Abstract
Background
Kidney stone disease is associated with hypertension, diabetes, metabolic syndrome, kidney function decline and increased cardiovascular (CV) events. However, its association with all-cause and CV mortality is unclear.
Methods
We used The Third National Health and Nutrition Examination Survey, a large US population-based study with mortality data through 2006 determined via linkage to the National Death Index to examine kidney stone disease in relation to all-cause and CV mortality risks.
Results
Among 14,879 men and women over 18 years of age who were eligible for analysis, 683 participants reported a history of kidney stones. There was a total of 3,590 all-cause and 1,608 CV deaths occurred during a median follow up of 14.9 years. Stone formers had a significantly higher risk for all-cause mortality (HR 1.95, 95% CI 1.64–2.33, p<0.0001), and CV mortality (HR 2.05, 95% CI 1.60–2.62, p<0.0001) in unadjusted analyses. However, after multivariate adjustment for age, gender, race and poverty, stone formers no longer had increased risk for all-cause mortality (HR 1.08, 95% CI 0.93–1.26, p=0.3) and CV mortality (HR 1.07, 95% CI 0.84–1.36, p=0.6). Results remain unchanged after further adjustment for other clinical variables including history of hypertension, diabetes, and CV disease.
Conclusion
The increased risk of all-cause and CV mortality in kidney stone formers is likely a reflection of unique demographics and associated co-morbidities. There is no independent association of prevalent kidney stone disease with all-cause and CV mortality.
Keywords: Mortality, Cardiovascular disease, Kidney stone disease
Introduction
Kidney stone disease is common in industrialized countries, affecting 10–15% males and 3–5% females in the United States [1], and its prevalence is still rising [2]. It causes significant morbidities and has a vast economic impact [3].
Kidney stone disease has been increasingly recognized as a complex systemic illness. Studies have linked kidney stone disease to a variety of cardiovascular (CV) risk factors including hypertension, diabetes, and metabolic syndrome [4, 5]. More recently, Reiner and colleagues analyzed data among participants in the CARDIA study, and demonstrated that young adults with a history of kidney stone had a higher prevalence of subclinical atherosclerosis [6]. Similarly, Rule and colleagues also show that stone formers had a significantly higher risk for myocardial infarction (MI) [7]. Furthermore, kidney stone disease has also been associated with an increased risk of kidney function decline and incident end-stage renal disease [8, 9].
Despite these strong associations between kidney stone disease and other systemic illnesses that are important and well established predictors of survival, no studies to date have examined the independent relationship between all-cause and CV mortality risk with kidney stone disease. Therefore, in this study we aim to examine the relationship between kidney stone disease and all-cause and CV mortality among the US adult population using the Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III).
Subjects and Methods
Study population
NHANES III is a national probability sample of the total non-institutionalized civilian population, two months of age or over in the United States. It is a cross-sectional study and was conducted from 1988 to 1994. The survey collected demographic, socioeconomic, dietary, and health-related information, in addition to the examination and laboratory data obtained by highly trained medical personnel.
There were a total of 33,994 participants in NHANES III, and our analyses were limited to 20,024 adult participants of 18 years or older who have mortality data. Among those, 19,597 responded yes or no to the question regarding the history of kidney stones. Responders, who did not have complete information on physical activity or poverty-income ratio (PIR), had missing body mass index (BMI), measurements of serum C-reactive protein (CRP), total cholesterol (TC), high density lipoprotein (HDL), and serum 25-hydroxy vitamin D (25(OH)D), as well as estimated glomerular filtration rate (eGFR) as measured by the Modification of Diet in Renal Disease (MDRD) equation, or had incomplete data for the history of hypertension, diabetes, and cardiovascular disease were excluded (n = 4,718). Thus, the final sample used in this study included 14,879 adult participants.
Primary predictor and outcome
The primary predictor or independent variable was prevalent kidney stone disease. It was extracted from the interview data file. “Have you ever had a kidney stone?” was the question asked during the standardized home interview. Adult participants who responded “yes” to the question were considered to have a history of kidney stone in life. Participants were also asked the number of different occasions they passed a kidney stone.
The outcome or dependent variable of interest was all-cause and CV mortality. To evaluate mortality we used the NHANES III linked mortality file. This uses probabilistic record matching with the National Death Index through December 31, 2006, to determine the mortality status among NHANES III eligible participants. International Classification of Diseases, Tenth Revision (ICD10) codes were used to identify participants for all-cause mortality, as well as for whom CV disease was listed as the underlying cause of death (ICD10 I00–I78). A detailed description of matching methodology is available [10].
Covariates
Age was defined as age at the time of the interview. Race/ethnicity was self-reported as non-Hispanic white (NHW), non-Hispanic black (NHB), Mexican American (MA) or other. A history of hypertension was defined as self-reported physician diagnosis of hypertension, or ever been asked to take blood pressure medication, or taking blood pressure medication at the time of interview. A history of diabetes was defined as self-reported physician diagnosis of diabetes or taking insulin at the time of interview. A history of CV disease was defined as having at least one of the following self-reported physician diagnoses of acute MI, stroke or heart failure. Physical activity assessment was part of the comprehensive interview. Participants were asked to identify various exercises during their spare time. They were asked to specify the number of times they engaged in an identified activity during the past month. The frequency of other exercises, sports or activities was also recorded. The physical activity was specified as the sum of intensity rating multiplied by times (of each activity) every month. PIR was obtained by dividing family income by the poverty threshold and was used as the indicator of socioeconomic status. A cut-off value of 2.0 was based on previously reported PIR value distribution among NHANES III population [11]. Of note, a PIR <2.0 corresponds to a low socioeconomic status. Family income was reported during the household interview. The poverty thresholds are adjusted for family size and are updated annually for inflation. BMI in kilograms per square meter was calculated from the weight and height measured during the physical examination. Serum CRP was quantified using latex enhanced nephelometry. Serum TC and HDL were measured enzymatically. Serum 25(OH)D concentrations were measured using a radioimmunoassay kit (DiaSorin Inc, Stillwater, MN) [12]. Serum creatinine was measured using the Jaffe modified kinetic method. The eGFR was calculated using the MDRD equation [13].
Statistical analysis
We used SAS (9.3) PROC SURVEYMEANS and SURVEYFREQ obtain descriptive statistics for the population. Characteristics of the population were compared between those with and without any history of kidney stone using the Rao-Scott Chi Square for categorical variables and ANOVA for continuous variables. SAS (9.3) PROC SURVEYPHREG were used to perform Cox Proportional-Hazards Regression analyses. Results were presented as hazard ratio (HR) with 95% confidence limits. In Cox Regression, we first examined unadjusted HR of all-cause mortality and CV mortality. Then for model 1, we added age (continuous variable), gender (male, female), race/ethnicity (NHW, NHB, MA, and other race), and PIR (<2.0 vs., ≥2.0). For model 2, we added BMI (continuous variable), history of diabetes (yes, no), history of hypertension (yes, no), history of cardiovascular disease (yes, no), TC (continuous variable), HDL (continuous variable), and physical activity (continuous variable) in addition to those in model 1. For model 3, we added additional variables including eGFR (continuous variable), CRP (continuous variable) and serum 25(OH)D concentration (continuous variable) to model 2. Due to the complex sample strategy of NHANES III, appropriate 6 year weights and strata were applied. Because SAS was unable to generate survey-adjusted Kaplan Meier plots, median follow-up times and separate models for males and females, R version 2.15.0 and its associated Survey package were used for these analyses. Previous analyses conducted in SAS were duplicated in R to confirm that all results were comparable.
Results
Out of 14,879 participants eligible for the final analysis, 683 reported a history of kidney stones. There were a total of 3,590 all-cause and 1,608 CV deaths during a median follow up of 14.9 years. As shown in table 1, stone formers tended to be older, male, non-Hispanic white, had a higher BMI, TC, and a lower HDL compared to non-stone formers. They were also more likely to have a history of hypertension, diabetes, and CV disease. In addition, they had a higher serum CRP concentration and lower kidney function as measured by eGFR. Serum 25(OH)D concentrations were not different between stone formers and non-stone formers. Of note, 91% of subjects in this study reported their income, and stone formers were more likely to have a higher socio-economic status based on income.
Table 1.
Baseline characteristics of the study population
| Stone formers (N=683) |
Non-stone formers (N=14,196) |
P value | |
|---|---|---|---|
| Age, year | 54 (±0.8) | 44 (±0.2) | <0.0001 |
| Men | 419 (61%) | 6558 (46%) | <0.0001 |
| Race-NHW | 479 (70%) | 5983 (42%) | <0.0001 |
| Poverty Income Ratio <2 | 244 (36%) | 6617 (47%) | <0.0001 |
| Hypertension | 275 (40%) | 3712 (26%) | <0.0001 |
| Diabetes | 80 (12%) | 1094 (8%) | 0.0001 |
| Cardiovascular disease | 120 (18%) | 1063 (8%) | <0.0001 |
| BMI, kg/m2 | 27.8 (±0.3) | 26.4 (±0.1) | <0.0001 |
| CRP, mg/dl | 0.48 (±0.03) | 0.41 (±0.01) | 0.04 |
| 25(OH)D, ng/ml | 29.6 (±0.6) | 30.0 (±0.1) | 0.5 |
| eGFR, ml/min/1.73m2 | 63.5 (±0.8) | 69.8 (±0.2) | <0.0001 |
| Total cholesterol, mg/dl | 213.7(±2.1) | 202.9 (±0.6) | <0.0001 |
| HDL cholesterol, mg/dl | 47.6 (±0.8) | 51.0 (±0.2) | <0.0001 |
| Physical activity | 97.2 (±5.8) | 114.3 (±1.8) | 0.005 |
Data were presented as mean ±SE, or number (weighted %); NHW=non-Hispanic white; BMI=body mass index; CRP=C-reactive protein; eGFR=estimated glomerular filtration rate; HDL=high density lipoprotein
In table 2 is depicted that kidney stone formers had significantly higher risk of all-cause mortality (HR: 1.95, 95% CI 1.64–2.33, p<0.0001) and CV mortality (HR: 2.05, 95% CI 1.60–2.62, p<0.0001) in unadjusted analysis (Figure 1 & 2). After adjustment for age, gender, race and PIR in model 1, stone formers no longer associated with increased all-cause mortality (HR: 1.08, 95% CI 0.93–1.26, p=0.3) and CV mortality (HR: 1.07, 95% CI 0.84–1.36, p=0.6) in comparison to non-stone formers. After adding additional confounding variables in model 2, including physical activity, history of hypertension, diabetes, CV disease, BMI, TC, and HDL, kidney stone disease was again not associated with increased hazard for all-cause mortality (HR: 1.00, 95% CI 0.85–1.17, p=1.0) or CV mortality (HR: 0.94, 95% CI 0.74–1.19, p=0.6). Finally, there were no significant differences in all-cause mortality (HR: 0.99, 95% CI 0.85–1.16, p=0.9) and CV mortality (HR: 0.94, 95% CI 0.75–1.19, p=0.6) between stone formers and non-stone formers when eGFR, serum CRP and 25(OH)D concentration were added to the final model (Model 3). The stratified analyses by gender revealed similar findings.
Table 2.
Association of kidney stone disease with all-cause and cardiovascular mortality
| Men | Women | Combined | |||||
|---|---|---|---|---|---|---|---|
| Regression Model | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality | Unadjusted | 2.03 (1.62–2.53) | <0.0001 | 1.81 (1.35–2.42) | <0.0001 | 1.95 (1.64–2.33) | <0.0001 |
| Model 1 | 1.03 (0.85–1.25) | 0.8 | 1.21 (0.95–1.55) | 0.1 | 1.08 (0.93–1.26) | 0.3 | |
| Model 2 | 0.99 (0.81–1.20) | 0.9 | 1.05 (0.82–1.34) | 0.7 | 1.00 (0.85–1.17) | 1.0 | |
| Model 3 | 0.96 (0.79–1.18) | 0.7 | 1.03 (0.80–1.31) | 1.0 | 0.99 (0.85–1.16) | 0.9 | |
| Cardiovascular mortality | Unadjusted | 2.05 (1.60–2.62) | <0.0001 | 2.06 (1.40–3.04) | 0.0002 | 2.05 (1.60–2.62) | <0.0001 |
| Model 1 | 0.96 (0.70–1.32) | 0.8 | 1.34 (0.94–1.90) | 0.1 | 1.07 (0.84–1.36) | 0.6 | |
| Model 2 | 0.88 (0.64–1.22) | 0.5 | 1.09 (0.79–1.51) | 0.6 | 0.94 (0.74–1.19) | 0.6 | |
| Model 3 | 0.88 (0.64–1.21) | 0.4 | 1.08 (0.78–1.48) | 0.7 | 0.94 (0.75–1.19) | 0.6 | |
Variables adjusted for: Model 1=age, gender, race, and poverty income ratio. Model 2=body mass index, total cholesterol, high density lipoprotein, physical activity, histories of hypertension, diabetes and cardiovascular disease, in addition to model 1. Model 3: serum C-reactive protein, serum 25-hydroxyvitamin D, and estimated glomerular filtration rate, in addition to model 2
Figure 1.
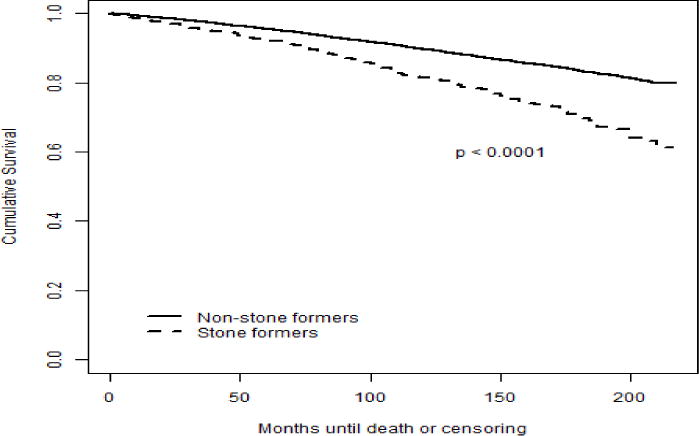
Kaplan-Meier estimates for all-cause mortality (unadjusted)
Figure 2.
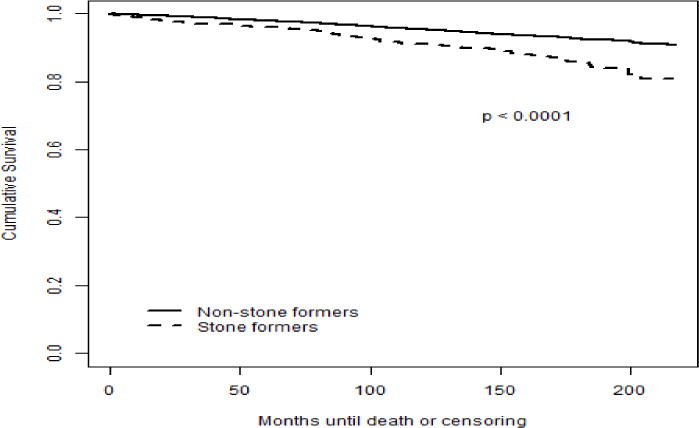
Kaplan-Meier estimates for cardiovascular mortality (unadjusted)
Among the demographic variables in the analyses, age was found to be an important modifier of the mortality risk in this study. To confirm this age effect, we added age to the unadjusted model between kidney stone disease and all-cause mortality, and found that kidney stone disease was no longer associated with a higher risk of all-cause mortality (HR: 1.15, 95% CI 0.99–1.33, p=0.07). As expected, age was a significant predictor of all-cause mortality in unadjusted analysis (HR per 10-year age increase: 2.40, 95% CI 2.31–2.49, p<0.0001). The additions of neither gender nor race alone into the unadjusted model resulted in any significant change in the association between kidney stone disease and all-cause mortality (data not shown).
In our multivariate Cox proportional hazard regression analyses, the following variables were found to have significant associations with all-cause mortality: older age (HR per 10-year age increase: 2.23, 95% CI 2.22–2.24, p<0.0001); male gender (HR: 1.69, 95% CI 1.53–1.88, p<0.0001); PIR<2 (HR: 1.53, 95% CI 1.37–1.71, p<0.0001); history of hypertension (HR: 1.29, 95% CI 1.16–1.42, p<0.0001); history of diabetes (HR: 1.68, 95% CI 1.47–1.92, p<0.0001); history of CV disease (HR: 1.65, 95% CI 1.47–1.85, p<0.0001); and higher serum CRP (HR per 1mg/dl increase: 1.20, 95% CI 1.15–1.26, p<0.0001). Of note, higher physical activity (HR per 1 unit increase: 0.99, 95% CI 0.99–0.99, p=0.008), increasing BMI (HR per 1 kg/m2 increase: 0.98, 95% CI 0.97–0.99, p=0.002), and higher serum 25(OH)D concentrations (HR per 1 ng/ml increase: 0.99, 95% CI 0.98–0.99, p=0.0003) were associated with decreased all-cause mortality. The following variables were also strong predictors of CV mortality: older age (HR per 10-year age increase: 2.55, 95% CI 2.53–2.57, p<0.0001); male gender (HR: 1.86, 95% CI 1.58–2.18, p<0.0001); PIR<2 (HR: 1.31, 95% CI 1.11–1.55, p=0.001); history of hypertension (HR: 1.52, 95% CI 1.31–1.76, p<0.0001); history of diabetes (HR: 1.66, 95% CI 1.37–2.01, p<0.0001); history of CV disease (HR: 2.28, 95% CI 1.95–2.67, p<0.0001); higher TC (HR per 10 mg/dl increase: 1.03, 95% CI 1.03–1.03, p=0.0003), and higher serum CRP (HR per 1mg/dl increase: 1.18, 95% CI 1.11–1.26, p<0.0001). Increasing BMI (HR per 1 kg/m2 increase: 0.98, 95% CI 0.97–0.99, p=0.04), higher physical activity (HR per 1 unit increase: 0.99, 95% CI 0.99–0.99, p=0.02), higher serum 25(OH)D concentration (HR per 1 ng/ml increase: 0.99, 95% CI 0.98–0.99, p=0.002), and a higher eGFR (HR per 5 ml/min/1.73 m2 increase: 0.96, 95% CI 0.95–0.96, p=0.01) were associated with decreased CV mortality.
Discussion
To our knowledge, this is the first study examining the independent association of prevalent kidney stone disease with all-cause and CV mortality in the US adult population. We showed a significant increase in all-cause and CV mortality risk among stone formers in unadjusted analysis. However, after adjusting for age, other demographic variables and vascular risk factors, the association of kidney stone disease with all-cause or CV mortality was no longer significant. This complete attenuation of the association suggests that the mortality risk among stone formers is likely a reflection of unique demographics and shared risk factors for mortality and kidney stone disease.
Consistent with findings from earlier studies [1, 5, 14–16], stone formers in our study were older and had a male predominance. They were also more likely to have hypertension, diabetes and obesity. Hypertension among stone formers has been linked to a reduction of urinary citrate excretion, thus promotes stone formation [17]. Both glucose intake and insulin administration can reduce calcium reabsorption [18–20], suggesting that high glucose and insulin state from insulin-resistance in diabetes and obesity can increase urinary calcium excretion. In addition, by suppressing renal tubular ammonium production [21], insulin resistance can lead to more acidified urine and increase risk of uric acid stone formation [22]. Prevalent chronic kidney disease (CKD) was also more common among stone formers in our study population, consistent with what has been reported by Gillen DL and other investigators [9, 23, 24]. It is unclear why stone formers develop CKD, as evidence suggesting CKD risk among stone formers is independent of traditional risk factors such as hypertension and diabetes [9]. Stone types (in particular, cystine and struvite stones), among others, could be the contributing factor for developing CKD, although potential mechanisms are not clear [25].
Because of the strong associations of above-mentioned CV disease risk factors with prevalent kidney stone disease, it is not surprising to see a significantly increased risk of CV disease among stone formers in our study population. This is consistent with the findings reported previously by Reiner AP et al who showed a strong association between kidney stone disease and carotid wall thickness among CARDIA study participants [6]. Rule AD et al compared 4,564 kidney stone formers to 10,860 matched controls from Olmsted County, Minnesota, and reported a 31% increased risk for new onset MI in stone formers after adjustment for CKD and other comorbidities [7].
Among all the demographic and clinical variables included in this study, older age, male gender, poverty, physical inactivity, history of hypertension, diabetes, cardiovascular disease, reduced body vitamin D store and inflammatory state were strong predictors of all-cause mortality and CV mortality. These are consistent with the mortality risk factors previously described in the general population [26–28]. Reduced kidney function was also noted to be a predictor for CV mortality, as it has been reported in the US adult population [29]. Furthermore, higher BMI appeared to have a mild protective effect on both all-cause mortality and CV mortality. This was unexpected considering the high mortality risk among overweight general population [30].
Limitations of our study need to be mentioned. First, the prevalent kidney stone cases were self-reported, and some participants may have kidney stone disease without self awareness or clinical diagnosis. This may lead to potential misclassification and are likely to be random with respect to case status. Therefore it could bias the study results toward the null. Second, we do not have information on stone composition, although ~80% of kidney stone in the general population like NHANES are calcium based. It is unclear whether patients with different type of kidney stones carry different mortality risks. Lastly, due to the observational study design and the nature of the NHANES survey database, we cannot establish any causal relationships.
In summary, the increased risk of all-cause and CV mortality among kidney stone formers is likely a reflection of unique demographics and associated comorbidities. There is no independent association of prevalent kidney stone disease with mortality. Future studies in other populations are needed to confirm these results.
Acknowledgments
This manuscript is supported in part by NIH/NIDDK grants 1R01DK094796 and 1R01DK081473-01.
Footnotes
All authors declare that they have no relevant financial interests.
References
- 1.Stamatelou KK, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–23. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68(4):1808–14. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 5.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68(3):1230–5. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Reiner AP, et al. Kidney stones and subclinical atherosclerosis in young adults: the CARDIA study. J Urol. 2011;185(3):920–5. doi: 10.1016/j.juro.2010.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule AD, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21(10):1641–4. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander RT, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rule AD, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(4):804–11. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, mortality follow-up through 2006: matching methodology. 2009 Available from: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf.
- 11.Sabanayagam C, Shankar A. Income is a stronger predictor of mortality than education in a national sample of US adults. J Health Popul Nutr. 2012;30(1):82–6. doi: 10.3329/jhpn.v30i1.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) reference manuals and reports [book on CD-ROM] Bethesda: National Center for Health Statistics; 2002. [Google Scholar]
- 13.Stevens LA, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo M, Laurenzi M. Elevated blood pressure and positive history of kidney stones: results from a population-based study. J Hypertens Suppl. 1988;6(4):S485–6. doi: 10.1097/00004872-198812040-00153. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens. 1999;17(7):1017–22. doi: 10.1097/00004872-199917070-00019. [DOI] [PubMed] [Google Scholar]
- 16.Madore F, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32(5):802–7. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 17.Taylor EN, et al. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47(5):780–9. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Lemann J, Jr, et al. Evidence that glucose ingestion inhibits net renal tubular reabsorption of calcium and magnesium in man. J Lab Clin Med. 1970;75(4):578–85. [PubMed] [Google Scholar]
- 19.Lemann J, Jr, Piering WF, Lennon EJ. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Engl J Med. 1969;280(5):232–7. doi: 10.1056/NEJM196901302800502. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, et al. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55(4):845–55. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chobanian MC, Hammerman MR. Insulin stimulates ammoniagenesis in canine renal proximal tubular segments. Am J Physiol. 1987;253(6 Pt 2):F1171–7. doi: 10.1152/ajprenal.1987.253.6.F1171. [DOI] [PubMed] [Google Scholar]
- 22.Asplin JR. Uric acid stones. Semin Nephrol. 1996;16(5):412–24. [PubMed] [Google Scholar]
- 23.Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67(2):685–90. doi: 10.1111/j.1523-1755.2005.67128.x. [DOI] [PubMed] [Google Scholar]
- 24.Vupputuri S, et al. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004;14(3):222–8. doi: 10.1016/S1047-2797(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 25.Worcester EM, et al. Renal function in patients with nephrolithiasis. J Urol. 2006;176(2):600–3. doi: 10.1016/j.juro.2006.03.095. discussion 603. [DOI] [PubMed] [Google Scholar]
- 26.Lee IM, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emerging Risk Factors, C et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123(16):1737–44. doi: 10.1161/CIRCULATIONAHA.110.005645. [DOI] [PubMed] [Google Scholar]
- 29.Muntner P, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–53. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 30.Hu G, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164(10):1066–76. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]


