Abstract
Interactions between species can alter selection on sexual displays used in mate choice within species. Here we study the epicuticular pheromones of two Drosophila species that overlap partially in geographic range and are incompletely reproductively isolated. Drosophila subquinaria shows a pattern of reproductive character displacement against Drosophila recens, and partial behavioral isolation between conspecific sympatric versus allopatric populations, whereas D. recens shows no such variation in mate choice. First, using manipulative perfuming experiments, we show that females use pheromones as signals for mate discrimination both between species and among populations of D. subquinaria. Second, we show that patterns of variation in epicuticular compounds, both across populations and between species, are consistent with those previously shown for mating probabilities: pheromone compositions differ between populations of D. subquinaria that are allopatric versus sympatric with D. recens, but are similar across populations of D. recens regardless of overlap with D. subquinaria. We also identify differences in pheromone composition among allopatric regions of D. subquinaria. In sum, our results suggest that epicuticular compounds are key signals used by females during mate recognition, and that these traits have diverged among D. subquinaria populations in response to reinforcing selection generated by the presence of D. recens.
Keywords: Mate choice, pheromones, reinforcement, sexual selection, speciation
In natural populations, it is common for individuals of one sex to prefer certain trait values over others when choosing mates. Although an important source of sexual selection within populations, these mate preferences, and the sexual signals or displays they target, are also thought to play a central role in the origin of new species. In particular, the divergence among populations of signal traits and preferences may be an important cause of behavioral isolation during speciation (Coyne and Orr 2004). For example, when two populations that have been diverging in allopatry come into secondary contact, reinforcing selection can favor a strengthening of premating isolation in areas of sympatry in response to reduced hybrid fitness (Dobzhansky 1951; Howard 1993; Servedio and Noor 2003; Coyne and Orr 2004). The expected outcome is a pattern of reproductive character displacement (RCD) in which there is greater divergence of traits involved in behavioral isolation in areas of sympatry than in areas of allopatry (Howard 1993), although subsequent gene flow out of sympatry may erase this pattern.
Most demonstrations of RCD are based on mating probabilities, where behavioral isolation is shown to be stronger between sympatric than between allopatric populations of two species (Coyne and Orr 2004). However, a comprehensive understanding of how divergent natural and/or sexual selection generate behavioral isolation in nature requires knowledge of the male signal traits and female preferences that underlie mate choice and that diverge during reinforcement (Mendelson and Shaw 2012). In some cases, the male signal trait of interest is obvious, for example in the Ficedula flycatchers where male plumage differs dramatically between sympatric and allopatric populations (reviewed in Saetre and Saether 2010). However, in many cases it can be challenging to identify the traits that underlie behavioral isolation in nature, in part because females often assess multiple traits when choosing mates (Candolin 2003; Chenoweth and Blows 2006).
One indication that a male signal trait contributes to behavioral isolation, and thus may be a target of reinforcing selection, is if it varies in a pattern concordant with that of both female mate preferences and with the observed behavioral isolation. In other words, one might expect to see displacement not only in the pattern of female discrimination, but also in the male characters and female preferences that target them. Furthermore, this pattern may suggest that females from sympatric populations use population-specific rather than species-specific cues for mate discrimination. This could also lead to an additional layer of reproductive isolation between conspecific allopatric and sympatric populations, where conspecific allopatric males are no longer considered to be suitable mates (reviewed in Ortiz-Barrientos et al. 2009; Hosken and Higgie 2010).
Here we focus on the sexual signals involved in RCD between the fly Drosophila subquinaria and its sister species, Drosophila recens. Drosophila subquinaria and D. recens occur in western and eastern North America, respectively. Their geographic ranges overlap for about 1200 km in central Canada, and it is thought that this zone of sympatry reflects a secondary contact event that has occurred within the last 20,000 years after the retreat of the last glacial maximum (Jaenike et al. 2006). The two species are morphologically identical except for the male genitalia (Wheeler 1960). Where these species overlap, there are no known ecological differences between them: both can be collected at the same time of year and on the same mushrooms, which they use as a food source and mating substrate. Like other mushroom-feeding Drosophila, both species are thought to be generalists on fleshy basidiomycetes.
Drosophila subquinaria females show a pattern of RCD against mating with D. recens: in the geographic region where the species overlap, D. subquinaria females discriminate strongly against D. recens males, whereas D. subquinaria females from populations outside the zone of sympatry (i.e., allopatric) will mate with D. recens males at a moderate rate (Jaenike et al. 2006). In contrast to D. subquinaria, populations of D. recens that are sympatric and allopatric with D. subquinaria do not show a pattern of RCD (Jaenike et al. 2006). This asymmetric pattern in RCD is similar to many other species of Drosophila (Yukilevich 2012). A potential selective origin for this asymmetric pattern in this system is a Wolbachia infection present only in D. recens. The infection causes offspring survival from crosses between D. subquinaria females from any population and D. recens males to be low due to interspecific cytoplasmic incompatibility, whereas the offspring from the reciprocal cross survive and F1 females are fertile, although hybrid males are always sterile (Shoemaker et al. 1999).
In addition to displaying a pattern of RCD against D. recens, sympatric D. subquinaria females also discriminate against males of their own species from allopatric populations, although they mate freely with the conspecific males with which they co-occur (Jaenike et al. 2006). Although the performance of hybrids in nature is not known, there are no known postzygotic effects of these intra-specific crosses in the lab based on the fertility of F1 and F2 individuals (Jaenike et al. 2006; K. A. Dyer, unpubl. data). This suggests that this isolation is primarily, if not entirely, behavioral. The presence of gene flow among sympatric and nearby allopatric (i.e., “inland” allopatric, see Materials and Methods) D. subquinaria populations suggests that the pattern of between-species mate discrimination is the result of a history of reinforcing selection on D. subquinaria females to avoid mating with D. recens males (Jaenike et al. 2006, E. R. Bewick and K. A. Dyer, unpubl. data). Furthermore, this change in female mate choice may have also caused the behavioral isolation between sympatric D. subquinaria females and allopatric D. subquinaria males as a by-product, consistent with a pattern of “cascade reinforcement” (Hosken and Higgie 2010).
In many species of Drosophila, as well as in many other insects, epicuticular compounds are known to be important signals during courtship and mate discrimination (reviewed in Ferveur 2005). Several lines of evidence point to the importance of epicuticular compounds as male signals for female mate choice within these species. First, after their antennae are ablated, the third segment of which is necessary for the perception of male pheromones in Drosophila (Grillet et al. 2006), female D. subquinaria almost never mate with conspecific males from their own population (Giglio and Dyer 2013). This effect is also seen in D. recens females, although it is not as strong. In contrast, the removal of the female’s arista, which is attached to the antennae and is necessary for hearing, or painting over the female’s eyes, have no effect on mating frequency in either species. Second, epicuticular compounds are shared between the species, several are sexually dimorphic, and a few are found only in males (Curtis et al. 2013). Furthermore, the relative amounts of many epicu-ticular compounds differ between D. recens and D. subquinaria males, but not between females, suggesting that these traits are targets of sexual selection. Finally, in both species, female binomial mate choice trials using males from the same species and population suggest there are differences in female mate preferences for these traits. Specifically, within each species there was strong directional sexual selection on male epicuticular profiles, and between species the sexual selection vectors differed significantly in multivariate trait space (Curtis et al. 2013).
As chemosensory cues are essential for D. subquinaria females to mate within species, and are apparent targets of sexual selection in both species, they may also underlie variation in behavioral discrimination across populations and species. Here we test whether male epicuticular compounds act as pheromonal signals used by sympatric D. subquinaria females to discriminate against both D. recens and their own allopatric D. subquinaria males. First, we use perfuming experiments to assay the contribution of divergent epicuticular profiles to the existing behavioral isolation both between the two species and between sympatric and allopatric populations within D. subquinaria. If these compounds are a target of female choice that underlies species and/or population discrimination, we hypothesize that perfuming normally unattractive D. recens males with D. subquinaria male pheromones will increase the receptivity of D. subquinaria females to these males, thereby increasing hybridization rates. In addition, perfuming normally unattractive allopatric D. subquinaria males with sympatric male pheromones will increase mating rates with sympatric D. subquinaria females. Second, we examine variation in male and female epicuticular compounds from populations throughout the ranges of D. subquinaria and D. recens. Using populations as replicates, we quantify variation in epicuticular compounds and test whether their composition follows the same geographic pattern as that of female mate discrimination. In particular, we hypothesize that if these traits act as sexual pheromones that are the subject of reinforcing selection, a pattern of RCD will be present in D. subquinaria but not in D. recens. Finally, given known genetic structuring among allopatric D. subquinaria populations on either side of the Coast Mountains (termed coastal vs. inland populations; Jaenike et al. 2006), we also test for differences between these regions.
Materials and Methods
DROSOPHILA STRAINS AND REARING
We used isofemale lines of D. subquinaria and D. recens collected from sympatric and allopatric populations during the summers of 2009 to 2010 (Table 1 and Fig. 1). In D. subquinaria, within the large allopatric region there is evidence for genetic differentiation among populations to the west (i.e., coastal) and east (i.e., inland) of the Coast Mountains (Jaenike et al. 2006). Thus, we sampled replicate sympatric, allopatric coastal, and allopatric inland populations of D. subquinaria (Table 1). Because D. subquinaria and D. recens are morphologically identical, flies were identified to species with multiple molecular markers, including Wolbachia infection, mtDNA COI haplotype, and Y-linked kl-3 haplotype. The number of isofemale lines of each species used in this study, as well as the overall species composition of each population, is shown in Table 1.
Table 1.
Summary of populations used in this study. Sympatric (sym) populations contain both species, and allopatric (allo) populations are where one species only is present, with the allopatric region (coast or inland) indicated for Drosophila subquinaria populations. For each population, the number of isofemale lines used in this study, as well abundance of D. subquinaria relative to Drosophila recens at the time of collection, is included.
| Population | Abbr. | Region | Year collected | No. of D. subquinaria lines | No. of D. recens lines | % D. subquinaria among wild flies |
|---|---|---|---|---|---|---|
| Portland, OR | POR | allo – coast | 2010 | 24 | 0 | 100 |
| Seattle, WA | SEA | allo – coast | 2010 | 24 | 0 | 100 |
| Missoula, MT | MIS | allo – inland | 2010 | 5 | 0 | 100 |
| Deary, ID | DEA | allo – inland | 2009 | 7 | 0 | 100 |
| Shuswap, BC | SHU | allo – inland | 2010 | 3 | 0 | 100 |
| Canmore, AB | CAN | sym | 2010 | 3 | 4 | 47 |
| Hinton, AB | HIN | sym | 2010 | 12 | 7 | 27 |
| Kawtikh, AB | KAW | sym | 2010 | 6 | 22 | 8 |
| Nordegg, AB | NOR | sym | 2010 | 0 | 1 | Unknown |
| Lake Nippising, ON | NIP | allo | 2010 | 0 | 7 | 0 |
| Peru, NY | PER | allo | 2009 | 0 | 9 | 0 |
| Smoky Mountains, TN | SMT | allo | 2009 | 0 | 15 | 0 |
Figure 1.
Map of population locations used in this study. Locations are noted by their abbreviation in Table 1.
We also created four “mixed” stocks by combining isofemale lines and allowing them to mass breed for at least four generations. These included two mixed D. subquinaria stocks: one made from four isofemale lines from three sympatric populations (Hinton, Canmore, Kawtikh), and the other made from six isofemale lines from four allopatric populations, which included flies from both coast (Portland, Seattle) and inland (Missoula, Shuswap) populations. The other two were mixed D. recens stocks. The first combined three isofemale lines from separate sympatric populations (Hinton, Canmore, Kawtikh) that were known to carry the Wolbachia infection, and the second was created from three isofemale lines all from the Kawtikh population, each of which was naturally uninfected with Wolbachia. (In D. recens, Wolbachia has a maternal transmission rate of ~98% [Shoemaker et al. 1999], and thus these rare uninfected lines are assumed to be the result of imperfect maternal transmission.) Because no Wolbachia are present in this second D. recens stock, and there is thus no cytoplasmic incompatibility, the offspring of D. subquinaria females and D. recens are expected to survive. We verified this by pairing D. subquinaria allopatric females with these uninfected male D. recens; of 15 observed matings, 11 of the mated females produced viable offspring.
All fly cultures were maintained on Instant Drosophila food (Carolina Biological, Burlington, NC) supplemented with commercial mushroom (Agaricus bisporus) and reared at 20°C on a 14:10 light:dark cycle and 60% relative humidity. Flies for experiments were reared at a controlled density. Virgins were collected using light CO2 anesthesia within 24 h of emergence and subsequently held separately by sex at 10–15 flies per vial.
EXTRACTION AND QUANTIFICATION OF EPICUTICULAR COMPOUNDS
Epicuticular compounds were extracted from single flies by washing individuals in 100 μL of hexane for approximately 3 min and then vortexing for 1 min, after which the fly was removed and discarded. All extractions were completed within 3 h of the lights turning on in the incubator, and were performed in a randomized block design to minimize effects from the time of day, day, and order of extraction. Extractions were stored at −20°C and were subsequently shipped from Athens, GA, to Ottawa, ON, for analysis.
Samples were analyzed on a dual-channel Agilent Technologies (Wilmington, DE) 6890 N fast gas chromatograph with flame ionization detector using the temperature program described in Curtis et al. (2013). Individual profiles were determined by integrating the area under 17 and 23 peaks in females and males, respectively, corresponding to those previously identified in Curtis et al. (2013) with the exception of hentria-n-n-contadiene (C31:2), a very low concentration hydrocarbon that was undetectable in many individuals and which was therefore not included. The integrated peaks included 17 long-chain hydrocarbons composed only of odd carbon numbers (C29, C31, C33, and C35) and consisting of several methyl-branched alkanes, alkenes, and alkadienes, all of which were present in both sexes of both species (Curtis et al. 2013). Also present in males only of both species was 11-cis-Vaccenyl acetate (cVa), along with five fatty acids, provisionally identified as tri-acylglycerides (Curtis et al. 2013).
After integration, the relative abundance of each compound was calculated by dividing the area under each peak by the total area of all peaks for that individual. Working with relative abundances corrects for substantial technical error associated with quantifying absolute amounts via gas chromatography. To break the unit-sum constraint inherent in such compositional data and thus allowing multivariate analyses to be performed, proportions were transformed into log-contrasts (Aitchison 1986) using 2-methyl octacosane as the common divisor, as described in Curtis et al. (2013). The resulting 23 and 17 log-contrast traits for males and females, respectively, were used in all subsequent analyses.
PERFUMING ASSAYS
We conducted two types of perfuming experiments, both of which used the mixed D. subquinaria and mixed D. recens stocks described above and employed females that were always derived from the D. subquinaria sympatric mixed stock. In the first experiment, allopatric D. subquinaria mixed males were perfumed with 25 or 50 males from either their same allopatric D. subquinaria mixed stock or from the sympatric D. subquinaria mixed stock. In the second experiment, sympatric D. recens males that did not carry the Wolbachia infection were perfumed with 50 males from either the same D. recens mixed stock or from the sympatric D. subquinaria mixed stock. We used naturally uninfected flies to reduce any confounding effects of antibiotic treatment on the microbiome of the fly, which may affect pheromone composition (Sharon et al. 2010).
To perfume males, a single target male with unclipped wings was placed with the donor males (with clipped wings) in a standard food vial, and the cotton plug was pushed down to leave about 2 cm of space for the flies to move around. All male flies were 0–3 days old at the beginning of perfuming, and flies were perfumed for 9 days. Each target male was removed from the perfuming vial by aspiration and added to a vial that contained five virgin 7- to 10-day-old females from the D. subquinaria sympatric mixed stock. All males used in the mating trials had experienced the crowded perfuming environment, thus controlling for any potential effects on male activity. Mating trials used 1-dram vials that contained a blended mushroom-agar food, and were started within 1 h of the incubator lights on. Vials were observed for 3 h, and the time until copulation and copulation duration(s) were recorded. For trials with D. recens males, flies were left in the mating vial for an additional 21 h following the observation period, after which the male was discarded. These females were then placed together in a standard food vial and two weeks later scored for the presence of offspring. A total of five blocks of mating trials were completed for each perfuming experiment; within each block we included an average of 21 replicate test crosses (range 10–51) for each type of perfumed male (Table 2).
Table 2.
Results of no-choice perfuming trials measuring the fraction of sympatric Drosophila subquinaria females that mated when confined with the target male for 3 h (with D. subquinaria) or 24 h (with Drosophila recens). Each target male had been perfumed with pheromones from either their own males or from sympatric D. subquinaria males.
| Target male | Block | Perfumed with target males | Perfumed with sympatric D. subquinaria males |
|---|---|---|---|
| Allopatric D. subquinaria | 1 | 7/23 (30%) | 16/30 (53%) |
|
| |||
| 2 | 3/16 (19%) | 6/12 (50%) | |
|
| |||
| 3 | 3/15 (20%) | 7/13 (54%) | |
|
| |||
| 4 | 3/20 (15%) | 4/13 (31%) | |
|
| |||
| 5 | 6/41 (15%) | 5/28 (18%) | |
| Total | 22/118 (19%) | 38/96 (40%) | |
| Sympatric D. recens | 1 | 0/18 (0%) | 1/18 (6%) |
|
| |||
| 2 | 0/15 (0%) | 0/24 (0%) | |
|
| |||
| 3 | 0/12 (0%) | 0/10 (0%) | |
|
| |||
| 4 | 0/27 (0%) | 2/21 (10%) | |
|
| |||
| 5 | 0/51 (0%) | 0/15 (0%) | |
|
| |||
| Total | 0/123 (0%) | 3/87 (3.4%) | |
To test whether female mating preferences differed when males were perfumed with attractive versus unattractive males, we used a logistic regression with the male treatment type and block as effects in the model. In the within-species perfuming experiment, more than one copulation occurred in some vials during the observation period, and thus we also tested whether the total number of copulations per male differed using a Wilcoxon rank sum test. The time to first copulation from being placed in the vial and the duration of the first copulation were also compared between male types using a Wilcoxon rank sum test. We combined the results for 25 vs. 50 perfuming males in the within species treatment because there was no effect of the number of perfuming males in a vial. Analyses were performed using JMP version 10 (SAS Institutes, Cary, NC).
We tested the extent to which perfuming altered the epicuticular compounds of the target males, specifically asking whether the cuticular hydrocarbon (CHC) profiles of these males changed to more closely resemble their respective donor males. This experiment was completed at a different time than the perfuming experiments, but used the same stocks. We completed the perfuming procedures exactly as for the mating trials, but instead of placing the perfumed male with females, we extracted and quantified each male’s epicuticular compounds as described above. We did this for both the within and between species perfuming experiments, and we also simultaneously extracted the epicuticular compounds from virgin nonperfumed males for reference. An average of 21 males (range 11–34) of each type was used in this experiment. To provide a simple, visual interpretation of the effects of perfuming, a canonical discriminate analysis was conducted on all log-contrast epicuticular compounds including only males from the three nonperfumed (i.e., pure) types: sympatric D. recens, allopatric D. subquinaria, and sympatric D. subquinaria. Perfumed individuals were then scored for the first two canonical variates and all individuals (perfumed and nonperfumed) were plotted in the resulting trait space, allowing the effects of perfuming to be examined within the context of the major axes of variation that distinguish the sympatric and allopatric forms of the two species (note that sympatric and allopatric D. recens do not differ, see Results).
Our perfuming experiments used D. recens that were not infected with Wolbachia, whereas most flies in the wild harbor the infection. We therefore also tested whether the presence of Wolbachia alone affected D. subquinaria female mating preferences. The assay involved placing a single 7- to 10-day-old virgin female from the D. subquinaria sympatric mixed stock and either an infected or uninfected D. recens virgin male from their respective mixed stocks together in a 1-dram vial that contained a blended mushroom-agar food. Each pair was observed for 3 h to determine whether copulation occurred.
VARIATION IN EPICUTICULAR COMPOUNDS
Epicuticular compounds were sampled from an average of 25 (range 21–28) virgin females and 26 (range 19–31) virgin males from a variable number of isofemale lines from each species and population, as outlined in Table 1. Extraction and quantification were performed as described above. All flies were 7–9 days postemergence. Analyses were conducted separately by sex because the suite of epicuticular compounds varies qualitatively between males and females. To visualize RCD within the context of the total among population and between species variation in epicuticular compounds, we extracted individual scores from a canonical discriminant analysis that differentiated among all combinations of species and populations, separately for each sex. We then plotted the first two discriminate functions for both males and females. Working with the canonical variates had the advantage not only of reducing dimensionality, allowing subsequent tests for RCD to be performed on the majority of the among-individual variation in epicuticular compounds, but also avoided statistical issues arising from a moderate degree of multicolinearity among the log-contrast traits. We therefore tested the effects of sympatry/allopatry on the first five canonical variates via multivariate analysis of variance (MANOVA), separately by sex, using the following linear model:
| (1) |
where sym and spp are the fixed effect of sympatry/allopatry and species (D. recens and D. subquinaria) respectively, and pop is the random effect of population nested within the fixed effects interaction. The sym×spp interaction was highly significant in both sexes, indicating species-specific effects of the presence versus absence of the other species. We therefore repeated the discriminate analysis separately for each sex and species, extracting the individual-level canonical scores for the first four canonical variates in each case, accounting for 92% or more of the total variation. Differences in epicuticular compounds between sympatry/allopatry on the first four canonical variates of each sex and species were then tested via MANOVA, with sympatry (i.e., sym) as a fixed effect and population (i.e., pop) as a random effect nested within sym. Finally, given the known genetic structuring of allopatric D. subquinaria populations into coastal versus inland groups, this analysis was also repeated in D. subquinaria males and females after replacing the sympatry/allopatry effect with the three-level designation of sympatric/allopatric-inland/allopatric-coastal, with population again as a random effect nested within this. The above analyses treat populations as the unit of replication. In doing so, we seek to demonstrate that any pattern of RCD detected is not specific to a particular population, but rather is detected across multiple populations. Separate populations are clearly not phylogenetically independent, and the presence of RCD across multiple populations does not imply that it evolved independently in each (i.e., multiple origins).
Results
PERFUMING ASSAYS
Perfuming of allopatric D. subquinaria males successfully altered their epicuticular profiles as expected. In particular, when these allopatric males were perfumed with sympatric D. subquinaria males, their epicuticular profiles shifted to more closely resemble that of the sympatric males (Fig. 2). In contrast, perfuming of these males with other allopatric D. subquinaria males as a control produced little change in their epicuticular profiles (Fig. 2). In response to this perfuming, the acceptance by sympatric D. subquinaria females of the allopatric D. subquinaria males approximately doubled, from 19% (n = 118) to 40% (n = 96) (Table 2), when these males were perfumed with sympatric as opposed to control (i.e., allopatric) males. In a logistic regression, male perfume type was highly significant (Likelihood ratio test [LRT]: χ2 = 9.5; df = 1; P = 0.002). Similarly, considering the total number of copulations per male during the 3-h observation period, allopatric D. subquinaria males perfumed with sympatric D. subquinaria pheromones attained twice as many copulations as did these males when perfumed with D. subquinaria allopatric pheromones (mean ± SE per male: allopatric = 0.40 ± 0.09, sympatric = 0.80 ± 0.12), a difference that is significant overall (Wilcoxon rank sum χ2 = 10.3; df = 1; P = 0.0013). The time to the first copulation did not vary significantly depending on which type of male was used to perfume (allopatric males = 34.2 ± 7.9 min, sympatric males = 25.3 ± 4.2 min; Wilcoxon rank sum χ2 = 0.46; df = 1; P = 0.5), nor did the duration of the first copulation (allopatric males = 6.9 ± 0.65 min, sympatric males = 8.3 ± 0.56 min; Wilcoxon rank sum χ2 = 2.3; df = 1, P = 0.12).
Figure 2.
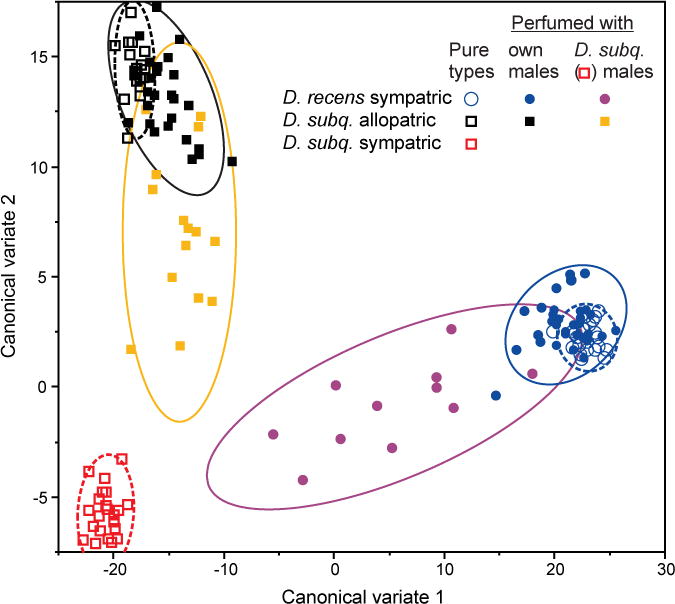
Individual variation in epicuticular compounds among male Drosophila recens (circles) and Drosophila subquinaria (squares) in response to perfuming. Pure (i.e., nonperfumed) individuals (open symbols) are shown in relation to perfumed individuals (filled symbols), with the latter including those individuals perfumed with their own (conspecific) males and those perfumed with sympatric D. subquinaria males. Ninety percent bivariate normal density ellipses are also shown for the various combinations of perfumed (solid lines) and nonperfumed (broken lines) groups.
The perfuming of D. recens males also altered their epicuticular profiles as expected. In particular, the D. recens males perfumed with sympatric D. subquinaria shifted to more closely resemble these latter males, whereas those in the control treatment that were perfumed with their own D. recens males showed little change (Fig. 2). The D. recens males that had been perfumed with sympatric D. subquinaria males were also found to be more attractive to sympatric D. subquinaria females than were the controls. In particular, none of the 123 D. recens males perfumed with their own D. recens male pheromones mated during the 3 h observation period, and none of these vials produced any offspring after an additional 21 h together. Of the 87 D. recens males perfumed with sympatric D. subquinaria pheromones, one mated during the 3-h observation period and an additional two vials produced offspring indicating that mating occurred during the following 21 h. This yielded a total of 3.4% mated across the 24-h mating period (Table 2). A logistic regression of the incidence of copulation indicates that the male perfume type was significant (LRT: χ2 = 4.61; df = 1; P = 0.032). Thus, there was a moderate, though significant, increase in female mate acceptance of heterospecific D. recens males when these males were perfumed with sympatric D. subquinaria male pheromones.
Finally, we found that Wolbachia infection itself did not have a significant effect on the patterns of female mate discrimination of D. subquinaria against D. recens. Of 38 trials in which sympatric D. subquinaria females were confined with Wolbachia-infected D. recens males, and 40 trials in which they were confined with Wolbachia-uninfected D. recens males, no copulations occurred within the 3-h observation period in either case (Fisher’s exact test, P = 1.0).
VARIATION IN EPICUTICULAR COMPOUNDS
In D. recens, there was little indication of any pattern of RCD of epicuticular compounds, with extensive phenotypic overlap among sympatric and allopatric populations for the first two canonical variates of the among-population variation in both males and females (Fig. 3B; see Table S1 for trait loadings). In D. subquinaria, however, a pattern of RCD was apparent in both sexes, with little to no overlap between sympatric and allopatric populations of the first two canonical variates (Fig. 3A; see Table S1 for trait loadings). In a multivariate test of the first five canonical variates of the among-population variation, accounting for 95.5% and 94.5% of the total variation in epicuticular compounds among females and males respectively, this contrasting pattern generated a highly significant species × sympatry/allopatry interaction overall in females (MANOVA: Pillai’s trace=0.957, F5,7 = 31.08, P<0.0001) and in males (MANOVA: Pillai’s trace = 0.937, F5,7 = 20.92, P = 0.0004), indicating that the effect of sympatry varied between species. Given this interaction, we repeated the discriminate analyses separately by species (and sex), extracting the first four canonical variates in each case and then testing for RCD in them in a multivariate analysis. In D. recens, there was again little indication of any consistent difference in epicuticular compounds between sympatric and allopatric populations in either sex (Table 3), demonstrating the absence of RCD of epicuticular compounds in this species. In contrast, in D. subquinaria, highly significant differences in epicuticular compounds were detected between sympatric and allopatric populations in both sexes, with allopatric populations also differing in conjunction with known genetic structuring on either side of the Coast Mountains (i.e., allopatric-coastal vs. allopatric inland; Table 3; Fig. S1).
Figure 3.
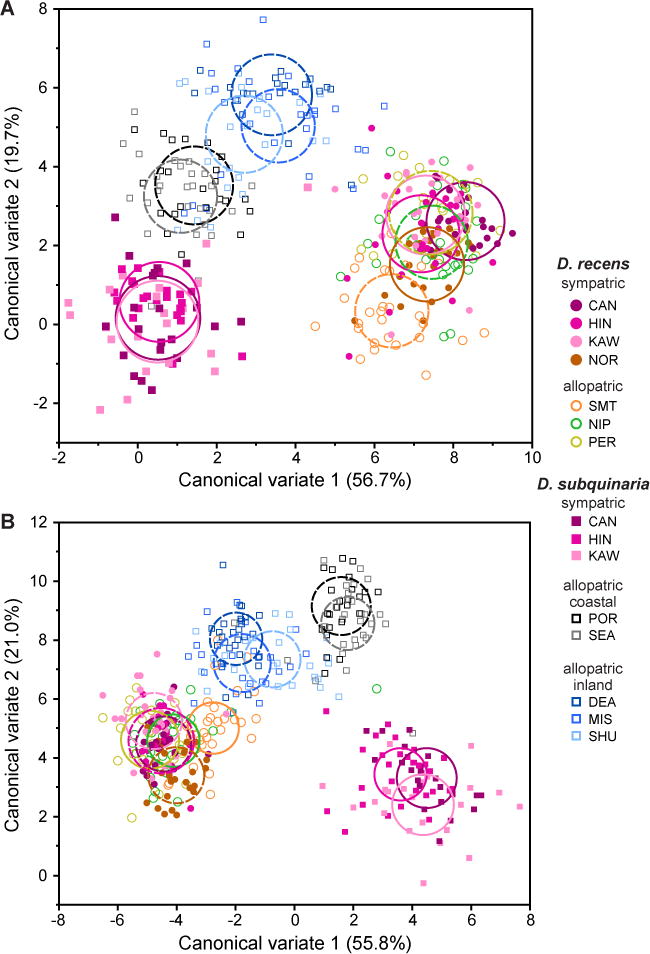
Individual variation in epicuticular compounds of (A) female and (B) male Drosophila recens (circles) and Drosophila subquinaria (squares) collected from sympatric (filled symbols) and allopatric (open symbols) locations. Axes are the first and second canonical variates from a discriminate function analysis, conducted separately by sex, which discriminated among individuals according to species and population. Circles depict the 95% confidence limits for the means of the various sympatric (solid line) and allopatric (broken line) populations.
Table 3.
Results of multivariate analyses of variance testing for differences in epicuticular compounds separately for male and female Drosophila recens and Drosophila subquinaria. Analyses were conducted on the first four canonical variates from a discriminate function analysis among individuals based on their population of origin.
| Species | Sex | Pillai’s trace | F | df | P | % variance1 |
|---|---|---|---|---|---|---|
| Sympatry vs. allopatry | ||||||
| D. recens | Female | 0.388 | 0.32 | 4,2 | 0.849 | 95.3 |
| D. recens | Male | 0.622 | 0.82 | 4,2 | 0.613 | 92.4 |
| D. subquinaria | Female | 0.992 | 98.17 | 4,3 | 0.002 | 95.9 |
| D. subquinaria | Male | 0.988 | 62.55 | 4,3 | 0.003 | 97.4 |
| Sympatry vs. allopatric inland vs. allopatric coastal | ||||||
| D. subquinaria | Female | 1.965 | 41.96 | 8,6 | <0.001 | 95.9 |
| D. subquinaria | Male | 1.976 | 62.44 | 8,6 | <0.001 | 97.4 |
Percent of the among-individual variance accounted for by the first four canonical variates.
Discussion
The divergence of mate recognition systems can generate the behavioral isolation that has long been thought to be key in both initiating and completing (i.e., reinforcing) speciation in nature (Howard 1993; Coyne and Orr 2004). Mate recognition systems are the product of the underlying mate preferences and the signal traits they target, and these can evolve in response to sexual selection within populations, due to ecological differences among populations, and in response to species interactions (e.g., hybridization and/or reproductive interference). How these different processes interact to affect the evolution of mate recognition systems, and the consequences this has for diversification, are not well understood (Andersson 1994; Coyne and Orr 2004; Hoskin and Higgie 2010; Mendelson and Shaw 2012). Here we were interested in identifying and characterizing variation in sexual signals for two sister species of Drosophila in which one of the pair (D. subquinaria) shows a pattern of RCD consistent with reinforcing selection to avoid mating with the other (D. recens), as well as a pattern consistent with cascade reinforcement (Jaenike et al. 2006). Previous work suggested that epicuticular compounds were critical for D. subquinaria to mate, and were also potential targets of sexual selection arising from female mate choice within each species (Giglio and Dyer 2013; Curtis et al. 2013). We tested whether these signals are also involved in discrimination among populations and between species, and characterized their variation among populations of both species.
From direct manipulations using perfuming experiments, our results suggest epicuticular compounds serve as pheromonal signals that contribute to behavioral isolation both among D. subquinaria populations and between the two species. Although in neither case did perfuming recover the mating rates seen within populations (e.g., about 80% of sympatric D. subquinaria females mate with conspecific sympatric males within a 2-h period; Jaenike et al. 2006; E. R. Bewick and K. A. Dyer, unpubl. data), our manipulations did increase the among-population conspecific mating rate by two-fold to about 40%, and the between species mating rate from zero to approximately 3.4% of pairs. Given the exceptionally strong behavioral isolation between sympatric D. subquinaria females and D. recens males, it is notable that any heterospecific matings occurred after perfuming. In another study that completed more than 700 mating trials between D. subquinaria sympatric females and D. recens males, we did not observe a single successful copulation within 2 h, and even when flies are crowded for an extended period of time it is difficult to get these flies to mate (E. R. Bewick and K. A. Dyer, pers. obs.). Perfuming studies are somewhat crude as pheromone profiles likely only transfer to a limited extent and will also mix with the existing profile of the recipient. In our case, perfuming did generate a substantial, but not complete, shift in multivariate epicuticular profile towards that of the donor males (Fig. 2), and therefore likely underestimate the contribution of these traits to mate choice. Our results are comparable to perfuming effects in other Drosophila in which epicuticular compounds have been shown to be important in mate choice. For example, in isolated populations of Drosophila mojavensis, perfuming males nearly doubled the rate of mating between populations (Etges and Ahrens 2001). Between species, Blows and Allan (1998) found that interspecific perfuming of D. serrata and D. birchii increased mating rates from 0% to 8% using 3-day-long mating trials. Furthermore, Coyne et al. (1994) found that perfuming D. simulans females with heterospecific D. sechellia females reduced the attractiveness of these females to D. simulans males, as the number of copulations fell from 10% to 3% during 30 min trials. In future studies of D. subquinaria, it will be interesting to perfume sympatric D. subquinaria males with D. recens males to ask if mating rates decrease, as was found by Coyne et al. (1994). As with other species, chemosensory signals are likely not the only sexual displays involved in mate choice, and between species there are likely additional layers of discrimination as even allopatric D. subquinaria females mate with D. recens only about 30% of the time (Jaenike et al. 2006; E. R. Bewick and K. A. Dyer, unpubl. data). Nevertheless, following past studies, our results demonstrate that epicuticular compounds contribute to this isolation.
We also used wild-derived isofemale lines to characterize the natural variation among populations of D. subquinaria and D. recens for both males and females from across the geographic range of each species. Before conducting our assays, all lines were raised in a common laboratory environment for several generations so that differences in trait means among populations could be attributed to genetic rather than to the environment differences. We found strong differences between the species, with almost no overlap in pheromonal profiles between them for either sex (Fig. 3). Within D. recens, epicuticular compounds were also very similar between populations that are allopatric versus sympatric with D. subquinaria, providing no evidence of any pattern of RCD (Fig. 3). In fact, there was little evidence of variation among populations from across the entire, geographically large, range of D. recens (Fig. 1), with the possible exception of a single population from the Smoky Mountains (Fig. 3). This allopatric population lies at the southern edge of the geographic distribution of D. recens and also exhibits moderate genetic differentiation from the rest of the range (Jaenike et al. 2006; Dyer et al. 2007). It is currently unknown whether there is any sexual isolation between D. recens from the Smokies and flies from the rest of the range, nor what has driven the differentiation of this population.
Finally, D. recens is infected with Wolbachia whereas D. subquinaria is not, and other studies have shown an effect of Wolbachia infection on mate preferences (Koukou et al. 2006; Miller et al. 2010). All of the isofemale lines of D. recens we assayed for CHCs were infected with Wolbachia, and we have not tested for a direct effect of Wolbachia on the CHC profile in this species. However, our experiment that compared mating rates of sympatric D. subquinaria females with infected versus uninfected D. recens males showed no increase in acceptance of uninfected males, indicating that if Wolbachia infection has an effect on CHC profile it is not a change that affects the patterns of behavioral discrimination by D. subquinaria females.
In contrast to D. recens, D. subquinaria showed substantial geographic variation in epicuticular profiles, with a striking and highly significant difference between populations that are sympatric versus allopatric with D. recens. Specifically, epicuticular composition was extremely similar among three replicate sympatric populations, and these differed consistently from the three replicate and nearby inland allopatric populations. This trait divergence was multivariate, involving the contribution of multiple epicuticular compounds (Table S1), and was substantial, even relative to the between-species differences for both sexes (Fig. 3). This pattern mirrors that previously shown for mate discrimination among sympatric vs. allopatric populations (Jaenike et al. 2006; E. R. Bewick and K. A. Dyer, unpubl. data) and is consistent with the presence of D. recens in sympatric populations generating reinforcing selection on these pheromones. Previous work on the genetic structure of these populations indicated the presence of gene flow between sympatric and these nearby inland allopatric populations (Jaenike et al. 2006; E. R. Bewick and K. A. Dyer, unpubl. data), suggesting the pattern observed in the CHCs is unlikely to be the product of genetic drift and must be maintained by some form of selection.
In addition to differences between sympatric and allopatric populations of D. subquinaria, we also found significant differences in epicuticular composition between allopatric populations that occur to the west (coastal) versus east (inland) of the Coast Mountains. There is no behavioral isolation between populations of D. subquinaria from these two regions (Jaenike et al. 2006; E. R. Bewick and K. A. Dyer, unpubl. data), at least that is detectable in no-choice mating trials, although as previously noted sympatric D. subquinaria females do differentiate between them. This inland-coastal divergence appears to have occurred in a multivariate combination of pheromones that is almost orthogonal to that separating the sympatric from the inland allopatric populations (Fig. S1), inconsistent with gene flow out of sympatry into nearby allopatric populations.
The origin of this allopatric inland-coastal divergence is currently unknown and there are several possible explanations. First, it may have arisen in allopatry as product of genetic drift, as this divergence is consistent with previous population genetic work that found a similar pattern of very strong genetic differentiation between these regions (Jaenike et al. 2006). Second, and potentially more likely given the magnitude of the differences, it may have arisen as a result of divergent selection caused from abiotic and/or biotic differences between the regions (e.g., Frentiu and Chenoweth 2010). Epicuticular hydrocarbons are well known to be important for desiccation resistance and temperature tolerance (reviewed in Howard and Blomquist 2005), and the coastal populations of D. subquinaria in Seattle, WA, and Portland, OR, experience much more rainfall than populations to the east of the Coast mountains, which are mesic in climate during the summer. However, D. recens occupies a broad range of climatic conditions yet shows no such variation among populations, suggesting that climatic conditions alone may be insufficient to explain these differences. In other species, desiccation selection also tends to favor increases in the relative concentration of the longest chain-length hydrocarbons (Kwan and Rundle 2010), but this is not the primary axis of trait differences between the two allopatric regions in D. subquinaria (Fig. S1). Alternatively, or in combination with other differences, this variation in epicuticular composition may be caused by the presence in only one of these regions of a third species, driving character displacement between the coastal and inland allopatric populations of D. subquinaria. Closely related species in this region include Drosophila suboccidentalis, Drosophila occidentalis, Drosophila rellima, and Drosophila falleni, although fine-scale distributions are not well characterized in this region and patterns of behavioral isolation with D. subquinaria have not been investigated. A separate RCD involving another species is therefore a distinct plausibility that will require further work to evaluate.
All else being equal, in D. subquinaria we expect that reinforcing selection should target male pheromones more strongly than female pheromones because the fitness consequences of hybridization are greater for females than for males. However, it is striking that both male and female epicuticular compounds vary in a fairly consistent pattern of RCD. This may simply represent a correlated response to selection, whereby reinforcing selection on males caused changes in pheromone profiles in females due to a shared genetic basis of the traits between the sexes. In D. subquinaria, all epicuticular compounds present in females are hydrocarbons and all are also found in males (Curtis et al. 2013). Although the intersexual correlations for these traits have not been measured in this species, they are sufficiently low in at least two other Drosophila to allow at least partially independent evolution of the sexes (e.g., Chenoweth et al. 2008; Bedhomme et al. 2011). An alternative explanation is that female epicuticular hydrocarbons may be under sexual selection arising from male mate choice. Male preferences for hydrocarbons in females have been shown in D. serrata and D. simulans (Coyne et al. 1994; Chenoweth and Blows 2006; Rundle and Chenoweth 2011), and the removal of the male antennae from sympatric D. subquinaria males, causing them to be unable to smell, was observed to decrease the mating rate (Giglio and Dyer 2013). It is not known whether sympatric males prefer their own sympatric females over allopatric conspecific females, although if this is the case it may contribute to maintenance of divergence in epicuticular composition between sympatric and allopatric populations of D. subquinaria. However, male mate choice likely does not contribute to the divergence in female epicuticular pheromones between inland and coastal allopatric populations of D. subquinaria, as there was no effect on mating rates after removing the antennae of allopatric D. subquinaria males (Giglio and Dyer 2013). Instead, as with the differences in males between these regions, genetic drift or divergent selection is more likely driving divergence in male epicuticular pheromones among these populations.
In summary, in combination with previous work in this system, our results suggest that females use epicuticular compounds as pheromonal signals during mate recognition and that this contributes to behavioral isolation between conspecific populations within D. subquinaria as well as between the species (this study; Giglio and Dyer 2013; Curtis et al. 2013). Epicuticular pheromones have also been shown to be critical to mate discrimination in other insect systems, often differing between closely related species (Jallon and David 1987; Howard et al. 1993; Coyne et al. 1994; Noor and Coyne 1996; Mullen et al. 2007; de Oliveira et al. 2011) and among recently diverged populations within a species (e.g., Etges and Ahrens 2001; Higgie and Blows 2007). Furthermore, in laboratory selection experiments these traits can evolve rapidly (e.g., Higgie et al. 2000; Rundle et al. 2005; Hunt et al. 2012), and in some cases are thought to be the target of reinforcing selection (Higgie et al. 2000; Ortiz-Barrientos et al. 2004). CHCs have also been shown to depend on diet (Etges et al. 2009; Delcourt and Rundle 2011; Gosden and Chenoweth 2011). Thus, although D. recens and D. subquinaria are thought to be generalists on mushrooms, a comprehensive understanding of the role of epicuticular compounds in mate choice and reproductive isolation in the wild will therefore also require knowledge of their diets and whether this varies among populations and between species. It will also be interesting to test whether epicuticular compounds vary depending on exposure to other species in the rearing substrate. Finally, characterizing among-population variation in mate preferences for these traits in D. subquinaria and D. recens, including testing for RCD of preferences, will be an additional important next step, with further studies aimed at providing a manipulative test of the origins of behavioral isolation, and the operation of reinforcement, in the wild.
Supplementary Material
Table S1. Loadings of the log-contrast transformed epicuticular compounds on the first two canonical variates (CV1, CV2), conducted separately by sex, discriminating among all combinations of populations and species.
Figure S1. Individual variation in epicuticular compounds of (A) female and (B) male Drosophila subquinaria collected from sympatric (filled symbols) and allopatric (open symbols) locations.
Acknowledgments
The authors thank M. Bray, D. Gaydos, C. Pinzone, and R. Webster for laboratory assistance. Funding was provided by National Science Foundation grants OISE-1132807 (KAD and HDR) and DEB-1149350 (KAD) and a grant from the Natural Sciences and Engineering Research Council of Canada (HDR).
Footnotes
DATA ARCHIVED
The doi for our data is 10.5061/dryad.17sr0.
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
LITERATURE CITED
- Aitchison J. The statistical analysis of compositional data. Chapman and Hall; Lond: 1986. [Google Scholar]
- Andersson M. Sexual selection. Princeton Univ. Press; Princeton, NJ: 1994. [Google Scholar]
- Bedhomme S, Chippindale AK, Prasad NG, Delcourt M, Abbott JK, Mallet MA, Rundle HD. Male-limited evolution suggests no extant intralocus sexual conflict over the sexually dimorphic cuticular hydrocarbons of Drosophila melanogaster. J Genet. 2011;90:443–452. doi: 10.1007/s12041-011-0109-3. [DOI] [PubMed] [Google Scholar]
- Blows MW, Allan RA. Levels of mate recognition within and between two Drosophila species and their hybrids. Am Nat. 1998;152:826–837. doi: 10.1086/286211. [DOI] [PubMed] [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Chenoweth SF, Blows MW. Dissecting the complex genetic basis of mate choice. Nat Rev Genet. 2006;7:681–692. doi: 10.1038/nrg1924. [DOI] [PubMed] [Google Scholar]
- Chenoweth SF, Rundle HD, Blows MW. Genetic constraints and the evolution of display trait sexual dimorphism by natural and sexual selection. Am Nat. 2008;171:22–34. doi: 10.1086/523946. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Crittenden AP, Mah K. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science. 1994;265:1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates; Sunderland, MA: 2004. [Google Scholar]
- Curtis S, Sztepanacz JL, White BE, Dyer KA, Rundle HD, Mayer P. Epicuticular compounds of Drosophila subquinaria and D. recens: identification, quantification, and their role in female mate choice. J Chem Ecol. 2013;39:579–590. doi: 10.1007/s10886-013-0284-1. [DOI] [PubMed] [Google Scholar]
- de Oliveira CC, Manfrin MH, Sene FD, Jackson LL, Etges WJ. Variations on a theme: diversification of cuticular hydrocarbons in a clade of cactophilic. Drosophila BMC Evol Biol. 2011;11:179. doi: 10.1186/1471-2148-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt M, Rundle HD. Condition dependence of a multicomponent sexual display trait in Drosophila serrata. Am Nat. 2011;177:812–823. doi: 10.1086/659949. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the origin of species. Columbia Univ. Press; New York: 1951. [Google Scholar]
- Dyer KA, Charlesworth B, Jaenike J. Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc Natl Acad Sci USA. 2007;104:1587–1592. doi: 10.1073/pnas.0605578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges WJ, Ahrens MA. Premating isolation is determined by larval-rearing substrates in cactophilic Drosophila mojavensis. V. Deep geographic variation in epicuticular hydrocarbons among isolated populations. Am Nat. 2001;158:585–598. doi: 10.1086/323587. [DOI] [PubMed] [Google Scholar]
- Etges WJ, de Oliveira CC, Ritchie MG, Noor MA. Genetics of incipient speciation in Drosophila mojavensis. II. Host plants and mating status influence cuticular hydrocarbon QTL expression and G × E interactions. Evolution. 2009;63:1712–1730. doi: 10.1111/j.1558-5646.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- Frentiu FD, Chenoweth SF. Clines in cuticular hydrocarbons in two Drosophila species with independent population histories. Evolution. 2010;64:1784–1794. doi: 10.1111/j.1558-5646.2009.00936.x. [DOI] [PubMed] [Google Scholar]
- Giglio EM, Dyer KA. Divergence of premating behaviors in the sister species Drosophila subquinaria and D. recens. Ecol Evol. 2013;3:365–374. doi: 10.1002/ece3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden TP, Chenoweth SF. On the evolution of heightened condition dependence of male sexual displays. J Evol Biol. 2011;24:685–692. doi: 10.1111/j.1420-9101.2010.02205.x. [DOI] [PubMed] [Google Scholar]
- Grillet M, Dartevelle L, Ferveur JF. A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci. 2006;273:315–323. doi: 10.1098/rspb.2005.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgie M, Blows MW. Are traits that experience reinforcement also under sexual selection? Am Nat. 2007;170:409–420. doi: 10.1086/519401. [DOI] [PubMed] [Google Scholar]
- Higgie M, Chenoweth S, Blows MW. Natural selection and the reinforcement of mate recognition. Science. 2000;290:519–521. doi: 10.1126/science.290.5491.519. [DOI] [PubMed] [Google Scholar]
- Hoskin CJ, Higgie M. Speciation via species interactions: the divergence of mating traits within species. Ecol Lett. 2010;13:409–420. doi: 10.1111/j.1461-0248.2010.01448.x. [DOI] [PubMed] [Google Scholar]
- Howard DJ. Reinforcement: origin, dynamics, and fate of an evolutionary hypothesis. In: Harrison RG, editor. Hybrid zones and the evolutionary process. Oxford Univ. Press; New York: 1993. pp. 46–69. [Google Scholar]
- Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- Howard RW, Jackson LL, Banse H, Blows MW. Cuticular hydrocarbons of Drosophila birchii and D. serrata: identification and role in mate choice in D. serrata. J Chem Ecol. 1993;29:961–976. doi: 10.1023/a:1022992002239. [DOI] [PubMed] [Google Scholar]
- Hunt J, Snook RR, Mitchell C, Crudgington HS, Moore AJ. Sexual selection and experimental evolution of chemical signals in Drosophila pseudoobscura. J Evol Biol. 2012;25:2232–2241. doi: 10.1111/j.1420-9101.2012.02603.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol. 2006;4:1852–1862. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon JM, David JR. Variations in cuticular hydrocarbons among the 8 species of the Drosophila melanogaster subgroup. Evolution. 1987;41:294–302. doi: 10.1111/j.1558-5646.1987.tb05798.x. [DOI] [PubMed] [Google Scholar]
- Koukou K, Pavlikaki H, Kilias G, Werren JH, Bourtzis K, Alahiotisi SN. Influence of antibiotic treatment and Wolbachia curing on sexual isolation among Drosophila melanogaster cage populations. Evolution. 2006;60:87–96. [PubMed] [Google Scholar]
- Kwan L, Rundle HD. Adaptation to desiccation fails to generate pre- and postmating isolation in replicate Drosophila melanogaster laboratory populations. Evolution. 2010;64:710–723. doi: 10.1111/j.1558-5646.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- Mendelson TC, Shaw KL. The (mis)concept of species recognition. Trends Ecol Evol. 2012;27:421–427. doi: 10.1016/j.tree.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Ehrman L, Schneider D. Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog. 2010;6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen SP, Mendelson TC, Schal C, Shaw KL. Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse Hawaiian crickets (Gryllidae : Trigonidiinae : Laupala) Evolution. 2007;61:223–231. doi: 10.1111/j.1558-5646.2007.00019.x. [DOI] [PubMed] [Google Scholar]
- Noor MAF, Coyne JA. Genetics of a difference in cuticular hydrocarbons between Drosophila pseudoobscura and D. persimilis. Genet Res. 1996;68:117–123. doi: 10.1017/s0016672300034005. [DOI] [PubMed] [Google Scholar]
- Ortiz-Barrientos D, Counterman BA, Noor MAF. The genetics of speciation by reinforcement. PLoS Biol. 2004;2:2256–2263. doi: 10.1371/journal.pbio.0020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Barrientos D, Grealy A, Nosil P. The genetics and ecology of reinforcement implications for the evolution of prezygotic isolation in sympatry and beyond. Year in Evol Biol. 2009;1168:156–182. doi: 10.1111/j.1749-6632.2009.04919.x. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Chenoweth SF. Stronger convex (stabilizing) selection on homologous sexual display traits in females than in males: a multipopulation comparison in Drosophila serrata. Evolution. 2011;65:893–899. doi: 10.1111/j.1558-5646.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Chenoweth SF, Doughty P, Blows MW. Divergent selection and the evolution of signal traits and mating preferences. PLoS Biol. 2005;3:1988–1995. doi: 10.1371/journal.pbio.0030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetre GP, Saether SA. Ecology and genetics of speciation in Ficedula flycatchers. Mol Ecol. 2010;19:1091–1106. doi: 10.1111/j.1365-294X.2010.04568.x. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol S. 2003;34:339–364. [Google Scholar]
- Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker DD, Katju V, Jaenike J. Wolbachia and the evolution of reproductive isolation between Drosophilla recens and Drosophila subquinaria. Evolution. 1999;53:1157–1164. doi: 10.1111/j.1558-5646.1999.tb04529.x. [DOI] [PubMed] [Google Scholar]
- Wheeler MR. New species of the quinaria group of Drosophila (Diptera, Drosophilidae) Southwestern Nat. 1960;5:1430–1446. [Google Scholar]
- Yukilevich R. Asymmetrical patterns of speciation uniquely support reinforcement in Drosophila. Evolution. 2012;66:1430–1446. doi: 10.1111/j.1558-5646.2011.01534.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Loadings of the log-contrast transformed epicuticular compounds on the first two canonical variates (CV1, CV2), conducted separately by sex, discriminating among all combinations of populations and species.
Figure S1. Individual variation in epicuticular compounds of (A) female and (B) male Drosophila subquinaria collected from sympatric (filled symbols) and allopatric (open symbols) locations.


