Abstract
The primary objective of this study was to determine the safety, toxicity, and maximum tolerated dose of nanoparticle albumin-bound (nab)-paclitaxel as part of biochemotherapy for metastatic melanoma and to determine whether substituting nab-paclitaxel for less potent agents could increase response rates and duration. Treatment consisted of intravenous cisplatin (20 mg/m2) on days 1–4, oral temozolomide (250 mg/m2) on days 1–3, subcutaneous interferon-α (5 × 106 IU/m2) on days 1–5, and continuous intravenous interleukin-2 (9 × 106 IU/m2) for 96 h on days 1–4. A standard 3 + 3 dose escalation method was used; the nab-paclitaxel starting dose was 100 mg/m2 on day 1 and 70 mg/m2 on day 5. The treatment cycle was repeated every 3 weeks and toxicity was assessed weekly. Ten patients were enrolled. Dose-limiting toxicities included diarrhea, transaminasemia, and neutropenia. The maximum tolerated dose was not identified because the nab-paclitaxel dose on day 1 at the lowest planned dose (80 mg/m2) caused dose-limiting toxicity in two of five patients. Of the nine patients who were evaluable for response, five had a partial response. The median time to disease progression was 5.30 months and the median overall survival was 8.73 months.
Six patients developed central nervous system metastasis at a median of 5.33 months after treatment initiation. Biochemotherapy including nab-paclitaxel according to the doses and schedule regimen used in the present study has significant toxicity. Substituting dacarbazine with temozolomide did not prevent central nervous system metastasis in patients with metastatic melanoma.
Keywords: biochemotherapy, brain metastasis, melanoma, nab-paclitaxel, temozolomide
Introduction
Since the 1990s, biochemotherapy – such as that using cisplatin-based chemotherapy regimens in combination with biological agents of interferon-α (IFN-α) and interleukin-2 (IL-2) – has been reported to have the highest response rates among various systemic therapies for metastatic melanoma. For example, in a phase II study in which 53 patients with metastatic melanoma received biochemotherapy [cisplatin, vinblastine, and dacarbazine (CVD) plus IFN-α and IL-2], 34 patients (64%) achieved objective responses (complete and partial response) [1]. However, the high response rates did not translate into improved overall survival (OS). In addition, despite the high response rates in their systemic metastases, patients in the study showed a high risk of developing brain metastasis.
Combining biochemotherapy with other active agents might further increase the response rate. Several studies have shown that Cremophor-based paclitaxel is active against metastatic melanoma [2–4]. In one phase II study, a brief intravenous (i.v.) infusion of paclitaxel elicited verifiable responses in melanoma metastasis in the liver of three patients in whom previous CVD treatment had failed [5]. A phase I/II trial of a CVD-based regimen in which paclitaxel was substituted for vinblastine paclitaxel showed improved times to tumor progression (TTP) and OS and fewer brain metastases [6]. However, combining paclitaxel with biochemotherapy is challenging because of the requirement of steroid premedication, which could potentially counteract the anticancer activity of IL-2.
Nanoparticle albumin-bound (nab)-paclitaxel, a modified version of paclitaxel, does not require steroid premedication and can thus be readily combined with biochemotherapy. In-vitro studies and clinical breast cancer studies have shown that nab-paclitaxel has higher bioavailability and results in a higher intratumoral paclitaxel concentration than traditional solvent-based taxanes [7,8]. In addition, unlike paclitaxel, nab-paclitaxel binds to secreted protein acidic and rich in cysteine, a protein that is overexpressed in melanoma cells [9], which may further increase intracellular accumulation of the drug. Nab-paclitaxel has shown favorable response rates, TTP, and OS in metastatic melanoma in phase II trials, both as a single agent and in combination with carboplatin [10,11]. To our knowledge, however, substitution of nab-paclitaxel for the less potent vinblastine in biochemotherapy has not been subjected to clinical trials, even though this change would be expected to augment the tumor response.
Temozolomide, a dacarbazine analogue, has the advantage of excellent central nervous system (CNS) penetration, and thus may be effective against melanoma CNS metastases and may even prevent the development of metastases. In a clinical trial conducted by González Cao and colleagues, 36 patients received temozolomide-based biochemotherapy. Only two patients showed the first evidence of disease progression in the form of brain metastasis, although at baseline, 23 patients had stage IV (metastasis grade M1c) disease and seven patients had received previous whole-brain irradiation for previous brain metastasis [12].
On the basis of this evidence, we hypothesized that the temozolomide–nab-paclitaxel biochemotherapy can prolong the duration of response and prevent the development of CNS metastasis. Thus, we conducted this phase I clinical trial to test whether substituting vinblastine and dacarbazine with nab-paclitaxel and temozolomide, respectively, could improve the antitumor activity of biochemotherapy and decrease the risk of brain metastasis from melanoma. Our primary objectives were to evaluate the safety and toxicity of this biochemotherapy combination and to determine the maximum tolerated dose (MTD) of nab-paclitaxel. The secondary objectives were assessment of the response rate, OS, and incidence of CNS metastasis associated with this regimen.
Patients and methods
Patient selection
The study protocol was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. All patients provided written informed consent before study enrollment.
Patients with histologically confirmed stage IV or unresectable stage III metastatic melanoma were enrolled in our study. Eligible patients were between 18 and 65 years of age, had an Eastern Cooperative Oncology Group performance status score of 2 or less, had disease that could be measured or evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) evaluation tool, version 1.0 [13], had adequate organ function (white blood cell count ≥ 3000/μl, absolute neutrophil count ≥ 1500/μl, platelet count ≥ 100 000 μl, serum creatinine < 1.1 and 1.4 mg/dl in women and men, respectively, serum bilirubin < 1.2 mg/dl, calcium level < 11 mg/dl), had no active or symptomatic brain metastases, were not receiving steroids, and were IL-2 and chemotherapy naíve. We considered that if a patient had received previous IFN-α treatment this may be a predictor for IFN nonresponsive tumors. All patients had to have adequate cardiopulmonary function as indicated by pulmonary function testing and ECG, chemical stress echocardiography, or a myocardial perfusion scan (left ventricular ejection fraction ≥ 55%, QT on ECG < 460 ms, forced expiratory volume ≥ 75% of that which was predicted).
Treatment plan
The treatment schedule is shown in Table 1. Treatment consisted of cisplatin (20 mg/m2) i.v. infusion on days 1–4, oral temozolomide (250 mg/m2) on days 1–3, and subcutaneous IFN-α2β (5 × 106 IU/m2) on days 1–5. Patients also received a continuous i.v. infusion of IL-2 (9 × 106 IU/m2) for 96 h on days 1–4. Intravenous nab-paclitaxel (Abraxane; Celgene, Summit, New Jersey, USA) was administered on days 1 and 5 of each cycle; the dose was split to avoid microtubule saturation, a phenomenon long observed with the use of taxanes. The starting nab-paclitaxel dose was 100 mg/m2 on day 1 and 70 mg/m2 on day 5. In this study, a higher daily dose of temozolomide over 3 days rather than traditional (150 mg/m daily for 5 days) was administered to achieve higher plasma concentration and DNA methylation in the brain [14]. A standard 3 + 3 dose escalation method was used to determine the nab-paclitaxel dose on day 1; no intrapatient dose escalation was allowed. Treatment was repeated in cycles of 21 days to a maximum of six cycles. Patients who developed CNS metastasis were excluded from the study, but were offered the option to continue the treatment described above off-protocol.
Table 1.
Nab-paclitaxel dose-escalation schedule
| Dose level | Nab-paclitaxel (i.v.; day 1) (mg/m2) |
Temozolomide (PO; days 1–3) (mg/m2) |
Cisplatin (i.v.; days 1–4) (mg/m2) |
IL-2 (CI; days 1–4) (106) (IU/m2) |
Nab-paclitaxel (i.v.; day 5) (mg/m2) |
IFN-α2b (SQ; days 1–5) (106) (IU/m2) |
|---|---|---|---|---|---|---|
| −1 | 80 | 250 | 20 | 9 | 70 | 5 |
| 0 | 100 | 250 | 20 | 9 | 70 | 5 |
| 1 | 120 | 250 | 20 | 9 | 70 | 5 |
| 2 | 140 | 250 | 20 | 9 | 70 | 5 |
CI, continuous intravenous infusion; IFN-α2b, interferon-α2b; IL-2, interleukin-2; i.v., intravenous; PO, per overall survival; SQ, subcutaneous injection.
IL-2 was withheld from patients who developed hypotension (despite support with dopamine at a dose of up to 5 mg/kg/min and i.v. fluids), liver dysfunction (bilirubin > 3 mg/dl), and/or kidney dysfunction (serum creatinine > 1.8 mg/dl). IFN-α2β was withheld from patients who developed thrombocytopenia (< 50 000 cells/ml3) or neutropenia (absolute neutrophil count r 300 cells/ml3). Cisplatin was held in patients who developed grade 2 hearing loss, peripheral neuropathy, kidney dysfunction (creatinine level ≥ 1.5 mg/dl), or thrombocytopenia (< 30 000 cells/ml3). Treatment was restarted after resolution of the above-mentioned limiting factors. Nab-paclitaxel on day 1 was held in patients who developed grade 3 neurotoxicity, but was restarted after neurotoxicity resolved or was reduced to grade 2 or less. Nab-paclitaxel on day 5 was restarted if the patient’s absolute neutrophil count was greater than 600 cells/ml3.
Toxicity evaluation
Patients were evaluated for toxicity using the US National Cancer Institute’s Common Terminology Criteria for Adverse Events [15]. Patients underwent a physical examination and comprehensive blood tests, including a complete blood count with differential, a complete metabolic panel, and measurement of lactate dehydrogenase before and during every treatment cycle to assess toxicity. Dose-limiting toxicity (DLT) was defined as grade 4 hematologic toxicity, grade 3 or higher nonhematologic toxicity, or any other toxicity that required dose reduction or that interrupted the patient’s treatment schedule for more than 1 week and that occurred during the first cycle. Treatment was withheld until toxicity resolved to less than or equal to grade 2. Prophylactic hematologic growth factors to prevent neutropenia were not allowed in the first cycle.
Response evaluation
MRI of the brain and computed tomography of the chest, abdomen, and pelvis were used to evaluate treatment response after every two cycles. Disease response or progression was defined according to RECIST version 1.0 [13]. Response duration was measured from the time of response until evidence of disease progression. TTP was measured from the time of treatment initiation to evidence of disease progression, and OS was measured from the time of treatment initiation to the time of death or last follow-up. Kaplan–Meier estimates were used to plot survival curves.
Results
Patient characteristics
Ten patients with stage IV or unresectable stage III metastatic melanoma were enrolled in the study between March 2010 and March 2011. One patient was evaluable for toxicity, but not for response and was excluded from the study after receiving one cycle of treatment because of a change in diagnosis. The patient’s initial biopsy was performed in an outside facility, which found pleomorphic cells with strong S-100 expression and focal melan-A expression; however, the repeat biopsy at the MD Anderson Cancer Center was negative for both S-100 and focal melan-A expression. Because the differential diagnosis included dedifferentiated chondrosarcoma with melanocytic features as well as metastatic melanoma with cartilaginous involvement, this patient was excluded from our study. Patient characteristics are shown in Table 2. Six and four patients had Eastern Cooperative Oncology Group performance status scores of 0 and 1, respectively. The mean age of the patients was 46 years and ranged from 19 to 61 years.
Table 2.
Characteristics of patients with metastatic melanoma (n=10)
| Characteristics | Number of patients [n (%)] |
|---|---|
| Sex | |
| Male | 7 (70) |
| Female | 3 (30) |
| Median age [years (range)] | 46 (19–61) |
| Prior IFN therapy | 2 |
| Eastern Cooperative Oncology Group performance status score | |
| 0 | 6 (60) |
| 1 | 4 (40) |
| Melanoma typea | |
| Cutaneous | 5 (50) |
| Mucosal | 1 (10) |
| Acrolentiginous | 1 (10) |
| Unknown | 2 (20) |
| Stagea | |
| M1a | 1 (10) |
| M1b | 0 |
| M1c | 8 (80) |
IFN, interferon.
Data exclude one patient with a questionable diagnosis of melanoma.
The patients’ risk factors for brain metastasis, including sex, thickness of melanoma, primary site of melanoma, ulceration status, number of involved lymph nodes, grade M1c disease status, and lactate dehydrogenase level at baseline, are described in Table 3.
Table 3.
Patient risk factors for brain metastasis
| Patients | Months to CNS metastasis |
Sex | Primary site |
Primary type |
Primary Clark level |
Primary Breslow score (mm) |
Primary ulceration status |
Number of involved LNs |
Metastasis grade |
LDH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.00 | Male | H&N | Cut | Unknown | Unknown | Unknown | > 3 | M1c | nl |
| 2 | NA | Male | Unknown | Unknown | Unknown | Unknown | Unknown | > 3 | M1a | nl |
| 3 | NA | Male | Nail | Acro | IV | 6.20 | Ulc | > 3 | M1c | High |
| 4 | 4.33 | Female | Chest wall |
Cut | IV | 0.80 | nUlc | > 3 | M1c | High |
| 5 | 2.86 | Male | H&N | Nodular | IV | 4.90 | nUlc | > 3 | M1c | High |
| 6 | 5.33 | Male | Trunk | Cut | IV | 4.30 | Ulc | 0 | M1c | High |
| 7 | 1.66 | Female | Trunk | Cut | Unknown | 4.75 | nUlc | > 3 | M1c | nl |
| 8 | 5.33 | Male | Sinus | Mucosal | Unknown | Unknown | Unknown | > 3 | M1c | nl |
| 9 | NA | Female | Unknown | Unknown | Unknown | Unknown | Unknown | > 3 | M1c | nl |
Arco, acrolentiginous; CNS, central nervous system; Cut, cutaneous; H&N, head and neck; LDH, lactate dehydrogenase; LN, lymph node; NA, not applicable (patient did not develop CNS yet); nl, normal; nUlc, nonulcerated; Ulc, ulcerated.
Two patients had received adjuvant IFN therapy previously; the first (patient no. 7 in Table 3) received IFN 3 years before developing widespread disease in the lymph nodes, lungs, subcutaneous tissue, omentum, mediastinum, and right kidney; the other patient (patient no. 3 in Table 3) received IFN 1 month before developing rapidly progressive disease in the lymph nodes, lungs, and left adrenal gland.
Treatment
The first two cohorts of patients were treated at dose level 0 (nab-paclitaxel on day 1 at 100 mg/m2 and on day 5 at 70 mg/m2). At dose level 0, two of five patients experienced DLT (grade 3 transaminasemia and grade 3 diarrhea). Therefore, the next three patients were treated at dose level – 1 (nab-paclitaxel on day 1 at 80 mg/m2 and 70 mg/m2 on day 5). One patient had DLT (prolonged grade 2 neutropenia that delayed the second cycle of therapy for more than 1 week). Of two additional patients treated at level 0, one had DLT (grade 4 transaminasemia). Because no additional patients were accrued to receive lower doses of nab-paclitaxel, the MTD of nab-paclitaxel was not identified and the lowest dose level evaluated was too toxic. In later cycles, three patients did not receive the day 5 dose of nab-paclitaxel (two in cycle 6, one in both cycle 4 and cycle 5) because of accumulative thrombocytopenia.
Toxicity
Treatment toxicities are presented in Table 4. Two incidents each of grade 3 or 4 neutropenia, transaminasemia infection, and diarrhea and one each of grade 3 or 4 hyponatremia, leukopenia, thrombocytopenia, and lymphopenia occurred during the first cycle of treatment. The most common grade 3 or 4 toxicities overall were neutropenia (nine patients), diarrhea (three patients), and infection (three patients). Accumulative toxicities included thrombocytopenia, anemia, peripheral neuropathy, and hearing loss. In addition to experiencing the common side effects of biochemotherapy (diarrhea, flu-like symptoms, fever, chills, malaise, nausea, vomiting, and anorexia), eight patients in our study experienced tinnitus and one patient had treatment discontinued because of grade 3 toxicity.
Table 4.
Treatment-related toxicity
| Toxicity | Total instances (% out of 46 cycles) [n (%)] |
Patients with toxicity grade 3/4 |
|---|---|---|
| Neutropenia | 20 (43) | 12 |
| Thrombocytopenia | 20 (43) | 4 |
| Anemia | 19 (41) | 2 |
| Lymphopenia | 3 (7) | 2 |
| Diarrhea | 36 (78) | 3 |
| Fatigue | 35 (76) | 2 |
| Nausea | 30 (65) | 0 |
| Skin rash | 25 (54) | 1 |
| Headache | 21 (46) | 0 |
| Hypotension | 20 (43) | 0 |
| Pruritus | 20 (43) | 0 |
| Constipation | 16 (35) | 0 |
| Taste alteration | 16 (35) | 0 |
| Neuropathy | 14 (30) | 1 |
| Alopecia | 14 (30) | 0 |
| Chills | 13 (28) | 0 |
| Vomiting | 13 (28) | 0 |
| Tinnitus | 8 (17) | 1 |
| Hypomagnesemia | 7 (15) | 0 |
| Anxiety | 7 (15) | 0 |
| Hearing loss | 4 (9) | 0 |
| Infection | 4 (9) | 3 |
| Alanine aminotransferase elevation |
4 (9) | 2 |
Clinical efficacy
The mean number of treatment cycles was five among the nine patients. Five patients completed all six planned cycles: four patients received level 0 and one received level – 1 of nab-paclitaxel. The median TTP was 5.3 months and the median OS duration was 8.73 months (Fig. 1a and b).
Fig. 1.
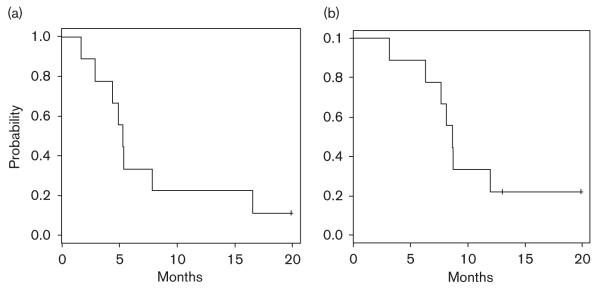
(a) Time to progression; (b) overall survival.
Of the nine patients, five (56%) had a partial response, three and two responders completed six and five cycles, respectively (three received level 0 and two received level – 1), three (33%) had progressive disease, and one (11%) had stable disease. No patient showed a complete response to treatment. We observed response in lymph nodes, soft tissue, liver, lung, and kidney metastatic sites. Although bone mestastases are not considered target lesions according to RECIST [13], we had evidence of responses in two patients and disease stabilization in another among the five patients with known bone metastasis.
Six patients developed melanoma metastasis in the CNS. Two patients developed brain metastasis while receiving treatment and two patients developed brain metastasis soon after completing treatment. The other two patients developed leptomeningeal metastasis after treatment. The median time from the start of treatment to the development of metastasis in the CNS in all patients with CNS involvement was 5.33 months.
At 18 months of follow-up, two patients were alive. One patient had a partial response to treatment and was disease free after undergoing surgical resection of the residual disease. The other patient had disease progression and was excluded from the study after two treatment cycles because of new brain metastasis. This patient received whole-brain radiation therapy, followed by two cycles of CVD with disease progression and was responding to treatment with a selective BRAF inhibitor at the time of last follow-up.
Discussion
Our study showed that biochemotherapy with nab-paclitaxel and temozolomide in this schedule resulted in significant toxicity in patients with metastatic melanoma. The MTD of day 1 nab-paclitaxel in this combination therapy could not be defined in this phase I study.
At dose level – 1, the combined dose of nab-paclitaxel administered on days 1 and 5 was 150 mg/m2, half the 300 mg/m2 MTD of nab-paclitaxel administered every 3 weeks in combination with carboplatin alone that was reported in the phase I study of Stinchcombe et al. [16]. In addition, three of the patients in our study received only the day 1 nab-paclitaxel without day 5 treatment in later cycles because of accumulative thrombocytopenia.
In our study, there were 30 incidents of grade 3 or 4 toxicity, which was unexpected, given the small number of patients. Nab-paclitaxel and biochemotherapy have several similar toxicities such as myelosuppression and neuropathy, which were especially evident in the late cycles of treatment. In addition, when the two therapies are used in combination, their common toxicities can have an additive effect in patients. In most patients who receive traditional biochemotherapy, diarrhea is not severe, lasts for 8 or 9 days, and rarely requires treatment cessation [17]. In the present study, however, two patients developed severe diarrhea that required delayed discharge and a third patient developed diarrhea as a DLT during the first cycle of treatment. Two patients had dose-limiting transaminasemia, but neither had liver metastasis, which suggests that the therapy had hepatic toxicity.
Biochemotherapy plus nab-paclitaxel and temozolomide had excellent antitumor activity, except in CNS disease. Reports of the biochemotherapy response rate have varied historically between 40 and 60% in multiple phase II studies [17], and were as low as 19.5% in a randomized phase III study [18]. In our study, five of nine patients had a partial response, with one patient from the five responders experiencing a durable remission after undergoing surgical resection of the residual disease.
The OS duration in the present study was shorter than those reported in previous studies of biochemotherapy. In their retrospective analysis, Bedikian et al. [19] reported that chemotherapy-naíve patients who received biochemotherapy had a median survival duration of 12.2 months. In another retrospective analysis, Keilholz et al. [20] reported that patients who received biochemotherapy that included IL-2 and IFN-α had a median survival duration of 11.4 months. In our study, however, the median OS duration was only 8.73 months, which was even shorter than the median OS duration (11.0 months) reported for patients who received a CVD regimen in which paclitaxel was substituted for vinblastine [7]. The shorter OS duration and TTP in our study can be attributed to the high incidence of melanoma metastasis in the CNS: in five of the six patients who developed melanoma metastasis in the brain or leptomeninges, CNS involvement was the first sign of disease progression, and these patients died shortly thereafter.
Reducing or preventing melanoma metastasis in the CNS remains a challenge. We found that temozolomide-containing biochemotherapy plus nab-paclitaxel did not prevent nor delay the occurrence of melanoma metastasis in the CNS. Although extracranial response or control was achieved in many patients, six of the nine patients in our study developed melanoma metastasis in the CNS during therapy or shortly thereafter. In comparison with the patients studied by González Cao et al. [12], our patients received temozolomide for 3 days each cycle instead of the standard 5 days, although our patients did receive a higher dose (250 mg/m2), rather than the standard 150 mg/m2 dose on the 21-day cycle, rather than every 28 days in the clinical trial of González Cao and colleagues. Our finding is in contrast to earlier reports that suggested that temozolomide-based biochemotherapy could prevent melanoma brain metastasis owing to temozolomide’s ability to penetrate the blood–brain barrier [12,21] and a previous study that suggested that replacing vinblastine with paclitaxel in a CVD regimen could decrease or prevent melanoma brain metastasis [7]. Chiarion-Sileni et al. [22], in a phase III randomized study, showed the inefficacy of temozolomide in preventing CNS metastasis from melanoma, although they suggested that temozolomide may delay the onset of CNS disease. In the present study, the median time to CNS involvement was only 5.33 months, which undermines that claim.
That the majority of patients in our study developed melanoma metastasis in the CNS was likely partly because of the fact that these patients were already at a high risk of developing brain metastasis [23]. Most patients had a thick primary melanoma (Clark level ≥ 3 or Breslow score ≥ 2 mm) and a primary lesion at an unknown site or on the trunk, head, or neck; these types of melanoma are associated with shorter intervals between the initial diagnosis and CNS metastasis [24].
Our study is limited by the fact that no MTD for day 1 nab-paclitaxel was found; however, it would likely be less than 80 mg/m2, considering the accumulative toxicity in later cycles of treatment. We speculate that a nab-paclitaxel dose for any phase II trial for this combination should be far less than that dose, which might even prevent adequate activity against the tumor itself. Another limiting factor in our analysis was the small number of patients enrolled in this study, which may have limited the accuracy of our efficacy/survival evaluation.
The new Food and Drug Administration-approved anti-cancer agents have opened the horizon in melanoma therapy. Combining biochemotherapy with new agents such as ipilimumab or selective BRAF inhibitors may prove to increase not only the response rate and response duration but also treatment tolerability.
Conclusion
Nab-paclitaxel can replace vinblastine in biochemotherapy for metastatic melanoma. However, the combination is very toxic to the liver, bone marrow, and gastrointestinal tract. In addition, although the combination elicited responses in extracranial metastasis, it did not prevent CNS involvement. We do not recommend nab-paclitaxel as a part of biochemotherapy for metastatic melanoma in the doses used in this study.
Acknowledgements
This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
This study was accepted to the American Society of Clinical Oncology Annual Meeting (ASCO), 1–5 June 2012, Chicago, IL (ASCO 2012, Abstract ID: e19009).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Legha S, Ring S, Eton O, Bedikian A, Buzaid AC, Plager C, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon-alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol. 1998;16:1752–1759. doi: 10.1200/JCO.1998.16.5.1752. [DOI] [PubMed] [Google Scholar]
- 2.Einzig AI, Hochster H, Wiernik PH, Trump DL, Dutcher JP, Garwski E, et al. A phase II study of Taxol in patients with malignant melanoma. Invest New Drugs. 1991;9:59–64. doi: 10.1007/BF00194546. [DOI] [PubMed] [Google Scholar]
- 3.Legha SS, Ring S, Papadopoulos N, Raber M, Benjamin RS. A phase II trial of Taxol in metastatic melanoma. Cancer. 1990;65:2478–2481. doi: 10.1002/1097-0142(19900601)65:11<2478::aid-cncr2820651114>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Walker L, Schalch H, King DM, Dietich L, Eastman M, Kwak M, et al. Phase II trial of weekly paclitaxel in patients with advanced melanoma. Melanoma Res. 2005;15:453–459. doi: 10.1097/00008390-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bedikian AY, Plager C, Papadopoulos N, Eton O, Ellerhorst J, Smith T. Phase II evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res. 2004;14:63–66. doi: 10.1097/00008390-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos NE, Bedikian A, Ring S, Kim KB, Hwu WJ, Gerber DL, et al. Phase I/II study of a cisplatin–Taxol–dacarbazine regimen in metastatic melanoma. Am J Clin Oncol. 2009;32:509–514. doi: 10.1097/COC.0b013e3181942a1f. [DOI] [PubMed] [Google Scholar]
- 7.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 9.Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol. 1997;108:210–214. doi: 10.1111/1523-1747.ep12334263. [DOI] [PubMed] [Google Scholar]
- 10.Hersh EM, O’Day SJ, Ribas A, Samlowski WE, Gordon MS, Shechter DE, et al. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116:155–163. doi: 10.1002/cncr.24720. [DOI] [PubMed] [Google Scholar]
- 11.Kottschade LA, Suman VJ, Amatruda T, III, McWilliams RR, Mattar BI, Nikcevich DA, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group Study, N057E(1) Cancer. 2011;117:1704–1710. doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González Cao M, Malvehy J, Martí R, Conill C, Sanchez M, Martin M, et al. Biochemotherapy with temozolomide, cisplatin, vinblastine, subcutaneous interleukin-2 and interferon-alpha in patients with metastatic melanoma. Melanoma Res. 2006;16:59–64. doi: 10.1097/01.cmr.0000195697.58013.b7. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Sherring-Plough Corp. Study. Sherring-Plough Corp; Kenilworth, NJ: 1998. Data on file; pp. C193–114. [Google Scholar]
- 15.National Cancer Institute . CTEP: NCI Guidance on CTC Terminology Applications. National Cancer Institute; Bethesda, MD: 2006. [Google Scholar]
- 16.Stinchcombe TE, Socinski MA, Walko CM, O’Neil BH, Collichio FA, Ivanova MJ, et al. Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol. 2007;60:759–766. doi: 10.1007/s00280-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzaid AC, Atkins M. Practical guidelines for the management of biochemotherapy-related toxicity in melanoma. Clin Cancer Res. 2001;7:2611–2619. [PubMed] [Google Scholar]
- 18.Atkins MB, Hsu J, Lee S, Cohen GI, Flaherty LE, Sosman JA, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedikian AY, Johnson MM, Warneke CL, Mcintyre S, Papadopoulos N, Hwu WJ, et al. Systemic therapy for unresectable metastatic melanoma: impact of biochemotherapy on long-term survival. J Immunotoxicol. 2008;5:201–207. doi: 10.1080/15476910802131519. [DOI] [PubMed] [Google Scholar]
- 20.Keilholz U, Conradt C, Legha SS, Khayat D, Scheibenbogen C, Thatcher N, et al. Results of interleukin-2-based treatment in advanced melanoma: a case record-based analysis of 631 patients. J Clin Oncol. 1998;16:2921–2929. doi: 10.1200/JCO.1998.16.9.2921. [DOI] [PubMed] [Google Scholar]
- 21.Ron IG, Sarid D, Rvvo L, Sapir EE, Schneebaum S, Mester U, et al. A biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, temozolomide (Temodal), interferon-alfa and interleukin-2 for metastatic melanoma: a phase II study. Melanoma Res. 2006;16:65–69. doi: 10.1097/01.cmr.0000183921.46031.93. [DOI] [PubMed] [Google Scholar]
- 22.Chiarion-Sileni V, Guida M, Ridolfi L, Romanini A, Del Bianco P, Piqozzo J, et al. Central nervous system failure in melanoma patients: results of a randomized, multicentre phase 3 study of temozolomide-and dacarbazine- based regimens. Br J Cancer. 2011;104:1816–1821. doi: 10.1038/bjc.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 24.Bedikian AY, Wei C, Detry M, Kim KB, Papadopoulos NE, Hwu WJ, et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am J Clin Oncol. 2011;34:603–610. doi: 10.1097/COC.0b013e3181f9456a. [DOI] [PubMed] [Google Scholar]


