Abstract
Purpose
To investigate the pharmacogenetic associations between the genetic risk variants of age-related macular degeneration (AMD) and long-term outcome after intravitreal anti-vascular endothelial growth factor (VEGF) treatment in Korean neovascular AMD patients.
Methods
This prospective study included 394 treatment-naïve patients (394 eyes) that underwent intravitreal anti-VEGF treatment for neovascular AMD for at least 12 months. Patients were genotyped for 17 single nucleotide polymorphisms within 13 AMD-relevant genes. Initially, patients underwent three monthly injections of intravitreal ranibizumab and were retreated as needed with ranibizumab or bevacizumab. For each candidate polymorphism, genotypic associations with treatment outcome measures at months 12 and 24, including mean change in best-corrected visual acuity (BCVA) from baseline, visual gain of ≥15 letters, mean change in central subfield macular thickness (CSMT) from baseline on spectral domain optical coherence tomography (OCT), presence of fluid on OCT, and mean number of injections, were investigated using logistic or linear regression models with adjustment for non-genetic covariates.
Results
At month 24, BCVA improved by 4.5 ± 22.5 letters and CSMT decreased by 69.4 ± 112.6 µm from baseline. Regression analysis with Bonferroni correction showed that the TT genotype for VEGFA rs3025039 was associated with a significantly higher chance of a visual gain of ≥15 letters at month 24 than other genotypes (odds ratio, 4.57; 95% confidence interval, 1.89 - 11.1; corrected p = 0.0434). As for tomographic outcome, the minor allele homozygotes for ARMS2 rs10490924 and HTRA1 rs1100638 (GG genotype for both) were associated with a larger CSMT reduction at month 12 than other genotypes, with borderline significance after Bonferroni correction (118.6 ± 132.7 µm versus 62.7 ± 89.7 µm, corrected p = 0.0656 for rs10490924; 115.7 ± 131.7 µm versus 63.6 ± 89.8 µm, corrected p = 0.0528 for rs11200638). No polymorphism showed a significant association with the number of injections.
Conclusions
In this Korean neovascular AMD cohort, treatment outcome after anti-VEGF was found to differ by the genotypes of VEGFA rs3025039, ARMS2 rs10490924, and HTRA1 rs11200638. Given more evidence of pharmacogenetic associations with the anti-VEGF agent, individualized therapeutic approaches based on genetic background could lead to optimal treatment in neovascular AMD.
Introduction
Age-related macular degeneration (AMD) is the major cause of irreversible vision loss in elderly people in developed countries. The neovascular (or exudative) form of AMD, characterized by choroidal neovascularization (CNV) formation and proliferation of fibrous tissue, represents only 10–15% of all AMD cases but is responsible for more than 90% of severe visual loss caused by AMD [1].
Treatment for neovascular AMD has dramatically improved since the introduction of intravitreal therapy with anti-vascular endothelial growth factor (VEGF) monoclonal antibody. Two pivotal randomized clinical trials showed that monthly ranibizumab enabled a visual gain of ≥15 letters in 30% to 40% of neovascular AMD patients [2,3]. The most widely used agents are the on-label anti-VEGF agent ranibizumab (Lucentis; Novartis, Basel, Switzerland) and the off-label agent bevacizumab (Avastin; Roche, Basel, Switzerland). In addition, a novel VEGF inhibitor, aflibercept (Eylea, Bayer HealthCare, Berlin, Germany), which is a recombinant fusion protein composed of components of both VEGF receptor 1 and receptor 2, showed comparable efficacy in visual improvement in neovascular AMD patients [4]. Recently, the “Comparison of Age-Related Macular Degeneration Treatments Trials” (CATT) and the “Alternative Treatments to Inhibit VEGF in Patients with Age-Related Choroidal Neovascularization” (IVAN) study groups reported that the effects of ranibizumab and bevacizumab on visual acuity were equivalent [5,6].
Although anti-VEGF treatment is effective in most neovascular AMD patients, some patients do not benefit from treatment, and 5% to 10% of patients lose ≥15 letters despite treatment [2,3,5,6]. Genetic profile seems to contribute to this variability in therapeutic responsiveness. To date, some studies have suggested that CFH Y402H, ARMS2 rs10490924, HTRA1 rs11206038, APOE, and several VEGFA polymorphisms were associated with outcomes after ranibizumab or bevacizumab treatment [7-21]. However, recent pharmacogenetic studies from two multicenter randomized trials (the CATT and IVAN trials) reported that no statistically significant association was found between genetic variants and anti-VEGF responsiveness [22-24]. Therefore, the genetic association with outcome after anti-VEGF treatment in neovascular AMD is still controversial.
On the other hand, the ethnic diversity in AMD-associated polymorphisms may contribute to therapeutic responsiveness to anti-VEGF in East Asian AMD patients [25,26]. However, there has been no large-scale pharmacogenetic study of anti-VEGF treatment in East Asian neovascular AMD populations. This prospective cohort study aimed to investigate whether the genetic variants previously reported to be associated with AMD susceptibility contribute to the therapeutic outcomes after anti-VEGF treatment in Korean neovascular AMD patients.
Methods
Study design and patient eligibility
The treatment protocol and design of this study were approved by the Institutional Review Board of Seoul National University Hospital and were performed in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients before participation. Outpatients who started intravitreal anti-VEGF treatment for active CNV secondary to AMD at the retina center of the Seoul National University Hospital between January 2009 and July 2011 were prospectively recruited. CNV was considered active when both leakage on fluorescein angiography (FA) and fluid on optical coherence tomography (OCT) were observed either within or below the retina.
Inclusion criteria were age of ≥50 years, subfoveal CNV secondary to AMD with an initial best corrected visual acuity (BCVA) of ≥5 letters using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Only patients of Korean descent who agreed to blood sampling for genetic analysis were included, and all angiographic lesion types were included in the study. Exclusion criteria were a myopic refractive error of >6 diopters, CNV secondary to causes other than AMD, polypoidal choroidal vasculopathy (PCV), presence of a disciform macular scar or atrophy, any prior treatment for neovascular AMD, previous history of vitrectomy, and follow-up loss within 12 months after the first injection. When both eyes of a patient were eligible at presentation, only the eye with shorter symptom duration was included. If a fellow eye developed CNV during the course of study, it was treated at the discretion of treating physicians but was not included in the study.
Clinical examination and anti-VEGF treatment protocol
At initial presentation, patient demographics and a peripheral blood sample for DNA extraction were collected after obtaining consent. Patients underwent a full ophthalmic examination including BCVA measurement, slit-lamp examination, intraocular pressure measurement, fundus examination, spectral domain OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA), FA, and indocyanine green angiography (ICGA). Visual acuity was measured using ETDRS charts at a distance of 4 m by well-trained testers after standardized refraction. With OCT, the central 1-mm subfield was analyzed using automated software to obtain central subfield macular thickness (CSMT). If there was an error in the automated segmentation of the inner limiting membrane or retinal pigment epithelium (RPE) by OCT algorithm, CSMT was manually corrected using a caliper provided by the software. Angiographic subtypes were defined using baseline FA as predominantly classic (>50% classic, well demarcated areas of hyperfluorescence appearing early in the angiogram followed by late leakage), minimally classic (<50% classic), or occult (leakage appearing only late in the angiogram). PCV was diagnosed based on the presence of branching vascular networks and polypoidal choroidal vascular lesions on ICGA images, and patients with PCV were excluded from the study. Using digitalized FA images and fundus photographs, the total area of CNV lesion, which includes CNV, thick blood, blocked fluorescence, and serous detachment of the RPE, was measured in disc areas (DA).
All patients underwent three monthly injections of ranibizumab (month 0, 1, 2), followed by treatment on an as-needed basis until month 24. During the as-needed phase, anti-VEGF re-treatments were performed at monthly visits when any of the following criteria were met: evidence of persistent fluid by OCT, BCVA loss of >5 letters or CSMT increase of >100 µm from the previous visit, or new macular hemorrhage. Bevacizumab was used for re-treatment in some patients whose medical insurance coverage for ranibizumab had been terminated. A dose of 0.5 mg ranibizumab or 1.25 mg bevacizumab, both in 0.05 ml solution, was injected intravitreally under standard sterile conditions.
DNA preparation and genetic analysis
Approximately 10 ml of peripheral blood was collected from each patient at initial presentation. Genomic DNA was prepared using a nucleic acid isolation device, QuickGene-mini80 (FUJIFILM, Tokyo, Japan). In this study, candidate genes for analysis were selected from previous reports of AMD-associated genes, including CFH, ARMS2/HTRA1, C2/CFB/ SKIV2L, C3, CFI, VEGFA, APOE, PEDF, SCARB1, and SYN3/TIMP3 [27-37]. Commonly evaluated polymorphisms of these genes were chosen based on a review of previous pharmacogenetic studies of anti-VEGF agents for neovascular AMD treatment [7-18,38]. In total, 17 candidate polymorphisms in 13 genes were determined. All genetic variants were genotyped using TaqMan SNP genotyping assays (Applied Biosystems Inc. [ABI], Foster City, CA) or SNaPshot Multiplex kit (ABI) according to the manufacturer’s recommendations. The characteristics, genotyping method, and overall genotyping results for candidate polymorphisms are listed in Table 1, and primer sequences for polymerase chain reaction are listed in Appendix 1. Hardy–Weinberg equilibrium (HWE) for genotypic distributions was evaluated using the HWE exact test.
Treatment outcome measures
Evaluation of treatment response was based on visual acuity, tomographic features assessed by OCT, and number of injections. Visual outcome variables were mean BCVA change from baseline and the proportion of patients with a gain of ≥15 letters from baseline. Tomographic outcome variables were mean CSMT change from baseline and the proportion of patients who showed dry status without fluid on OCT. The checking and correction of CSMT measurements by OCT and a qualitative assessment of dry status on OCT were performed independently by two retinal specialists (UCP and JHP) unaware of the patients’ personal information, genotypes, and visual outcome variables. Discrepancies were resolved by consensus.
Statistical analysis
Non-genetic covariates included in the analysis were age, sex, smoking status (ever versus never), CNV lesion type (predominantly classic versus others), AMD status of fellow eye (presence of CNV at baseline or development during follow-up versus absence), and baseline BCVA, CSMT, and CNV lesion size. A linear regression model was used to determine the influence of non-genetic covariates on continuous outcome variables (i.e., mean changes in BCVA and CSMT from baseline). When investigating the genetic influence of each polymorphism, results were adjusted for all non-genetic covariates.
The associations between the genotypes of candidate polymorphisms and treatment outcome variables at months 12 and 24 were evaluated using regression models. Analyses were performed for each genetic variant independent of other variants using allelic, dominant, recessive, and additive genetic models. A linear regression model was used to analyze continuous outcome variables (i.e., mean changes in BCVA and CSMT from baseline). In the analysis of categorical outcome variables (i.e., visual improvement of ≥15 letters or dry status on OCT), an ordinary logistic regression model was used to calculate odds ratio (OR) and 95% confidence interval (CI). Correction for multiple testing was performed using the Bonferroni method. For variants showing a significant association, any change of the associated treatment outcome variable throughout the entire 24-month follow-up was investigated with independent regression analyses at each time point to verify the influences of the genotype.
Statistical analyses were performed using PLINK software version 1.07 and SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL). For the APOE gene, allelic variants of ε2, ε3, or ε4 resulting from the combination of two polymorphisms (rs429358, rs7412) were used in the analysis. The effect of the presence of the ε2 (1 or 2 versus no ε2 allele) or the ε4 allele on treatment response was investigated respectively. For all statistical tests, corrected p values of <0.05 were considered statistically significant.
Results
Cohort characteristics and anti-VEGF treatment outcome
Of the 443 patients who met the inclusion/exclusion criteria, the genetic samples of six patients did not yield sufficiently high-quality DNA, and 43 patients failed to complete 12 months of follow-up. Accordingly, 394 patients (394 eyes) were available for analysis after 12 months of follow-up and 366 patients were available after 24 months.
Patient demographics and baseline characterizations of AMD phenotypes are presented in Table 2. Mean age at baseline was 69.4 ± 7.9 years, and mean baseline BCVA was 46.4 ± 21.1 ETDRS letters (approximate Snellen equivalent 20/118). Mean BCVA improvements from baseline were 7.8 ± 18.8 and 4.5 ± 22.5 letters at month 12 and 24, respectively. The proportions of patients who showed visual improvement of ≥15 letters from baseline were 29.2% at month 12 and 30.9% at month 24. Patients received on average 6.7 ± 1.9 injections in the first 12 months and 3.8 ± 2.3 injections in the second 12 months. Sixty-five patients (16.5%) were treated with only ranibizumab, and the remaining 329 (83.5%) received one or more injections of bevacizumab during follow-up.
Table 2. Demographic and baseline clinical features of the patients.
| Variables | Total |
|---|---|
| Number of patients |
394 |
| Mean Age (years) |
69.4 ± 7.9 (51-91) |
| Male / Female |
219 (55.6%) / 175 (44.4%) |
| Smoking status | |
| Never |
235 (59.6%) |
| Ever (current / ex-smoker) |
159 (40.4%) |
| Angiographic lesion type | |
| Predominantly classic lesion |
81 (20.6%) |
| Minimally classic lesion |
112 (28.4%) |
| Occult lesion |
201 (51.0%) |
| Fellow eye neovascular AMD status | |
| Presence at baseline |
49 (12.4%) |
| Development during follow-up |
52 (13.2%) |
| Baseline Visual Acuity, ETDRS letter |
46.4 ± 21.1 letters |
| Baseline Central Subfield Macular Thickness, µm |
322.0 ± 95.3 µm |
| Baseline CNV lesion area, DA | 3.6 ± 4.1 |
ETDRS = Early Treatment Diabetic Retinopathy Study; CNV = Choroidal neovascularization; DA = Disc area
Regression coefficients (β) and corresponding 95% CI and p values for non-genetic covariates are presented in Table 3. The regression model revealed that younger patients were more likely to achieve greater improvements both in BCVA and CSMT. Lower baseline BCVA and higher baseline CSMT were associated with a greater increase in BCVA and a greater decrease in CSMT, respectively. Baseline CNV lesion size was found to be significantly inversely associated with BCVA change from baseline, but gender, smoking, lesion type, and AMD status of fellow eye showed no significant association with continuous outcome variables. The results of the pharmacogenetic analyses were adjusted for all non-genetic covariates.
Table 3. Regression coefficients (β) and P values for association of non-genetic covariates with continuous outcome variables at month 12 and 24.
| Age | Sex | Smoking | Baseline BCVA | Baseline CSMT | Baseline CNV lesion size | Lesion type | AMD status of fellow eye | ||
|---|---|---|---|---|---|---|---|---|---|
| BCVA change from baseline |
at month 12 |
-0.244 ± 0.124 |
0.165 ± 2.224 |
3.846 ± 2.342 |
-0.395 ± 0.065 |
-0.004 ±0.01 |
-0.433 ± 0.210 |
0.477 ±2.313 |
2.056 ±1.721 |
| (P = 0.035) |
(P = 0.931) |
(P = 0.089) |
(P < 0.001) |
(P = 0.709) |
(P = 0.046) |
(P = 0.830) |
(P = 0.452) |
||
| at month 24 |
-0.461 ± 0.138 |
0.661 ± 2.683 |
2.796 ± 2.717 |
-0.434 ± 0.054 |
0.002 ± 0.011 |
-0.726 ± 0.267 |
-0.561± 2.791 |
2.627 ±1.071 |
|
| (P = 0.001) |
(P = 0.805) |
(P = 0.304) |
(P < 0.001) |
(P = 0.836) |
(P = 0.007) |
(P = 0.841) |
(P = 0.605) |
||
| CSMT change from baseline |
at month 12 |
1.341 ± 0.452 |
-3.947 ± 9.116 |
-4.692 ± 9.230 |
0.163 ± 0.192 |
-0.728 ± 0.139 |
1.324 ± 0.895 |
16.833± 9.367 |
-3.112 ±5.212 |
| (P = 0.004) |
(P = 0.653) |
(P = 0.618) |
(P = 0.376) |
(P < 0.001) |
(P = 0.134) |
(P = 0.075) |
(P = 0.385) |
||
| at month 24 | 1.452 ± 0.545 |
-34.408 ± 10.623 |
-30.274 ± 10.758 |
-0.312 ± 0.214 |
-0.796 ± 0.045 |
2.176 ± 1.057 |
-7.058± 11.049 |
-4.772 ±3.832 |
|
| (P = 0.008) | (P = 0.101) | (P = 0.105) | (P = 0.146) | (P < 0.001) | (P = 0.140) | (P = 0.523) | (P = 0.152) |
BCVA = Best-corrected visual Acuity; CSMT = Central subfield macular thickness; CNV = Choroidal neovascularization; AMD = Age-related macular degeneration
Pharmacogenetic analysis
The overall genotyping rate was 99.6% and missing data was less than 2% for each candidate variant. The distribution of genotypes for each genetic variant was consistent with the HWE (p>0.05), with the exception of the ARMS2 and HTRA1 variants. The ARMS2 (A69S, rs10490924) and HTRA1 (−625A/G, rs11200638) variants are highly associated with AMD risk in East Asian populations, so it is not surprising that these variants deviated from HWE in a population completely comprising Korean neovascular AMD subjects. In this cohort, no subject carried the rare allele for the C3 rs2230199 variant, so pharmacogenetic analysis was performed for 16 SNPs in the 12 genes (C3 rs2230199 was excluded). The nominal p value for statistical significance should be less than 0.0031 (= 0.05 / 16) when applying the Bonferroni method to correct for multiple testing.
Regarding regression analyses for pharmacogenetic associations with treatment outcome variables, the genetic variants that reached at least nominal significance (uncorrected p<0.05) are summarized in Table 4. Nominally significant associations were found usually under the recessive model, and the associations between all candidate polymorphisms and visual and tomographic outcome variables and the number of injections as determined by the recessive and additive models are listed in Appendix 2, Appendix 3, and Appendix 4, respectively. For polymorphisms with an extremely low minor allele homozygote frequency of <3%, that is, CFH rs1061170, C2 rs9332739, CFB rs641153, SKIV2L rs429608, and SYN3/TIMP3 rs9621532, results of the dominant model are shown.
Table 4. Pharmacogenetic association with anti-vascular endothelial growth factor treatment outcome in neovascular age-related macular degeneration patients.
| Outcome parameters | Gene | Variant | Genotype (N)* | N (%) or Mean* | Genetic model | OR (95% CI)** | Uncorrected P - value† | Corrected P - value†† | |
|---|---|---|---|---|---|---|---|---|---|
| ≥ 15-letter gain |
12mo |
- |
- |
- |
|
- |
- |
- |
- |
| |
24mo |
VEGFA |
rs3025039 |
CC(219) / CT(124) / TT(23) |
64(29.2) / 39(29.0) / 13(56.5) |
Recessive |
4.57 (1.89-11.1) |
0.0027 |
0.0434 |
| BCVA change from baseline |
12mo |
- |
- |
- |
|
- |
- |
- |
- |
| |
24mo |
- |
- |
- |
|
- |
- |
- |
- |
| Dry status on OCT |
12mo |
- |
- |
- |
|
- |
- |
- |
- |
| |
24mo |
- |
- |
- |
|
- |
- |
- |
- |
| CSMT change from baseline |
12mo |
ARMS2 |
rs10490924 |
TT(196) / TG(150) / GG(47) |
-62.9 / -62.4 / -118.6 (µm) |
Additive |
- |
0.0141 |
0.2256 |
| |
|
|
|
|
Recessive |
- |
0.004 |
0.0656 |
|
| |
HTRA1 |
rs11200638 |
AA(202) / AG(144) / GG(48) |
-63.0 / -62.4 / -115.7 (µm) |
Additive |
- |
0.0141 |
0.2256 |
|
| |
|
|
|
|
Recessive |
- |
0.0033 |
0.0528 |
|
| 24mo |
ARMS2 |
rs10490924 |
TT(183) / TG(139) / GG(44) |
-63.2 / -60.9 / -126.2 (µm) |
Additive |
|
0.0384 |
0.6144 |
|
| |
|
|
|
|
|
Recessive |
- |
0.0258 |
0.4128 |
| |
|
HTRA1 |
rs11200638 |
AA(188) / AG(133) / GG(45) |
-62.3 / -62.4 / -123.7 (µm) |
Additive |
- |
0.0323 |
0.5168 |
| |
|
|
|
|
|
Recessive |
- |
0.0293 |
0.4688 |
| Number of injections |
12mo |
ARMS2 |
rs10490924 |
TT(196) / TG(150) / GG(47) |
6.8 / 6.8 / 6.1 |
Recessive |
- |
0.0344 |
0.5504 |
| |
|
HTRA1 |
rs11200638 |
AA(202) / AG(144) / GG(48) |
6.8 / 6.7 / 6.1 |
Recessive |
- |
0.0304 |
0.4864 |
| |
24mo |
VEGFA |
rs3025039 |
CC(219) / CT(124) / TT(23) |
10.7 / 10.4 / 9.0 |
Additive |
- |
0.0286 |
0.4576 |
| |
|
|
|
|
|
Recessive |
- |
0.015 |
0.2352 |
| |
|
ARMS2 |
rs10490924 |
TT(183) / TG(139) / GG(44) |
10.5 / 10.9 / 9.4 |
Recessive |
- |
0.0338 |
0.5408 |
| HTRA1 | rs11200638 | AA(188) / AG(133) / GG(45) | 10.5 / 10.9 / 9.4 | Recessive | - | 0.0275 | 0.4403 | ||
OR = Odds ratio; CI = Confidence interval; BCVA = Best-corrected visual acuity; OCT = Optical Coherence Tomography; CSMT = Central subfield macular thickness *: Major allele homozygote / Heterozygote / Minor allele homozygote **: Odds ratio calculated for minor allele homozygote †: Uncorrected P - value from logistic regression model (categorical outcomes) or linear regression model (continuous outcomes); Polymorphisms with uncorrected P value < 0.05 are listed ††: P - value corrected for multiple testing with Bonferroni method.
For visual outcome measures, VEGFA rs3025039 was the only polymorphism significantly associated with visual improvement of ≥15 letters. According to the recessive model, patients with the TT genotype at VEGFA rs3025039 were 4.6 times (OR, 4.57; 95% CI, 1.89 – 11.1; uncorrected p = 0.0027; corrected p = 0.0434) more likely to achieve visual improvement of ≥15 letters at month 24 compared to patients carrying other genotypes (Figure 1). Additional evaluation for other time points (every three months till month 24) showed nominal significance (uncorrected p<0.05) at all time points, except months 12 and 18.
Figure 1.
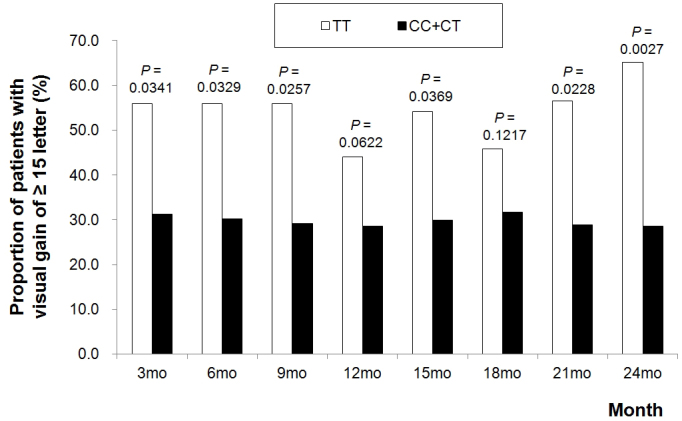
The proportion of patients who showed a visual improvement of 15 or more letters from baseline according to the VEGFA gene rs3025039 genotype under the recessive genetic model. Uncorrected p values from ordinary logistic regression are <0.05 at all time points, except months 12 and 18.
For tomographic outcome measures, no candidate polymorphism showed statistically significant pharmacogenetic association, but genetic variants at the 10q26 locus (ARMS2 rs10490924, HTRA1 rs11200638) showed possible associations with mean CSMT change under the recessive model. At month 12, mean CSMT reduction from baseline in patients homozygous for the minor allele (GG genotype for both polymorphisms) was greater than in patients with other genotypes (118.6 ± 132.7 µm versus 62.7 ± 89.7 µm, uncorrected p = 0.0040, corrected p = 0.0656 for rs10490924; 115.7 ± 131.7 µm versus 63.6 ± 89.8 µm, uncorrected p = 0.0033, corrected p = 0.0528 for rs11200638). The variants were highly correlated (r2 coefficient of linkage disequilibrium = 0.949), and for both variants, the greater CSMT reduction observed in minor allele homozygotes persisted through to month 24 (uncorrected p<0.05 at all time points, except month 3; Figure 2).
Figure 2.
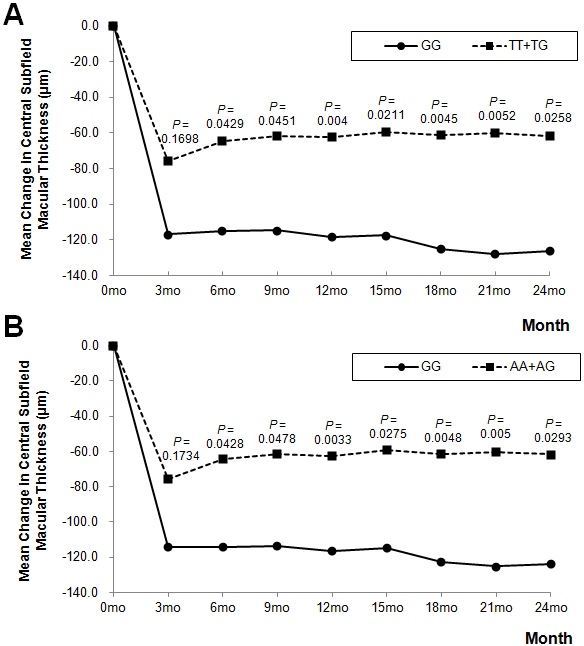
Mean change in central subfield macular thickness during 24-month follow-up according to the 10q26 locus polymorphisms (A: ARMS2 rs10490924, B: HTRA1 rs11206038) under the recessive model. Uncorrected p values from linear regression are <0.05 at all time points, except month 3.
There was no polymorphism with a statistically significant association with the number of injections at month 12 or at month 24. Minor allele homozygotes of 10q26 locus variants at both 12 and 24 months and minor allele homozygotes of VEGFA rs3025039 at 24 months received fewer injections compared to other genotypes (uncorrected p<0.05), but this did not survive Bonferroni correction (Table 4).
Discussion
This is the first large-scale, long-term pharmacogenetic study of anti-VEGF treatment for neovascular AMD in an East Asian population. The mean visual acuity gain of 4.5 letters after two years of anti-VEGF treatment was comparable to the two-year results of the as-needed treatment groups in the CATT and IVAN clinical trials, which were 5.87 and 3.5 letters, respectively [5,6]. Although monthly follow-up and as-needed anti-VEGF treatment improved vision and retinal morphology on average, not all patients showed favorable outcomes. In this cohort of 394 Korean neovascular AMD patients, treatment response was found to be different according to the genotypes of some polymorphisms in VEGFA and ARMS2/HTRA1.
In this study, minor allele homozygotes for VEGFA rs3025039 showed a significantly higher proportion of visual gains of ≥15 letters at month 24 compared to the other genotype groups. Although the association was significant only at month 24 when corrected for multiple testing, the consistent trend during the 24-month study period supports the relevance of rs3025039 to visual response after anti-VEGF treatment. The rs3025039 (936C>T) polymorphism located in the 3′-untranslated region leads to loss of a potential binding site for transcription factor AP-4 and is reported to inhibit the transcription of VEGF [39]. Plasma VEGF levels were reported to be lower in carriers of the T allele compared to non-carriers. One could hypothesize that the greater therapeutic effect of anti-VEGF agents observed in the TT genotype group in this study might be attributable to their inherently lower levels of VEGF production [39,40].
Previously, there have been several reports of VEGFA gene associations with anti-VEGF response in neovascular AMD patients. These associations have been reported for a range of VEGFA variants, including rs3025000, rs3024997, rs2010963, rs833069, rs1413711, and rs699947 [10,11,16,19-21,41]. To our knowledge, this is the first pharmacogenetic study to report an association between rs3025039 and treatment outcome after anti-VEGF in neovascular AMD. In addition, a recent study a showed significant association between polymorphisms in the VEGF receptor 2 (VEGFR2) gene and visual outcome after ranibizumab treatment in neovascular AMD patients [42]. Although there are conflicting reports of no association between VEGFA polymorphisms and response to anti-VEGF therapy [8,18,24,43,44], we cannot exclude the possible role of genetic factors related to the VEGF pathway in anti-VEGF responsiveness in neovascular AMD.
Regarding tomographic outcome variables, polymorphisms at the 10q26 locus showed an association with mean CSMT change at month 12, which was marginally significant after correction. In this study cohort, minor allele homozygosity for 10q26 variants seemed to have a favorable influence on CSMT reduction over the entire study period. Reports of ARMS2 and HTRA1 associations with anti-VEGF response have been inconsistent to date [7,8,10,11,13,18,20,22,38,43,45]. A recent prospective cohort study, which included 224 neovascular AMD patients, reported that these two polymorphisms are associated with visual outcome after anti-VEGF treatment [9], but larger robust pharmacogenetic studies from CATT or IVAN trials found no evidence of association [22,23]. Genetic variants at 10q26 locus have shown strong evidence for AMD susceptibility [46], but their biologic mechanism is still not fully understood. Although marginally significant, the present data implies a need for further investigation of the potential influence of 10q26 polymorphisms on anti-VEGF responsiveness.
In this study, the number of injections was included as a treatment outcome variable. In an as-needed treatment protocol, a higher injection frequency generally implies a more persistent or recurrent nature of a CNV lesion. Although it was not significant after Bonferroni correction, the minor allele homozygotes of 10q26 variants and VEGFA rs3025039 required fewer injections compared to other genotypes, and this corresponds to their better results in visual and tomographic outcomes. However, the number of injections is thought to be less representative of therapeutic responsiveness than visual acuity or retinal thickness because some patients who developed fovea-involving neurosensory retinal atrophy or subfoveal fibrosis without fluid during follow-up did not satisfy the retreatment criteria and were likely to receive fewer frequent injections, although their treatment response was poor. On the contrary, there are other patients who maintained relatively good visual acuity and dry macula with frequent injections.
Although previous pharmacogenetic studies reported significant associations of anti-VEGF agents for neovascular AMD treatment, in many studies, the sample sizes were small and the treatment protocols and outcome measures varied. In this long-term study, a standardized as-needed treatment protocol for treatment-naïve neovascular AMD patients minimized possible bias due to variations in the treatment regimen. In addition, we evaluated treatment response multilaterally using both visual and tomographic parameters. Visual acuity is a subjective measure that is influenced by the entire visual system and has been reported to be weakly correlated with retinal morphology as evaluated by OCT [47]. We included tomographic parameters as treatment outcome measures to reflect anatomic change after anti-VEGF therapy. This can be helpful in minimizing the error resulting from using vision as the only measure of treatment response.
The findings of this study do not concur with those of recent pharmacogenetic studies using a well-defined cohort of neovascular AMD patients from multicenter randomized trials (CATT and IVAN), which found no significant association between studied genetic variants and anti-VEGF response [22-24]. Although such studies have provided robust results for large populations, our study differs in several respects. First, they included both monthly and as-needed treatment groups in their pharmacogenetic analyses, whereas our cohort included only an as-needed treatment regimen and was followed-up for a longer period. Second, among the candidate polymorphisms examined during the present study, only CFH rs1061170, ARMS2 rs10490924, HTRA1 rs11200638, CFI rs10033900, CFB rs641153, VEGFA rs699947, and SYN3/TIMP3 rs9621532 were evaluated in the pharmacogenetic studies from the CATT and IVAN trials [22-24].
Furthermore, our cohort included only Korean patients and may reveal unique pharmacogenetic associations of anti-VEGF agents that are most relevant for East Asian neovascular AMD patients. If a certain nucleotide has an influence on treatment response, the pharmacogenetic association could differ by ethnicity when the allele distribution of the candidate polymorphism varies among races. For example, ARMS2 rs10490924 T allele frequency is 0.431 in Han Chinese and 0.394 in Japanese, while it is 0.199 in Europeans (HapMap). In addition, a recent multi-center study reported that the risk of major AMD variants such as CFH rs1061170 and ARMS2 rs10490924 in Europeans cannot be generalized to a non-European population [48].
The major weakness of this study is a possible bias introduced by patients who did not complete 12 months of follow-up visits. As compared with patients who showed good response following anti-VEGF treatment, the patients with an unfavorable outcome were more likely to have discontinued follow-up. Although we performed interim analyses using the treatment outcomes of patients who completed 12 months of follow-up to minimize possible selection bias, the final cohort of this study at month 24 would represent a relatively better responding group compared to the initial cohort at baseline. Another limitation is that we used central 1-mm subfield thickness obtained from macular thickness maps as a tomographic outcome variable, and this involves only the distance between the internal limiting membrane and RPE. The distance between the internal limiting membrane and Bruch’s membrane centered on the fovea may reflect the morphologic change of the fovea more accurately because it also includes the height of CNV and RPE elevation. Finally, gene–gene or gene–environment interactions were not fully evaluated in the present study. To check for possible gene–gene or gene–age interaction, we performed a multifactor dimensionality reduction analysis, which adopts a simple algorithm for detecting genetic interaction of common human diseases, but we found no significant interaction (data not shown). Considering that AMD is a complex disease and its pathogenesis involves various biologic pathways, there still remains the possibility that therapeutic response might be affected by interaction among multiple genetic variants or environmental factors.
In conclusion, this study investigated the pharmacogenetic associations between AMD-relevant genetic variants and long-term treatment outcomes following a two-year as-needed anti-VEGF regimen in Korean neovascular AMD patients. Treatment response was evaluated using visual and tomographic outcomes and number of injections, and the results suggested that polymorphisms in the VEGFA and ARMS2/HTRA1 may influence long-term response to anti-VEGF therapy. In addition, there remains the possibility that a genetic variant that has not yet been investigated may be associated with anti-VEGF response. Given further supportive evidence, genetic background could be used to individualize anti-VEGF therapy in neovascular AMD patients, identifying those most likely to achieve optimal response.
Table 1. Characteristics of candidate genetic markers.
| Chr | Gene | Location | dbSNP ID | Major/Minor Allele | MAF | Genotyping Method | HWE P - value | Genotyping rate |
|---|---|---|---|---|---|---|---|---|
| 1 |
CFH |
I62V |
rs800292 |
G/A |
0.281 |
SNaPshot |
0.503 |
100% |
| 1 |
CFH |
Y402H |
rs1061170 |
T/C |
0.097 |
SNaPshot |
1 |
99.0% |
| 1 |
CFH |
IVS14–543A/G |
rs1410996 |
G/A |
0.314 |
Taqman |
0.213 |
99.8% |
| 6 |
CFI |
64060C/T |
rs10033900 |
T/C |
0.302 |
Taqman |
0.633 |
99.8% |
| 6 |
C2 |
E318D |
rs9332739 |
G/C |
0.014 |
Taqman |
0.098 |
100% |
| 6 |
CFB |
R32Q |
rs641153 |
G/A |
0.063 |
SNaPshot |
1 |
99.5% |
| 6 |
SKIV2L |
3493G/A |
rs429608 |
G/A |
0.071 |
Taqman |
0.144 |
99.5% |
| 6 |
VEGFA |
C-2578A |
rs699947 |
C/A |
0.298 |
SNaPshot |
0.087 |
99.5% |
| 6 |
VEGFA |
C936T |
rs3025039 |
C/T |
0.234 |
Taqman |
0.401 |
100% |
| 10 |
ARMS2 |
A69S |
rs10490924 |
T/G |
0.313 |
Taqman |
0.043 |
99.8% |
| 10 |
HTRA1 |
−625A/G |
rs11200638 |
A/G |
0.315 |
Taqman |
0.008 |
100% |
| 12 |
SCARB1 |
A350A |
rs5888 |
C/T |
0.262 |
Taqman |
0.425 |
99.8% |
| 17 |
PEDF |
Met72Thr |
rs1136287 |
C/T |
0.489 |
Taqman |
1 |
99.5% |
| 19 |
C3 |
R80G |
rs2230199* |
C/G |
0 |
Taqman |
1 |
99.7% |
| 19 |
APOE |
Cys112Arg |
rs429358 |
T/C |
0.095 |
Taqman |
0.336 |
98.7% |
| 19 |
APOE |
Arg158Cys |
rs7412 |
C/T |
0.063 |
Taqman |
1 |
98.6% |
| 22 | SYN3/TIMP3 | IVS5–91789G/T | rs9621532 | T/G | 0.027 | SNaPshot | 0.508 | 99.8% |
SNP=Single nucleotide polymorphism (dbSNP ID); MAF=Minor allele frequency; HWE=Hardy–Weinberg Equilibrium; IVS=Intervening sequence *: Excluded from analysis because all patients showed CC genotype
Acknowledgments
The authors thank Dr. Jong Eun Lee and Sujin Kim for their technical support of genetic analyses. This study was funded by GlaxoSmithKline Medicines Research Centre and supported in part by grant No. 04–2011–1160 from the SNUH Research Fund.
Appendix 1.
Polymerase chain reaction sequencing primers of candidate polymorphisms. To access the data, click or select the words “Appendix 1.”
Appendix 2. Association of candidate genetic variants with visual outcome variables
OR = Odds ratio; CI = Confidence interval; BCVA = Best-corrected visual acuity *: Major allele homozygote / Heterozygote / Minor allele homozygote **: All odds ratio are calculated with respect to minor allele †: Uncorrected P-value from logistic regression model. When applying Bonferroni correction, uncorrected P-value for significance should be less than 0.0031. ††: Uncorrected P-value from linear regression model. When applying Bonferroni correction, uncorrected P-value for significance should be less than 0.0031. §: Analyzed under dominant model because of extremely low proportion of minor allele homozygotes §§: ε2+ = ε2 carrier, ε4+ = ε4 carrier. To access the data, click or select the words “Appendix 2.”
Appendix 3. Association of candidate genetic variants with tomographic outcome variables
OCT = Optical Coherence Tomography; OR = Odds ratio; CI = Confidence interval; CSMT = Central subfield macular thickness *: Major allele homozygote / Heterozygote / Minor allele homozygote **: All odds ratio are calculated with respect to minor allele †: Uncorrected P-value from logistic regression model. When applying Bonferroni correction, uncorrected P-value for significance should be less than 0.0031. ††: Uncorrected P-value from linear regression model. When applying Bonferroni correction, uncorrected P-value for significance should be less than 0.0031. §: Analyzed under dominant model because of extremely low proportion of minor allele homozygotes §§: ε2+ = ε2 carrier, ε4+ = ε4 carrier. To access the data, click or select the words “Appendix 3.”
Appendix 4.
Association of candidate genetic variants with number of injection. OR = Odds ratio; CI = Confidence interval *: Major allele homozygote / Heterozygote / Minor allele homozygote **: Uncorrected P-value from linear regression model. When applying Bonferroni correction, uncorrected P-value for significance should be less than 0.0031. †: Analyzed under dominant model because of extremely low proportion of minor allele homozygotes ††: ε2+ = ε2 carrier, ε4+ = ε4 carrier. To access the data, click or select the words “Appendix 4.”
References
- 1.Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47:171–88. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC, IVAN study investigators Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–67. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 7.Brantley MA, Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114:2168–73. doi: 10.1016/j.ophtha.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Kloeckener-Gruissem B, Barthelmes D, Labs S, Schindler C, Kurz-Levin M, Michels S, Fleischhauer J, Berger W, Sutter F, Menghini M. Genetic association with response to intravitreal ranibizumab in patients with neovascular AMD. Invest Ophthalmol Vis Sci. 2011;52:4694–702. doi: 10.1167/iovs.10-6080. [DOI] [PubMed] [Google Scholar]
- 9.Abedi F, Wickremasinghe S, Richardson AJ, Islam AF, Guymer RH, Baird PN. Genetic influences on the outcome of anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Ophthalmology. 2013;120:1641–8. doi: 10.1016/j.ophtha.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Imai D, Mori K, Horie-Inoue K, Gehlbach PL, Awata T, Inoue S, Yoneya S. CFH, VEGF, and PEDF genotypes and the response to intravitreous injection of bevacizumab for the treatment of age-related macular degeneration. J Ocul Biol Dis Infor. 2010;3:53–9. doi: 10.1007/s12177-010-9055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smailhodzic D, Muether PS, Chen J, Kwestro A, Zhang AY, Omar A, Van de Ven JP, Keunen JE, Kirchhof B, Hoyng CB, Klevering BJ, Koenekoop RK, Fauser S, den Hollnader AI. Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2012;119:2304–11. doi: 10.1016/j.ophtha.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 12.McKibbin M, Ali M, Bansal S, Baxter PD, West K, Williams G, Cassidy F, Inglehearn CF. CFH, VEGF and HTRA1 promoter genotype may influence the response to intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Br J Ophthalmol. 2012;96:208–12. doi: 10.1136/bjo.2010.193680. [DOI] [PubMed] [Google Scholar]
- 13.Teper SJ, Nowinska A, Pilat J, Palucha A, Wylegala E. Involvement of genetic factors in the response to a variable-dosing ranibizumab treatment regimen for age-related macular degeneration. Mol Vis. 2010;16:2598–604. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AY, Raya AK, Kymes SM, Shiels A, Brantley MA., Jr Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2009;93:610–3. doi: 10.1136/bjo.2008.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nischler C, Oberkofler H, Ortner C, Paikl D, Riha W, Lang N, Patsch W, Egger SF. Complement factor H Y402H gene polymorphism and response to intravitreal bevacizumab in exudative age-related macular degeneration. Acta Ophthalmol (Copenh) 2011;89:e344–9. doi: 10.1111/j.1755-3768.2010.02080.x. [DOI] [PubMed] [Google Scholar]
- 16.Boltz A, Ruiss M, Jonas JB, Tao Y, Rensch F, Weger M, Garhofer G, Frantal S, El-Shabrawi Y, Schmetterer L. Role of vascular endothelial growth factor polymorphisms in the treatment success in patients with wet age-related macular degeneration. Ophthalmology. 2012;119:1615–20. doi: 10.1016/j.ophtha.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Wickremasinghe SS, Xie J, Lim J, Chauhan DS, Robman L, Richardson AJ, Hageman G, Baird PN, Guymer R. Variants in the APOE gene are associated with improved outcome after anti-VEGF treatment for neovascular AMD. Invest Ophthalmol Vis Sci. 2011;52:4072–9. doi: 10.1167/iovs.10-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Qin X, Fang K, Chen Q, Hou J, Li J, Yu W, Chen D, Hu Y, Li X. Association of genetic polymorphisms with response to bevacizumab for neovascular age-related macular degeneration in the Chinese population. Pharmacogenomics. 2012;13:779–87. doi: 10.2217/pgs.12.53. [DOI] [PubMed] [Google Scholar]
- 19.Abedi F, Wickremasinghe S, Richardson AJ, Makalic E, Schmidt DF, Sandhu SS, Baird PN, Guymer RH. Variants in the VEGFA gene and treatment outcome after anti-VEGF treatment for neovascular age-related macular degeneration. Ophthalmology. 2013;120:115–21. doi: 10.1016/j.ophtha.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Chang W, Noh DH, Sagong M, Kim IT. Pharmacogenetic association with early response to intravitreal ranibizumab for age-related macular degeneration in a Korean population. Mol Vis. 2013;19:702–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Nakata I, Yamashiro K, Nakanishi H, Tsujikawa A, Otani A, Yoshimura N. VEGF gene polymorphism and response to intravitreal bevacizumab and triple therapy in age-related macular degeneration. Jpn J Ophthalmol. 2011;55:435–43. doi: 10.1007/s10384-011-0061-z. [DOI] [PubMed] [Google Scholar]
- 22.Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Callanan DG, Kim IK, Klein ML, Maguire MG, Martin DF, Comparison of AMD Treatments Trials Research Group Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2013;120:593–9. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotery AJ, Gibson J, Cree AJ, Downes SM, Harding SP, Rogers CA, Reeves BC, Ennis S, Chakravarthy U, Alternative Treatments to Inhibit VEGF in Patients with Age-Related Choroidal Neovascularisation (IVAN) Study Group Pharmacogenetic Associations with Vascular Endothelial Growth Factor Inhibition in Participants with Neovascular Age-related Macular Degeneration in the IVAN Study. Ophthalmology. 2013;120:2637–43. doi: 10.1016/j.ophtha.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Maguire MG, Martin DF, Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group VEGFA and VEGFR2 Gene Polymorphisms and Response to Anti-Vascular Endothelial Growth Factor Therapy: Comparison of Age-Related Macular Degeneration Treatments Trials (CATT). JAMA Ophthalmol. 2014;132:521–7. doi: 10.1001/jamaophthalmol.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotoh N, Yamada R, Hiratani H, Renault V, Kuroiwa S, Monet M, Toyoda S, Chida S, Mandai M, Otani A, Yoshimura N, Matsuda F. No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet. 2006;120:139–43. doi: 10.1007/s00439-006-0187-0. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Guan N, Xu J, Yang X, Ma K, Zhou H, Zhang F, Snellingen T, Jiao Y, Liu X, Wang N, Liu N. Association of CFH, LOC387715, and HTRA1 polymorphisms with exudative age-related macular degeneration in a northern Chinese population. Mol Vis. 2008;14:1373–81. [PMC free article] [PubMed] [Google Scholar]
- 27.Andreoli MT, Morrison MA, Kim BJ, Chen L, Adams SM, Miller JW, DeAngelis MM, Kim IK. Comprehensive analysis of complement factor H and LOC387715/ARMS2/HTRA1 variants with respect to phenotype in advanced age-related macular degeneration. Am J Ophthalmol. 2009;148:869–74. doi: 10.1016/j.ajo.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, McCarty CA, Guymer RH. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD). Hum Mutat. 2006;27:337–42. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- 29.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopplin LJ, Igo RP, Jr, Wang Y, Sivakumaran TA, Hagstrom SA, Peachey NS, Francis PJ, Klein ML, SanGiovanni JP, Chew EY, Pauer GJ, Sturgill GM, Joshi T, Tian L, Xi Q, Henning AK, Lee KE, Klein R, Klein BE, Iyengar SK. Genome-wide association identifies SKIV2L and MYRIP as protective factors for age-related macular degeneration. Genes Immun. 2010;11:609–21. doi: 10.1038/gene.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, Tsai CH, Tseng SH, Tsai FJ. Vascular endothelial growth factor gene polymorphisms in age-related macular degeneration. Am J Ophthalmol. 2008;145:1045–51. doi: 10.1016/j.ajo.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, Tsai CH, Tseng SH, Tsai FJ. Pigment epithelium-derived factor gene Met72Thr polymorphism is associated with increased risk of wet age-related macular degeneration. Am J Ophthalmol. 2008;145:716–21. doi: 10.1016/j.ajo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Bhangale TR, Fagerness J, Ripke S, Thorleifsson G, Tan PL, Souied EH, Richardson AJ, Merriam JE, Buitendijk GH, Reynolds R, Raychaudhuri S, Chin KA, Sobrin L, Evangelou E, Lee PH, Lee AY, Leveziel N, Zack DJ, Campochiaro B, Campochiaro P, Smith RT, Barile GR, Guymer RH, Hogg R, Chakravarthy U, Robman LD, Gustafsson O, Sigurdsson H, Ortmann W, Behrens TW, Stefansson K, Uitterlinden AG, van Duijn CM, Vingerling JR, Klaver CC, Allikmets R, Brantley MA, Jr, Baird PN, Katsanis N, Thorsteinsdottir U, Ioannidis JP, Daly MJ, Graham RR, Seddon JM. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet. 2011;20:3699–709. doi: 10.1093/hmg/ddr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerbib J, Seddon JM, Richard F, Reynolds R, Leveziel N, Benlian P, Borel P, Feingold J, Munnich A, Soubrane G, Kaplan J, Rozet JM, Souied EH. rs5888 variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS ONE. 2009;4:e7341. doi: 10.1371/journal.pone.0007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Zhao P, Tang S, Lu F, Hu J, Lei C, Yang X, Lin Y, Ma S, Yang J, Zhang D, Shi Y, Li T, Chen Y, Fan Y, Yang Z. Association study of complement factor H, C2, CFB, and C3 and age-related macular degeneration in a Han Chinese population. Retina. 2010;30:1177–84. doi: 10.1097/IAE.0b013e3181cea676. [DOI] [PubMed] [Google Scholar]
- 36.Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP, Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N. N Keilhauer C, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Saïd S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Léveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR; AMD Gene Consortium. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–9. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arakawa S, Takahashi A, Ashikawa K, Hosono N, Aoi T, Yasuda M, Oshima Y, Yoshida S, Enaida H, Tsuchihashi T, Mori K, Honda S, Negi A, Arakawa A, Kadonosono K, Kiyohara Y, Kamatani N, Nakamura Y, Ishibashi T, Kubo M. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat Genet. 2011;43:1001–4. doi: 10.1038/ng.938. [DOI] [PubMed] [Google Scholar]
- 38.Orlin A, Hadley D, Chang W, Ho AC, Brown G, Kaiser RS, Regillo CD, Godshalk AN, Lier A, Kaderli B, Stambolian D. Association between high-risk disease loci and response to anti-vascular endothelial growth factor treatment for wet age-related macular degeneration. Retina. 2012;32:4–9. doi: 10.1097/IAE.0b013e31822a2c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443–8. doi: 10.1159/000054076. [DOI] [PubMed] [Google Scholar]
- 40.Al-Habboubi HH, Sater MS, Almawi AW, Al-Khateeb GM, Almawi WY. Contribution of VEGF polymorphisms to variation in VEGF serum levels in a healthy population. Eur Cytokine Netw. 2011;22:154–8. doi: 10.1684/ecn.2011.0289. [DOI] [PubMed] [Google Scholar]
- 41.Dos Reis Veloso CE, Frota de Almeida LN, Recchia FM, Pelayes D, Nehemy MB. VEGF gene polymorphism and response to intravitreal ranibizumab in neovascular age-related macular degeneration. Ophthalmic Res. 2014;51:1–8. doi: 10.1159/000354328. [DOI] [PubMed] [Google Scholar]
- 42.Hermann MM, van Asten F, Muether PS, Smailhodzic D, Lichtner P, Hoyng CB, Kirchhof B, Grefkes C, den Hollander AI, Fauser S. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014;121:905–10. doi: 10.1016/j.ophtha.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 43.Hautamaki A, Kivioja J, Vavuli S, Kakko S, Savolainen ER, Savolainen MJ, Liinamaa MJ, Seitsonen S, Onkamo P, Järvelä I, Immonen I. Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. 2013;33:1815–27. doi: 10.1097/IAE.0b013e318285cf92. [DOI] [PubMed] [Google Scholar]
- 44.Wang VM, Rosen RB, Meyerle CB, Kurup SK, Ardeljan D, Agron E, Tai K, Pomykala M, Chew EY, Chan CC, Tuo J. Suggestive association between PLA2G12A single nucleotide polymorphism rs2285714 and response to anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration. Mol Vis. 2012;18:2578–85. [PMC free article] [PubMed] [Google Scholar]
- 45.Park UC, Shin JY, Kim SJ, Shin ES, Lee JE, McCarthy LC, Newcombe PJ, Xu CF, Chung H, Yu HG. Genetic factors associated with response to intravitreal ranibizumab in Korean patients with neovascular age-related macular degeneration. Retina. 2014;34:288–97. doi: 10.1097/IAE.0b013e3182979e1e. [DOI] [PubMed] [Google Scholar]
- 46.Fisher SA, Abecasis GR, Yashar BM, Zareparsi S, Swaroop A, Iyengar SK, Klein BE, Klein R, Lee KE, Majewski J, Schultz DW, Klein ML, Seddon JM, Santangelo SL, Weeks DE, Conley YP, Mah TS, Schmidt S, Haines JL, Pericak-Vance MA, Gorin MB, Schulz HL, Pardi F, Lewis CM, Weber BH. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14:2257–64. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 47.Moutray T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U. Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisation. Br J Ophthalmol. 2008;92:361–4. doi: 10.1136/bjo.2007.123976. [DOI] [PubMed] [Google Scholar]
- 48.Restrepo NA, Spencer KL, Goodloe R, Garrett TA, Heiss G, Bůžková P, Jorgensen N, Jensen RA, Matise TC, Hindorff LA, Klein BE, Klein R, Wong TY, Cheng CY, Cornes BK, Tai ES, Ritchie MD, Haines JL, Crawford DC. Genetic Determinants of Age-Related Macular Degeneration in Diverse Populations from the PAGE Study. Invest Ophthalmol Vis Sci. 2014;55:6839–50. doi: 10.1167/iovs.14-14246. [DOI] [PMC free article] [PubMed] [Google Scholar]


