Abstract
Objective. An underuse of oral anticoagulants (OAC) in patients with atrial fibrillation (AF) has been suggested, as only 50% of all patients with AF receive OAC treatment. Whether this is due to contraindications, lack of an indication to treat, or an expression of underuse is sparsely investigated. This study therefore aimed to characterize individuals without OAC treatment in a real-life population of patients with AF. Design. Retrospective cross-sectional study. The medical records were scrutinized in order to identify the type of AF, risk factors for embolism and bleeding, and other factors of importance for OAC treatment. Setting. The municipalities of Skellefteå and Norsjö, northern Sweden. Subjects. A total of 2274 living residents with at least one verified episode of AF on or before December 31, 2010. Main outcome measures. Prevalence of treatment with OAC and documented reasons to withhold OAC treatment. Results. Among all 2274 patients with AF, 1187 (52%) were not treated with OAC. Of the untreated patients, 19% had no indication or had declined or had experienced adverse effects other than bleeding on warfarin treatment. The most common reason to withhold OAC was presence of risk factors for bleeding, found in 38% of all untreated patients. Furthermore, a documented reason could be identified to withhold OAC in 75%. Conclusions. Among patients with AF without OAC treatment a reason could be identified to withhold OAC in 75%. The underuse of OAC is estimated to be 25%.
Key Words: Atrial fibrillation, anticoagulation, epidemiology, general practice, risk factors, thromboembolic risk, thromboembolism, Sweden
Patients with atrial fibrillation may benefit from treatment with oral anticoagulants; however, many patients do not receive such treatment.
52% of patients with atrial fibrillation were not treated with oral anticoagulants.
Most patients with atrial fibrillation not treated with oral anticoagulants had a documented reason as to why treatment was being withheld.
The most common reason to withhold treatment with oral anticoagulants was presence of risk factors for bleeding.
Introduction
Patients with atrial fibrillation or atrial flutter (AF) at high risk of stroke, transient ischaemic attack (TIA), or arterial embolism should be recommended treatment with oral anticoagulants (OAC) [1]. Current guidelines advocate the use of the CHA2DS2-VASc risk score. Clinical factors considered in this risk score are: congestive heart failure, hypertension, age ≥ 75 years (scores 2 points), diabetes mellitus, previous stroke/TIA/arterial embolism (scores 2 points), vascular disease, age 65–74 years, and female sex [2–5]. According to ESC guidelines patients with CHA2DS2-VASc ≥ 2 points should be recommended OAC treatment if no contraindications are present. For men with CHA2DS2-VASc = 1, OAC treatment can also be considered. Similarly, bleeding risk depends on many different factors and should be assessed concomitantly when OAC treatment is considered [2,6]. Several studies have shown that adherence to OAC treatment guidelines in patients with AF is suboptimal. These studies have been based predominantly on large registries or selected populations [7–11]. These studies suggest that only 41–54% of the patients at high risk for stroke were treated with OAC [7,12]. Known patient characteristics associated with withholding OAC in patients with AF are advanced age and previous major bleeding [12–15].
To our knowledge, a detailed characterization of patients with AF not treated with OAC has not been performed in a real-life population. This information would be valuable in identifying situations where treatment guidelines are not followed despite clear indications and a lack of contraindications. The aim of this study is to characterize the individuals without OAC treatment in a real-life population of patients with AF.
Material and methods
Setting and case finding
The study was performed in two municipalities (Skellefteå and Norsjö), in northern Sweden with a total population of 75 945. Details on the study population have previously been published [16]. In short, all living residents in Skellefteå and Norsjö with at least one verified episode of AF on or before December 31, 2010 were included in the study. All patients with at least one ECG in the regional ECG database with computer-interpreted AF or a corresponding diagnosis of AF (ICD-10 code I48) in the National Patient Register between January 1, 2004 and December 31, 2010 were considered as potential cases. All cases were then manually verified. AF was confirmed in 2274 patients giving an overall prevalence of 3.0% (3.4% in men and 2.6% in women). The prevalence rose steadily with age, and was 16.8% in patients aged 75 years and older.
Type of AF and risk factors for embolism and bleeding
We scrutinized all individual medical records for data on the type of AF and documented risk factors for embolism and bleeding from all 11 primary health care centres in the region and from hospital records from the Department of Internal Medicine, General Surgery, and Orthopaedics. All available data up to study inclusion were reviewed retrospectively. Information on OAC treatment (only warfarin and coumarin were available in 2010), risk factors for embolism, risk factors for bleeding, and whether the patient had had an adverse effect or declined OAC treatment were registered. All ECGs were manually verified and all individuals with AF were classified as having either an isolated episode of AF, paroxysmal AF, persistent AF, or permanent AF. For detailed definitions, please see online Supplementary Appendix 1 and 2 available online at http://informahealthcare.com/doi/abs/10.3109/02813432.2014.984952.
We considered an absolute reason to withhold OAC to be present if the patient had declined warfarin treatment, had had previous adverse effects of warfarin other than bleeding or had a CHA2DS2-VASc score of 0 (males) or 1 (females). We considered relative reasons to withhold OAC to be terminal illness, predisposition for bleeding, inability to comply with INR monitoring, liver or renal failure, alcohol abuse, dementia, other type of cognitive dysfunction, or an isolated episode of AF.
Statistical analysis
Mean, numbers, and proportions were calculated for baseline data.
Ethics
The study was approved by the Regional Ethics Review Board of Umeå University.
Results
Characteristics of the individuals treated and not treated with OAC
Characteristics of the 2274 individuals with verified AF are given in Table I. Of these, 1087 patients were treated and 1187 (52%) were not treated with OAC. The mean age was 74.9 in the group treated with OAC and 74.4 in the group not treated with OAC. The proportion of women and men were similar in the two groups. An isolated episode of AF and paroxysmal AF were more common in the group of patients not treated with OAC. Risk factors for embolism were more common in the group of patients treated with OAC, but 86% of the group not treated with OAC had one or more risk factor for embolism. A previous event of stroke, TIA, or arterial embolism was more common in the patients treated with OAC. Predisposition for bleeding was less common (20%) in the patients treated with OAC compared with patients not treated with OAC (33%).
Table I.
Characteristics of all 2274 patients with atrial fibrillation, n (%).
| OAC treatment (n = 1087) | No OAC treatment (n = 1187) | |
| Age, years: | ||
| Age 0–64 years | 140 (12.9) | 260 (21.9) |
| Age 65–74 years | 320 (29.4) | 251 (21.1) |
| Age 75–89 years | 607 (55.8) | 547 (46.1) |
| Age 90 years and more | 20 (1.8) | 129 (10.9) |
| Female sex | 473 (43.5) | 496 (41.8) |
| Type of AF: | ||
| Isolated episode of AF | 89 (8.2) | 310 (26.1) |
| Paroxysmal AF | 122 (11.2) | 202 (17.0) |
| Persistent AF | 191 (17.6) | 184 (15.5) |
| Permanent AF | 673 (61.9) | 466 (39.3) |
| Unclassified type of AF | 12 (1.1) | 25 (2.1) |
| Risk factors for embolism: | ||
| Congestive heart failure | 305 (28.1) | 230 (19.4) |
| Hypertension | 812 (74.7) | 720 (60.7) |
| Diabetes mellitus | 200 (18.4) | 189 (15.9) |
| Previous stroke or TIA or arterial embolism | 301 (27.7) | 204 (17.2) |
| Vascular disease | 238 (21.9) | 240 (20.2) |
| One or more risk factors for embolism | 1063 (97.8) | 1025 (86.4) |
| Risk factors for bleeding: | ||
| Renal failure or liver failure | 14 (1.3) | 19 (1.6) |
| Predisposition for bleedinga | 216 (19.9) | 389 (32.8) |
| Inability to comply with INR monitoring | 4 (0.4) | 96 (8.1) |
| Persistent uncontrolled hypertension | 1 (0.1) | 2 (0.2) |
| Alcohol abuse | 3 (0.3) | 29 (2.4) |
| No risk factors for bleeding | 858 (78.9) | 733 (61.8) |
| Other indications for OAC treatmentb | 126 (11.6) | 25 (2.1) |
Notes: OAC = oral anticoagulant. AF = atrial fibrillation. a = Previous intracranial haemorrhage, previous gastrointestinal bleeding, anaemia, thrombocytopenia, bleeding disorder, or other reasons for predisposition for bleeding (see Appendix). b = Chronic venous thromboembolism, mechanical or biological heart valve or planned cardioversion without other indications for OAC.
Documented reasons to withhold OAC treatment
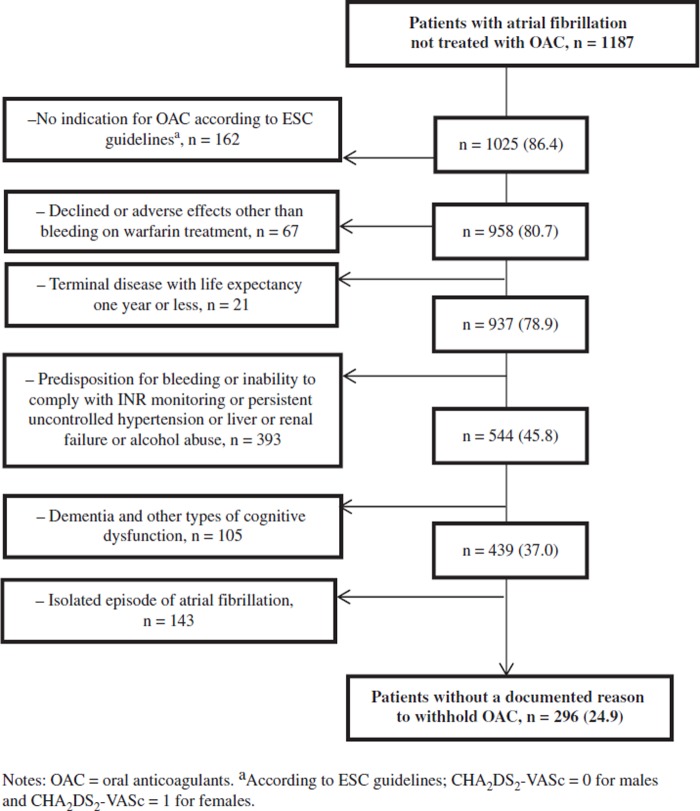
Among all 1187 patients not treated with OAC absolute reasons to withhold OAC were present in 229 (19%) and relative reasons in 662 (56%) as shown in Figure 1. An isolated episode of AF was documented in 143 patients and among these 73 had a reversible cause associated with the commencement of AF (i.e. coronary artery bypass grafting, myocardial infarction, or pulmonary embolism). We could not identify any reason to withhold OAC in 296 (25%) of the patients not treated with OAC. The mean age was 75.2 years and 65% were men. Permanent AF was found in 41% and previous stroke or TIA or arterial embolism was found in 10% (see Table II). Among patients with indication and no reason to withhold treatment 72% were treated with OAC. Treatment with OAC was not given to 93% of the patients with indication and absolute reasons to withhold OAC.
Figure 1.
Documented reasons to withhold oral anticoagulants in individuals with atrial fibrillation in Skellefteå and Norsjö not treated with oral anticoagulants, n (%).
Table II.
Characteristics of 296 patients without a documented reason to withhold oral anticoagulants.
| n (%) | |
| Age, years: | |
| Age 0–64 years | 47 (15.9) |
| Age 65–74 years | 95 (32.0) |
| Age 75–89 years | 128 (43.2) |
| Age 90 years and more | 26 (8.9) |
| Female sex | 103 (34.8) |
| Type of AF: | |
| Paroxysmal AF | 94 (31.8) |
| Persistent AF | 73 (24.7) |
| Permanent AF | 120 (40.5) |
| Unclassified type of AF | 9 (3.0) |
| Risk factors for embolism: | |
| Congestive heart failure | 63 (21.3) |
| Hypertension | 199 (67.2) |
| Diabetes mellitus | 44 (14.9) |
| Previous stroke or TIA or arterial embolism | 31 (10.5) |
| Vascular disease | 74 (25.0) |
Notes: OAC = oral anticoagulant. AF = atrial fibrillation
Discussion
An underuse of OAC in almost 50% of all patients with AF has been suggested [7,11,12]. These studies have predominantly considered the presence of an indication for OAC as a discriminating factor in determining suitability for treatment. However, OAC is not appropriate for all patients with AF. One must also consider the clinical features constituting contraindications for OAC treatment (e.g. elevated bleeding risk). However, patients with a high risk of bleeding may also have a high risk of stroke [17,18]. This complicates the assessment of the risk–benefit ratio in patients with AF.
An inpatient study performed in the United States found that factors associated with a lower probability of being treated with OAC were age ≥ 75 years, female sex, hepatic or renal disease, aspirin use, and previous fractures [14]. A Swedish study showed that age was associated with increased bleeding risk [19]. A registry-based study of newly diagnosed inpatients and outpatients with AF aged 65 years or more showed that the probability of being treated with OAC did not differ among the various strata of embolic risk according to the CHADS2 risk score [15]. This study also showed that dementia, previous gastrointestinal bleeding, and anaemia were associated with a reduced probability of being treated with OAC. A meta-analysis found that factors correlated with increased OAC use included a history of a stroke, heart failure, and male gender [13]. As was the case in our study, factors shown by other studies to be associated with decreased OAC use were alcohol or drug abuse and previous gastrointestinal or intracranial haemorrhage [13,20].
There are two specific issues concerning the characterization of patients with AF not treated with OAC that should be addressed. First, to what extent the patients can be considered to be undertreated, and second, the fact that a patient can still benefit from OAC treatment despite a presumed risk for bleeding complications.
Regarding the first point, patients with AF not treated with OAC can be divided into three sub-categories: those who would benefit from treatment, those who have no indication for treatment or an unacceptable bleeding risk, and an intermediate category. In our study, 25% of the patients not treated with OAC would benefit from treatment according to guidelines. These patients suffer more often from paroxysmal AF and are less likely to have had a previous embolic event, such as stroke, TIA or arterial embolism.
In our study, one fifth of the individuals not treated with OAC had an absolute reason to withhold treatment, usually the lack of a treatment indication according to current guidelines. In a nationwide Swedish study, 17% of all patients with AF had no indication for OAC defined as CHA2DS2-VASc < 2 [10].
About three fifths of the patients not treated with OAC had a relative reason to withhold treatment. Increased risk of bleeding and cognitive dysfunction were considered the most common reasons to withhold OAC. Appropriate assistance and oversight can make OAC treatment both possible and beneficial in patients with cognitive dysfunction. Novel OACs with fixed dosage can also make OAC treatment possible in patients with cognitive dysfunction, as the variable dose of warfarin often presents a challenge for these patients. To reduce bleeding risk, it is important to consider drug–drug interactions. A Norwegian study showed that warfarin was sometimes prescribed in combination with other drugs such as antidepressants, which can increase the bleeding risk [21,22].
Current guidelines recommend OAC treatment for all patients with paroxysmal, persistent or permanent AF. One isolated episode of AF was found in 143 patients without any other reason to withhold OAC (see Figure 1). Of these, 51% had an identifiable reversible cause such as coronary artery bypass grafting or pulmonary embolism associated with the commencement of AF and more than 80% had experienced the isolated episode of AF more than one year prior to study conclusion. The benefit of treatment in this patient group is less well studied when compared to other types of AF and is not discussed adequately in guidelines. Defining all patients with an isolated episode of AF as eligible for OAC would have increased the proportion of undertreated patients from 25% to 37%.
There are several strengths of this study. The population-based study design, which spanned all age groups and types of AF, was thorough and inclusive. All cases of AF were manually verified and the medical records of included cases were reviewed. The detailed information on risk factors for bleeding and embolism made it possible for us to identify reasons to withhold OAC and thus estimate the extent of underuse. The main limitation of the retrospective design used in this study is that it is dependent on the completeness and accuracy of what is documented in the medical records. For example, it is difficult to quantify the severity and the extent of cognitive dysfunction and the extent of alcohol abuse as detailed documentation may be lacking. On the other hand, data on previous bleeding, terminal disease, a patient's decision to decline OAC, and the possibility to classify type of AF are usually available in medical records. The proportion of undiagnosed AF, predominantly asymptomatic AF, is unknown in our study. A recent systematic review reported that screening can identify unknown AF in 1.4% of the population over 65 years [23]. A Swedish study showed that among persons aged 75 years with two or more risk factors for stroke 7.4% were diagnosed with paroxysmal AF [24]. The prevalence of AF is similar in our study to that in other recent Swedish studies [8–10] but higher than in international studies especially in persons 80 years and older. The proportion of patients with AF and OAC treatment is within the range of that in other studies [7,12].We believe that our population and results are representative of Swedish conditions. However, the generalizability to other populations may be limited.
Conclusions
Our study shows that half of the patients with AF are not treated with OAC and the majority of these have a documented reason to withhold OAC, predominantly predisposition for bleeding. The underuse of OAC in our study was at least 25%, but many patients with reasons to withhold OAC could benefit from OAC treatment. An individual assessment must always be performed and patient preferences onsidered.
Acknowledgments
This study was supported by generous grants from the County Council of Västerbotten, the Joint Committee of Northern Sweden Health Care Region and the Foundation for Medical Research in Skellefteå. The authors would like to thank Thomas Suh, Skellefteå, for invaluable English language assistance.
Ethics, funding, and declaration of conflicting interests
The study was approved by the Regional Ethics Review Board of Umeå University. This study was supported by generous grants from the County Council of Västerbotten, the Joint Committee of Northern Sweden Health Care Region and the Foundation for Medical Research in Skellefteå. The authors declare that there is no conflict of interest.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the contents and writing of the paper.
Supplementary material available online
Supplementary Appendices 1 and 2.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 3.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 6.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 7.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: Who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study). Eur Heart J. 2006;27:1954–64. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 8.Andersson P, Londahl M, Abdon NJ, Terent A. The prevalence of atrial fibrillation in a geographically well-defined population in northern Sweden: Implications for anticoagulation prophylaxis. J Intern Med. 2012;272:170–6. doi: 10.1111/j.1365-2796.2012.02519.x. [DOI] [PubMed] [Google Scholar]
- 9.Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: A population-based study. Stroke. 2013;44:3103–8. doi: 10.1161/STROKEAHA.113.002329. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461–8. doi: 10.1111/joim.12114. [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am J Med. 2010;123:638–45. e634. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165:93–101. e101. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: A systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal S, Bennett D, Smith DJ. Predictors of warfarin use in atrial fibrillation patients in the inpatient setting. Am J Cardiovasc Drugs. 2010;10:37–48. doi: 10.2165/11318870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: The risk–treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97:2046–50. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 16.Norberg J, Bäckström S, Jansson J-H, Johansson L. Estimating the prevalence of atrial fibrillation in a general population using validated electronic health data. Clin Epidemiol. 2013;5:475–81. doi: 10.2147/CLEP.S53420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poli D, Testa S, Antonucci E, Grifoni E, Paoletti O, Lip GY. Bleeding and stroke risk in a real-world prospective primary prevention cohort of patients with atrial fibrillation. Chest. 2011;140:918–24. doi: 10.1378/chest.10-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcucci M, Nobili A, Tettamanti M, Iorio A, Pasina L, Djade CD, et al. Joint use of cardio-embolic and bleeding risk scores in elderly patients with atrial fibrillation. Eur J Intern Med. 2013;24:800–6. doi: 10.1016/j.ejim.2013.08.697. [DOI] [PubMed] [Google Scholar]
- 19.Wallvik J, Själander A, Johansson L, Bjuhr O, Jansson JH. Bleeding complications during warfarin treatment in primary healthcare centres compared with anticoagulation clinics. Scand J Prim Health Care. 2007;25:123–8. doi: 10.1080/02813430601183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Immonen S, Valvanne J, Pitkälä KH. The prevalence of potential alcohol–drug interactions in older adults. Scand J Prim Health Care. 2013;31:73–8. doi: 10.3109/02813432.2013.788272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakken MS, Ranhoff AH, Engeland A, Ruths S. Inappropriate prescribing for older people admitted to an intermediate-care nursing home unit and hospital wards. Scand J Prim Health Care. 2012;30:169–75. doi: 10.3109/02813432.2012.704813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran KA, Cavallari LH, Shapiro NL, Bishop JR. Bleeding incidence with concomitant use of antidepressants and warfarin. Ther Drug Monit. 2011;33:433–8. doi: 10.1097/FTD.0b013e318224996e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: A systematic review. Thromb Heamost. 2013;110:213–22. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 24.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: Implications for stroke prevention. Circulation. 2013;127:930–7. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendices 1 and 2.