Abstract
Detection and analysis of Babesia gibsoni infection were performed with whole-blood samples collected between July 2002 and July 2003 from 945 and 137 dogs from the Aomori and Okinawa Prefectures of Japan, respectively, by PCR and loop-mediated isothermal amplification (LAMP). On the basis of the criterion for positivity by PCR, 3.9% (37 of 945) and 10.9% (15 of 137) of the dogs had B. gibsoni DNA. All 37 positive animals from Aomori Prefecture were male Tosa dogs (Japanese mastiff). The 15 dogs from Okinawa Prefecture with positive PCR assay results were of various breeds, ages, and sexes. The 18S ribosomal DNA (18S rDNA) sequences from all samples showed 100% homology to each other and to published B. gibsoni sequences. The limits of detection of B. gibsoni parasitemia by the PCR and LAMP methods with an 18S rDNA-based primer set were 0.0005% each. A comparison of the PCR and LAMP methods with microscopic examination for the detection of B. gibsoni infections in blood samples from 945 field dogs in Aomori Prefecture and 137 field dogs in Okinawa Prefecture showed that 37 and 15 dogs, respectively, were positive by the PCR and LAMP methods and that 16 and 12 dogs, respectively, were positive by light microscopic examination. All samples found to be positive by microscopic examination were also positive by the PCR and LAMP methods. The results of the PCR and LAMP methods agreed for samples with positive results by either method. Moreover, nonspecific reactions were not observed by the LAMP method. These results suggest that the LAMP method provides a useful tool for the detection of B. gibsoni infections in dogs.
Babesia gibsoni is a tick-borne hemoprotozoan parasite that causes remittent fever, progressive anemia, hemoglobinuria, and marked splenomegaly and hepatomegaly in dogs and, in some cases, the death of infected animals. However, chronically infected animals may have significant, minimal, or no clinical signs. The parasite was first identified in 1910 in dogs and jackals from India and is now considered endemic in Asia, Africa, Australia, Europe, and the United States (5, 14, 15, 17, 18, 20). This disease in the dog has long been problematic in Japan, especially in the western region. Recently, the geographical range of infection has spread to the eastern region of Japan (19). However, no recent detailed data are available for B. gibsoni-infected dogs in Japan.
B. gibsoni infection has frequently been found in companion dogs, creating a clinical problem (2, 8). The clinical severity of babesiosis in dogs is influenced to an extent by the age and the immune status of the affected individual: B. gibsoni infection causes progressive anemia in infected animals after surgery or when the animals are receiving immunosuppressive therapy and in general tends to be more severe in young dogs, with a subclinical carrier state arising after recovery from infection. Moreover, host immune responses to the parasite and changes in erythrocyte membrane proteins lead to the development of antierythrocyte antibodies, immune-mediated erythrocyte destruction, and a combination of intravascular and extravascular hemolysis (1, 6), with evidence of immune-mediated hemolysis in blood smears and with many animals having a positive Coomb's test (13). Thrombocytopenia is also commonly reported, along with variable leukocyte changes, influenced by the effects of stress, systemic inflammation, and bone marrow stimulation (3, 20). Therefore, the diagnosis of this disease and the detection of dogs that are carriers or that have a chronic form of this disease are very important.
The definitive means of diagnosis of babesiosis is by the identification of Babesia parasites in Giemsa-stained thin-film blood smears examined by microscopy. However, the detection of Babesia parasites is difficult in dogs with unapparent or chronic infections since the level of parasitemia is very low. Therefore, the development of a highly specific and sensitive system for the diagnosis of B. gibsoni infection is required. It has become possible to detect B. gibsoni infection in dogs by PCR methods (9, 14). PCR offers the advantages of high degrees of sensitivity and specificity. Recently, the introduction of loop-mediated isothermal amplification (LAMP) has allowed the development of rapid, sensitive, specific, and simple methods for the diagnosis of mycobacteriosis (7, 12). Moreover, detection of B. gibsoni by the LAMP method is specific and rapid, with the specificity and time to detection being comparable to those of PCR (16). The development of rapid and simple tests for the diagnosis of B. gibsoni infections in the animal hospital is required. However, the LAMP method has not yet been evaluated for the diagnosis of B. gibsoni infection. In this study, we determined the prevalence of B. gibsoni infection in dogs in two prefectures in Japan: Aomori Prefecture (in northern Japan) and Okinawa Prefecture (in southern Japan). Moreover, we developed a LAMP method for the detection of B. gibsoni infection in dogs and evaluated its sensitivity and specificity with blood from field dogs by comparison of the results obtained by the LAMP method with those obtained by microscopic examination and a PCR method.
MATERIALS AND METHODS
Blood samples.
From July 2002 to July 2003, 945 and 137 blood samples were obtained from dogs at a veterinary teaching hospital (Kitasato University) and from dogs at private animal hospitals in various parts of Aomori and Okinawa Prefectures in Japan, respectively. Whole-blood samples were collected from dogs, with EDTA or heparin used as the anticoagulant, and were stored at 4°C until further analysis. Thin smears were made from blood samples positive by PCR and were stained with Giemsa for evaluation for intracellular parasites by standard microscopic methods.
DNA preparation.
For each sample, DNA was extracted from 100 μl of whole blood by using the GFX Genomic Blood DNA Purification kit (Amersham Biosciences, Branchburg, N.J.), according to the instructions of the manufacturer. Extracted DNAs were stored at 4°C until use.
PCR assay.
The universal canine Babesia-specific primer set, primers B18S-F (5′-TGGTTGATCCTGCCAGTA-3′) and B18S-R (5′-CTTCTCCTTCCTTTAAGTGA-3′), was designed to amplify 1,665 bp of 18S ribosomal DNA (18S rDNA) (GenBank accession nos. AF205636, AY072926, and AY072925). The B. gibsoni-specific primer set of primer Bg.Pd3 (5′-TCCGTTCCCACAACACCAGC-3′) and primer Bg.Pd4 (5′-TCCTCCTCATCATCCTCATTCG-3′) was designed to amplify a 182-bp portion of the B. gibsoni P18 gene (9). All extracted DNAs were used for both PCRs. PCR was performed in 50 μl of a mixture containing 1 μl of the extracted DNA, 50 pmol of each primer, a 200 μM concentration of each deoxynucleoside triphosphate, and 1.25 U of Taq Gold DNA polymerase (Applied Biosystems, Foster City, Calif.) in 1× buffer (10 mM Tris-HCl, 1.5 mM MgCl2, 0.001% gelatin; Applied Biosystems). PCR was performed for 10 min at 95°C to activate the Taq Gold DNA polymerase. The reaction with the B18S-F and B18S-R primer set was repeated for 30 cycles under the following conditions: 30 s of denaturation at 94°C, 2 min of annealing at 55°C, and 2 min of extension at 72°C. The reaction with the Bg.Pd3 and Bg.Pd4 primer set was repeated for 30 cycles under the following conditions: 30 s of denaturation at 94°C, 1 min of annealing at 54°C, and 1 min of extension at 72°C. A 5-min extension at 72°C was performed after the last cycle in the reactions with both sets of primers. Ten microliters of each of the PCR products was analyzed by electrophoresis on 1.5 and 2.0% agarose gels, followed by ethidium bromide staining and photography.
DNA sequence.
The 18S rDNA PCR product was ligated into the pCR 2.1-TOPO vector by using the TOPO TA Cloning kit (Invitrogen, Carlsbad, Calif.). The entire ligation reaction was used to transform Escherichia coli DH5α competent cells. Plasmid DNA from two positive transformants was used to sequence the DNA of the insert. Both strands of the plasmid insert DNA were sequenced by using the Dye Terminator Cycle Sequencing kit, supplied by Applied Biosystems, with the following five primers: B18S-F, B18S-R, B18S-1 (5′-GGGAGGTAGTGACAAGAAA-3′), B18S-2 (5′-TTCCCCGTGTTGAGTCAAA-3′), and B18S-3 (5′-GTCTCGTTCGTTAACGGAA-3′). Analysis was done with an ABI PRISM 310 DNA sequencer (Applied Biosystems). The sequencing analysis was performed with the computer program GENETYX-MAC (version 10; Software Development, Co. Ltd., Tokyo, Japan).
LAMP method.
A B. gibsoni-specific LAMP primer set consisting of primers B18S-F3 (5′-TCCCTTGGTTTTCGGTGATT-3′), B18S-B3 (5′-GCTGCCTTCCTTAGATGTGG-3′), B18S-FIP (5′-CCCTACCGTCAAGCTGATGGGTACTCGCGAATCGCTTTTAGC-3′), and B18S-BIP (5′-TATTGGCCTACCGAGGCAGCATTCTCAGGCTCCCTCTCC-3′) was designed by using the PrimerExplorer program (http://venus.netlaboratory.com/partner/lamp/index.html) to amplify the 18S rDNA from GenBank (accession no. AF205636). LAMP was performed in 25 μl of a mixture containing 2 μl of the extracted DNA, 40 pmol (each) of primers B18S-FIP and B18S-BIP, 5 pmol (each) of primers B18S-F3 and B18S-B3, and 1 μl of Bst DNA polymerase (Eiken Chemical Co., Ltd., Tokyo, Japan) in 1× buffer [20 mM Tris-HCl (pH 8.8), 10 mM KCl, 8 mM MgSO4, 10 mM (NH4)2SO4, 0.1% Tween 20, 0.8 M gelatin, a 1.4 mM concentration of each deoxynucleoside triphosphate; Eiken Chemical Co.]. The LAMP reaction was performed for 60 min at 63°C. Inactivation for 2 min at 80°C was performed after the last cycle. Twenty microliters of each of the LAMP products was analyzed by electrophoresis with 1.5% agarose gels, followed by ethidium bromide staining and photography.
Nucleotide sequence accession number.
The sequence of the 18S rDNA gene of B. gibsoni (Aomori isolate) has been submitted to the GenBank database under accession no. AB118032.
RESULTS
Detection of B. gibsoni.
All blood samples that were positive by PCR with the B18S-F and B18S-R primer set specific for canine Babesia were also positive by PCR with the B. gibsoni-specific primer set, primers Bg.Pd3 and Bg.Pd4. The results were positive for 37 of the 945 dogs from Aomori Prefecture and 15 of the 137 dogs from Okinawa Prefecture. All 37 positive dogs from Aomori Prefecture were male Tosa dogs (Japanese mastiff) used for dogfights. Sixteen of these PCR-positive dogs had microscopic evidence of parasitemia and various clinical symptoms, including anemia and hemoglobinuria (Table 1). Eight of the 11 Tosa dogs positive by PCR but with no microscopic evidence of parasitemia were clinically normal. No microscopic data were available for the remaining 10 Tosa dogs with positive PCR assay results; 7 of these 10 dogs were clinically normal. The 15 dogs from Okinawa Prefecture with positive PCR assay results were of various breeds, ages, and sexes (Table 2). Among these dogs, 12 had microscopic evidence of parasitemia and various clinical symptoms. Two of the three dogs with positive PCR assay results but no microscopic evidence of parasitemia were clinically normal.
TABLE 1.
Comparison of the PCR and LAMP methods and microscopic examination for detection of B. gibsoni infection in field dogs from Aomori Prefecture, Japan
| Dog no.a | Age (yr) | Clinical manifestations of Babesia infection | Microscopy result (% parasitemia) | PCR result | LAMP result |
|---|---|---|---|---|---|
| 1 | 2.3 | + | + (<0.1) | + | + |
| 3 | 2 | − | NDb | + | + |
| 4 | ND | − | ND | + | + |
| 5 | 2 | − | ND | + | + |
| 7 | 3 | − | ND | + | + |
| 12 | 2.5 | − | − | + | + |
| 16 | 1 | + | + (ND) | + | + |
| 17 | 4 | + | ND | + | + |
| 21 | 2 | − | ND | + | + |
| 22 | ND | + | ND | + | + |
| 30 | ND | + | + (1.1) | + | + |
| 33 | 2 | − | ND | + | + |
| 34 | 3 | − | ND | + | + |
| 35 | 2.7 | + | + (2.3) | + | + |
| 58 | 2 | + | + (0.7) | + | + |
| 65 | 1 | + | + (<0.1) | + | + |
| 69 | 3 | + | + (<0.1) | + | + |
| 74 | 4 | + | + (ND) | + | + |
| 77 | 3 | + | ND | + | + |
| 84 | 4 | − | − | + | + |
| 85 | 2.1 | + | + (0.5) | + | + |
| 86 | 3 | − | − | + | + |
| 204 | 1 | + | + (1.3) | + | + |
| 205 | 1.5 | + | + (0.3) | + | + |
| 218 | 2.4 | + | − | + | + |
| 285 | ND | + | − | + | + |
| 316 | 2.8 | + | + (<0.1) | + | + |
| 317 | 4 | + | − | + | + |
| 318 | 3 | − | − | + | + |
| 321 | ND | + | + (1.1) | + | + |
| 360 | 2 | − | − | + | + |
| 406 | 0.9 | + | + (3.9) | + | + |
| 428 | 2 | + | + (8.0) | + | + |
| 458 | 2.5 | + | + (0.3) | + | + |
| 499 | 5 | − | − | + | + |
| 500 | 7 | − | − | + | + |
| 501 | 7 | − | − | + | + |
All dogs were male Tosa dogs.
ND, no data.
TABLE 2.
Comparison of the PCR and LAMP methods and microscopic examination for detection of B. gibsoni infection in field dogs from Okinawa Prefecture, Japan
| Dog no. | Breed of dog | Sex | Age (yr) | Clinical manifestations of Babesia infection | Microscopy (% parasitemia) | PCR result | LAMP result |
|---|---|---|---|---|---|---|---|
| 12 | ND | NDa | ND | + | + (ND) | + | + |
| 13 | Golden retriever | Male | 4.5 | + | + (ND) | + | + |
| 14 | Mongrel | Male | 2.6 | − | − | + | + |
| 15 | Mongrel | Female | 2.6 | − | − | + | + |
| 16 | Mongrel | Female | 1.3 | + | − | + | + |
| 17 | Mongrel | Female | 1.3 | − | + (0.2) | + | + |
| 18 | Mongrel | Female | 1.4 | + | + (1.6) | + | + |
| 30 | Mongrel | Female | ND | + | + (ND) | + | + |
| 79 | Golden retriever | Male | 5 | + | + (10.1) | + | + |
| 116 | Miniature pinscher | Male | 0.8 | + | + (1.9) | + | + |
| 117 | Mongrel | Male | 4 | + | + (<0.1) | + | + |
| 118 | Poodle | Male | 11 | + | + (<0.1) | + | + |
| 122 | Tibetan spaniel | Male | 2.3 | + | + (0.2) | + | + |
| 136 | Labrador retriever | Male | 8 | + | + (0.6) | + | + |
| 137 | Mongrel | Male | 9 | + | + (<0.1) | + | + |
ND, no data.
DNA sequence analysis.
The 18S rDNA amplified from all PCR-positive samples from both prefectures was sequenced. The 18S rDNA sequences (1,617 bp, without the primer region) showed 100% homology to the sequences from all other samples and previously published sequences of B. gibsoni strains from dogs in Asia, Oklahoma, North Carolina, and Okinawa (GenBank accession nos. AF175300, AF205636, AF271081, and AF271082, respectively) (data not shown).
Comparison of PCR and LAMP assays.
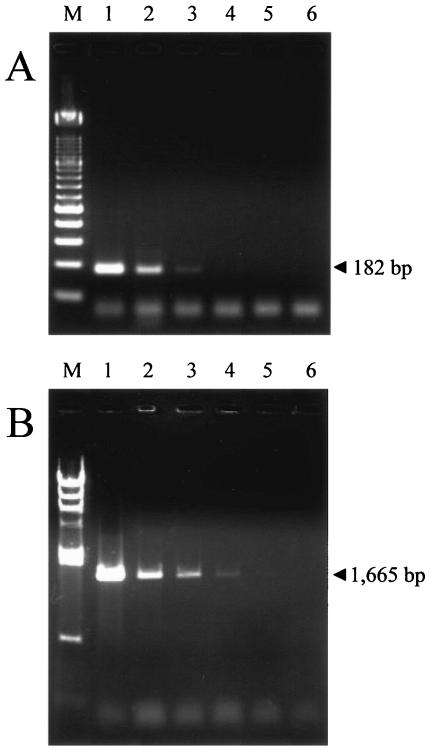
B. gibsoni-infected erythrocytes with 5% parasitemia were subjected to 10-fold serial dilution with erythrocytes from healthy dogs, and DNA was extracted from each diluted sample to test the sensitivities of the PCR and LAMP methods. The PCR used in this study was able to detect an estimated 0.005% parasitemia with the B. gibsoni-specific primer set of primers Bg.Pd3 and BgPd4 and 0.0005% parasitemia with the universal canine Babesia-specific primer set of primers B18S-F and B18S-R (Fig. 1A and B).
FIG. 1.
Sensitivities of the PCR methods determined by agarose gel electrophoresis of the PCR products from samples serially diluted 10-fold and ethidium bromide staining. Primers Bg.Pd3 and Bg.Pd4 (A) and primers B18S-F and B18S-R (B) were used in PCRs to detect B. gibsoni. Lanes 1, dilution of 10−1 with 0.5% parasitemia; lanes 2, dilution of 10−2 with 0.05% parasitemia; lanes 3, dilution of 10−3 with 0.005% parasitemia; lanes 4, dilution of 10−4 with 0.0005% parasitemia; lanes 5, dilution of 10−5 with 0.00005% parasitemia; lanes 6, dilution of 10−6 with 0.000005% parasitemia; lane M (A), 100-bp DNA ladder marker; lane M (B), HindIII-digested bacteriophage lambda marker.
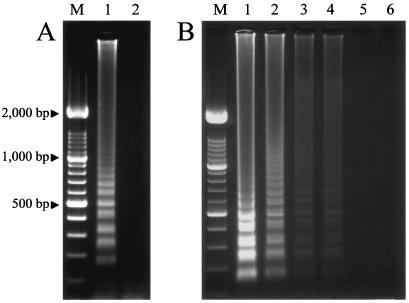
The LAMP method was examined by using DNA extracted from B. gibsoni-infected dog blood with 5% parasitemia and leukocytes from healthy dogs. As shown in Fig. 2A, the positive LAMP reaction produced a smear of DNA between the well and 1,000 bp, with many bands of different sizes from 100 to 1,000 bp (Fig. 2A). The LAMP method used in this study was able to detect an estimated 0.0005% parasitemia with the B. gibsoni-specific primer set of primers B18S-F3, B18S-B3, B18S-FIP, and B18S-BIP (Fig. 2B). The PCR and LAMP methods with the primer set specific for 18S rDNA showed a detection limit of 0.0005% for B. gibsoni.
FIG. 2.
(A) Agarose gel electrophoresis of LAMP products from B. gibsoni-infected dog blood (lane 1) and leukocytes from healthy dogs (lane 2) stained with ethidium bromide. (B) Sensitivity of the LAMP method determined by agarose gel electrophoresis of LAMP products from 10-fold serial diluted samples and ethidium bromide staining. Target B. gibsoni DNA was detection by the LAMP reaction. Lane 1, dilution of 10−1 with 0.5% parasitemia; lane 2, dilution of 10−2 with 0.05% parasitemia; lane 3, dilution of 10−3 with 0.005% parasitemia; lane 4, dilution of 10−4 with 0.0005% parasitemia; lane 5, dilution of 10−5 with 0.00005% parasitemia; lane 6, dilution of 10−6 with 0.000005% parasitemia; lanes M, 100-bp DNA ladder marker.
Detection of B. gibsoni from field samples.
In a comparison of the PCR and LAMP methods with microscopic examination for the detection of B. gibsoni infection in blood samples from 945 field dogs in Aomori Prefecture and 137 field dogs in Okinawa Prefecture, 37 and 15 dogs, respectively, were positive by the PCR and LAMP methods and 16 and 12 dogs, respectively, were positive by light microscopic examination (Tables 1 and 2). All samples found to be positive by microscopic examination were also positive by the PCR and LAMP methods. All samples positive by PCR were also positive by the LAMP method. Moreover, nonspecific reactions were not observed by the LAMP method.
DISCUSSION
In the present study, the B18S-F and B18S-R primer set specific for canine Babesia species and the Bg.Pd3 and Bg.Pd4 primer set specific for B. gibsoni were used for PCR detection of B. gibsoni in blood samples from 945 dogs from Aomori Prefecture and 137 dogs from Okinawa Prefecture in Japan. On the basis of the criterion for positivity described above, 3.9% (37 of 945) and 10.9% (15 of 137) of the dogs from Aomori and Okinawa Prefectures, respectively, had B. gibsoni infection. However, these results do not indicate the actual canine Babesia infection rate in each prefecture, because these samples not only were from randomly sampled animals but also were from animals that were brought to animal hospitals and sampled as a result of clinical manifestations of Babesia infection. The cases of canine babesiosis reported in Okinawa Prefecture have been considered to be caused by B. gibsoni and B. canis (21). Recently, B. gibsoni and B. canis DNAs were detected by PCR from ticks in Aomori and Okinawa Prefectures (11). However, in this study, all positive dogs had B. gibsoni infection and B. canis infection was not seen. This situation may change as larger numbers of dogs are sampled, and examination of the infecting species will be necessary in future studies. Nevertheless, it is of interest that all 37 positive dogs among the 945 dogs sampled in Aomori Prefecture were male Tosa dogs. The general mode of B. gibsoni transmission has been reported to be by ticks (4). Furthermore, Macintire et al. (14) suggested the following four possible modes of B. gibsoni transmission: (i) transplacental transmission, (ii) the direct transmission of blood during dogfights, (iii) direct transmission of blood by iatrogenic means, and (iv) shipping of dogs from areas of endemicity. Transmission was probably not due to ticks, as all other breeds of dogs were negative. Moreover, although the sample number was small, all eight samples from female Tosa dogs were negative. Male Tosa dogs are mainly used in dogfights in Aomori Prefecture, indicating that transmission of B. gibsoni infection in Aomori Prefecture is due to the direct transmission of blood during dogfights. However, it will be necessary to examine the time dependence of B. gibsoni infections in Tosa dogs and to perform detailed epidemiological studies in other geographical areas in order to clarify this. Moreover, additional studies are needed to determine whether the Tosa dog is genetically predisposed to B. gibsoni infection and to determine the mode of transmission. In Okinawa Prefecture, the means of transmission of B. gibsoni infection is mainly by ticks, because in this study dogs of various breeds, ages, and sexes were positive and because the tick vector (Rhipicephalus sanguineus) is known to exist in Okinawa Prefecture (10).
The sequences of the 18S rDNA amplified from blood samples from dogs in Aomori and Okinawa Prefectures in the present study showed 100% homology to the sequences from all other samples and matched the sequences isolated from Asia, Oklahoma, North Carolina, and Okinawa. Therefore, the primers used for the LAMP method were based on 18S rDNA, for which there is no regional genetic variation. The usefulness of this method for diagnosis was examined. On examination of 945 field dogs from Aomori Prefecture and 137 field dogs from Okinawa Prefecture, PCR and LAMP detected parasite DNA in 11 and 3 samples, respectively, that were negative by microscopic examination, indicating that the sensitivities of the PCR and LAMP methods are higher than the sensitivity of microscopic examination for the diagnosis of B. gibsoni infection in dogs. The sensitivities of the LAMP and PCR methods were equivalent, with detection limits of 0.0005% each. However, at this level of dilution, positive LAMP results could easily be determined by visual examination of the DNA smear and the pattern of many DNA bands of different sizes. In addition, the reaction time of the LAMP method is 1 h, which is about 3 h less than the amount of time required for PCR.
We used a novel DNA amplification method, LAMP, to detect B. gibsoni in whole blood. DNA amplification methods, such as PCR, have been developed to allow the sensitive and specific detection of B. gibsoni infections (9, 14). In this regard, the LAMP method has the advantages of a rapid and accurate reaction and simple operation. The LAMP method will be a promising method for wider use in animal hospitals. However, when the LAMP method is used, care must be taken to avoid contamination during sample preparation and DNA extraction and appropriate controls must be used to avoid cross-contamination. Moreover, a small number of samples was used in this study; thus, further study of the LAMP method with a larger number of samples and with the blood of animals implicated in B. gibsoni transmission is necessary to determine the best DNA target for B. gibsoni detection and to ascertain whether this rapid, simple, and highly sensitive test can be applied to the detection of B. gibsoni infection in dogs in the field.
Acknowledgments
This study was supported by a Grant-in-Aid for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science; and the Kitasato University Research Grant for Young Researchers and Encouragement of Young Scientists.
REFERENCES
- 1.Adachi, K., M. Tateishi, Y. Horii, H. Nagatomo, T. Shimizu, and S. Makimura. 1995. Immunologic characteristics of antierythrocyte membrane antibody produced in dogs during Babesia gibsoni infection. J. Vet. Med. Sci. 57:121-123. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, K., C. Ueno, and S. Makimura. 1993. Immunosuppression in dogs naturally infected with Babesia gibsoni. J. Vet. Med. Sci. 55:503-505. [DOI] [PubMed] [Google Scholar]
- 3.Birkenheuer, A. J., M. G. Levy, K. C. M. Savary, R. B. Gager, and E. B. Breitschwerdt. 1999. Babesia gibsoni infections in dogs from North Carolina. J. Am. Anim. Hosp. Assoc. 35:125-128. [DOI] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B. 1984. Babesiosis, p. 796-805. In C. E. Green (ed.), Clinical microbiology and infectious diseases of the dog and cat. The W. B. Saunders Co., Philadelphia, Pa.
- 5.Casapulla, R., L. Baldi, V. Avallone, R. Sannino, L. Pazzanese, and V. Mizzoni. 1998. Canine piroplasmosis due to Babesia gibsoni: clinical and morphological aspects. Vet. Rec. 142:168-169. [DOI] [PubMed] [Google Scholar]
- 6.Conrad, P., J. Thomford, I. Yamane, J. Whiting, L. Bosma, T. Uno, H. J. Holshuh, and S. Shelly. 1991. Hemolytic anaemia caused by Babesia gibsoni infection in dogs. J. Am. Vet. Med. Assoc. 199:601-605. [PubMed] [Google Scholar]
- 7.Enosawa, M., S. Kageyama, K. Sawai, K. Watanabe, T. Notomi, S. Onoe, Y. Mori, and Y. Yokomizo. 2003. Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 41:4359-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farwell, G. E., E. K. LeGrand, and C. C. Cobb. 1982. Clinical observations on Babesia gibsoni and Babesia canis infections in dogs. J. Am. Vet. Med. Assoc. 180:507-511. [PubMed] [Google Scholar]
- 9.Fukumoto, S., X. Xuan, S. Shigeno, E. Kimbita, I. Igarashi, H. Nagasawa, K. Fujisaki, and T. Mikami. 2001. Development of a polymerase chain reaction method for diagnosing Babesia gibsoni infection in dogs. J. Vet. Med. Sci. 63:977-981. [DOI] [PubMed] [Google Scholar]
- 10.Inokuma, H., D. Raoult, and P. Brouqui. 2000. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 38:4219-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inokuma, H., Y. Yoshizaki, Y. Shimada, Y. Sakata, M. Okuda, and T. Onishi. 2003. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 41:3494-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto, T., T. Sonobe, and K. Hayashi. 2003. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 41:2616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen, L. S., and I. A. Clark. 1994. The pathophysiology of canine babesiosis: new approaches to an old puzzle. J. S. Afr. Vet. Assoc. 65:134-145. [PubMed] [Google Scholar]
- 14.Macintire, D. K., M. K. Boudreaux, G. D. West, C. Bourne, J. C. Wright, and P. A. Conrad. 2002. Babesia gibsoni infection among dogs in the southeastern United States. J. Am. Vet. Med. Assoc. 220:325-329. [DOI] [PubMed] [Google Scholar]
- 15.Muhlnickel, C. J., R. Jefferies, U. M. Ryan, and P. J. Irwin. 2002. Babesia gibsoni infection in three dogs in Victoria. Aust. Vet. J. 80:606-610. [DOI] [PubMed] [Google Scholar]
- 16.Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino, and T. Hase. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patton, W. S. 1910. Preliminary report on a new piroplasm found in the blood of hounds of the Madras-Hunt and subsequently discovered in the blood of the jackal. Bull. Soc. Pathol. Exot. 3:274-280. [Google Scholar]
- 18.Wozniak, E. J., B. C. Barr, J. W. Thomford, I. Yamane, S. P. McDonough, P. F. Moore, D. Naydan, T. W. Robinson, and P. A. Conrad. 1997. Clinical, anatomic and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris). J. Parasitol. 83:692-699. [PubMed] [Google Scholar]
- 19.Wulansari, R., A. Wijaya, H. Ano, Y. Horii, and S. Makimura. 2003. Lymphocyte subsets and specific IgG antibody levels in clindamycin-treated and untreated dogs experimentally infected with Babesia gibsoni. J. Vet. Med. Sci. 65:579-584. [DOI] [PubMed] [Google Scholar]
- 20.Yamane, I., P. A. Conrad, and I. Gardner. 1993. Babesia gibsoni infections in dogs. J. Protozool. Res. 3:111-125. [PubMed] [Google Scholar]
- 21.Yonamine, H., H. Ichiki, M. Hamakawa, T. Shimabukuro, M. Sugiyama, and M. Isoda. 1984. Studies on canine babesiosis in Okinawa Island. Jpn. J. Vet. Sci. 46:511-518. [DOI] [PubMed] [Google Scholar]