Abstract
BACKGROUND
Previous studies have shown remarkable decrease in size of the left ventricle after left ventricular assist device (LVAD) implantation due to mechanical unloading. However, a certain number of patients continue to have significant mitral regurgitation (MR) under LVAD support. We investigated pre-operative echocardiographic features associated with persistent MR after LVAD implantation.
METHODS
We retrospectively reviewed 82 consecutive patients undergoing continuous-flow LVAD implantation between 2007 and 2010. We obtained echocardiograms performed within 2 weeks before and 1 week after surgery. We investigated the pre operative echocardiographic findings associated with significant MR post-LVAD and compared 1-year mortality after LVAD surgery between patients with and without significant MR post-LVAD.
RESULTS
MR was significant in 43 patients (52.4%) before LVAD surgery. Among those, 5 underwent concomitant mitral valve repair (MVr) at the time of LVAD implantation. Of the remaining 38 patients, 25 (65.8%) showed improvement of MR, whereas 13 patients (34.2%) continued to have significant MR post-LVAD. Multivariate analysis revealed that posterior displacement of the coaptation point of mitral leaflets was significantly associated with significant MR post-LVAD (hazard ratio, 1.335; 95% confidence interval, 1.035–1.721; p = 0.026) even after adjusting for the amount of pre operative MR flow. Post-LVAD 1-year survival of patients with and without significant MR post-LVAD was not significantly different (92.3% vs 89.1%, p = 0.826).
CONCLUSIONS
Pre-LVAD posterior displacement of mitral leaflets may be indicative of post-operative significant MR, which would help identify echocardiographic features of functional MR refractory to simple volume reduction of the ventricle.
Keywords: heart failure, mitral regurgitation, ventricular assist device, echocardiography
The left ventricular (LV) assist device (LVAD) has evolved into a standard therapy for patients with advanced heart failure (HF).1-3 The management of native valve dysfunction in LVAD recipients has been discussed mainly with regards to aortic valve and tricuspid valve dysfunction.4-8 Aortic insufficiency after LVAD implantation limits forward cardiac output.4-6 The effect on outcome of concomitant tricuspid valve repair in patients with significant tricuspid regurgitation undergoing LVAD implantation is still controversial7,8
A large number of patients with advanced HF develop mitral regurgitation (MR) as a result of the tethering of the mitral valve (MV) leaflets secondary to LV dilatation and a change in the geometry from an elliptical to spherical shape.9-12 LV chamber size decreased after mechanical unloading by pulsatile-flow or continuous-flow LVAD support.13 Holman et al14 reported that mechanical unloading of pulsatile-flow LVAD decreased not only LV dimension but also MR jet area and that the effect persisted for several months under LVAD support. As a result of the marked decrease in LV size by mechanical unloading, a number of patients with pre-implant moderate to severe MR showed improvement in the grade of MR after LVAD implantation without concomitant MV surgery. In contrast, there were also patients showing persistent significant MR after LVAD implantation. The effect of post-LVAD MR on post-operative outcome in patients undergoing continuous-flow LVAD remains unknown. In the present study, we investigated pre-implant echo findings associated with significant MR after LVAD implantation to distinguish patients who persistently have significant MR after LVAD implantation from those showing improvement of MR severity due to mechanical unloading alone.
Methods
The Columbia University Institutional Review Board approved the data collection protocol used in this study. The protocol complied with the Health Insurance Portability and Accountability Act and all ethical guidelines outlined by the 1975 Declaration of Helsinki.
Study patients and data collection
We retroactively reviewed 82 consecutive patients undergoing continuous flow LVAD (HeartMate II; Thoratec Corp, Pleasanton, CA) implantation at Columbia University Medical Center between June 2007 and August 2010. Patients undergoing emergent LVAD implantation due to cardiogenic shock or those with previous MV surgery were excluded. We also excluded patients with known MR for organic reasons such as MV prolapse before surgery. Echocardiograms performed within 2 weeks before and 1 week after surgery were analyzed. We adjusted the device speed according to the recommendations by Topilsky et al,15 including target mean atrial pressure > 65 mm Hg, middle interventricular septum portion, and intermittent aortic valve opening.
First, we classified patients according to their pre operative MR grade as those with none or mild MR (mild or lower MR) and those with moderate or more severe MR (moderate or more MR). Patients with moderate or more MR pre operatively were further divided into those who underwent MV repair (MVr) concomitantly at the time of LVAD placement (moderate or more MR + MVr, defined as Group MVr) and those who underwent LVAD surgery alone (moderate or more MR + No MVr). None of the patients with mild or lower MR underwent concomitant MVr with LVAD implantation.
Next, we reviewed the echocardiograms recorded 1 week after the surgery and the post-operative MR severity was graded. Patients who showed moderate or severe MR post-LVAD were classified as Group significant-Post-MR. Patients who showed none or only mild MR post-LVAD were classified as Group None/Mild Post-MR.
We compared clinical characteristics, pre operative laboratory measurements closest to the LVAD surgery, and pre operative echocardiographic findings between Group significant-Post-MR and Group None/Mild Post-MR. Patients who underwent concomitant MVr were not included in the comparison analysis. We then analyzed the pre operative factors associated with significant MR after surgery in patients who did not undergo concomitant MVr. For analysis of post-operative outcome, we compared 1-year mortality between Group significant Post-MR and Group None/Mild Post-MR.
Echocardiographic assessment of MR and LV geometry
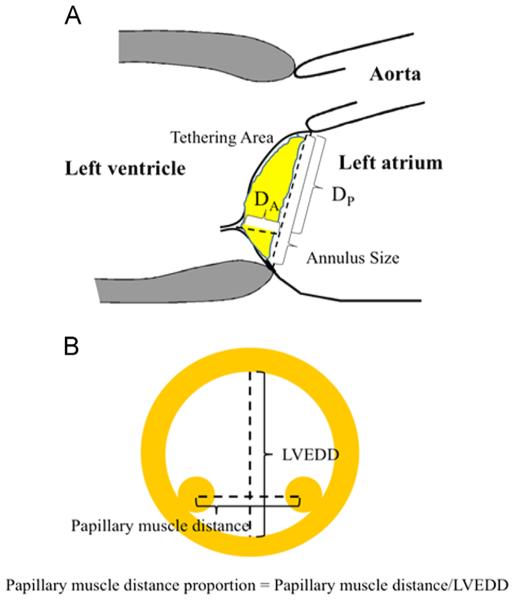
We assessed echocardiographic parameters, including LV dimension, LV ejection fraction (LVEF), percentage of fractional shortening (%FS), left atrial size, severity of MR, mitral annulus size, papillary muscle (PM) distance proportion, the degree of mitral annulus calcification, and the mitral leaflet configuration. Parasternal long axis and short axis images were used for measurement of LV end diastole (LVEDD) and end systole dimensions (LVESD). The LVEF was determined by the modified biplane Simpson method.16 Left atrial size was assessed by left atrial area (LAA). MR was evaluated quantitatively using 4-point grading scales based on the MR color flow jet reaching the left atrial cavity, as previously described.17-19 We defined moderate to severe MR, considered to be significant MR, as MR grade ≥3 by 4 point grading scales. Mitral annulus size was defined as the diameter from the anterior root of the anterior mitral leaflet to the posterior root of the posterior mitral leaflet in parasternal long axis images (Figure 1A). The distance between the anterior and posterior PMs was measured using parasternal short axis images. We defined the PM distance proportion as the ratio of the distance to LVEDD, which is expressed by percentage (Figure 1B).
Figure 1.
The measurements of echocardiographic parameters to quantify the mitral leaflet configurations are shown in the parasternal 2 dimensional echocardiogram (A) long axis and (B) short axis views. DA, apical displacement; DP, posterior displacement; LVEDD, left ventricular end diastolic dimension.
Mitral annulus calcification was assessed qualitatively by 3 visual grading scales (0/none, +1/mild, and +2/moderate or more).20 Mitral leaflet configuration was quantified by posterior (DP) and apical (DA) displacements of the coaptation point of the mitral leaflets using mid-systolic parasternal long axis images, as previously described (Figure 1A) 21,22 Tethering proportion was defined as the ratio of DA to DP and expressed by percentages. The change (Δ) of each parameter before and after LVAD implantation was calculated using the formula Δ pre LVAD value – post-LVAD value. A positive Δ indicates a decrease in the value, and a negative Δ indicates an increase in the value after LVAD surgery.
Statistical analysis
Data are presented as mean ± standard deviation and frequency. Normality was evaluated on the basis of normal distribution plots and histograms. Student’s unpaired t-test and 1-way analysis of variance were used to compare the variables among the groups. Categoric variables were compared using the chi square test. A p-value of < 0.05 was considered statistically significant. The correlations of the changes (Δ) in echocardiographic parameters were analyzed by Pearson coefficient. Univariate and multivariate logistic regression analysis were performed to identify pre-LVAD factors associated with significant MR after surgery. The variables included in the logistic regression analysis were LVEDD, LVESD, LVEF, LAA, DA, DP, tethering area, annulus size, PM distance proportion, tethering proportion, and annulus calcification.
Variables that achieved statistical significance in the univariate analysis were subsequently included in a multivariate analysis. The ratio of MR area to LAA was included in the multivariate analysis to adjust for the effect of pre operative MR on significant MR post-LVAD. Post-operative mortality at 1 year was assessed by Kaplan-Meier analysis with log rank test. Interobserver and intraobserver variability for echocardiographic parameters was determined using 10 randomly selected patients by 2 independent observers (S.K. and T.S.K.) and the interclass correlation coefficient was calculated. Statistical analyses were performed using SPSS 20.0 software (IBM Corp, Armonk, NY).
Results
Pre-LVAD and post-LVAD MR grade and associated patients’ characteristics
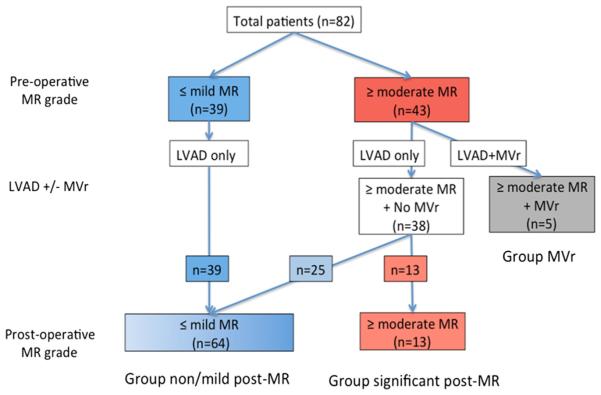
Figure 2 illustrates the distribution of patients with and without MR before and after surgery. Among the 82 patients studied, 39 (47.6%) had none or mild MR before LVAD implantation, and 43 (52.4%) had moderate or severe MR pre-operatively. None of the patients with pre-operative none/mild MR underwent concomitant MVr with LVAD implantation, and none developed worsening or newly developed significant MR after surgery. Among the 43 patients who had pre-operative moderate or severe MR, 5 (11.6% [6.1% of total patients]) underwent concomitant MVr with LVAD implantation (Group MVr), consisting of 4 MV repairs and 1 MV replacement. Therefore, 38 patients had moderate or more MR before surgery but did not receive concomitant MVr. Among those, 25 patients showed a marked decrease in MR severity to the grade of mild or none after LVAD surgery (58.1% of those with pre-operative moderate or more MR [30.5% of total patients]), and the remaining 13 patients still had moderate or severe MR even after LVAD implantation (30.2% of those with pre-operative moderate or severe MR [15.9% of total patients]). Sixty-four patients were classified as Group None/Mild-Post-MR and the remaining 13 were classified as Group significant-Post-MR.
Figure 2.
The prevalence of mitral regurgitation (MR) before and after left ventricular assist device (LVAD) implantation. The diagram indicates the number of patients in each group according to the classification based on the pre LVAD and post-LVAD MR severity. MVr, mitral valve repair.
Clinical characteristics and laboratory data of each group are summarized in Table 1. None of the variables differed significantly among the groups. We compared the length of stay in the intensive care unit after surgery as well as the amount of required intraoperative blood transfusion between patients with and without concomitant MV surgery and found that both parameters were not significantly different between those with and without concomitant surgery (12.4 ± 11.7 VS 12.8 ± 15.3 days [p = 0.958] and 0.80 ± 1.30 VS 0.88 ± 1.47 units [p = 0.912], respectively).
Table 1.
Characteristics of Candidates for Left Ventricular Assist Device Implantation
| Variablea | All (n = 82) |
Group Significant-Post-MR (n = 13) |
Group None/Mild-Post-MR (n = 64) |
Group MVr (n = 5) |
p-value |
|---|---|---|---|---|---|
| Age, y | 56.6 ± 13.7 | 50.2 ± 15.0 | 57.6 ± 13.4 | 59.8 ± 12.0 | 0.204 |
| Male gender | 59 (72.0) | 9 (69.2) | 47 (73.4) | 3 (60.0) | 0.926 |
| Blood pressure, mm Hg | |||||
| Pre-LVAD | 76 ± 11 | 79 ± 8 | 75 ± 11 | 74 ± 11 | 0.365 |
| Post-LVAD | 82 ± 11 | 79 ± 10 | 83 ± 11 | 82 ± 10 | 0.474 |
| Ischemic heart disease | 60 (73.2) | 12 (92.3) | 45 (70.3) | 3 (60.0) | 0.209 |
| Diabetes | 26 (31.7) | 1 (7.7) | 23 (35.9) | 2 (40.0) | 0.126 |
| Hypertension | 42 (51.2) | 6 (46.2) | 34 (53.1) | 2 (40.0) | 0.787 |
| Hyperlipidemia | 28 (34.1) | 4 (30.8) | 22 (34.4) | 2 (40.0) | 0.931 |
| Cerebrovascular disease | 16 (19.5) | 1 (7.7) | 14 (21.9) | 1 (20.0) | 0.5 |
| WBC, × 103 / μl | 8.51 ± 3.36 | 8.32 ± 2.64 | 8.77 ± 3.50 | 6.18 ± 2.68 | 0.443 |
| Platelets, × 103 / μl | 19.1 ± 7.3 | 20.9 ± 7.1 | 18.7 ± 7.1 | 19.7 ± 10.0 | 0.471 |
| BUN, mg/dl | 33.6 ± 16.8 | 27.2 ± 11.3 | 35.4 ± 17.8 | 28.2 ± 11.3 | 0.175 |
| Creatinine, mg/dl | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.3 ± 0.4 | 0.7 |
| AST, IU/liters | 49.2 ± 74.5 | 51.1 ± 69.0 | 50.7 ± 79.2 | 28.2 ± 8.0 | 0.815 |
| ALT, IU/liters | 88.8 ± 250.3 | 54.9 ± 105.8 | 101.4 ± 280.4 | 27.2 ± 9.2 | 0.716 |
| Total bilirubin, mg/dl | 1.6 ± 0.9 | 1.9 ± 1.2 | 1.6 ± 0.9 | 1.0 ± 0.5 | 0.13 |
| Albumin, g/dl | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.6 | 0.908 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LVAD, left ventricular assist device; MR, mitral regurgitation; MVr, mitral valve repair; WBC, white blood cell.
Categoric variables are shown as number (%) and continuous variables as mean ± standard deviation.
Echocardiographic findings of patients without concomitant MVr
Among the 82 patients studied, 75 (91.5% of total) showed a significant reduction of LV dimension after LVAD implantation compared with the value before surgery (p < 0.001). Table 2 summarizes the echocardiographic findings of patients who did not have concomitant MVr at the time of LVAD implantation. Pre-operative and post-operative LV and LA sizes were both greater in Group Significant-Post-MR than in Group None/Mild-Post-MR, but the changes before and after surgery were not different between the groups. Regarding the displacement of the coaptation points of mitral leaflets, pre-operative Dp was greater in Group Significant-Post-MR than Group None/Mild-Post-MR. The ratio of MR area to LAA was larger in Group Significant-Post-MR than in Group None/Mild-Post-MR both before and after LVAD; however, its change before and after surgery was only significant in Group None/Mild-Post-MR. There was no difference in LVAD pump speed between the groups (9059 ± 482 rpm Group None/Mild-Post-MR vs 9036 ± 427 rpm Group Significant-Post-MR, p = 0.885).
Table 2.
Echocardiographic Parameters in Patients Without Concomitant Mitral Surgerya
| Variable | Group Significant-Post-MR (n = 13) Mean ± SD |
Group None/Mild-Post-MR (n = 64) Mean ± SD |
p-value |
|---|---|---|---|
| LVEDD, pre/post mm | 76.7 ± 8.5/67.5 ± 7.2 | 66.7 ± 11.4/55.6 ± 12.5 | 0.004/0.001 |
| LVESD, pre/post mm | 70.2 ± 7.5/62.3 ± 7.6 | 59.9 ± 12.1/49.7 ± 13.7 | <0.001/<0.001 |
| ΔLVEDD, mm | 9.2 ± 8.2 | 11.1 ± 10.5 | 0.533 |
| LVEF, pre/…b % | 16.2 ± 4.2 /… | 15.7 ± 6.5 /… | 0.819/… |
| %FS, pre/post % | 8.3 ± 4.9/7.8 ± 2.9 | 10.4 ± 6.1/11.1 ± 11.0 | 0.243/0.283 |
| Δ%FS, % | −0.5 ± 6.1 | 0.7 ± 11.7 | 0.711 |
| IVS, pre/post mm | 9.2 ± 2.8/11.2 ± 2.4 | 9.8 ± 2.9/10.2 ± 3.3 | 0.515 /0.302 |
| LAA, pre/post mm2 | 36.3 ± 10.5/26.5 ± 9.1 | 26.7 ± 6.6/20.3 ± 6.3 | 0.007/0.004 |
| ΔLAA, mm2 | 9.8 ± 9.9 | 6.5 ± 6.1 | 0.265 |
| Annulus size, pre/post mm | 40.1 ± 4.9/36.9 ± 4.6 | 36.8 ± 5.0/31.6 ± 5.0 | 0.033/0.001 |
| ΔAnnulus size, mm | 3.2 ± 5.4 | 5.7 ± 5.7 | 0.147 |
| DA, pre/post mm | 17.4 ± 3.0/16.3 ± 3.6 | 16.0 ± 3.9/13.7 ± 3.8 | 0.218/0.027 |
| ΔDA, mm | 1.2 ± 3.7 | 2.5 ± 4.4 | 0.302 |
| DP, pre/post mm | 29.8 ± 6.8/25.9 ± 5.1 | 24.2 ± 5.0/18.7 ± 5.5 | 0.001/<0.001 |
| ΔDP, mm | 3.9 ± 3.1 | 5.7 ± 6.2 | 0.118 |
| Tethering area, pre/post mm2 | 353 ± 90/306 ± 97 | 299 ± 97/215 ± 80 | 0.065/0.001 |
| ΔTethering area, mm2 | 47 ± 105 | 83 ± 87 | 0.196 |
| PM distance, pre/post mm | 48.9 ± 9.4/42.8 ± 8.0 | 42.5 ± 7.9/35.5 ± 7.2 | 0.012/0.001 |
| ΔPM distance, mm | 6.2 ± 8.7 | 8.2 ± 9.8 | 0.492 |
| PM distance proportion, pre/post % | 63.8 ± 9.5/64.7 ± 9.6 | 63.5 ± 10.3/64.6 ± 15.2 | 0.758/0.803 |
| ΔPM distance proportion, % | 0.3 ± 11.9 | 0.2 ± 15.5 | 0.985 |
| Tethering proportion, pre/post % | 60.9 ± 15.0/65.0 ± 17.8 | 68.9 ± 21.6/79.6 ± 32.0 | 0.209/0.117 |
| ΔTethering proportion, % | −4.1 ± 15.8 | −11.2 ± 31.3 | 0.236 |
| Calcification score | 0.23 ± 0.44 | 0.14 ± 0.43 | 0.496 |
| Ratio of MRA to LAA, pre/post % | 43.8 ± 15.4/43.0 ± 11.4 | 27.3 ± 17.9/6.5 ± 9.3 | <0.001/<0.001 |
| ΔRatio of MRA to LAA, % | 0.8 ± 13.4 | 20.8 ± 17.0 | <0.001 |
Δ, change of the value before and after LVAD implantation; DA, apical displacement; DP, posterior displacement; FS, fractional shortening; IVS, interventricular septum wall thickness; LAA, left atrial area; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MRA, mitral regurgitation area; PM, papillary muscle; post, value obtained after surgery; pre, value obtained before surgery.
Data shown in bold type are statistically significant.
Postoperative LVEF was not measurable by modified Simpson's methods due to the left ventricular assist device inflow cannula.
Concomitant aortic valve closure at the time of LVAD implantation was performed in 12 of our study patients and none had aortic insufficiency (AI) after LVAD surgery. Post-LVAD echocardiograms showed trace to mild AI in 24 LVAD recipients and moderate AI in 1 recipient among those who did not receive concomitant aortic valve closure. Moderate or severe tricuspid regurgitation was found in 10 LVAD recipients post-LVAD.Neither the severity of Al nor tricuspid regurgitation was significantly different between patients with and without significant MR post-LVAD.
Intra-observer reproducibility for the parameters was sufficient with the intraclass correlation coefficient being 0.91, 0.88, 0.85, and 0.88 for DA and Dp, PM distance proportion, and mitral annulus calcification score, respectively. Similar results were obtained for inter-observer reproducibility with intraclass correlation coefficient values of 0.88, 0.87, 0.90, and 0.89, respectively.
Correlation between LV dimensional change, the amounts of MR flow, and echocardiographic parameters of MV configuration
Correlation analysis between the changes in LVEDD (ΔLVEDD) and MV configuration revealed that the ΔLVEDD was significantly correlated with aannulus size (r = 0.362, p < 0.001), ΔDA (r = 0.323, p < 0.001), ΔDP (r = 0.343, p < 0.001), atethering area (r = 0.431, p < 0.001), and ΔPM distance proportion (r = 0.453, p < 0.001), but not with Δtethering proportion (r = 0.065, p = 0.572).
Similarly, the change in MR flow expressed as the ratio of MR area to LAA before and after surgery (ΔMR amount) was correlated with ΔLVEDD (r = 0.389, p < 0.001), Δannulus size (r = 0.321, p = 0.006), ΔDp (r = 0.346, p = 0.002), and Δtethering area (r = 0.288, p = 0.01), but not with ΔDA (r = 0.195, p = 0.087), ΔPM distance proportion (r = 0.005, p = 0.963), or Δtethering proportion (r = 0.212, p = 0.067).
Pre-operative echocardiographic parameters associated with significant MR after LVAD implantation
Pre-implant echocardiographic parameters associated with significant MR after LVAD implantation in patients without concomitant MVr were investigated by univariate and multivariate logistic regression analysis (Table 3). Univariate analysis revealed that pre-implant LVEDD, LVESD, LAA, DP, annulus size, and the ratio of MR area and LAA were significantly associated with post-LVAD significant MR. Multivariate analysis revealed that pre-operative DP and the ratio of MR area to LAA were independently associated with post-LVAD significant MR.
Table 3.
Pre-operative Echocardiographic Parameters Associated With Device Implantationa Significant Mitral Regurgitation After Left Ventricular Assist
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| LVEDD, mm | 1.091 (1.024–1.163) | 0.007 | 0.908 (0.717–1.150) | 0.425 |
| LVESD, mm | 1.080 (1.020–1.144) | 0.008 | 1.126 (0.903–1.404) | 0.294 |
| Ejection fraction, % | 1.011 (0.920-1.111) | 0.816 | ||
| Left atrial area, mm2 | 1.154 (1.057-1.260) | 0.001 | 1.101 (0.990-1.223) | 0.075 |
| Displacement, mm | ||||
| Apical | 1.108 (0.941–1.305) | 0.218 | ||
| Posterior | 1.227 (1.073–1.403) | 0.003 | 1.335 (1.035–1.721) | 0.026 |
| Annulus size, mm2 | 1.152 (1.007–1.317) | 0.039 | 0.889 (0.690–1.144) | 0.360 |
| Tethering area, mm2 | 1.006 (0.999–1.012) | 0.071 | ||
| PM distance proportion, % | 0.990 (0.929–1.055) | 0.754 | ||
| Tethering proportion, % | 0.106 (0.003–3.462) | 0.207 | ||
| Annulus calcification | 1.525 (0.454–5.123) | 0.495 | ||
| Ratio of MR area to LAA, % | 1.054 (1.015–1.094) | 0.006 | 1.094 (1.013–1.181) | 0.022 |
CI, confidence interval; HR, hazard ratio; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; MR, mitral regurgitation; PM, papillary muscle.
Data shown in bold type are statistically significant.
Comparison of post-LVAD survival
Kaplan-Meier analysis revealed that post-LVAD 1-year survival of patients with and without significant MR was not significantly different (92.3% vs 89.1%, p = 0.826). During the observation period, no patient in Group MVr died.
Discussion
In this study we demonstrate (1) more than 50% of patients undergoing LVAD implantation had moderate or severe MR before surgery, (2) more than 30% of patients without concomitant MVr with LVAD implantation continued to have significant MR post-LVAD, and (3) pre-operative posterior displacement of mitral leaflets was independently associated with persistent significant MR post-LVAD.
The mechanism of functional MR has been reported to be augmented mitral leaflet tethering caused by the outward displacement of PMs secondary to LV remodeling or dilatation.21,22 As a result of LV dilatation and the geometric change from an elliptical to spherical shape, the MV annulus dilates with tethered PM and chordae tendineae resulting in the displacement of the coaptation point of mitral leaflets. The mitral displacement reduces MV leaflet coaptation reserve, which eventually causes an increase in MR.10-12,23,24
LV chamber size reduction is a promising effect of LVAD implantation, as reported previously13,25 and shown in this study. Even patients who still had significant MR after surgery showed a remarkable decrease in LVEDD by more than 10 mm after surgery, which was equivalent to the decrease of LVEDD in patients without significant MR post-LVAD (Table 2). Irrespective of the remarkable decrease of LV size by mechanical unloading, more than 30% of LVAD recipients with pre-operative significant MR continued to have significant MR after surgery. Of note, a remarkable decrease of LV size in LVAD recipients resulted in a decrease in their annulus size, both anterior and posterior mitral displacements, tethering area, and PM distance proportion. Therefore, this raises the question of which pre-operative parameter is most significantly and independently associated with significant MR post-LVAD in patients without concomitant LV surgery.
In our observations, the change in posterior mitral displacement before and after surgery (ΔDp) was correlated with the change of MR severity, but the correlation was not seen between the changes in apical displacement (ΔDA) and severity of MR. In addition, pre-operative Dp (but not DA) was a noticeable independent predictor of significant MR post-LVAD.
Nakai et al26 recently reported that apical and posterior displacement of coaptation points were both correlated with LV sphericity in patients with LV dysfunction with EF < 50% but that the correlation was more significant in posterior mitral displacement. The PM displacement vector contains 3 components: the mediolateral, posterior, and apical directions. Previous studies reported that the PM mainly displaces in the mediolateral and posterior directions.27-29 Therefore, the pre-operative severe posterior displacement may be related to severe tethering resulting in its irreversibility. We speculate that patients with more significant displacement of the coaptation points toward the posterior direction had more severe LV remodeling before surgery, and this could not be corrected, even after remarkable LV size reduction, due to mechanical unloading by LVAD. In other words, pre-operative mitral leaflets displacement toward posterior direction may more sensitively reflect the severity of LV remodeling than its displacement toward apical direction. This may explain why the DA before surgery was not significantly different between Group significant-Post-MR and Group Mild/None-Post-MR, but Dp before surgery was greater in Group significant-Post-MR. Furthermore, Kuwahara et al22 reviewed patients with and without persistent MR after annuloplasty and found that Dp and DA decreased early after surgery in both groups, but in patients with recurrent post-operative MR, only DP (but not DA) significantly increased again after surgery. This would support our findings that the Dp, rather than DA, reflected less coaptation reserve in patients undergoing LVAD surgery.
One interesting finding from this study was that the post-operative outcome was not different between Group significant-Post-MR and Group None/Mild-Post-MR, although the number of patients in each group was small. We speculate that patients in Group significant-Post-MR consisted of patients whose LV remodeling was too advanced to gain a benefit of LV size reduction by LVAD causing improvement of MR and that patients who benefited from LV size reduction experienced an increase in LV contractility that balanced out the MR reduction. The mixture of patients in this group, whose conditions were too severe to reverse MR and who had enough LV functional recovery to generate MR flow after LVAD surgery, may bring complexity to interpretations of the effect of MR severity on post-LVAD outcome. The long-term effect of significant MR post-LVAD on exercise capacity or prognosis/comorbidity should be further investigated. According to our small cohort of patients, concomitant MVr did not affect the duration of intensive care unit stay or further requirements of peri-operative care; however, investigation of a larger number of patients would be necessary. In addition, a prospective study would be required to determine which patients would get the most benefit from concomitant MVr.
The study contains several limitations worth noting due to its retrospective nature based on a small cohort of patients from a single center. The major limitation of the analysis is that we could not obtain echocardiograms recorded in a chronic phase after surgery, such as 3 months after surgery, from all patients. Because of this we used the echocardiograms recorded 1 week after the surgery. Post-operative optimization of volume status may typically take more than 1 week from the time of surgery, and echocardiograms at 1 week post-operatively may not represent the physiology under adequate mechanical unloading by LVAD; therefore, our analysis of MR may be overestimated.
Further, we could not obtain enough post-operative information such as exercise tolerance and quality of life indices. Therefore, the effect of persistent MR on post-operative functional statuses other than survival was not fully assessed.
The decision of whether to perform concomitant MVr in our cohort was made by the surgeons according to the pre-operative clinical information and peri-operative transesophageal echocardiographic findings. Our cohort included patients with ischemic and non-ischemic etiology. In addition, we did not fully assess the configuration of MV leaflet and the quantitative assessment of MR flow. Using 3-dimensional echocardiography or cardiac magnetic resonance imaging could allow us to evaluate MV configuration in more detailed manner.30,31
Finally, our study included patients who did not have pre-operative MR in order to find the features associated with post-operative MR. Indeed, none of these patients developed MR post-operatively. However, we included these patients because we initially considered the possibility that the grade of MR might worsen if a patient’s LV contraction dramatically improved or LV dyssynchrony became obvious under LVAD support, even in a patients without MR before LVAD.
In conclusion, pre-LVAD posterior displacement of the coaptation points of mitral leaflets detected by echocardiograms was associated with persistent MR after LVAD implantation. Our observation may help to identify morphologic features of the MV associated with functional MR refractory to volume reduction of the LV.
Footnotes
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long term use of a left ventricular assist device for end stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Heart Mate II Investigators. Advanced heart failure treated with continuous flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Birks EJ, Yacoub MH, Banner NR, Khaghani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–53. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Rao V, Slater JP, Edwards NM, Naka Y, Oz MC. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg. 2001;71:1448–53. doi: 10.1016/s0003-4975(01)02479-1. [DOI] [PubMed] [Google Scholar]
- 5.Pak SW, Uriel N, Takayama H, et al. Prevalence of de novo aortic insufficiency during long term support with left ventricular assist devices. J Heart Lung Transplant. 2010;29:1172–6. doi: 10.1016/j.healun.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Goda A, Takayama H, Pak SW, et al. Aortic valve procedures at the time of ventricular assist device placement. Ann Thorac Surg. 2011;91:750–4. doi: 10.1016/j.athoracsur.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Krishan K, Nair A, Pinney S, Adams DH, Anyanwu AC. Liberal use of tricuspid valve annuloplasty during left ventricular assist device implantation. Eur J Cardiothorac Surg. 2012;41:213–7. doi: 10.1016/j.ejcts.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saeed D, Kidambi T, Shalli S, et al. Tricuspid valve repair with left ventricular assist device implantation: is it warranted? J Heart Lung Transplant. 2011;30:530–5. doi: 10.1016/j.healun.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Pino PG, Galati A, Terranova A. Functional mitral regurgitation in heart failure. J Cardiovasc Med (Hagerstown) 2006;7:514–23. doi: 10.2459/01.JCM.0000234770.88701.75. [DOI] [PubMed] [Google Scholar]
- 10.Di Salvo TG, Acker MA, Dec GW, Byrne JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol. 2010;55:271–82. doi: 10.1016/j.jacc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 11.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–8. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 12.Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J. 1992;123:961–6. doi: 10.1016/0002-8703(92)90703-x. [DOI] [PubMed] [Google Scholar]
- 13.Kato TS, Chokshi A, Singh P, et al. Effects of continuous flow versus pulsatile flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail. 2011;4:546–53. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman WL, Bourge RC, Fan P, Kirklin JK, Pacifico AD, Nanda NC. Influence of left ventricular assist on valvular regurgitation. Circulation. 1993;88:II309–18. [PubMed] [Google Scholar]
- 15.Topilsky Y, Hasin T, Oh JK, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging. 2011;4:648–61. doi: 10.1161/CIRCIMAGING.111.965335. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Thomas JD, Anconina J, et al. Impact of impinging wall jet on color Doppler quantification of mitral regurgitation. Circulation. 1991;84:712–20. doi: 10.1161/01.cir.84.2.712. [DOI] [PubMed] [Google Scholar]
- 18.Enriquez Sarano M, Tajik AJ, Bailey KR, Seward JB. Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol. 1993;21:1211–29. doi: 10.1016/0735-1097(93)90248-y. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 20.Salarifar M, Rezvanfard M, Sadeghian H, Safir-mardanloo A, Shafii N. Mitral annular calcification predicts immediate results of percutaneous transvenous mitral commissurotomy. Cardiovasc Ultrasound. 2011;9:29. doi: 10.1186/1476-7120-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu F, Otsuji Y, Yotsumoto G, et al. Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation. 2005;112(9 Suppl):I396–401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara E, Otsuji Y, Iguro Y, et al. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation. 2006;114(1 Suppl):I529–34. doi: 10.1161/CIRCULATIONAHA.105.000729. [DOI] [PubMed] [Google Scholar]
- 23.Tsukiji M, Watanabe N, Yamaura Y, et al. Three dimensional quantitation of mitral valve coaptation by a novel software system with transthoracic real time three dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:43–6. doi: 10.1016/j.echo.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah HN, Kono T, Stein PD, Mancini GB, Goldstein S. Left ventricular shape changes during the course of evolving heart failure. Am J Physiol. 1992;263:H266–70. doi: 10.1152/ajpheart.1992.263.1.H266. [DOI] [PubMed] [Google Scholar]
- 25.Kato TS, Chokshi A, Singh P, et al. Markers of extracellular matrix turnover and the development of right ventricular failure after ventricular assist device implantation in patients with advanced heart failure. J Heart Lung Transplant. 2012;31:37–45. doi: 10.1016/j.healun.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Nakai H, Kaku K, Takeuchi M, et al. Different influences of left ventricular remodeling on anterior and posterior mitral leaflet tethering. Circ J. 2012;76:2481–7. doi: 10.1253/circj.cj-11-1527. [DOI] [PubMed] [Google Scholar]
- 27.Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 28.Chaput M, Handschumacher MD, Guerrero JL, et al. Mitral leaflet adaptation to ventricular remodeling: Prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120(11 Suppl):S99–103. doi: 10.1161/CIRCULATIONAHA.109.844019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito K, Okura H, Watanabe N, et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging. 2012;5:337–45. doi: 10.1016/j.jcmg.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Rukosujew A, Klotz S, Welp H, et al. Surgery of secondary mitral insufficiency in patients with impaired left ventricular function. J Cardiothorac Surg. 2009;4:36. doi: 10.1186/1749-8090-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitner J, Bhumireddy GP, Crowley AL, et al. Clinical application of cine MRI in the visual assessment of mitral regurgitation compared to echocardiography and cardiac catheterization. PLoS One. 2012;7:e40491. doi: 10.1371/journal.pone.0040491. [DOI] [PMC free article] [PubMed] [Google Scholar]