Abstract
We describe the first case of Epstein-Barr virus (EBV)-associated thymic carcinoid tumor found by in situ hybridization (ISH) on paraffin-embedded sections. ISH revealed that both tumor cells and infiltrated lymphocytes were EBV positive, while a few EBV-infected lymphocytes were detected in 2 of 11 thymuses and 1 of 11 thymomas.
CASE REPORT
A 72-year-old Japanese man with a 3-week history of back pain initially presented with a right upper anterior mediastinal mass discovered by chest roentgenography and computed tomography in Shimonoseki-saiseikai Hospital, Shimonoseki, Japan. There was no evidence of Cushing's syndrome, definite carcinoid syndrome, or myasthenia gravis in his medical history. Laboratory examinations showed all data to be in normal ranges, except for elevated values of serum lactic dehydrogenase and alkaline phosphatase. A mass arising from the right lobe of the thymus to the right side of the mediastinum was observed when a thoracotomy via midline sternotomy was performed. The tumor had invaded the right upper and middle lobes of the lung, the phrenic nerve, and the mediastinal lymph nodes. Even though the patient received appropriate postoperative chemotherapy, he suffered vertebral metastasis that induced paralysis of the lower extremities, and he eventually died of progression of the disease.
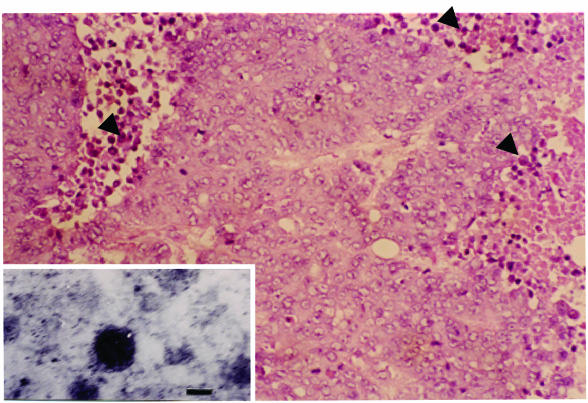
The surgically removed thymic tumor (8 by 4 by 4 cm) was fixed in formalin, embedded in paraffin, and sectioned for routine histological examination. A piece of the thymic tumor was subjected to transmission electron microscopy. The tumor was histologically composed of solid nests of polygonal cells in a characteristic rosette formation. Mitosis was seen occasionally, and focal to geographic necrosis was evident in the tumor tissue. A considerable number of lymphocytes were distributed among the tumor nests. Transmission electron microscopy revealed that distinct electron-dense granules with a diameter of 15 to 40 nm surrounded by a halo were seen in the cytoplasm of most tumor cells, indicating structures corresponding to neurosecretory granules (Fig. 1). Grimelius staining revealed that a considerable number of tumor cells had dark-brown granules in their cytoplasm.
FIG. 1.
Photomicrograph of the tumor showing that the tumor is composed of solid nests of polygonal cells with necrosis and lymphocyte infiltration. These tumor cells have hyperchromatic and prominent nucleoli. The tissue sections were stained by hematoxylin-eosin-staining. Arrowheads point to infiltrated lymphocytes. Magnification, ×20. (Inset) Transmission electron micrograph showing that neurosecretory granules are clearly seen in the cytoplasm of a tumor cell. Inset magnification, ×20,000. Bar, 50 nm.
Immunohistochemical staining with 11 commercially available antibodies—EBNA2 (Dako Japan Co., Ltd., Kyoto, Japan), ZEBRA (Dako), virus capsid antigen (VCA) (Dako), latent membrane protein (LMP) (Dako), CD45RO (UCHL1) (Nichirei, Tokyo, Japan), CD20 (L26) (Dako), CD7 (Dako), neuron-specific enolase (NSE) (Dako), chromogranin A (Dako), Bcl-2 (Dako), and p53 (Oncogene Science, Cambridge, Mass.)—was performed, demonstrating that the tumor cells reacted with p53, NSE, and chromogranin A antibodies. Thus, the tumor was determined to be a thymic carcinoid tumor. The identities of Epstein-Barr virus (EBV)-related proteins above follow. EBNA2 is necessary to provide the growth stimulus for B cells in vitro and in classical posttransplant lymphoproliferative disease. ZEBRA, the BZLF1-encoded protein, is not expressed during latent infection; however, activation of the immediate-early gene BZLF1 is required to switch from latent to lytic infection in host cells (6). Antibody against VCA produced in lytic infection is routinely used to diagnose EBV infection by serological examination.
Next, to identify EBV-related genes and transcripts in the tumor, in situ hybridization (ISH) with four alkaline phosphatase-labeled EBV gene-specific antisense oligoprobes, including EBV-encoded small RNA 1 (EBER1), BamHI-W, latent membrane protein 1 (LMP1), and gp350/220 (a component of VCA) (Iatron Laboratories, Chiba, Japan), and two digoxigenin-labeled EBV gene-specific riboprobes, BZLF1 and BHRF1, generated by in vitro transcription (Boehringer, Mannheim, Germany) was performed as described previously (17, 26, 27). ISH with four oligoprobes revealed that cells that were almost neoplastic and contiguous lymphocytes were positive only for EBER1 (Fig. 2), indicating the presence of an EBV-associated carcinoid tumor. EBV positivity on the section has been determined primarily by ISH with the specific probe EBER1. There were abundant EBER1 transcripts (approximately 107 copies per cells), which exist as ribonucleoprotein particles complexed with the cellular La antigen in EBV-infected cells and EBV-associated tumors, except for oral hairy leukoplakia in which no EBER1 was detected (12).
FIG. 2.
ISH reveals that tumor cells and lymphocytes are positive for EBER1. Magnification, ×20. (Inset) Cells show no signal against EBER1 sense oligoprobe utilized as a negative control. The arrowhead point to infiltrated lymphocytes. Magnification, ×20.
Furthermore, according to previously published methods (26, 27), the nucleic extracts (DNA and RNA) obtained from sectioned tissues were subjected to PCR for EBV genome, reverse transcription-PCR for EBER1, and Southern blot hybridization with appropriate primers and probes, demonstrating that EBV genomic DNA and EBER1 were presented. Immunohistochemical examination showed that the EBV-infected lymphocytes reacted with CD45RO (UCHL1) and CD7 antibodies, indicating that the lymphocytes were NK or T lymphocytes. These results are summarized in Table 1. Taken together, EBV-infected tumor cells expressed p53 protein but not LMP1 or Bcl-2, and the status of EBV detected in both tumor cells and NK or T lymphocytes in tissue surrounding the tumor was not replication phase, since neither VCA nor the BZLF1 transcript, whose product mediates lytic replication of EBV (6), was detected.
TABLE 1.
Summary of immunohistochemical and ISH results
| Stain, antibody, or targeted DNA or transcript | Result or reaction of:
|
|||
|---|---|---|---|---|
| Tumor cell | Lymphocyte | |||
| Grimelius stain | + | − | ||
| Immunohistochemical staining antibodies
|
||||
| UCHL1 | − | + | ||
| CD7 | − | + | ||
| L26 | − | − | ||
| EBNA2 | − | − | ||
| LMP1 | − | − | ||
| ZEBRA | − | − | ||
| VCA | − | − | ||
| p53 | + | − | ||
| Bcl-2 | − | − | ||
| NSE | + | − | ||
| Chromogranin A | + | − | ||
| ISH targets | ||||
| EBV genomic DNAa | − | − | ||
| Transcripts | ||||
| EBER1 | + | + | ||
| LMP1 | − | − | ||
| gp350/220 (VCA) | − | − | ||
| BHRF1 | + | + | ||
| BZLF1 | − | − | ||
Results shown were obtained with oligoprobe BamHI-W.
To determine the distribution of EBV-infected cells in the thymus, ISH with EBER1 probe was performed on 20 consecutive sections each from 11 normal thymuses (7 males and 4 females; average age of 79 years) obtained from Shimonosekishi-ishikai Hospital, and 11 thymomas without myasthenia gravis obtained from both Shimonosekishi-ishikai and Shimonoseki-saiseikai Hospitals. Only a very small number of lymphocytes infected with EBV were detected in two normal thymuses and one thymoma.
EBV, a B-lymphotropic gammaherpesvirus, is associated with a number of human malignancies, such as B-cell, T-cell, and NK lymphomas, nasopharyngeal carcinoma, and gastric carcinoma as well as disorders in the thymus (5, 19, 20, 21). EBV in the thymus and various thymic tumors has not always been detected in southern Chinese (21), European (2), and Western subjects (16). ISH with EBER1 probe showed EBV in malignant epithelial cells in one case of thymic lymphoepithelioma-like carcinoma but not in thymic carcinoma, normal thymus, thymoma, or thymic lymphoid hyperplasia (20). Studies of Taiwanese subjects by PCR and ISH with EBER1 probe showed that lymphoepithelioma-like thymic carcinoma was more often associated with the virus than other thymic tumors (5), indicating that EBV-associated thymic diseases may be linked to epidemiologic factors or genetic predisposition; otherwise, the sensitivity of the methodology used may affect these results. The occurrence of carcinoid tumor in the thymus is rare and defined on the basis of mitotic activity and severity of tumor invasion and has a better prognosis than tumors accompanied by necrosis or infiltrated lymphocytes, as in the case presented here. However, the reason for such different prognoses has not been explained. The EBV-associated neoplasms generally have relatively worse prognoses and more aggressive behavior compared to non-EBV-associated neoplasms, depending on the virus status, such as profiles of viral gene expression which eventually affect signal transduction in the infected cell (8, 13, 22).
In this case of EBV-associated thymic carcinoid, EBV was detected in tumor cells and contiguous infiltrated lymphocytes, and the EBV status and characteristics of both EBV-infected cells are determined. The fact that EBV in most of the morphologically malignant cells was in a nonreplicating stage was interpreted to mean that the virus must have invaded the target cells prior to neoplastic transformation and clonal proliferation; if EBV infection had been a late event, it is highly unlikely that these tumor cells would have become infected.
The pathogenesis and interrelationships of neuroendocrine carcinomas are not well understood. Mutation of the beta-catenin gene (10), Int-2 allelic imbalance (9), and mitogen-activated protein kinase activation (18) of carcinoid tumors have been described. Studying the methylation profiles of carcinoid tumors revealed that methylation at MGMT (O6-methyl-guanine methyltransferase), THBS1 (thrombospondin 1), p14, and RARβ (retinoic acid receptor β 2) loci was more frequent in carcinoid tumors, which reflect molecular pathogenesis (4).
On the other hand, it is well-known that LMP1, which was not detected in this study, induces activation of several signaling pathway by NF-κB-, JNK-, and STAT-mediated transcription (8, 13, 22), leading to the onset of neoplasms at an early stage. At earlier stages of carcinogenesis, LMP1 might be transiently expressed immediately after an incident of EBV infection and transform primary neuroendocrine cells. EBV LMP1 is an immunodominant antigen, so disease caused by it, such as infectious mononucleosis, can be cured spontaneously by cytotoxic T lymphocytes. In this case, EBV-infected cells might escape from immunosurveillance in the host due to the absence of LMP1. Among the carcinoid tumors accompanied by infiltrated lymphocytes, the EBV-associated one might exist, as in this case.
An in vivo study utilizing transgenic mice in which adenovirus genes (E1A or E1B) were chromosomally integrated documented that such viral genes caused the early onset of bowel carcinoid tumors with high levels of N-myc and c-jun mRNA (25). In our case, it is unclear whether EBV was integrated into the chromosome or colocalized in the chromosome as a plasmid form. However, we were not able to exclude the possibility that viral gene expression of EBV stably exists in the nucleus, comparable to adenovirus, interfering with the transcriptional regulation of the promoter region of nuclear oncogene activation and with transformation and/or exerting CpG island methylation.
Furthermore, fluorescence ISH with locus-specific DNA probes demonstrated a high incidence of deletion of the tumor suppressor genes p53 and retinoblastoma (Rb), indicating that structural genomic alterations are frequent in neuroendocrine lung carcinomas and that their occurrence may be underestimated by immunohistochemical studies alone (14). Some EBV-infected cells possessed BHRF1 transcript, and the BHRF1 sequence was homologous to the bcl-2 oncogene (24), whose product (Bcl-2) is a major negative regulator of apoptosis.
In addition, p53 was immunohistochemically detected in tumor cells, indicating that EBV effects abnormal cell proliferation via evasion of apoptosis. Tumors expressing Bcl-2 generally correlate with a propensity for more aggressive biological behavior and worse prognosis. The patient in this case did not respond well to treatment, even though there was a lack of Bcl-2 expression. However, BHRF1 transcript was detected, suggesting that the putative viral protein from BHRF1, instead of Bcl-2, may reflect such clinical features with mutation in the p53 gene (7, 15). It should be noted that the sensitivity of available methods on tissue sections is limited. To clarify a causal relationship between EBV infection and carcinoid tumor might demand further improvements in methodology on tissue sections such as in situ PCR (17, 27).
The results from studies of ISH with EBER1 probe using 11 thymuses and 11 thymomas demonstrated that a few EBV-positive lymphocytes in 2 normal thymuses and one thymoma were detected, indicating that there are certainly EBV-infected lymphocytes in the thymus. Evidence supporting the presence of the EBV genome in NK or T lymphocytes has been reported previously (1, 11, 23, 28). Regarding our case, we thus hypothesize that the thymic carcinoid tumor may have arisen in a peculiar microenvironment, such as proliferation of EBV-infected NK or T lymphocytes or immature cells. The EBV produced from such cells might more efficiently infect cells, including neuroendocrine cells or primitive cell-like pluripotential endodermal cells in the early phase of tumorigenicity due to alteration of virus tropism (3).
In conclusion, in this report, we described the first case of EBV-associated thymic carcinoid and investigated the EBV status in this patient and examined the distribution of EBV-infected cells in the thymuses and thymomas of other patients. These results imply that EBV infection may have contributed to the development of the tumor in this case. However, more investigation with a larger number of clinical cases and molecular-based analyses both in vitro and in vivo are needed to elucidate the potential contribution of EBV to the pathogenesis of this very rare tumor type.
REFERENCES
- 1.Ban, S., Y. Goto, K. Kamada, M. Takahama, H. Watanabe, T. Iwahori, and H. Takeuchi. 1999. Systemic granulomatous arteritis associated with Epstein-Barr virus infection. Virchows Arch. 434:249-254. [DOI] [PubMed] [Google Scholar]
- 2.Borisch, B., T. Kirchner, A. Marx, and H. K. Muller-Hermelink. 1990. Absence of the Epstein-Barr virus genome in the normal thymus, thymic epithelial tumors, thymic lymphoid hyperplasia in a European population. Virchows Arch. B Cell. Pathol. Incl. Mol. Pathol. 59:359-365. [DOI] [PubMed] [Google Scholar]
- 3.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nature Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 4.Chan, A. O., S. G. Kim, A. Bedeir, J. P. Issa, S. R. Hamilton, and A. Rashid. 2003. CpG island methylation in carcinoid and pancreatic endocrine tumors. Oncogene 22:924-934. [DOI] [PubMed] [Google Scholar]
- 5.Chen, P. C., C. C. Pan, A. H. Yang, L. S. Wang, and H. Chiang. 2002. Detection of Epstein-Barr virus genome within thymic epithelial tumours in Taiwanese patients by nested PCR, PCR in situ hybridization, and RNA in situ hybridization. J. Pathol. 197:684-688. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, R. H., and N. Raab-Traub. 1994. Alterations of the p53 gene in Epstein-Barr virus-associated immunodeficiency-related lymphomas. J. Virol. 68:1309-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos, A. G., S. M. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein, S. D., T. Hasegawa, T. Colby, and S. A. Yousem. 1999. 11q13 allelic imbalance discriminates pulmonary carcinoids from tumorlets. A microdissection-based genotyping approach useful in clinical practice. Am. J. Pathol. 155:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimori, M., S. Ikeda, Y. Shimizu, M. Okajima, and T. Asahara. 2001. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor. Cancer Res. 61:6656-6659. [PubMed] [Google Scholar]
- 11.Gaal, K., L. M. Weiss, W. G. Chen, Y. Y. Chen, and D. A. Arber. 2002. Epstein-Barr virus nuclear antigen (EBNA)-1 carboxy-terminal and EBNA-4 sequence polymorphisms in nasal natural killer/T-cell lymphoma in the United States. Lab. Investig. 82:957-962. [DOI] [PubMed] [Google Scholar]
- 12.Gilligan, K., P. Rajadurai, L. Resnick, and N. Raab-Traub. 1990. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc. Natl. Acad. Sci. USA 87:8790-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gugger, M., E. Burckhardt, A. Kappeler, H. Hirsiger, J. A. Laissue, and L. Mazzucchelli. 2002. Quantitative expansion of structural genomic alterations in the spectrum of neuroendocrine lung carcinomas. J. Pathol. 196:408-415. [DOI] [PubMed] [Google Scholar]
- 15.Henderson, S., D. Huen, M. Rowe, C. Dawson, G. Johnson, and A. Rickinson. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA 90:8479-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inghirami, G., M. Chilosi, and D. M. Knowles. 1990. Western thymomas lack Epstein-Barr virus by Southern blotting analysis and by polymerase chain reaction. Am. J. Pathol. 136:1429-1436. [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, R., H. Takeuchi, M. Sasaki, M. Hasegawa, and K. Hirai. 1998. Detection of Epstein-Barr virus infection in the epithelial cells and lymphocytes of non-neoplastic tonsils by in situ hybridization and in situ PCR. Arch. Virol. 143:803-813. [DOI] [PubMed] [Google Scholar]
- 18.Launay, J. M., G. Birraux, D. Bondoux, J. Callebert, D. S. Choi, S. Loric, and L. Maroteaux. 1996. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J. Biol. Chem. 271:3141-3147. [DOI] [PubMed] [Google Scholar]
- 19.Leyvraz, S., W. Henle, A. P. Chahinian, C. Perlmann, G. Klein, R. E. Gordon, M. Rosenblum, and J. F. Holland. 1985. Association of Epstein-Barr virus with thymic carcinoma. N. Engl. J. Med. 312:1296-1299. [DOI] [PubMed] [Google Scholar]
- 20.Mann, R. B., T. C. Wu, E. M. MacMahon, Y. Ling, P. Charache, and R. F. Ambinder. 1992. In situ localization of Epstein-Barr virus in thymic carcinoma. Mod. Pathol. 5:363-366. [PubMed] [Google Scholar]
- 21.McGuire, L. J., D. P. Huang, R. Teoh, M. Arnold, K. Wong, and J. C. Lee. 1988. Epstein-Barr virus genome in thymoma and thymic lymphoid hyperplasia. Am. J. Pathol. 131:385-390. [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell, T., and B. Sugden. 1995. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima, K., Q. Liu, T. Koga, J. Suzumiya, and M. Kikuchi. 2002. Classification of cell lineage and anatomical site, and prognosis of extranodal T-cell lymphoma-natural killer cell, cytotoxic T lymphocyte, and non-NK/CTL types. Virchows Arch. 440:425-435. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, G. R., J. Luka, L. Petti, J. Sample, M. Birkenbach, D. Braun, and E. Kieff. 1987. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology 160:151-161. [DOI] [PubMed] [Google Scholar]
- 25.Sagara, M., F. Sugiyama, H. Horiguchi, H. Kamma, T. Ogata, K. Yagami, K. Murakami, and A. Fukamizu. 1995. Activation of the nuclear oncogenes N-myc and c-jun in carcinoid tumors of transgenic mice carrying the human adenovirus type 12 E1 region gene. DNA Cell Biol. 14:95-101. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi, H., R. Kobayashi, M. Hasegawa, and K. Hirai. 1996. Detection of latent infection by Epstein-Barr virus in peripheral blood cells of healthy individuals and in non-neoplastic tonsillar tissue from patients by reverse transcription-polymerase chain reaction. J. Virol. Methods 58:81-89. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, H., R. Kobayashi, M. Hasegawa, and K. Hirai. 1997. Detection of latent Epstein-Barr virus (EBV) DNA in paraffin sections of nasopharyngeal carcinomas expressing no EBV-encoded small RNAs using in situ PCR. Arch. Virol. 142:1743-1756. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto, T., Y. Nakamura, K. Kishimoto, H. Takeuchi, M. Shirakata, T. Mitsuya, and K. Hirai. 1999. Epstein-Barr virus (EBV)-infected cells were frequently but dispersely detected in T-cell lymphomas of various types by in situ hybridization with an RNA probe specific to EBV-specific nuclear antigen 1. Virus Res. 65:43-55. [DOI] [PubMed] [Google Scholar]