Abstract
Pancreatic cancer often has a poor prognosis, even when diagnosed early. Pancreatic cancer typically spreads rapidly and is rarely detected in its early stages, which is a major reason it is a leading cause of cancer death. Signs and symptoms may not appear until pancreatic cancer is quite advanced, and complete surgical removal is not possible. Furthermore, pancreatic cancer responds poorly to most chemotherapeutic agents. The importance of integrins in several cell types that affect tumor progression has made them an appealing target for cancer therapy. Some of the proteins found in the snake venom present a great potential as anti-tumor agents. In this study, we summarize the activity of two integrins antagonist, recombinant disintegrins mojastin 1 and viridistatin 2, on human pancreatic carcinoma cell line (BXPC-3). Both recombinant disintegrins inhibited some essential aspects of the metastasis process such as proliferation, adhesion, migration, and survival through apoptosis, making these proteins prominent candidates for the development of drugs for the treatment of pancreatic cancer.
Keywords: pancreatic cancer, cancer therapy, integrins antagonist, disintegrins
1. Introduction
The pancreas is an organ that has endocrine and exocrine functions. As an endocrine gland, it produces important hormones, including insulin, glucagon, somatostatin, and pancreatic polypeptide. These hormones play an imperative role in glucose metabolism and regulation of blood glucose concentration. As an exocrine gland, the pancreas secretes pancreatic juice containing digestive enzymes that assist the absorption of nutrients and the digestion in the small intestine. These enzymes help to further break down carbohydrates, proteins, and lipids (Hegyi and Petersen, 2013; Mastracci and Sussel, 2012). Pancreatic cancers can arise from the exocrine and endocrine parts of the pancreas; approximately 95% of them develop from the exocrine portion. In general, there are three basic types: ductal adenocarcinoma (more than 90% of pancreatic cancers), neuroendocrine tumors, and cystic neoplasm (Saif, 2011).
Ductal adenocarcinoma of the pancreas (PDA), commonly referred to as pancreatic cancer, is a frequent and lethal disease ranking the fourth cause in cancer-related death in western countries. PDA is a genetic disease caused by the successive accumulation of mutations in key oncogenes and tumor suppressor genes that once established is a quite complex, heterogeneous and genetically unstable disease (Hidalgo, 2012). The incidence of and number of deaths caused by pancreatic cancer have been gradually rising. Despite developments in detection and management of pancreatic cancer, only about 4% of patients will live 5 years after diagnosis. The only potentially curative therapy for pancreatic cancer is surgical resection. Unfortunately, 80–85% of patients present with advanced unresectable disease. Furthermore, pancreatic cancer responds poorly to most chemotherapy agents (Vincent et al., 2011). The developments of novel and more effective chemotherapeutic agents for patients with advance disease are needed to improve the prognosis of this disease.
Disintegrins represent a family of low molecular weight (40–100 amino acids), cystein-rich polypeptides released in viper venoms by proteolytic processing of PII snake venom metaloprotease (SVMP) precursors. Disintegrins bind specifically to integrins expressed on platelets and other cells, including vascular endothelial cells and some tumor cells, leading to inhibition of platelet aggregation, inhibition of cell adhesion, migration and angiogenesis (Calvete, 2013; Marcinkiewicz, 2013). Since integrins are intimately involved in cancer cell motility, invasion, and other processes critical to cancer progression and metastasis, disintegrins hold significant potential for cancer therapy (Calvete, 2013). For this reason, the objective of this study was to determine the effect of two recombinant disintegrins, r-mojastin 1 and r-viridistatin 2, derived from Crotalus scutulatus scutulatus and Crotalus viridis viridis, respectively on human pancreatic adenocarcinoma cancer cells (BXPC-3).
2. Materials and methods
2.1. Preparation of recombinant disintegrins
r-Mojastin 1 and r-viridistatin 2 were expressed in E.coli and further purified by two-step chromatography, using the method of Sánchez et al. (2010) and Lucena et al. (2012), respectively. Briefly, E. coli BL21 cells were grown, induced by 0.5 mM of isopropyl β-D thiogalactoside (IPTG) and centrifuged. After bacterial cell disruption with a Branson Sonifier 450 (Danbury, CT), the cell debris was removed by centrifugation and the crude lysate was incubated with glutathione Sepharose 4B (GS4B) (Amersham Biosciences). Recombinant disintegrins peptides were cleaved and eluted from glutathione S-transferase (GST) bound to GS4B by thrombin (80 U/mL, GE Healthcare Life Sciences, USA). Thrombin was removed from r-mojastin 1 and r-viridistatin 2 using a 5 mL HiTrap™ Benzamidine Sepharose 4 Fast Flow column (Amersham Biosciences). Purity of recombinant disintegrins was determined by using a 10–20% Tricine gel (Schägger and von Jagow, 1987) in an XCell SureLock Mini-Cell (Invitrogen Life Technologies, USA).
2.2. Cells lines and culture conditions
The human pancreatic adenocarcinoma (BXPC-3) cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The BXPC-3 cells were maintained with RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS) and 50 U/mL penicillin, 50μg/mL streptomycin. The cells were maintained in a humidified 5% CO2 air incubator at 37°C.
2.3. Proliferation inhibition studies
Anti-proliferation activity on BXPC-3 cells of recombinant disisntegrins, r-mojastin 1 and r-viridistatin 2 were performed by measuring cell proliferation using MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide). Two hundred microliters of cells were cultured on 96-well flat-bottom microtiter plates at 105 cells/well, in triplicate, and incubated at 37°C in 5% CO2 for 24 h.
Twenty microliters of each recombinant disintegrin (r-mojastin 1 and r-viridistatin 2) at various concentrations were added to the BXPC-3 cell suspension at 37°C for 48 h. Then, 10 μL of MTT (5 mg/mL) was added to each well. After incubation for 4 h at 37°C, MTT was aspirated and 100 μL of DMSO was added to lyse the cells. The absorbance at 570 nm was read using a Beckman Coulter™ model AD 340 reader. Doxorubicin, paclitaxel, gemcitabine, and 5-fluorouracil, anticancer drugs used in the treatment of prancreatic cancer and known to induce apoptosis, were used as positive controls (Neesse et al, 2014; Kratz et al, 2013; Saif, 2013). The negative control was cells treated with PBS buffer, pH 7.4. The percentage of cell proliferation was calculated relative to the negative control, which was defined as 100%. The 50% cytotoxic concentration (CC50) of sample is defined as the protein concentration, which reduced 50% of proliferation. The values of the percentages of cell proliferation inhibition were plotted against disintegrins concentrations, and the CC50 was determined. Experiments were performed in triplicate.
2.4. Cellular adhesion inhibition assay
r-Viridistatin 2 and r-mojastin 1 were used to inhibit the binding of BXPC-3 cells on two extracellular matrix proteins (laminin 1 and vitronectin at 10 μg/mL) coated plates (Sánchez et al., 2009). The negative control consisted of BPXC-3 cells incubated with PBS. The negative controls allowed binding of cells to extracellular matrix proteins. The percent inhibition was calculated by the following formula: .
2.5. Cellular adhesion inhibition using anti-integrin antibodies assay
To determine which integrins on the BXPC-3 cell surface are the targets for r-mojastin 1 and r-viridistatin 2, adhesion inhibition assays were performed using α5 (VC5 clone), α2 (AK-7 clone), α6 (GoH3 clone), α3 (C3 II.1 clone), β4 (439-9B clone), αvβ3 (23C6 clone), and β1 (MAR4 clone) monoclonal anti-integrin antibodies (BD Biosciences), and β6 (437211 clone) monoclonal anti-integrin antibody (R&D System). Duplicate wells of a 96-well plate (Falcon® Tissue Culture Plate) were coated with 0.1 mL of r-mojastin 1 and r-viridistatin 2 at a concentration of 40 μg/mL, in 0.01 M Phosphate buffer saline (PBS), pH 7.4, and incubated overnight at 4°C. Cells were harvested, counted and resuspended in medium containing 1% BSA at 5 × 105 cells/mL. Anti-integrins antibodies (α5, α2, α6, α3, β4, β6, αvβ3 and β1) were added to the cell suspension at 10 μg/mL and allowed to incubate at 37°C for 1 h. The positive control of adhesion consisted of BXPC-3 cells incubated with PBS (No IgG). Also, a control with murine IgG was used (mIgG). Negative control of adhesion was performed with the plate coated with BSA at 40 μg/mL.
In order to provide proof of the specific interaction between integrin α3β1 with r -mojastin 1 and r-viridisitatin 2, a binding assay of BXPC-3 to immobilized monoclonal anti-integrins α3 and β1 was performed. In this experiment, duplicate wells of a 96-well plate (Falcon® Tissue Culture Plate) were coated with 0.1 mL of monoclonal anti-integrins α3 and β1 at a concentration of 40 μg/mL, in 0.01 M Phosphate buffer saline (PBS), pH 7.4, and incubated overnight at 4°C. Cells were harvested, counted and resuspended in medium containing 1% BSA at 5 × 105 cells/mL. Recombinant disintegrins were added to the cell suspension at 5 μM and allowed to incubate at 37°C for 1 h. The positive control of adhesion consisted of BXPC-3 cells incubated with PBS (no disintegrin). Negative control of adhesion was performed with the plate coated with murine IgG (mIgG) at 40 μg/mL.
2.6. Cellular migration inhibition assay
BXPC-3 cell migration was measured after scraping cells from the bottom of the well as described by Galán et al., 2008. The negative control consisted of BXPC-3 cells incubated with PBS, which allowed cell migration to occur. Cells were then incubated in a CO2 chamber and were only removed from the incubator for microscopy images at times 0, 3, 6, 12, and 24 h after recombinant disintegrins incubation. The concentration of r-disintegrins used were 5, 2.5 and 1.25 μM. Percent of closure was calculated by the following equation: , where C is the units of distance of cell edge (mm) at zero time for the control, and E is the distance from the cell edge (mm) at the final incubation time for the disintegrin.
2.7. Apoptosis detection
BXPC-3 cells were cultured in 24 well plates. Five hundred thousand cells were added to 1 mL of media for each well and grown for 24 h. Experiments were performed in triplicates. After initial incubation, cells were treated with 1.25, 2.5, and 5 μM recombinant disintegrins and incubated for 24 h. An untreated control was also performed. Cells were then detached from the plate surface using 0.05% trypsin-EDTA. Trypsin was neutralized with culture media. Cells were centrifuged, washed twice with cold PBS, and resuspended in 250 μL of 1X apoptosis-binding buffer (BD Biosciences). Then, 100 μL were removed and exposed to 5 μL each of Annexin V-FITC and Propidium Iodide (PI). The cells were then incubated at room temperature, in the dark for 15 min. Four hundred microliters of 1X apoptosis-binding buffer was added and 10,000 events per sample were analyzed using a Becton Dickinson Accuri C6 flow cytometer and Accuri C6 software.
2.8. Statistical analyses
Results of proliferation and adhesion were expressed as the mean ± standard deviation (n=3), and analyzed using the two-tailed t-test, using the software program Graph Pad Prism. A one way-analysis of variance test followed by Newman-Keuls Multiple Comparison Test was used to determine the significance of both recombinant disintegrins and the control in inhibiting migration and inducing apoptosis. Differences were statistically significant if p value was less than 0.05. Experiments were performed in triplicate.
3. Results
3.1. Proliferation studies
r-Viridistatin 2 and r-mojastin 1 inhibited the proliferation of BXPC-3 cells with a CC50 of 10.6 and 8.7 μM, respectively. The combination of our recombinant disintegrins inhibited the proliferation with a CC50 of 8.0 μM. No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p>0.05). The combination of our recombinant disintegrins did not improve the anti-proliferation activity compared with the disintegrins alone (p>0.05). Doxorubicin, paclitaxel, and 5-fluorouracil inhibited the proliferation of human pancreatic carcinoma cells with a CC50 of 3.1, 42 and 1114.5 μM, respectively. Gemcitabine did not inhibited BXPC-3 cells proliferation under the conditions used in this study. Doxorubicin was more potent in inhibited the BXPC-3 cell proliferation than r-viridistatin 2 and r-mojastin 1 (p<0.05). Recombinant disintegrins were more potent than paclitaxel and 5-fluorouracil in inhibiting the BXPC-3 cell proliferation (p<0.01) (Table 1).
Table 1.
Inhibition of human pancreatic carcinoma (BXPC-3) cells proliferation in presence of recombinant disintegrins (r-mojastin 1 and r-viridistatin 2).
| SAMPLES | BXPC-3‡ |
|---|---|
|
| |
| r-Mojastin 1 | 8.7± 0.1 μM |
| r-Viridistatin 2 | 10.6 ± 0.5 μM |
| r-mojastin 1 + r-viridistatin 2 | 8.0 ± 0.6 μM |
| Doxorubicin | 3.1± 0.2 μM |
| Paclitaxel | 42 ± 2.8 μM |
| 5-Fluorouracil | 1114.5 ± 78 μM |
| Gemtabicine | NA |
The results are expressed as CC50.
NA: No activity
3.2. Cell adhesion inhibition studies
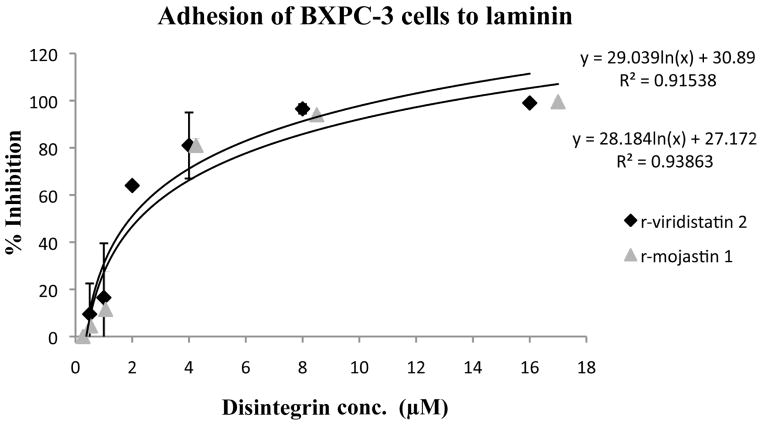
r-Viridistatin 2 and r-mojastin 1 inhibited BXPC-3 cell adhesion to laminin in a dose-dependent manner, with an IC50 of 1.93 and 2.25 μM, respectively. No statistical difference in the inhibition of BXPC-3 cell adhesion to laminin among r-viridistatin 2 and r-mojastin 1 was observed (p>0.05). On the other hand, r-viridistatin 2 and r-mojastin 1 inhibited BXPC-3 cell adhesion to vitronectin in a dose-dependant manner, with an IC50 of 0.24 of and 3.66 μM, respectively. r-Viridistain 2 was more potent than r-mojastin 1 in inhibiting the adhesion of BXPC-3 cell to vitronectin (p<0.05) (Figure 1A&1B).
Figure 1.
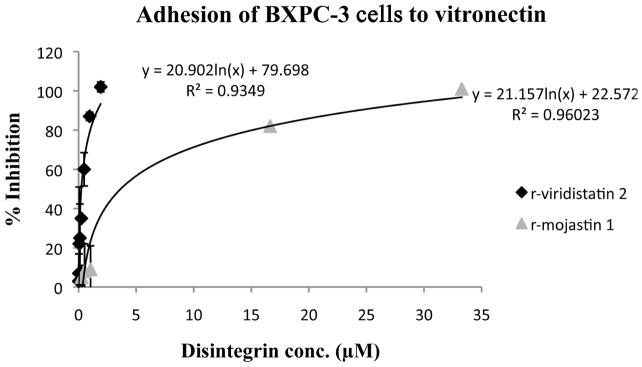
Inhibition of BXPC-3 cells adhesion to laminin (A) and vitronectin (B) in vitro by r-viridistatin 2 and r-mojastin 1. BXPC-3 cells (5×105 cells/mL, 0.2 mL) were treated with different concentrations of r-disintegrins for 1 h at 37 °C, and then added to a 96 well-plate coated with extracellular matrix protein (10 μg/mL). After removal of non-bind cells, the remaining cells were quantified using 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenltetrazolium bromide (MTT) and the IC50 values calculated as described in methods section. Graph equation: y: 50% proliferation; x: disintegrin concentration; R2: square of coefficient of correlation.
3.3. Cellular adhesion inhibition using anti-integrin antibodies assay
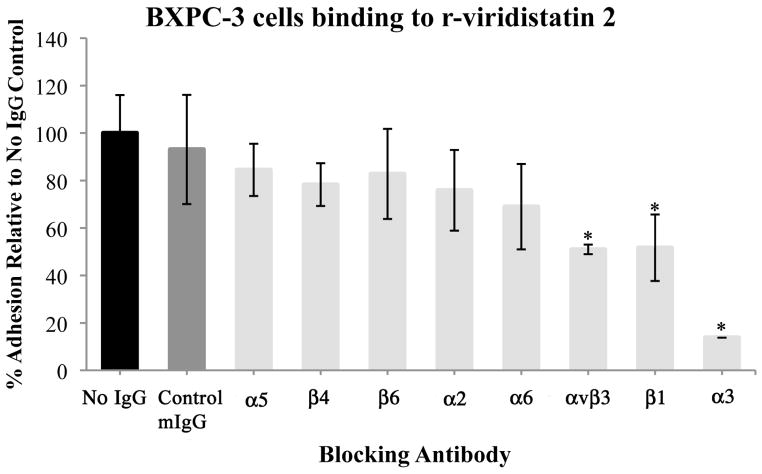
Incubation of BXPC-3 cells with control murine IgG did not inhibit the BXPC-3 cells adhesion to r-disintegrins. Whereas, preincubation of BXPC-3 cells with monoclonal antibodies to the integrins αvβ3, α3, and β1, significantly decreased BXPC-3 cell adhesion to r-viridistatin 2 and r-mojastin 1 (p<0.05). Monoclonal antibodies to integrins subunits α5, α2, α6, β4, and β6, had no significant effect on BXPC-3 cell adhesion to r-disintegrins (Figure 2A&2B).
Figure 2.

Identification of the integrin specificity of r-viridistatin 2 (A) and r-mojastin 1 (B). BXPC-3 cells were preincubated with PBS (no IgG), control murine IgG (mIgG) or specific anti-integrin antibodies, and then added to a 96 well-plate coated with r-disintegrins (40 μg/mL), followed by a quantitative inhibition adhesion assay as described in methods section. Results are expressed as mean ± S.D for percentage adhesion relative to the no IgG control (* p < 0.05). Inhibition assay with immobilized monoclonal antibodies to α3 and β1(C). BXPC-3 cells were preincubated with PBS (control), or recombinant disintegrins (5μM), and then added to a 96 well-plate coated with monoclonal antibodies to α3 and β1 (40 μg/mL), followed by a quantitative inhibition adhesion assay as described in methods section. Results are expressed as mean ± S.D for percentage adhesion relative to the control (* p < 0.05). Negative control of adhesion was performed with the plate coated with murine IgG (mIgG) at 40 μg/mL.
Taking into account that the interaction between monoclonal antibodies with their antigens are highly specific, and in order to corroborate the specific interaction between α3β1 integrin on BXPC-3 cells and r-disintegrins, an inhibition assay with immobilized monoclonal antibodies to α3 and β1 was performed. Incubation of BXPC-3 cells with PBS buffer did not inhibit the BXPC-3 cells adhesion to monoclonal antibodies for α3 and β1 integrin subunits. However, preincubation of BXPC-3 cells with r-mojastin 1 and r-viridistatin 2 (5μM) significantly decreased BXPC-3 cell adhesion to immobilize monoclonal antibodies for α3, and β1 integrin subunits (Figure 2C).
3.4. Migration inhibition assay
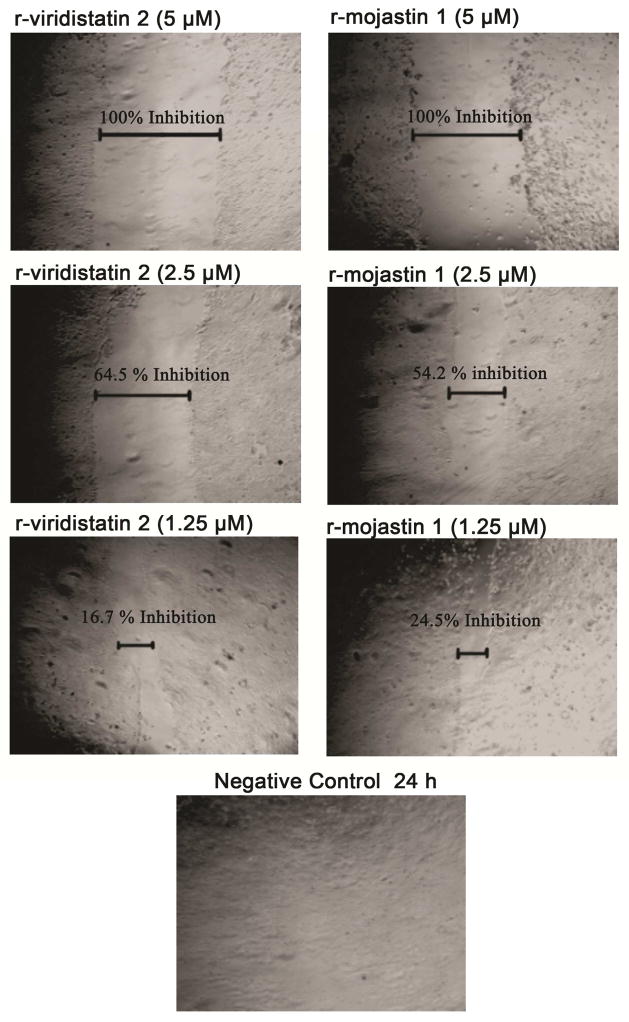
r-Viridistatin 2 and r-mojastin 1 inhibited BXPC-3 cell migration in a dose dependent manner after 24 h of incubation. r-viridistatin 2 inhibited BXPC-3 cell migration by 100, 64.5 and 16.7% at 5, 2.5 and 1.25 μM, respectively. On the other hand, r-mojastin 1 inhibited BXPC-3 cell migration by 100, 54.2 and 24.5% at the same concentrations. In comparison to the negative control, a statistically significant difference with r-viridistatin 2 and r-mojastin 1 (p < 0.05) was observed at 5 and 2.5 μM. No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p>0.05) (Figure 3).
Figure 3.
Inhibition cell migration of BXPC-3 cells. A confluent monolayer of cells was maintained in medium, and a line was scraped through the monolayer of cells with a plastic, sterile pipette tip. The cultures were allowed to migrate for 24 h at 37°C in the presence or absence of recombinant disintegrins at 1.25, 2.5, and 5 μM. The extent of wound closure was quantified by multiple measurements of the width of the scrape space for each cell line.
3.5. Apoptosis studies
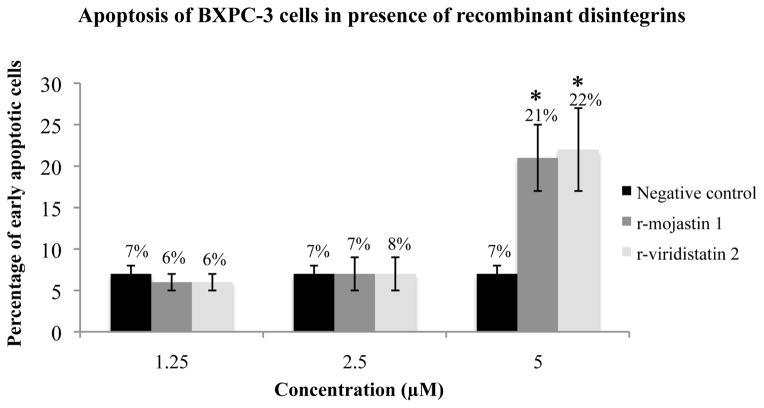
Annexin V binding assay was used to determine if r-viridistatin 2 and r-mojasrin 1 has the ability to induce apoptosis of BXPC-3 cells after 24 h of treatment. r-Viridistatin 2 and r-mojastin 1 induced apoptosis of BXPC-3 cells by 22 and 21 %, respectively. No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p>0.05). Recombinant disintegrins at 2.5 and 1.25 μM were not able to induce apoptosis of BXPC-3 cells (Figure 4).
Figure 4.
Induction of BXPC-3 apoptosis by r-viridistatin 2 and r-mojastin 1. BXPC-3 cells were incubated with r-disintegrins at 1.25, 2.5, and 5 μM for 24 h. Untreated cells were used as negative control. Results are expressed as mean ± S.D for percentage of early apoptotic cells compared to the negative control (* p < 0.05).
4. Discussion
Pancreatic adenocarcinoma remains a treatment-refractory cancer. Management of pancreatic cancer remains the most challenging task in oncology. Though pancreatic cancer represents only 2–3% of all cancers, it is the most lethal one accounting for the 6% of all cancer- related death after lung, prostate and colorectal cancer since the 1970s in the USA (Saif, 2013). The past few decades have seen practically no treatment advances for patients with metastatic pancreatic cancer. Clinical features of PDA include late symptom onset, invasive growth, early liver and lymph node metastasis, and resistance to available chemotherapies (Neesse et al., 2014). Drug targeting with non-invasive techniques would be desirable, aiming at increased local drug concentration and reduced systemic side effects (Tinkov et al., 2010).
In our study, doxorubicin, a common drug used in the treatment of pancreatic cancer (Kratz et al, 2013), was approximately three times more potent that our recombinant disintegrins in inhibiting the proliferation of pancreatic cancer cells (Table 1). However, an important factor to consider is that the effectiveness of anticancer treatments is often hampered by the serious side effects due to toxicity of anticancer drugs and their undesirable uptake by healthy cells in vivo. Among the anticancer drugs, doxorubicin is well known for its cardiotoxicity and myelosupression effects (Kaiserová et al., 2006). Paclitaxel, has been associated with unwanted effects such as hypersensitivity reactions, myelosuppresion, and neurotoxicity, among others. Moreover, chemotherapy might turn inefficient due to acquired chemoresistance as exemplified in the case of Gemcitabine-prime therapeutic used to treat pancreatic cancer (Marelli et al., 2013). In this current study, a maximum proliferation inhibition on BXPC-3 cells of 45.5 % was observed with the higher concentration of gemcitabine used (1112.3 μM). Although doxorubicin was more potent that r-viridistatin 2 and r-mojastin 1, the possibility that our recombinant proteins are less toxic than doxorubicin exists, and this is due to the fact that disintegrins are non-enzymatic venom components, therefore having the tendency to be less invasive. Yet, they display interesting biological properties, such as modulate cell interaction with extracellular matrix (ECM) proteins. Furthermore, disintegrins have been shown to be non-toxic in therapeutic doses in rodent and avian models (Marcinkiewicz, 2013; Walsh and Marcinkiewicz, 2011). Moreover, r-viridistatin 2 and r-mojastin 1 were significantly more potent than paclitaxel and 5-fluorouracil, other common drugs used in the treatment of pancreatic cancer (Neesse et al., 2014; Saif, 2013).
PDA is characterized by a dense and desmoplastic stroma composed of fibrillar elements such as collagen I, fibronectin, laminin, and activated fibroblasts among others (Mahadevan and Von Hoff, 2007). Recombinant disintegrins inhibited the adhesion of pancreatic cancer cells to laminin and vitronectin. A remarkable result was the inhibition of BXPC-3 adhesion to vitronectin in presence of r-viridistatin 2, which was 15 times more potent than r-mojastin 1 (Figure 1). Taking account the high affinity of r-viridistatin 2 towards vitronectin, perhaps r-viridistatin 2 can be used as candidates for treat some cancers that contain connective tissue rich in vitronectin. The connective tissue is a not just a mechanical barrier but constitutes a dynamic compartment critically involved in the process of tumor formation, progression, invasion, and metastasis (Hidalgo, 2012; Jay et al., 2010). For example, high-grade glioblastomas abundantly express the extracellular matrix protein vitronectin along with the integrin αvβ3, and this interaction affects tumor cell survival and invasion. The relatively large quantity of vitronectin present in the brain’s microenvironment surrounding glioblastomas might explain why these tumors are susceptible to the αvβ3 inhibitor, cilengitide (Jay et al., 2010). Affinity and selectivity for RGD-disintegrins is determined by the amino acid adjacent to the active motif on the C-terminus (Walsh and Marcinkiewicz, 2011). r-Viridistatin 2 is a monomeric, medium size disintegrin with an RGDNP-motif region from the Prairie rattlesnake, while r-mojastin 1 is a monomeric, medium size disintegrin with an RGDWN-motif region from the Mohave rattlesnake (Lucena et al., 2014). There is a possibility that presence of different amino acids in their RGD-loop significantly changed the affinity and activity of these disintegrins to antagonize the binding of BXPC-3 cells to vitronectin. Interestingly, no differences between r-viridistatin 2 and r-mojastin 1 were found in the other biological activities performed in the current and in previous study (Lucena et al., 2014).
One important function of integrins is the promotion of cell migration by virtue of their binding to extracellular matrix components. This phenomenon is responsible for the process of tumor proliferation, migration, invasion, and metastasis (Marelli et al., 2013). r-Mojastin 1 and r-viridistatin 2 at 5 and 2.5 μM inhibited the migration of BXPC-3 cells after 24 h of incubation. In recent years, combined treatment of conventional anticancer agents with natural compounds has been a focus of study due to the fact that natural compounds are multi-targeted compared with designed mono-target agents and hence can overcome intrinsic cancer cell resistance to apoptosis (Wang and Yuan, 2013). Snake venom toxins contributed significantly to the treatment of many medical conditions. There are many published studies describing the anti-cancer potential of snake venom proteins (Vyas et al., 2013). In particular, the effect of RGD-disintegrins on various cancer cells has been largely studied in vitro and in vivo. Cancer metastasis has been studied in context of tumoral cell adhesion to the extracellular matrix, as well as cell migration and proliferation (Marcinkiewicz, 2013).
PDA express a certain pattern of functionally active integrins that enable interaction with most matrix proteins. For instance, BXPC-3 express α2, α3, α5, α6, αv as well as β3, β4, β1, and β6 (Lee et al., 2012; Löhr et al., 1996). Different antibodies against several integrins expressed on BXPC-3 were used to identify which receptor was recognized by the recombinant disintegrins. Adhesion of BXPC-3 cells to r-mojastin 1 and r-viridistatin 2 was inhibited by monoclonal antibodies against integrins αvβ3, α3, and β1, which suggest that these integrins on BXPC-3 cells can be a target for both recombinant disintegrins. The integrin αvβ3 is expressed in carcinomas with lymph node metastases, and it has been reported that the integrin interacts with c-Src kinase to process cellular signaling to metastatic tumor progression in humans (Rathinam and Alahari, 2010). In addition, expression of integrin αvβ3 is correlated with disease progression in pancreatic carcinoma (Hosotani et al., 2002). We had previously described the interaction of r-mojastin 1 and r-viridistatin 2 to αvβ3 integrin (Lucena et al., 2014). In mammalian cells, α3 subunit is associated with β1 subunit to form integrin α3β1. Among integrins, α3β1 is particularly interesting due to its role in development, wound healing, tumorigenesis, and metastasis (Desgrosellier and Cheresh, 2010; Janik et al., 2010). In addition, α3β1 integrin has been proposed to be involved with cell migration in different cellular systems including BXPC-3 cells (Tani et al., 1997). The laminin from Engelbreth-Holm-Swarm (EHS) tumor used in this study is usually considered laminin-1 (Patarroyo et al., 2002). Integrin α3β1 was originally identified as a promiscuos receptor for a range of ligands including collagen, fibronectin, entactin, laminin-5, thrombospondin-1 and the laminin used in this study, lamini-1 (Ren et al., 2014). Most integrins bind to several ECM molecules, and most ECM bind to more than one integrin heterodimer. However, specific combinations of integrin α and β subunit result in different binding specificity. Despite α3β1 being considered as a non-RGD dependent integrin, it can bind to some ECM such as fibronectin through the RGD motif (Xu and Mosher, 2011). Being considered a promiscuous receptor, it is no surprise that α3β1 integrin may be a target for recombinant mojastin 1 and viridistatin 2.
Cancer is characterized by uncontrolled cell division, cell transformation, invasion, angiogenesis, metastasis, and escape of apoptosis. Induction of apoptosis is the most important mechanism of many anticancer agents (Vyas et al., 2013). Both recombinant disintegrins, viridistatin 2 and r-mojastin 1, effectively induced apoptosis of pancreatic cancer cells (Figure 4). This can be the mechanism responsible for the inhibition of proliferation observed in the BXPC-3 cells in presence of the recombinant disintegrins, making these proteins prominent candidates for the development of drugs for the treatment of pancreatic cancer.
Conclusion
Current clinical approaches to treat cancer in general still fail to treat highly aggressive cancer, such as pancreatic cancer. Our study summarize the activity of two recombinant disintegrins on human pancreatic carcinoma, with special focus on some functional aspects of the metastasis process such as proliferation, adhesion, migration and survival through apoptosis. Tumor targeted drug-delivery, represents a promising approach to overcome some of the limitations found with the conventional treatments. In this regard, ligands that recognize specific integrins, such as r-mojastin 1 and r-viridistatin 2, can be excellent candidates for the conjugation to drugs or drug carrier systems that can be targeted to pancreatic cancer. Studies in vivo using a mouse model needs to be done in order to consider r-mojastin 1 and r-viridistatin 2 relevant for further development for the treatment of pancreatic cancer.
Supplementary Material
Highlights.
Recombinant venom disintegrins inhibit processes of pancreatic carcinoma (BXPC-3) metastasis
r-Viridistatin 2 and r-mojastin 1 inhibited the proliferation of BXPC-3 cells
r-Viridistatin 2 and r-mojastin 1 inhibited BXPC-3 cell migration in a dose dependent manner
r-Viridistatin 2 and r-mojastin 1 induced apoptosis of BXPC-3 cells by 22 and 21%, respectively
Acknowledgments
Funding for the project was provided by the NIH/Biological Materials Resource Grant, Viper Resource Grant #s 3P40OD010960-10S1 and 2P40OD010960-11A1 (NNTRC, Texas A&M University-Kingsville, Dr. E.E. Sanchez). Additional support was provided by the PPOHA instrumentation grant (Dr. E.E. Sánchez), the Robert A. Welch Foundation Department Grant, Grant number AC-0006 (TAMUK-Department of Chemistry), the United States Department of Agriculture STEP-UP Grant #2011-38422-30826 (Dr. Shad Nelson (TAMUK) and Dr. Jonda Halcomb (Del Mar College)) and the National Science Foundation, ATE Grant REVISION,DUE 1205059 (Dr. John Hatherill and Dr. Daisy Zhang), and Department of Education Title V grant DUE P031C110077. We would also like to thank Dr. Daisy Zhang (Del Mar College), Nora Diaz De Leon and Mark Hockmuller (NNTRC serpentarium curator) and all the NNTRC personnel.
Footnotes
Conflict of Interests
Authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JA, Sánchez EE, Rodríguez-Acosta A, Soto JG, Bashir S, McLane MA, Paquette-Straub C, Pérez JC. Inhibition of lung tumor colonization and cell migration with the disintegrin crotatroxin 2 isolated from the venom of Crotalus atrox. Toxicon. 2008;51:1186–1196. doi: 10.1016/j.toxicon.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi P, Peterson OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30. doi: 10.1007/112_2013_14. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23:135–138. doi: 10.1093/annonc/mds313. [DOI] [PubMed] [Google Scholar]
- Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphavbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- Kaiserová H, den Hartog GJM, Simunek T, Schröterová L, Kvasnicková E, Bast A. Iron is not involved in oxidative stress-mediated cytotoxicity of doxorubicin and bleomycin. Br J Pharmacol. 2006;149:920–930. doi: 10.1038/sj.bjp.0706930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik ME, Litynska A, Vereecken P. Cell migration-The role of integrin glycosylation. Biochim Biophys Acta. 2010;1800:545–555. doi: 10.1016/j.bbagen.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Jay S, Desgrosellier S, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz F, Azab S, Zeisig R, Fichtner I, Warnecke A. Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model. Int J Pharm. 2013;441:499–506. doi: 10.1016/j.ijpharm.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Lee CN, Heidbrink JL, McKinnon K, Bushman V, Olsen H, FitzHugh W, Li A, Van Orden K, He T, Ruben SM, Moore PA. RNA interference characterization of proteins discovered by proteomic analysis of pancreatic cancer reveals function in cell growth and survival. Pancreas. 2012;41:84–94. doi: 10.1097/MPA.0b013e3182236385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr M, Trautmann B, Göttler M, Peters S, Zauner I, Maier A, Klöppel G, Liebe S, Kreuser ED. Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996;12:248–259. doi: 10.1097/00006676-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Lucena SE, Jing Yia, Soto JG, Parral J, Cantu E, Brannon J, Lardner K, Ramos CJ, Seoane AI, Sánchez EE. Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60:31–39. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena SE, Romo K, Suntravat M, Sánchez EE. Anti-angiogenic activities of two recombinant disintegrins derived from the Mohave and Prairie rattlesnakes. Toxicon. 2014;78:10–17. doi: 10.1016/j.toxicon.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int J Biochem Cell Biol. 2013;45:1974–1986. doi: 10.1016/j.biocel.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor targeting via integrin ligands. Front Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TL, Sussel L. The endocrine pancreas: insights into development, differentiation, and diabetes. WIREs Dev Biol. 2012;1:609–628. doi: 10.1002/wdev.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neesse A, Michl P, Tuveson DA, Ellenrieder V. nab-Paclitaxel: Novel Clinical and Experimental Evidence in Pancreatic Cancer. Z Gastroenterol. 2014;52:360–366. doi: 10.1055/s-0034-1366002. [DOI] [PubMed] [Google Scholar]
- Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Sem Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- Ren Y, Hao P, Law SK, Sze SK. Hypoxia-induced changes to integrin alpha 3 glycosylation facilitate invasion in epidermoid carcinoma cell line A431. Mol Cell Proteomics. 2014 doi: 10.1074/mcp.M114.038505. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MW. Advancements in the management of pancreatic cancer: 2013. JOP. 2013;14:112–118. doi: 10.6092/1590-8577/1481. [DOI] [PubMed] [Google Scholar]
- Saif MW. Pancreatic Neoplasm in 2011: An Update. JOP J Pancreas. 2011;12:316–321. [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galán JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the Mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb Res. 2010;126:e211–e219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez EE, Rodriguez AA, Palomar R, Lucena SE, Bashir S, Soto JG, Pérez JC. Colombistatin: A disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-MEL-28 cell adhesion. Arch Toxicol. 2009;83:271–27. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am J Pathol. 1997;151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
- Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: In-vivo characterization. J Control Release. 2010;148:368–372. doi: 10.1016/j.jconrel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas VK, Brahmbhatt K, Bhatt H, Parmar U. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pac J Trop Biomed. 2013;3:156–162. doi: 10.1016/S2221-1691(13)60042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EM, Marcinkiewicz C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon. 2011;58:355–362. doi: 10.1016/j.toxicon.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Yuan Z. Gambogic Acid Sensitizes Ovarian Cancer Cells to Doxorubicin Through ROS-Mediated Apoptosis. Cell Biochem Biophys. 2013;67:199–206. doi: 10.1007/s12013-013-9534-7. [DOI] [PubMed] [Google Scholar]
- Xu J, Mosher D. Fibronectin and other adhesive glycoproteins. In: Mecham RP, editor. The extracellular matrix: an overview. Spring; New York: 2011. pp. 41–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.