Abstract
Objective
How best to capture heterogeneity in attention-deficit/hyperactivity disorder (ADHD) using biomarkers has been elusive. This study evaluated whether emotion reactivity and regulation provide a means to achieve this.
Method
Participants were classified into three groups: children with ADHD plus low prosocial behavior (hypothesized to be high in callous/unemotional traits; n = 21); children with ADHD with age-appropriate prosocial behavior (n = 54); and typically developing children (n = 75). Children completed a task with four conditions: negative induction, negative suppression, positive induction, and positive suppression of affect. The task required children to view an emotion-laden film clip, while either facially mimicking (induction) or masking (suppression) the emotion of the main character. Parasympathetic and sympathetic nervous system activity were assessed via respiratory sinus arrhythmia (RSA) and cardiac pre-ejection period (PEP), respectively. Symptoms of anxiety, conduct, and oppositional defiant disorders were treated as covariates.
Results
The ADHD-typical-prosocial group displayed atypically elevated parasympathetic reactivity (emotion dysregulation) during positive induction, along with increased sympathetic activity (elevated arousal) across conditions. In contrast, the ADHD-low-prosocial group displayed reduced parasympathetic reactivity and reduced sympathetic activity (low emotional arousal) across baseline and task conditions. Thus, both ADHD groups had altered patterns of autonomic functioning, but in two distinct forms.
Conclusion
Although ADHD is heterogeneous clinically, results suggest that ADHD is also heterogeneous with regard to physiological indices of emotion and regulation. Future studies of emotion, regulation, and ADHD should take this into account. Further study of physiological responding in ADHD may yield clinically and etiologically distinct domains or groups.
Keywords: attention-deficit/hyperactivity disorder, autonomic nervous system, callous/unemotional traits, emotionality, emotion regulation
Attention-deficit/hyperactivity disorder (ADHD) is a clinically heterogeneous condition; however, its presumed biological heterogeneity remains in need of elucidation.1–3 Emotion reactivity and dysregulation may provide a promising way to achieve this.4–8 The present study evaluated the heterogeneity in ADHD using physiological indicators of emotion reactivity and regulation.
One suggestion for how to conceptually organize the interpretation of emotion and regulation data was put forward by Beauchaine.9 This formulation suggests that respiratory sinus arrhythmia (RSA) can be understood as an index of parasympathetic-based regulation, and cardiac pre-ejection period (PEP) as an index of sympathetic arousal. The idea that RSA reactivity is an index of parasympathetic regulation, albeit heuristic, has been proposed by several theories9–13 and has received empirical support.14–17 The idea that PEP is an index of sympathetic arousal, although less extensively suggested in the literature, has support with regard to its sympathetic and β-adrenergic origins18,19 and sensitivity to reward.20 We have adopted this perspective because of its heuristic value in the current study.
These measures of autonomic reactivity in child psychopathology have been examined before. Indeed, an association of externalizing behavior with reduced sympathetic activity and altered parasympathetic response during emotion-based tasks and reward is rather well established.22–27 However, it cannot be assumed that these same results will hold for ADHD. One study concluded that ADHD was also associated with reduced sympathetic activity during baseline.21 In a separate study of preschoolers, autonomic responses to reward tasks were characterized by lengthened PEP, as well as parasympathetic withdrawal (dys-regulation) in children with both ADHD and oppositional defiant disorder (ODD), suggesting that emotion dysregulation may be associated with both ADHD and ODD in young children.26 Furthermore, altered parasympathetic nervous system reactivity has also been observed during both the induction and suppression of negative and positive affect in ADHD, with larger effects in the positive domain.29 Thus, it is possible that physiological indices may help to clarify the heterogeneity in ADHD and reconfirm previous findings of elevated reactivity in response to emotional challenge.
In addition to the emerging role that emotion dysregulation has been hypothesized to play in ADHD, a particular ambiguity in ADHD concerns whether ADHD is characterized by over- or underarousal.8,9,26–29 One group of children who reliably show underarousal is children with callous/unemotional traits (CU).30–35 These children are deficit in prosocial emotions and behaviors, including low empathy, lack of a sense of guilt or remorse, shallow or blunted affect, and physiological underarousal.30–35 In this study, we examine children with ADHD who differed on their prosocial behavioral phenotype (an empirically valid, and perhaps less stigmatizing proxy for CU traits) and validate this physiologically in an effort to clarify differences in arousal and emotion regulation.
In previous studies, CU traits have been studied primarily in the context of conduct disorder and antisocial behavior,30–35 but, to a lesser degree, in children with ADHD. Experts have called for more careful consideration of CU in relation to ADHD.36–38 Indeed, CU traits are associated with ADHD even after controlling for comorbid conduct disorder.36–40 Thus, CU traits (and low prosocial behavior) are clinically important and perhaps theoretically informative for understanding heterogeneity of emotional arousal and regulation in ADHD.
It was hypothesized that variation in prosocial behavior will index biologically distinctions in ADHD on the basis of emotional arousal and regulation. To test this hypothesis, we assessed prosocial behavior and physiological indicators of emotional arousal and regulation in children with ADHD, excluding children with comorbid conduct disorder. We tested a double dissociation that may resolve prior contradictory conclusions about arousal and may also clarify the nature of emotion regulation variation in ADHD. Specifically, children with ADHD with age-typical pro-social behavior (here termed “ADHD”) were predicted to have a pattern of parasympathetic reactivity that is characteristic of ADHD per se, which is to say, elevated parasympathetic activity from baseline across the affective and regulatory demands of an emotional task. If so, this would support prior findings of atypical regulation in response to emotional challenge among children with ADHD.21,26,27 In contrast, we hypothesized that children with ADHD and low prosocial behaviors (here termed “ADHD-low-prosocial”) would show a pattern more similar to that of past research on antisocial youths with CU traits—namely, reduced parasympathetic reactivity across task conditions along with reduced sympathetic activity (lower arousal).30,31
METHOD
Participants
Overview
Participants were 150 children 7 to 11 years of age (mean age = 7.60 years, SD = 0.56 years); 75 met DSM-IV41 criteria for ADHD combined type, and 75 were typically developing comparison youth (Table 1). Of the ADHD group, we assigned 21 to the ADHD-low-prosocial group (criteria outlined below). None of the control group had atypical prosocial behavior scores. By design, none of the children in met DSM-IV criteria for conduct disorder.
TABLE 1.
Descriptive and Diagnostic Statistics for Attention-Deficit/Hyperactivity Disorder (ADHD) and Control Groups
| Variable | Control (n = 75) | ADHD
|
|
|---|---|---|---|
| ADHD Only (n = 54) | ADHD + Low Prosocial (n = 21) | ||
| Demographic | |||
| Age, mo, mean (SD) | 97.41 (6.91) | 97.30 (7.08) | 97.63 (8.83) |
| Gender, % male | 49.3a | 52.6a | 70.4b |
| Race/ethnicity, % white | 88.0 | 86.2 | 89.5 |
| Family income, $K, mean (SD) | 100.35 (46.23) | 84.81 (41.43) | 98.06 (51.48) |
| % Two-parent homes | 86.7 | 79.6 | 75.7 |
| Stimulant med, % on med | 0.00a | 29.6b | 26.3b |
| WISC-IV FSIQ, mean (SD) | 109.11 (5.19) | 107.14 (6.84) | 104.85 (5.91) |
| SDQ Subscales–Parent, mean (SD) | |||
| Emotional symptoms | 1.34 (1.57) | 1.86 (2.04) | 2.11 (1.60) |
| Conduct problems | 0.76 (1.28)a | 2.26 (1.60)b | 2.89 (1.91)b |
| Hyperactivity | 2.32 (2.40)a | 7.72 (2.07)b | 7.67 (2.93)b |
| Peer problems | 0.81 (1.26)a | 2.18 (2.11)b | 2.28 (1.45)b |
| Prosocial behavior | 8.97 (1.43)a | 8.24 (1.90)a | 4.67 (1.46)b |
| Total difficulties | 5.23 (5.04)a | 14.02(5.22)b | 14.94 (4.68)b |
| Impact/impairment score | 0.23 (0.82)a | 2.65 (1.98)b | 3.12 (2.04)b |
| SDQ Subscales–Teacher, mean (SD) | |||
| Emotional symptoms | 1.23 (1.54) | 1.44 (1.87) | 1.56 (1.95) |
| Conduct problems | 0.33 (1.11)a | 1.83 (1.91)b | 2.22 (2.77)b |
| Hyperactivity | 1.51 (1.93)a | 7.15 (2.38)b | 6.50 (3.17)b |
| Peer problems | 0.77 (1.06)a | 1.91 (1.94)b | 2.20 (2.21)b |
| Prosocial behavior | 8.19 (1.83)a | 7.37 (2.47)a | 4.29 (1.88)b |
| Total difficulties | 3.43 (3.95)a | 12.23 (5.15)b | 14.79 (7.13)b |
| Impact/impairment score | 0.14 (0.65)a | 1.83 (1.45)b | 1.89 (1.88)b |
| Comorbid Disorders, %, K-SADSc | |||
| Mood disorder (lifetime) | 2.7 | 3.7 | 3.7 |
| Anxiety disorder | 21.3a | 23.9a | 4.7b |
| CD | 0.0 | 0.0 | 0.0 |
| ODD | 8.1a | 24.7b | 23.8b |
| Tic disorder | 0.0 | 3.7 | 0.0 |
| Sleep disorder | 5.4 | 7.1 | 4.7 |
| CD symptoms, mean (SD), K-SADS | 0.02 (0.13)a | 0.09 (0.25)ab | 0.18 (0.42)b |
| ODD symptoms, mean (SD), K-SADS | 0.44 (1.15)a | 1.29 (1.86)b | 1.42 (1.1.84)b |
| Total anxiety Sx, mean (SD), K-SADS | 1.31 (1.59) | 2.31 (4.19) | 1.19 (2.45) |
Note: CD = conduct disorder; FSIQ = Full-Scale Intelligence Quotient (estimated); K-SADS = Kiddie Schedule of Affective Disorders and Schizophrenia; med. = medication; ODD = oppositional defiant disorder; Sx = symptoms; WISC-IV = Wechsler Intelligence Scales for Children.
Differing superscripts indicate pairwise comparisons that were significant after a modified Bonferroni correction for multiple group comparisons (α = 0.025) for continuous variables, including: age, family income, estimated full-scale IQ, Strengths and Difficulties Questionnaire (SDQ) parent and teacher subscales, and comorbid symptoms; and χ2comparisons for categorical variables, including gender, race, parent marital status, child stimulant medication status, and presence of comorbid disorders.
None (0%) of the sample had autism, eating disorders, learning disorders, post-traumatic stress disorder, psychosis, or substance use disorders.
Families were recruited from the community through advertisements and mailings. The local Institutional Review Board approved the study. All procedures conformed to the Ethical Principles of Psychologists and Code of Conduct.42 Parents provided written informed consent, and children provided written assent.
Recruitment and Identification
Sample recruitment, assessment, and diagnostic assignment followed procedures identical to those described in more detail elsewhere.27 In brief, volunteers passed through a multi-gate screening process to establish eligibility and group assignment. After completing a clinical structured diagnostic interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version [KSAD-S-E]), parent and teacher standardized ratings, and an IQ screen, a clinical diagnostic team comprising a board-certified psychiatrist and licensed clinical psychologist independently reviewed all case information to arrive at diagnoses using DSM-IV criteria. Agreement rates were acceptable (κ > 0.70 for all disorders with base rate of greater than 5%, including ADHD). Cases were excluded if agreement was not readily achieved.
Exclusion Criteria
Exclusion criteria for included the use of long-acting psychoactive medications (except stimulants), neurological impairments, seizures, traumatic brain injury, major medical conditions, mental retardation, pervasive developmental disorders, conduct disorder, current mood disorder, lifetime psychosis, or current learning disability. Other disorders were free to vary.
Identification of Social–Emotional Groups
A parent and a teacher of eligible youth completed the 25-item Strengths and Difficulties Questionnaire (SDQ), a well-validated, well-normed survey of child problems and impairment.43 The Prosocial Behavior scale (inverted) served as a measure of social–emotional maladjustment; it has been previously shown to be a reliable index of CU traits and to load on the same factor in factor analyses of CU measures.44,45 We specifically chose the SDQ because we wished to evaluate clinical severity in identifying our subgroups, as it has extensive published norms.43 To confirm the convergent validity of the measure of prosocial behavior in our sample, a subsample of parents (n = 90; 52 ADHD, 38 control) completed the Inventory of Callous/Unemotional Traits (ICU).46 Total score on the ICU was correlated negatively with the parent SDQ Prosocial Behavior domain at r = 0.742, p < .001 (reliability-corrected r = 0.853, p < .001). We therefore used the SDQ to identify our clinically low-prosocial group.
To be classified as ADHD-low-prosocial behavior, both the parent and teacher had to endorse a lack of prosocial behaviors in the 90th percentile. This was intended to select children who likely had clinically significant problems in this area. Clinical and demographic features of the groups are provided in Table 1.
Medication Washout
All children who were prescribed stimulant medications were required to complete a washout period of at least five half-lives. All children were stimulant medication free at the time of testing. Twenty-three ADHD children (31% of the ADHD group) were prescribed stimulants. Stimulant prescription status (present or absent) was treated as a covariate to remove the effect of the medication wash-out (e.g., rebound effects), with no effect on results. Results are presented without covarying medication status.
Emotion Induction and Suppression Procedure
The emotion task and physiological recording procedures were identical to those reported by Musser et al.,27 where they are described in detail. In brief, each child underwent an emotion induction and suppression procedure using both negative and positive emotion-laden film clips.
Four experimental conditions were presented in the same order for all children. In the induction condition, children were to facially mimic the emotion of the main character. In the suppression condition, children were to mask (suppress) the emotion. Each child had the same sequence as follows: negative induction, negative suppression, positive induction, and positive suppression of emotion.
To confirm that “positive” and “negative” film clips had the intended valence, all children completed the Self-Assessment Manikin (SAM) valence and arousal scales47 for each clip; all groups equally strongly rated the four conditions as differing in valence and arousal (Table S1, available online).
Baseline Conditions
A resting baseline of 2 minutes was presented before the task. A neutral baseline of 2 minutes was presented between the negative and positive task conditions. The neutral baseline consisted of observing a set of 10 neutral pictures from the International Affective Picture System (IAPS).48 Children again completed the SAM ratings (of the IAPS neutral pictures), and groups did not differ in their SAM ratings of the neutral pictures, all F < 1.0; all p > .10. All three groups rated them as more neutral than the positive and negative conditions (Table S1, available online).
Physiological Recording
Overview
Disposable silver or silver chloride electrodes were placed in an electrocardiography (ECG) and impedance cardiography (ICG) configuration. The ECG electrodes were placed at the right collar bone and the 10th-left rib with a ground electrode placed at the 10th-right rib. For ICG, two voltage electrodes were placed below the suprasternal notch and xiphoid process, and two current electrodes were placed on the back 3 to 4 cm outside the voltage electrodes. ECG and ICG recordings were made throughout each of the baselines and task epochs. The R-R series was sampled at 1,000 Hz. Interbeat interval and respiration rate data were derived using the ECG and ICG data.
Cardiac Pre-ejection Period
The cardiac pre-ejection period (PEP) was derived from ECG and ICG, in 60-second epochs, using MindWare Impedance Cardiography V. 2.6.49 PEP was indexed as the time interval in milliseconds from the onset of the Q-wave to the B-point of the dZ/dt wave.50 Artifacts were examined and removed using the completed by two raters (κ > 0.85 for each epoch). There were no between-group differences in the rate of artifacts (all p > .50).
Respiratory sinus arrhythmia
Respiratory sinus arrhythmia (RSA) was indexed by extracting the high frequency component (>0.15 Hz) of the R-R series. R-R waves were examined for artifacts and outliers using MindWare Heart Rate Variability software V. 2.6.51 Artifacts were removed using the software by two raters (all κ > 0.91). Again, there were no group differences in the rate of artifacts (all p > .50). RSA was derived using spectral analysis, in 60-second epochs. Spectral analysis was performed on the R-R time series from the ECG. The time series was detrended and submitted to a Fourier transformation. The high-frequency band (ln [ms2]) was set over the respiratory frequency band of 0.24 to 1.040 Hz. Respiratory rates and amplitudes were derived from the impedance cardiograph signal (Z0).
Analytic Decisions and Plan
Main Hypothesis Tests of Group Comparisons
Group comparisons were conducted using mixed-model, repeated-measures analysis of covariance (ANCOVA). Thus, simple effects for RSA and PEP were tested only when justified by the results of higher-order effects, and no further corrections were implemented.
Group Versus Dimensional Analysis
Analyses were also completed with ADHD group status and continuous prosocial behavior scores as independent variables (as prosocial behavior or CU may be continuous traits, and group cutoffs were a heuristic). The overall results of the analyses did not differ when prosocial behavior was treated as a continuous variable. Although creating groups rather than using continuous scores may reduce power, we chose to present the findings using group assignments because the overall outcomes did not differ, and because the group assignments enable visualization across different subgroups within ADHD. (The results for a dimensional approach are available to interested readers upon request to the first author. Further validity checks are provided in Supplement 1, available online.)
RESULTS
Preliminary Analyses
Descriptive and Diagnostic Overview of Sample
Descriptive statistics and comparisons are reported in Table 1. Groups did not differ with respect to age, race/ethnicity, family income, parent marital status, or IQ, nor did inclusion of any of these variables as covariates affect the results reported. Results are therefore reported without these variables treated as covariates. Groups differed in gender ratio. Gender was unrelated to physiological parameters. However, to remove ambiguity, gender was covaried in all results.
Clinical characteristics are provided in Table 1. Although the ADHD groups had more ODD than the control group, the two ADHD groups did not differ on incidence of ODD. The ADHD-low-prosocial behavior group had significantly fewer anxiety disorders than either the control or ADHD-only group, consistent with past findings that anxiety and CU traits are inversely related.52,53 The inclusion of comorbid disorders as covariates did not affect any of the main study results, nor did covarying of total ODD symptoms, total conduct disorder (CD) symptoms, or total anxiety symptoms (K-SADS-E). For clarity, we present results with CD, ODD, and anxiety symptoms covaried (as well as gender). Results from models without covariates are available upon request and did not differ from results presented here.
Primary Analyses: Effects of Emotion Induction and Suppression on PEP and RSA
RSA and PEP data for all task conditions and baselines are listed in Table 2.
TABLE 2.
Respiratory Sinus Arrhythmia (RSA; ms2) and Pre-Ejection Period (PEP; ms) in Task Epochs for Attention-Deficit/Hyperactivity Disorder (ADHD) and Control Groups
| Variable | Control (n = 75) | ADHD
|
|
|---|---|---|---|
| ADHD Only (n = 54) | ADHDþCU (n = 21) | ||
| Baseline Physiology Data | |||
| Rest baseline | |||
| RSA | 7.07 (0.86) | 7.22 (0.93) | 7.23 (0.83) |
| PEP | 98.64 (8.07) | 96.02 (8.27) | 99.55 (6.91) |
| Picture baseline 1 | |||
| RSA | 6.66 (0.86) | 6.93 (0.93) | 6.99 (0.83) |
| PEP | 97.38 (5.58) | 93.04 (6.24) | 101.10 (5.14) |
| Task Physiology Raw Scores | |||
| Negative induction | |||
| RSA | 6.98 (0.80) | 7.21 (0.67) | 7.28 (0.67) |
| PEP | 97.59 (7.49) | 94.40 (8.02) | 101.17 (5.73) |
| Negative suppression | |||
| RSA | 7.12 (0.75) | 7.25 (0.75) | 7.00 (0.66) |
| PEP | 97.45 (7.59) | 95.09 (7.71) | 99.31 (5.44) |
| Positive induction | |||
| RSA | 6.61 (0.79) | 7.09 (0.83) | 6.98 (0.71) |
| PEP | 98.18 (7.33) | 94.21 (7.55) | 98.04 (5.35) |
| Positive suppression | |||
| RSA | 6.90 (0.79) | 7.23 (0.82) | 7.04 (0.74) |
| PEP | 98.18 (7.45) | 95.51 (8.27) | 98.34 (6.32) |
| Task Physiology Change Scores | |||
| Negative induction | |||
| RSA | 0.33 (0.48) | 0.25 (0.28) | 0.06 (0.33) |
| PEP | 0.61 (4.82) | 0.98 (4.22) | 1.58 (4.96) |
| Negative suppression | |||
| RSA | 0.43 (0.52) | 0.32 (0.58) | 0.06 (0.48) |
| PEP | 0.59 (4.59) | 1.58 (4.62) | 0.62 (4.34) |
| Positive induction | |||
| RSA | 30.05 (1.12) | 0.15 (0.33) | 30.05 (0.35) |
| PEP | 0.88 (3.75) | 1.51 (3.74) | 30.71 (4.55) |
| Positive suppression | |||
| RSA | 0.25 (0.58) | 0.26 (0.58) | 30.06 (0.54) |
| PEP | 1.16 (4.09) | 1.68 (4.49) | 30.11 (3.86) |
Note: Repeated-measures analysis of variance was used to examine group differences in RSA and PEP reactivity scores across the emotion induction and suppression conditions. CU = callous/unemotional traits.
Effects on Sympathetic Arousal (PEP)
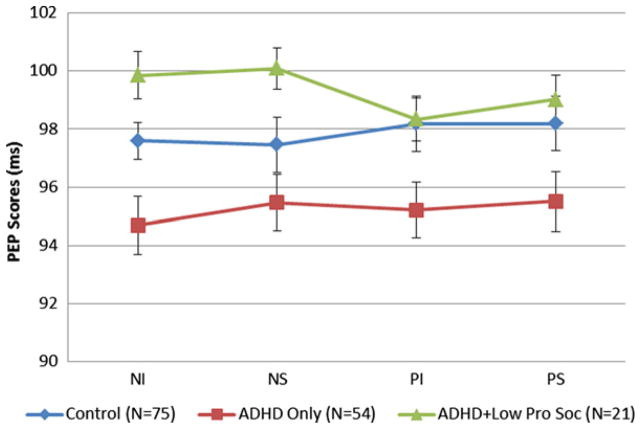
A 2×2×3 repeated-measures ANCOVA examined the effects of task condition on raw scores for PEP with covariates as noted. The interaction of valence (negative versus positive) by condition (induction versus suppression) by group (control versus ADHD versus ADHD-low-prosocial) was nonsignificant (F < 1.0, p > .4). However, there was a group main effect (F1,149 = 3.16, p < .05, η2 = 0.05). Specifically, the grand mean of PEP for the control group (97.85, SD = 7.42) was longer than for the ADHD group (95.22, SD = 7.91), but shorter than for the ADHD-low-prosocial behavior group (99.10, SD = 5.71), consistent with the hypothesis that low prosocial behavior would be associated with lower sympathetic arousal. These data are presented in Table 2 and Figure 1.
FIGURE 1.
Mean cardiac pre-ejection period (PEP) raw scores for each task epoch: negative induction (NI), negative suppression (NS), positive induction (PI), and positive suppression (PS) for control, attention-deficit/ hyperactivity disorder (ADHD), and ADHD-low-prosocial behavior groups. Note: Standard error estimates were obtained via analysis of variance.
Effects on Parasympathetic-Based Emotional Regulation (RSA)
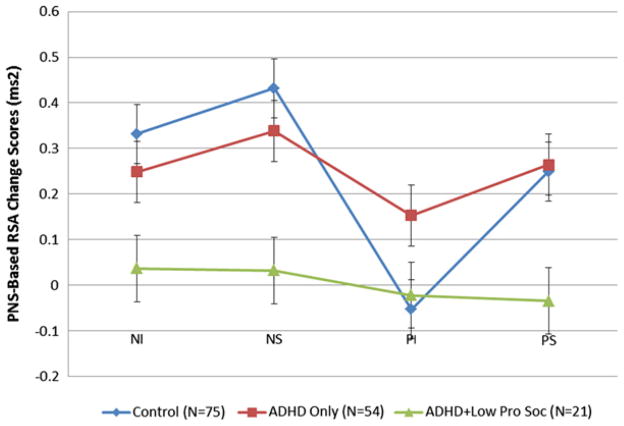
The mixed-model, 2×2×3, repeated-measures ANCOVA (with gender, CD, ODD, and anxiety symptoms treated as covariates) revealed a significant three-way interaction of valence (negative versus positive) by condition (induction versus suppression) by group (control versus ADHD versus ADHD-low-prosocial) (F1,149 = 3.748, p < .01, η2 = 0.04). Simple effects revealed that the ADHD-low-prosocial group showed a smaller increase than the ADHD and control group during negative induction (t[91] = 2.97, p < .05 and t[74] = 2.41, p < .05, respectively; Figure 2) and negative suppression (t[91] = 3.07, p < .05 and t[74] = 2.08, p < 0.05, respectively; Figure 2). Thus, the children in the ADHD-low-prosocial group did not increase their parasympathetic regulation, whereas both the ADHD and typically developing children did.
FIGURE 2.
Mean respiratory sinus arrhythmia (RSA) change scores from baseline to each of the task epochs: negative induction (NI), negative suppression (NS), positive induction (PI), and positive suppression (PS) for control, attention-deficit/hyperactivity disorder (ADHD), and ADHD-low-prosocial behavior groups. Note: Standard error estimates were obtained via analysis of variance.
Furthermore, in the positive induction condition, the ADHD group s RSA increased from baseline (suggesting regulatory demand was activated), while the control and ADHD-low-prosocial behavior group’s RSA decreased from baseline (suggesting no regulatory demand), suggesting that children with ADHD (but not low-prosocial) had a more challenging time regulating positive emotions (t[129] = 1.96, p < .05 and t[74] = 2.25, p < .05, respectively; Figure 2). A similar picture emerged during the positive suppression condition: RSA increased from baseline for the control and ADHD group, and this differed from that in the ADHD-low-prosocial behavior group, which did not change from baseline (t[129] = 2.29, p < .05 and t[74] = 2.27, p < .05, respectively; Figure 2). All other simple effects comparisons were nonsignificant (all t values < 1.0).
DISCUSSION
Grouping children with ADHD on the basis of a clinical indicator of atypical levels of prosocial behavior revealed distinct patterns of autonomic reactivity during emotion regulation demands, suggesting that the autonomic response validates this aspect of behavioral heterogeneity in ADHD. The cut-point of 90th percentile received physiological validation: however, other cut-points were not examined. Performance at different cut-points is a future direction. However, results did not depend on this cut-point, as the same results were observed using dimensional analyses. These results also were not explained by associated conduct problems, conduct disorder, other comorbid symptoms or disorders, sex, age, prescription medication status, or conceivable methodological artifacts (i.e., order effects). Thus, the most parsimonious conclusion is that variation in emotional processing distinguishes among children with ADHD and is a promising way forward for capturing heterogeneity in ADHD.
Children with ADHD and clinically significant low prosocial behavior displayed blunted parasympathetic and sympathetic activity both at baseline and across task conditions, consistent with tonic reduction of autonomic arousal, even though these children did not have conduct disorder and the effects held after covarying conduct symptoms. This reduced autonomic activity is consistent with past research on low-prosocial children with antisocial behavior. In contrast, children with ADHD and age-typical prosocial behavior displayed elevated sympathetic activity (i.e., high arousal).
These children also had the strongest activation of their parasympathetic system (largest parasympathetic increase from baseline of all the groups), during the positive emotion induction condition, suggesting that the experience of positive emotions introduces additional regulatory demand for these children. In fact, that this occurred most during the positive condition, may support theories of approach (or reward processing) alteration in ADHD.29,54,55
Several alternative interpretations of these data were ruled out, in addition to ruling out effects of comorbid disorders and symptoms. As described above, sympathetic nervous system activity differed across the groups during baseline conditions, and these differences held regardless of task condition. This is consistent with the view that sympathetic nervous system activity/arousal is reduced tonically in individuals with CU traits.56,57 In contrast, differences in parasympathetic nervous system activity held even after controlling for baseline effects, suggesting that these differences were not due to pre-existing parasympathetic differences in homeostatic functioning but rather to regulatory differences. Furthermore, children’s self-reported emotion valence and arousal levels during the emotionally neutral task suggested no pre-existing differences in emotion or task engagement among the groups.
These results are broadly consistent with past research findings in children with conduct disorder and co-occurring CU traits, in which CU traits have been shown to moderate emotional responding.53 However, this study breaks new ground in isolating the effects of prosocial behavior (i.e, CU traits) in ADHD. This design was intended to facilitate parsing the unique heterogeneity of ADHD by examining a specific subgroup of children with ADHD and altered social–emotional functioning. These findings build upon other studies that report differences in sympathetic functioning among children with ADHD and comorbid conduct problems when compared to typically developing youth, but which found attenuated sympathetic activity in response to reward-based tasks.26,27,58
Results should be interpreted in light of key limitations. First, the sample size, albeit larger than most physiological studies of ADHD, was not designed to have the power to test diagnosis-by-sex interactions or other subgroups of ADHD, including those with comorbid disorders. It will be interesting to examine children with comorbid CD and ADHD or comorbid anxiety and ADHD, for example. Furthermore, as this sample was recruited from the community, the clinical severity of both prosocial behavior deficits and ADHD may be less than a sample recruited from clinics or forensic samples, although the prosocial behavior levels used to designate a distinct group were quite low for the general population in that very few children would be rated above the 90th percentile in this domain by both parents and teachers. To evaluate the clinical meaningfulness of this finding, it will be of interest whether the patterns observed are stable over time or are predictive of course, impairment, response to treatment, the development of comorbid disorders, and other clinical outcomes. From a clinical and public health perspective, caution should be used in diagnosing children low in prosocial behaviors who do not have conduct disorder, until more research is done to determine their level of clinical impairment.37
In conclusion, this study revealed that when children with ADHD are divided according to social–emotional functioning (in this case, indexed by the prosocial scale of the SDQ), distinct patterns of autonomic responding within the ADHD population may be revealed. This clarifies that emotion reactivity and regulation alterations are features of ADHD differentially across children with the syndrome, and this variation can be validated and characterized physiologically. Two groups of children with ADHD had altered autonomic functioning compared with typically developing youth, but the two ADHD groups differed from typically developing children in quite different and easily distinguished ways. ADHD was associated with disruptions in emotional processing during positive emotions (as indexed by overactive parasympathetic activity), but this effect was masked in a subgroup who also had low levels of prosocial behavior. In turn, the latter group displayed a pattern of blunted autonomic functioning in both branches. Future studies of ADHD and emotional regulation must take into account this dramatic heterogeneity.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health grant R01 MH59105 (Principal Investigator: J.T.N.).
Footnotes
Disclosure: Drs. Frick and Nigg, Ms. Musser, and Ms. Galloway-Long report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Ms. Erica D. Musser, The Oregon Health and Science University, The University of Oregon
Ms. Hilary S. Galloway-Long, The Oregon Health and Science University
Dr. Paul J. Frick, The University of New Orleans
Dr. Joel T. Nigg, The Oregon Health and Science University
References
- 1.Barkley RA. ADHD and the Nature of Self-Control. New York, NY: Guilford Press; 1997. 2006. [Google Scholar]
- 2.Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 3.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry. 2010;49:503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Martel MM. A new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models [research review] J Child Psychol Psychiatry. 2009;50:1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 6.Martel MM, Nigg JT. Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. J Child Psychol Psychiatry. 2006;47:1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 7.Anastopoulos AD, Sommer JL, Schatz NK. ADHD and family functioning. Current Attention Disorders Reports. 2009;1:167–170. [Google Scholar]
- 8.Marx I, Domes G, Havenstein C, Berger C, Schulze L, Herpertz SC. Enhanced emotional interference on working memory performance in adults with ADHD. World J Biol Psychiatry. 2011;12:70–75. doi: 10.3109/15622975.2011.599213. [DOI] [PubMed] [Google Scholar]
- 9.Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 10.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 11.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. a polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 12.Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. In: Fox NA, editor. Monographs of the Society for Research in Child Development. Vol. 59. Boston: Blackwell; 1994. pp. 167–186. [PubMed] [Google Scholar]
- 13.Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 15.Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Dev. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- 16.Frazier TW, Strauss ME, Steinhauer SR. Respiratory sinus arrhythmia as an indicator of emotional response in young adults. Psychophysiology. 2004;41:75–83. doi: 10.1046/j.1469-8986.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 17.Sloan DM, Epstein EM. Respiratory sinus arrhythmia predicts written disclosure outcome. Psychophysiology. 2005;42:611–615. doi: 10.1111/j.1469-8986.2005.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. Noninvasive indices and basal responses as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 19.Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 20.Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- 22.Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg N, Guthrie IK, Fabes RA, et al. The relations of regulation and emotionality to resiliency and competent social functioning in elementary school children. Child Dev. 1997;68:295–311. [PubMed] [Google Scholar]
- 24.Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. Am J Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- 25.Marsh P, Beauchaine TP, Williams B. Dissociation of sad facial expressions and autonomic nervous system responding in boys with disruptive behavior disorders. Psychophysiology. 2008;45:100–110. doi: 10.1111/j.1469-8986.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/ hyperactivity disorder and oppositional defiant disorder in pre-school children. J Abnorm Psychol. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- 27.Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) J Abnorm Child Psychol. 2011;39:841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Meere JJ. The role of attention. In: Sandberg S, editor. Monographs on Child and Adolescent Psychiatry: Hyperactivity Disorders of Childhood. Cambridge, UK: Cambridge University Press; 1997. pp. 109–146. [Google Scholar]
- 29.Van der Meere J, Marzocchi GM, De Meo T. Response inhibition and attention deficit hyperactivity disorder with and without oppositional defiant disorder screened from a community sample. Dev Neuropsychol. 2005;28:459–472. doi: 10.1207/s15326942dn2801_1. [DOI] [PubMed] [Google Scholar]
- 30.Barry CT, Frick PJ, DeShazo TM, McCoy M, Ellis M, Loney BR. The importance of callous-unemotinoal traits for extending the concept of psychopathy to children. J Abnorm Psychol. 2000;109:335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- 31.Marsh AA, Finger EC, Schechter JC, Jurkowitz ITN, Reid ME, Blair RJ. Adolescents with psychopathic traits report reductions in physiological responses to fear. Journal of Child Psychol Psychiatry. 2010;52:834–841. doi: 10.1111/j.1469-7610.2010.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhani S, Blair RJR. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry. 2005;46:972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimonis ER, Frick PJ, Skeem JL, et al. Assessing callous-unemotional traits in adolescent offenders: validation of the inventory of callous-unemotional traits. Int J Law Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Pardini D. Perceptions of social conflicts among inarcerated adolescents with callous-unemotional traits: ‘You’re going to pay. It’s going to hurt, but I don’t care. J Child Psychol Psychiatry. 2011;52:248–255. doi: 10.1111/j.1469-7610.2010.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frick PJ, Ellis M. Callous-unemotional traits and subtypes of conduct disorder. Clin Child Fam Psychol. 1999;2:149–168. doi: 10.1023/a:1021803005547. [DOI] [PubMed] [Google Scholar]
- 36.Moffitt TE, Lynam DR, Caspi A, Wikstrom PH, Loeber R, Novak S. The interaction between impulsivity and neighborhood context on offending: effects of impulsivity are stronger in poorer neighborhoods. J Abnorm Psychol. 2000;109:563–574. doi: 10.1037//0021-843x.109.4.563. [DOI] [PubMed] [Google Scholar]
- 37.Frick PJ, Nigg JT. Current issues in the diagnosis of attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu Rev Clin Psychol. 2012;8:77–107. doi: 10.1146/annurev-clinpsy-032511-143150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hass SM, Waschbusch DA. Callous-unemotional traits and their relevance to ADHD. ADHD Rep. 2012;20:5–8. [Google Scholar]
- 39.Moran P, Ford T, Butler G, Goodman R. Callous and unemotional traits in children and adolescents living in Great Britan. Br J Psychiatry. 2008;192:65–66. doi: 10.1192/bjp.bp.106.034876. [DOI] [PubMed] [Google Scholar]
- 40.Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J Abnorm Child Psychol. 2003;31:457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 2000. Revised. [Google Scholar]
- 42.American Psychological Association. Ethical principles of psychologists and code of conduct. Am Psychol. 2002;57:1060–1073. [PubMed] [Google Scholar]
- 43.Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire (SDQ) J Am Acad Child Adolesc Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Dadds MR, Fraser J, Frost A, Hawes DJ. Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: a community study. J Consult Clin Psychol. 2005;73:400–410. doi: 10.1037/0022-006X.73.3.400. [DOI] [PubMed] [Google Scholar]
- 45.Pasalich DS, Dadds MR, Hawes DJ, Brennan J. Do callous-unemotional traits moderate the relative importance of parental coercion versus warmth in child conduct problems? An observational study. J Child Psychol Psychiatry. 2011;52:1308–1315. doi: 10.1111/j.1469-7610.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- 46.Kimonis ER, Frick PJ, Skeem JL, et al. Assessing callous-unemotional traits in adolescent offenders: validation of the inventory of callous-unemotional traits. Int J Law Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Expression Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 48.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): technical manual and affective ratings. Gainsville. FL: University of Florida; 1999. [Google Scholar]
- 49.Mind Ware Impedance Cardiography [computer program]. Version 2.6 System. Gahanna, OH: MindWare Technologies; 2008. [Google Scholar]
- 50.Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 51.Mind Ware Heart Rate Variability [computer program]. Version 2.6 System. Gahanna, OH: MindWare Technologies; 2008. [Google Scholar]
- 52.Frick PJ, White SF. The importance of callous-unemotional traits for developmental modes of aggressive and antisocial behavior [research review] J Child Psychol Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 53.Frick PJ, Viding EM. Antisocial behavior from a developmental psychopathology perspective. Dev Psychopathol. 2009;21:1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- 54.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 55.Nigg JT, Goldsmith HH, Sachek J. Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. J Clin Child Adolesc Psychol. 2004;33:42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- 56.Raine A, Venables R, Mednick S. Low resting heart rate at age three years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Proejct. J Am Acad Child Adolesc Psychiatry. 1997:361457–361464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- 57.Raine A. Annotation: the role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. J Child Psychol Psychiatry. 2002;43:417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- 58.Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.