable. Normal saline was added to the ketorolac vial to make the volume in each vial identical. In order to maintain allocation concealment, a nurse, also blinded, placed the two vials from each bag in a 50cc bag of normal saline for administration to the patient as an intravenous drip over 15 minutes (200cc/hr). We chose to use 20 milligrams of metoclopramide rather than a more standard 10 mg dose to avoid failure to detect a benefit of the drug due to under-dosing. Because akathisia is common among patients who receive higher doses of intravenous metoclopramide, we co-administered diphenhydramine to all patients who received it10.
Methods and Measurements
After obtaining informed written consent, research associates performed a brief pain assessment using a structured questionnaire (Appendix). The nurse then administered the intravenous solution. The research associates returned every thirty minutes to ascertain the patient’s pain level. At one and two hours after medication administration, the research associates asked a more detailed series of questions. Patients who required additional analgesia after one hour had elapsed were administered medication at the discretion of the treating physician. We contacted patients by telephone 24 hours after ED discharge to ascertain headache status, satisfaction with treatment, and occurrence of adverse events.
Outcomes
As a primary endpoint, we utilized an 11 point numerical rating scale 11. This scale asks patients to assign their pain a number between zero and ten, with zero representing no pain and ten representing the worst pain imaginable. The primary outcome was the between-group difference in the one-hour change in this scale. Secondary outcome measures included: 1) response to the question “Do you want to receive the same medication the next time you come to the ED with a headache?”; 2) headache freedom achieved in the ED without the use of rescue medication; 3) receipt of rescue medication at any time during the ED visit, defined as any medication administered specifically to alleviate headache; 4) sustained headache freedom, defined as achieving headache freedom in the ED and maintaining it for 24 hours without rescue medication; 5) use of rescue medication during the 24 hours following initial medication administration; and 6) percent improvement in pain score between baseline and one hour, defined as: (baseline pain score – 1hr pain score)/baseline pain score.
One hour after medication administration, we asked patients if they felt drowsy, and had them choose one of the following three options: 1) no drowsiness; 2) a little bit drowsy but able to function; or 3) too drowsy to function. At the follow-up phone call, we asked patients if they felt restless at any time after receiving the intravenous medication in the ED and had them choose one of the following three options: 1) no restlessness; 2) a little bit restless; or 3) very restless. We also asked them at one hour, two hours, and at the 24-hour follow-up interview if they experienced any other symptoms. If they answered in the affirmative, their symptoms were elicited with an open-ended question.
Research associates collected data using paper data collection forms. The principal investigator, who remained blinded to allocation assignment during this process, then transcribed the data into SPSS v.19.
Analysis
Based on previous work,12,13 our sample size calculation assumed normal distribution and a conservative alpha, and was driven by the need to identify statistically significant between-group differences in the sub-group of patients with tension-type headache. We estimated that a sample size of 44 patients in each arm would give us a power of 0.8 to detect a between-group difference in improvement in pain score of 2.0 units, a difference considered a robust clinical difference.14 We estimated that enrolling 88 patients with tension-type headache would require enrolling 50% more patients, i.e., about 130 patients with bland headache, but planned to stop as soon as we had obtained complete data on the subset of 88 patients with tension-type headache.
When analyzed, the continuous outcome data did not distribute normally, so we presented these data as medians with inter-quartile range and used the Hodges-Lehman estimate to construct 95%CI for difference between medians. We expressed between-group differences in dichotomous outcomes as proportions bounded by 95% CI’s and report for these the Number Needed to Treat (NNT), that is, the number of patients that would need to be treated with the more efficacious medication rather than the less efficacious one in order for a single patient to achieve the target outcome of interest.
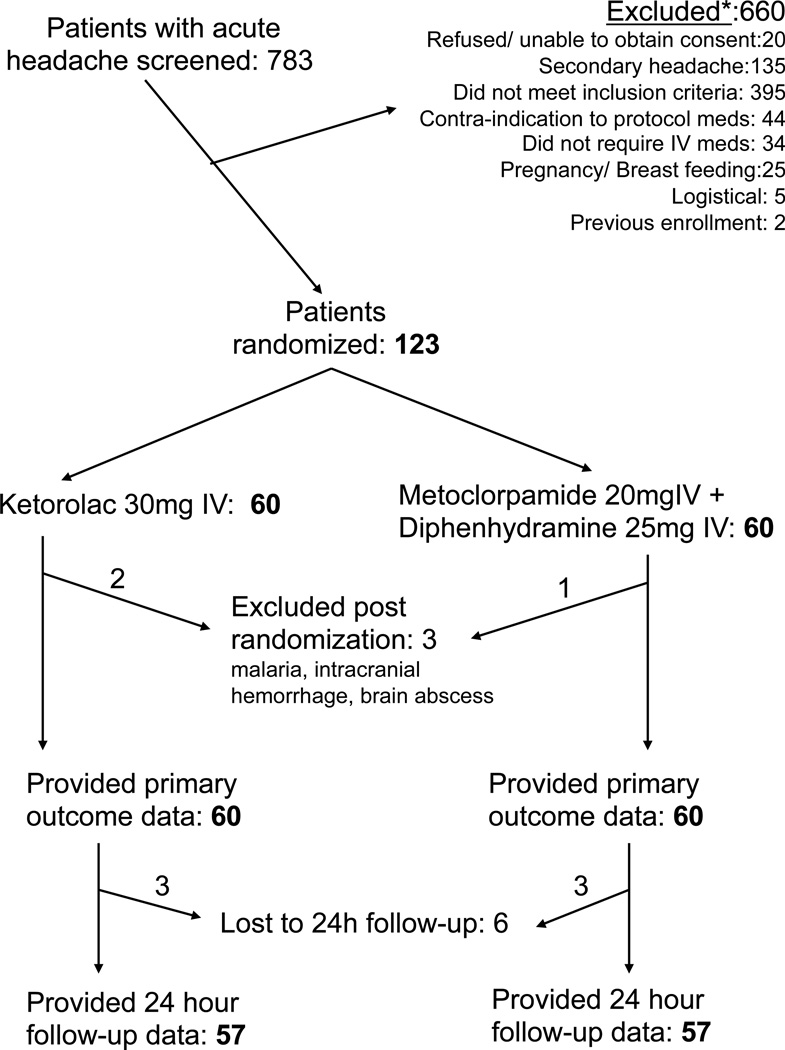
We analyzed data using a per protocol analysis. This seemed to us more clinically sensible than an intention-to-treat strategy, since, upon review of the dataset prior to unblinding, three randomized patients clearly were enrolled in error based upon their ultimate diagnoses: subarachnoid hemorrhage, brain abscess, and malaria. Thus, as shown in the CONSORT diagram (Figure 1), we excluded these patients from further analysis.
Results
Enrollment for this study began in November of 2009 and continued for 35 months. During this time, we approached 783 patients for participation and included 120 in the analysis (Figure 2). Of the 120 patients enrolled with bland headache, 89 of these met criteria for tension-type headache.
Figure 2.
CONSORT flow diagram
Baseline characteristics were comparable between the two groups (Table 1).
Table 1.
Baseline characteristics of entire study population
| Characteristic | Ketorolac (n=60) | Metoclopramide + diphenhydramine (n=60) |
|
|---|---|---|---|
| Median age in years (IQR) | 38 (26, 46) | 38 (29, 48) | |
| Female, n (%) | 48 (80%) | 42 (70%) | |
| Race/ ethnicity, n (%) | |||
| Asian | 0 | 0 | |
| Black | 11 (18%) | 17 (28%) | |
| Latino | 40 (67%) | 34 (57%) | |
| White | 1 (2%) | 2 (3%) | |
| Mixed | 3 (5%) | 4 (7%) | |
| Other | 5 (8%) | 2 (3%) | |
| Refused | 0 | 1 (2%) | |
| Median duration of headache in hours (IQR) | 72 (48, 168) | 72 (24, 144) | |
| Median number of days with headache over the previous 3 months (IQR) | 5 (2, 10) | 5 (2,10) | |
| Past medical history of migraine headaches n (%) | 16 (27%) | 11 (18%) | |
| Median baseline NRS pain score, on a scale from 0–10 with 0= no pain and 10= worst imaginable (IQR) | 8 (7, 10) | 8 (7, 9) | |
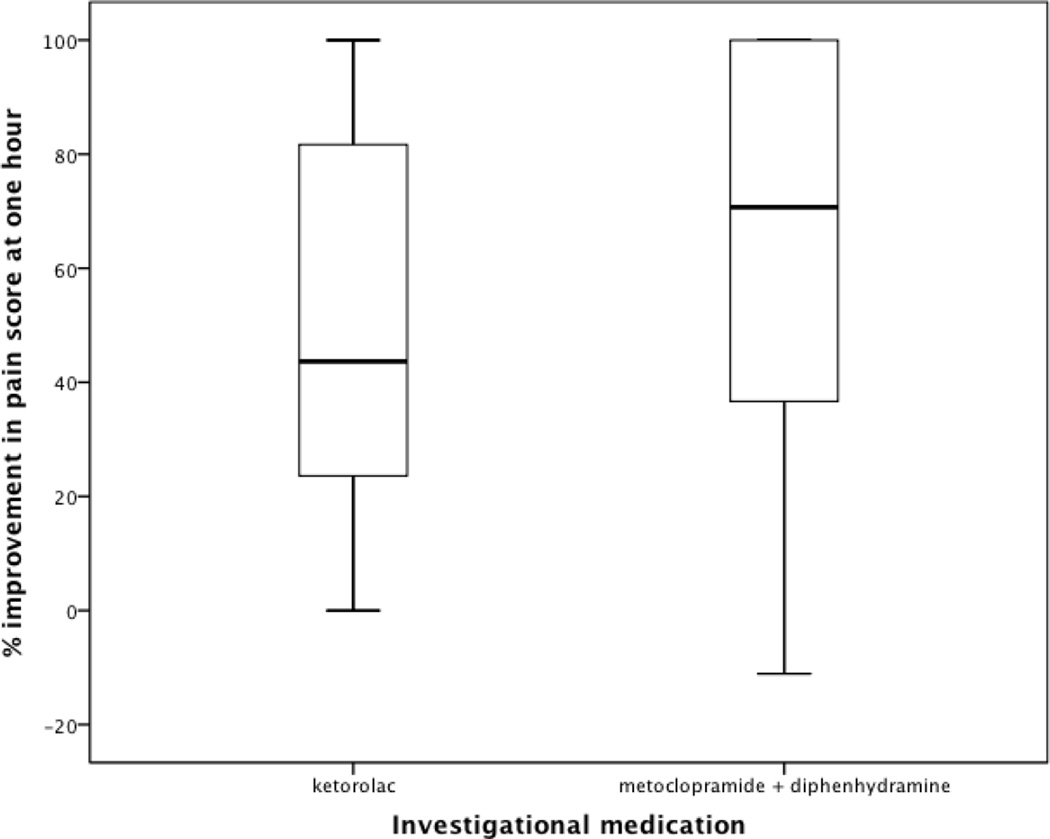
Patients with non-migraine, non-cluster recurrent headache who received the metoclopramide combination had greater pain relief than those randomized to ketorolac, as measured by change in pain scores (Table 2, Figure 3). The patients who received the metoclopramide combination were also more likely to achieve headache freedom in the ED, experience sustained headache freedom throughout the 24 hours following medication administration, and report wanting the same medication if treated again in the ED for similar headache (Table 3). These patients were less likely to require rescue medications (Table 3). At one hour, patients who received who received the metoclopramide combination improved by a median of 71% (IQR 35, 100%), while those who received ketorolac improved by a median of 44% (IQR 23, 83%) (Figure 4). These findings were nearly identical to the outcome data for the subset of patients with tension-type headache (Tables 2 and 4).
Table 2.
Change in numerical rating scale between baseline and one hour post baseline
| Population | Ketorolac Median Improvement (IQR), N |
Metoclopramide + diphenhydramine Median Improvement (IQR), N |
95%CI for difference between medians* |
|---|---|---|---|
| Non-migraine, non-cluster recurrent headache | 3 (2, 6), 60 | 5 (3, 7), 60 | 0, 3 |
| Tension-type headache | 3 (2, 6), 46 | 5 (3, 7), 43 | 0, 3 |
Independent samples Hodges-Lehman estimate
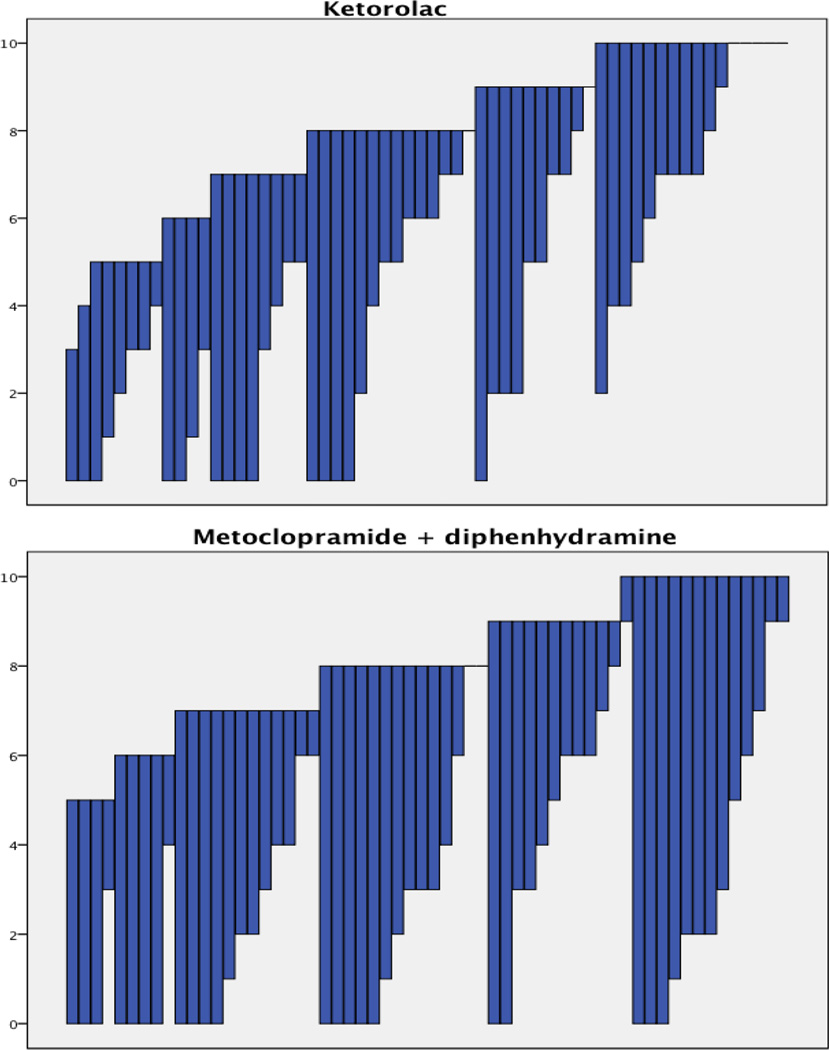
Figure 3.
Each line depicts the baseline and 1hr pain score for an individual. Data are sorted by baseline pain score and then 1hr pain score, so the one patient who worsened after receiving the metoclopramide combination (from 9 to 10) appears in the figure after all of the other patients with a baseline score of 9.
Table 3.
Categorical out comes among all patients with non-migraine non-cluster recurrent headache
| Outcome | Ketorolac | Metoclopramide + diphenhydramine |
Difference (95%CI) |
Number Needed to Treat (95%CI) |
|---|---|---|---|---|
| Would want to receive the same medication during the next ED visit for headache | 45/57 (79%) | 53/57 (93%) | 14% (2, 27%) | 7 (4, 65) |
| Achieved headache freedom in the ED without requiring rescue medication | 16/60 (27%) | 27/60 (45%) | 18% (1, 35%) | 6 (3, 67) |
| Required rescue medication in the ED | 27/60 (45%) | 8/60 (13%) | 32% (16, 47%) | 3 (2, 6) |
| Achieved headache freedom in the ED without requiring rescue medication and maintained headache freedom for 24 hours | 5/60 (8%) | 16/60 (27%) | 19% (5, 32%) | 6 (3, 20) |
| Required analgesic medication within 24 hours of ED discharge | 27/57 (47%) | 20/57 (35%) | 12% (−6, 30%) | Insufficient difference between groups—unable to calculate NNT |
Figure 4.
Boxplots demonstrating % improvement in 0– 10 pain score one hour after medication administration.
Table 4.
Categorical outcomes among all patients with tension-type headache
| Outcome | Ketorolac | Metoclopramide + diphenhydramine |
Difference (95%CI) |
Number Needed to Treat (95%CI) |
|---|---|---|---|---|
| Would want to receive the same medication during the next ED visit for headache | 34/ 43 (79%) | 37/ 40 (93%) | 14% (−1, 28%) | Insufficient difference between groups—unable to calculate NNT |
| Achieved headache freedom in the ED without requiring rescue medication | 10/ 46 (22%) | 20/ 43 (47%) | 25% (6, 44%) | 5 (2, 18) |
| Required rescue medication in the ED | 20/ 46 (44%) | 6/ 43 (14%) | 30% (12, 47%) | 4 (2, 8) |
| Achieved headache freedom in the ED without requiring rescue medication and maintained headache freedom for 24 hours | 4/ 46 (9%) | 11/ 43 (26%) | 17% (2, 32%) | 6 (3, 66) |
| Required analgesic medication within 24 hours of ED discharge | 21 / 43 (49%) | 16/ 40 (40%) | 9% (−12, 30%) | Insufficient difference between groups—unable to calculate NNT |
There were no serious or unexpected adverse events. The development of new symptoms after investigational medication administration was reported by 14/60 (23%) patients in the ketorolac arm and 12/60 (20%) patients in the metoclopramide arm (95%CI for difference of 3%: −11, 18%). These mostly consisted of evolving headache descriptions such as pulsating pain, severe headache, and facial pressure. Drowsiness at one hour was more common among those who received the metoclopramide combination, although drowsiness sufficient to impair function was uncommon in both groups (Table 5). Restlessness after receiving the investigational medications was evenly distributed between the two groups (Table 5). In general the medications were very well tolerated. Of the 16 patients who reported they would not want to receive the same medication at the next visit, all cited lack of efficacy rather than side effects as their rationale. Other infrequent adverse events are listed in Table 5.
Table 5.
Adverse events among entire study population
| Adverse event | Ketorolac (n=60) |
Metoclopramide + Diphenhydramine (n=60) |
Difference (95%CI) |
|
|---|---|---|---|---|
| Drowsy at one hour | For no drowsiness: 29% (12, 47%) | |||
| No | 38 (64%) | 21 (35%) | ||
| A little bit drowsy but able to function | 18 (31%) | 38 (63%) | ||
| Too drowsy to function | 3 (5%) | 1 (2%) | ||
| Not sure/Did not answer | 1 | 0 | ||
| Restless after receiving intravenous medication | For no restlessness: 1% (−13, 13%) | |||
| No | 47 (85%) | 48 (86%) | ||
| A little bit restless | 7 (13%) | 6 (11%) | ||
| Very restless | 1 (2%) | 2 (4%) | ||
| Lost-to-followup | 3 | 3 | ||
| Not sure/Did not answer | 2 | 1 | ||
| Other adverse events | ||||
| Dizziness | 2 | 2 | ||
| Epigastric pain | 1 | 1 | ||
| Nausea | 2 | 1 | ||
| Neck/ back pain | 1 | 2 | ||
| Palpitations* | 1 | 0 | ||
| Abnormal olfaction* | 0 | 1 | ||
One patient who received ketorolac reported a rapid heartbeat after ED discharge, for which the patient did not seek medical attention. One patient who received metoclopramide reported a self-limited change in sense of smell.
Limitations
We sought to exclude patients with migraine from this study based on strict application of International Headache Society criteria to the patient’s self-described headache characteristics at the time of enrollment. However, during their time in the ED, some patients developed nausea or had their headache evolve into a typical migraine headache. This is a relatively common phenomenon that has been reported previously. 15 The effect of this may have been to dilute our “homogenous” population of tension-type headache, potentially causing misclassification bias, which tends to drive outcomes toward the null.
A second limitation, which is common to most single-site studies, is that, in spite of the internal validity of our findings, we conducted this research in one urban ED in the Bronx, NY, caring for a largely non-white, under-served population. This necessarily limits any claims of external validity or generalizability
Finally, it took us nearly three years to enroll enough patients to meet our sample size requirements. We believe this reflects the clinical reality that the vast majority of recurrent headache disorders seen in emergency practice are migraine or probable migraine. In spite of the prevalence of tension-type headache in the population, acute episodes of severe or functionally disabling tension-type headache are relatively uncommon in the ED.15
Discussion
The preponderance of data from this study suggests that the intravenous combination of metoclopramide 20 mg + diphenhydramine 25 mg is more efficacious than 30 mg of intravenous ketorolac for treatment of acute non-migraine, non-cluster recurrent headaches and for tension-type headache. Patients who received metoclopramide were significantly more likely than patients who received ketorolac to achieve headache relief in the ED, experience sustained headache freedom during the 24 hours following medication administration, and report wanting the same medication if treated again in the ED for similar headache. They were also three times less likely to require rescue medication than patients who received ketorolac.
Both treatments used in this study were well tolerated. Restlessness, a common akathetic side effect of metoclopramide, seems to have been prevented successfully by the co-administration of diphenhydramine. The metoclopramide combination caused mild drowsiness in two-thirds of the patients who received it compared to about one-third of patients who received ketorolac. However, of the patients who reported some level of drowsiness, very few reported being “too drowsy to function.” A lower dose of metoclopramide may lessen the rate of drowsiness, although this may also lessen the efficacy. The choice of any treatment reflects a tradeoff between efficacy and side effects. In this case the consistency and the magnitude of the findings supporting the metoclopramide combination over ketorolac coupled with the patients’ frequently stated desire to receive this medication again, suggests that the benefits of the metoclopramide + diphenhydramine outweigh the harm.
Others have demonstrated that intravenous chlorpromazine, another dopamine antagonist, and intravenous metoclopramide are more effective than placebo for tension-type headache. Bigal et.al. tested chlorpromazine, dosed at 0.1 mg/kg, versus placebo in a randomized, double blind study conducted in public health clinics in Brazil. These authors reported an NNT of two versus placebo for achieving a pain free state by 60 minutes.2 Cicek et.al. randomized 140 patients with acute tension-type headache to receive either metoclopramide 10 mg IV alone, metoclopramide 10 mg IV + pethidine (meperidine) 50 mg IM, pethidine 50 mg IM alone, or placebo. With regard to need for rescue medication, the authors reported an NNT of two for metoclopramide versus placebo and an NNT of 3.5 for metoclopramide versus pethidine.3
The fact that both migraine and tension-type headache appear to respond well to metoclopramide, a medication without inherent analgesic properties, raises intriguing questions about headache nosology.16 It may be that tension-type headache and migraine are unique disease processes with a common final nociceptive pathway where metoclopramide may act. Alternatively, it may be that these two headache types share a similar pathophysiology, which presents with multiple phenotypes. To the best of our knowledge, there are no pharmacodynamic or mechanistic data that explain metoclopramide’s efficacy in acute headache.
During this study, once migraine and cluster headache had been excluded, we did not seek to classify any additional headache disorders other than tension type headache. We assumed homogeneity of response among the various uncommon headaches that do not meet migraine, cluster, or tension-type criteria, such as nummular headache,17 non-infectious rhino-sinusitis like headache,18 and hemicrania continua,9 an assumption that may not be strictly correct. Hemicrania continua, for example, is defined by its response to indomethacin9 and thus may be more likely to respond to ketorolac. However, the subset of patients with tension-type headache responded identically to each medication, when compared to the study population as a whole. This leads us to conclude that these less common headache types either were underrepresented in our study population, or alternatively, responded to the investigational medications in a manner comparable to tension-type headache.
In conclusion, for adults presenting to an ED with tension-type headache or with non-migraine, non-cluster recurrent headache, IV metoclopramide + diphenhydramine provided more headache relief than IV ketorolac.
Acknowledgments
Please include the following acknowledgement: This publication was supported in part by the CTSA Grant UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. The authors wish to thank Professor Hillel W. Cohen, DrPH,, MPH, for help with the statistical analysis
Footnotes
Meetings at which this work has been presented: None
Conflicts of interest: None
Author contributions:
BWF, CS, DE, PEB and EJG conceived and designed the study. BWF, VA and CC reviewed data for integrity and to confirm diagnosis. BWF and DE supervised the conduct of the trial and data collection. BWF analyzed the data. BWF drafted the manuscript, and all authors contributed substantially to its revision. BWF takes responsibility for the paper as a whole.
This trial was registered at http://cliincaltrials.gov NCT01011673
References
- 1.Loder E, Rizzoli P. Tension-type headache. BMJ. 2008;336:88–92. doi: 10.1136/bmj.39412.705868.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the acute treatment of episodic tension-type headache: a randomized, placebo controlled, double-blind study. Arq Neuropsiquiatr. 2002;60:537–541. doi: 10.1590/s0004-282x2002000400004. [DOI] [PubMed] [Google Scholar]
- 3.Cicek M, Karcioglu O, Parlak I, et al. Prospective, randomised, double blind, controlled comparison of metoclopramide and pethidine in the emergency treatment of acute primary vascular and tension type headache episodes. Emerg Med J. 2004;21:323–326. doi: 10.1136/emj.2002.000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman BW, Hochberg ML, Esses D, et al. Applying the International Classification of Headache Disorders to the Emergency Department: An Assessment of Reproducibility and the Frequency With Which a Unique Diagnosis Can be Assigned to Every Acute Headache Presentation. Ann Emerg Med. 2007 doi: 10.1016/j.annemergmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Miner JR, Fish SJ, Smith SW, Biros MH. Droperidol vs. prochlorperazine for benign headaches in the emergency department. Acad Emerg Med. 2001;8:873–879. doi: 10.1111/j.1553-2712.2001.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 6.Jones J, Sklar D, Dougherty J, White W. Randomized double-blind trial of intravenous prochlorperazine for the treatment of acute headache. Jama. 1989;261:1174–1176. [PubMed] [Google Scholar]
- 7.Baden EY, Hunter CJ. Intravenous dexamethasone to prevent the recurrence of benign headache after discharge from the emergency department: a randomized, double-blind, placebo-controlled clinical trial. Cjem. 2006;8:393–400. doi: 10.1017/s1481803500014184. [DOI] [PubMed] [Google Scholar]
- 8.Cady R, Schreiber C, Farmer K, Sheftell F. Primary headaches: a convergence hypothesis. Headache. 2002;42:204–216. doi: 10.1046/j.1526-4610.2002.02053.x. [DOI] [PubMed] [Google Scholar]
- 9.Classification subcommittee of the International Headache Society. The International Classification of Headache Disorders- 2nd Edition. Cephalalgia. 2004;24:1–151. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman BW, Bender B, Davitt M, et al. A randomized trial of diphenhydramine as prophylaxis against metoclopramide-induced akathisia in nauseated emergency department patients. Ann Emerg Med. 2009;53:379–385. doi: 10.1016/j.annemergmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 12.Cameron JD, Lane PL, Speechley M. Intravenous chlorpromazine vs intravenous metoclopramide in acute migraine headache. Acad Emerg Med. 1995;2:597–602. doi: 10.1111/j.1553-2712.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- 13.Corbo J, Esses D, Bijur PE, Iannaccone R, Gallagher EJ. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001;38:621–627. doi: 10.1067/mem.2001.119424. [DOI] [PubMed] [Google Scholar]
- 14.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5:1086–1090. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the spectrum study. Neurology. 2002;58:S27–S31. doi: 10.1212/wnl.58.9_suppl_6.s27. [DOI] [PubMed] [Google Scholar]
- 16.Kaniecki RG. Migraine and tension-type headache: An assessment of chaleenges in diagnosis. Neurology. 2002;58:S15–S20. doi: 10.1212/wnl.58.9_suppl_6.s15. [DOI] [PubMed] [Google Scholar]
- 17.Grosberg BM, Solomon S, Lipton RB. Nummular headache. Curr Pain Headache Rep. 2007;11:310–312. doi: 10.1007/s11916-007-0209-1. [DOI] [PubMed] [Google Scholar]
- 18.Eross E, Dodick D, Eross M. The Sinus, Allergy and Migraine Study (SAMS) Headache. 2007;47:213–224. doi: 10.1111/j.1526-4610.2006.00688.x. [DOI] [PubMed] [Google Scholar]