Abstract
Normal aging is accompanied by changes in hypothalamic functions including autonomic and endocrine functions and circadian rhythms. The rhesus monkey provides an excellent model of normal aging without the potential confounds of incipient Alzheimer's disease inherent in human populations. This study examined the hypothalamus of 51 rhesus monkeys (23 male, 18 female, 6.5–31 years old) using design-based stereology to obtain unbiased estimates of neuron and glia numbers and the Cavalieri method to estimate volumes for eight reference spaces: total unilateral hypothalamus, suprachiasmatic nucleus (SCN), supraoptic nucleus (SON), paraventricular nucleus (PVN), dorsomedial nucleus (DM), ventromedial nucleus (VM), medial mammillary nucleus (MMN), and lateral hypothalamic area (LHA). The results demonstrated no age-related difference in neuron number, glia number, or volume in any area in either sex except the PVN of male monkeys, which showed a significant increase in both neuron and glia numbers with age. Comparison of males and females for sexual dimorphisms revealed no significant differences in neuron number. However, males had more glia overall as well as in the SCN, DM, and LHA and had a larger hypothalamic volume overall and in the SCN, SON, VM, DM, and MMN. These results demonstrate that hypothalamic neuron loss cannot account for age-related deficits in hypothalamic function and provides further evidence of the absence of neurode-generation and cell death in the normal aging rhesus monkey.
Keywords: glia number, hypothalamus volume, paraventricular nucleus (PVN), sexual dimorphism
In contrast to age-related neurodegenerative diseases like Alzheimer's disease or Parkinson's disease, where extensive neuron loss in specific brain regions can explain much of the observed neurological dysfunction, the causes of the subtle neurological changes that accompany normal aging remain largely unknown. While early studies asserted a mild but pervasive loss of neurons with normal aging (Peng and Lee, 1979; Finch, 1993), over the last two decades the use of animal models, especially the rhesus monkey (Peters et al., 1996), as well as the adoption of the counting and sampling procedures of design-based stereology (West et al., 1991; West, 1993a), have demonstrated that counting biases or sampling deficiencies likely explain the claims of global neuron “loss” in the aging cortex (Pakkenberg and Gundersen, 1988; Korbo et al., 1990). Stereological studies of the hippocampus, entorhinal cortex, and/or neocortical areas in both rats (Rapp and Gallagher, 1996), and monkeys (Peters et al., 1994, 1998a; Morrison and Hof, 1997; Gazzaley et al., 1997; Merrill et al., 2000; Keuker et al., 2003) have provided strong evidence that cortical neurons are not lost in the process of normal aging.
Similar studies in humans are more difficult and have produced divergent results. Some well-controlled studies have reported neuron preservation in humans (Freeman et al., 2008), while others have reported both a generalized loss of neurons in cortex (Pakkenberg and Gundersen, 1997) and regional losses in specific subfields of the hippocampus (West, 1993b; Simic et al., 1997). It is worth noting that the human studies that reported neuron loss used “normal” human subjects “free from dementia,” but did not include formal neuropsychological assessments that could exclude early stage neurodegenerative diseases like Alzheimer's, where neuron loss does occur. This is important because carefully controlled studies in humans have demonstrated that there is no loss of neurons in cognitively normal individuals from age 60 to 90, while those with the mildest detectable cognitive impairment show a 32% loss (Gomez-Isla et al., 1996). In contrast, subjects who had severe dementia had cell loss in some areas of as much as 90% (Gomez-Isla et al., 1996). Hence, studies of “normal aging humans” that have not conducted complete neuropsychological assessments to detect mild cognitive impairment (MCI) may well include subjects who have incipient Alzheimer's disease, and hence have neuron loss that is not the result of normal aging.
While most studies of normal aging in the rhesus monkey as well as other species have focused on the hippo-campus or neocortex because of their importance for higher cognitive functions, including learning, memory, and executive function, normal aging is accompanied by changes in circadian rhythms including sleep (Dijk and Duffy, 1999; Dijk et al., 2001; Yoon et al., 2003; Zhdanova et al., 2011) as well as changes in a variety of regulatory systems, including autonomic (Huang et al., 2007; Barantke et al., 2008) and endocrine functions (Touitou, 1995; Goncharova and Lapin, 2002; Woller et al., 2002; Nakamura et al., 2003; Schlatt et al., 2008; Sorwell and Urbanski, 2010). All of these functions depend on the integrity of various hypothalamic circuits.
Studies of age-related changes have been undertaken in the hypothalamus of rodents (Hsu and Peng, 1978; Peng and Hsu, 1982; Sartin and Lamperti, 1985; Roozendaal et al., 1987; Chee et al., 1988; Woods et al., 1993; Madeira et al., 1995, 2001) and humans (Morton, 1969; Swaab and Fliers, 1985; Hofman et al., 1988, 1990; Goudsmit et al., 1990; Vogels et al., 1990; Raadsheer et al., 1994; Zhou et al., 1995) focusing mostly on the suprachiasmatic nucleus (SCN), supraoptic nucleus (SON), and paraventricular nucleus (PVN). Stereological counts in the rodent suprachiasmatic nucleus (SCN) demonstrated no age-based differences in neuron number but a possible change in volume (Woods et al., 1993; Madeira et al., 1995). In humans, studies have reported decreases (Goudsmit et al., 1990), increases (Hofman et al., 1990), and no change (Hofman et al., 1988) in cell numbers in specific hypothalamic areas as well as changes in volumes with age that paralleled cell losses or gains. Manaye et al. (2005) demonstrated a 50% neuron loss in the PVN in patients suffering from major depressive disorder. However, to the best of our knowledge there are no published reports assessing global neuron number in the hypothalamus in relation to normal aging in any species, including monkeys.
To determine if generalized or global changes in neuron numbers occur in the hypothalamus in normal aging rhesus monkeys, the present study used unbiased, design-based stereology to assess the numbers of neurons and glia in the total hypothalamus and in specific subdivisions. The monkeys studied were part of a larger project focused mainly on the cerebral cortex and higher cognitive functions, so extensive behavioral and other neuro-biological information was available. The specific regions studied included the SCN, SON, PVN, ventromedial nucleus (VM), dorsomedial nucleus (DM), medial mammillary nucleus (MMN), lateral hypothalamic area (LHA), as well as the residual hypothalamic areas not included in these nuclei. The specific nuclei were chosen for two reasons. First, they could be reliably defined in thionin-stained sections. Second, these areas have known, or hypothesized, functions that may play a role in the aging process.
MATERIALS AND METHODS
Animals
The subjects were 18 female and 23 male rhesus monkeys, ranging in age from 6.5–31 years of age (Table 1). All animals were obtained from the Yerkes National Primate Research Center (YNPRC) at Emory University and had complete health records and a known birth date. Tigges et al. (1988) reported that rhesus monkeys at the Yerkes Center have a mean life expectancy (50% survival) of between 16 and 17 years of age with a maximum of 35 years of age. A similar study at the Wisconsin Primate Center (Dyke et al., 1986) used a somewhat different method of modeling life expectancy and found similar results but reported that rhesus monkeys there may have a mean life expectancy closer to 18 years of age and could live up to 40 years. A conservative interpretation of these reports suggests that the relationship of rhesus monkey age to humans is about 1:3, so that a 5-year-old monkey, that is just sexually mature, likely corresponds to a 15-year-old human, while a 30-year-old monkey most likely corresponds to a 90-year-old human.
TABLE 1.
Summary of Subjects Used
| Animal # | Age (years) | Sex | Hemisphere | Brain weight (g) | Fixative | Areas NOT available to count |
|---|---|---|---|---|---|---|
| AM205 | 6.2 | M | Right | 82.2 | 4% PFA | |
| AM247 | 6.9 | M | Left | 89.6 | 4% PFA | |
| AM245 | 7.0 | M | Right | 87.5 | 4% PFA | |
| AM093 | 7.6 | M | Right | 79.5 | 4% PFA | |
| AM128 | 7.9 | M | Right | 84.7 | 4% PFA | |
| BM085 | 8.1 | M | Right | N/A | 4% PFA | SCN, PVN, VM, DM, MMN, Rem |
| AM229 | 8.3 | M | Right | 82.6 | 4% PFA | |
| AM138 | 12.0 | M | Right | 86.0 | 4% PFA | |
| AM137 | 12.6 | M | Left | 84.3 | 4% PFA | |
| AM143 | 15.7 | M | Right | 92.7 | PFA+Glut | SCN, SON |
| AM239 | 17.9 | M | Right | 78.0 | 4% PFA | SCN |
| AM233 | 19.0 | M | Right | 83.0 | 4% PFA | PVN, VM, DM, Rem |
| AM209 | 19.2 | M | Right | 87.7 | PFA+Glut | SCN |
| AM153 | 19.4 | M | Right | 84.2 | 4% PFA | |
| AM216 | 19.7 | M | Right | 94.2 | 4% PFA | |
| AM124 | 19.9 | M | Left | 94.1 | 4% PFA | MMN |
| AM193 | 20.1 | M | Right | 88.6 | 4% PFA | |
| AM037 | 21.5 | M | Right | 91.5 | 4% PFA | |
| AM189 | 24.5 | M | Right | 80.7 | 4% PFA | |
| AM064 | 24.9 | M | Right | 92.1 | 4% PFA | |
| AM110 | 25.8 | M | Right | 90.4 | 4% PFA | |
| AM207 | 28.7 | M | Right | 88.4 | 4% PFA | |
| AM121 | 30.2 | M | Right | 82.5 | 4% PFA | |
| AM188 | 6.5 | F | Right | 71.3 | 4% PFA | |
| AM198 | 7.8 | F | Right | 68.7 | 4% PFA | |
| AM202 | 10.0 | F | Left | 75.0 | 4% PFA | |
| AM199 | 10.6 | F | Right | 80.4 | 4% PFA | |
| AM194 | 11.9 | F | Left | 76.6 | 4% PFA | |
| AM195 | 12.1 | F | Right | 80.2 | 4% PFA | |
| AM190 | 18.0 | F | Right | 73.1 | 4% PFA | |
| AM161 | 19.2 | F | Left | 67.2 | 4% PFA | |
| AM125 | 19.8 | F | Right | 61.3 | 4% PFA | |
| AM149 | 19.8 | F | Right | 90.9 | 4% PFA | |
| AM159 | 19.8 | F | Left | 79.2 | 4% PFA | |
| AM177 | 20.9 | F | Right | 74.3 | 4% PFA | MMN, LHA, Rem |
| AM200 | 21.0 | F | Right | 65.0 | 4% PFA | |
| AM162 | 22.3 | F | Right | 74.2 | 4% PFA | |
| AM179 | 23.7 | F | Right | 79.6 | 4% PFA | SCN |
| AM181 | 27.5 | F | Right | 63.5 | 4% PFA | |
| AM180 | 29.6 | F | Right | 70.6 | 4% PFA | |
| AM119 | 31.0 | F | Right | 86.8 | 4% PFA |
N/A: not available; PFA: paraformaldehyde; PFA+Glut: paraformaldehyde and glutaraldehyde; DM: dorsomedial nucleus; LHA: lateral hypothalamic area; MMN: medial mammillary nucleus; PVN: paraventricular nucleus; Rem: hypothalamic remainder; SCN: suprachiasmatic nucleus; SON: supraoptic nucleus; VM: ventromedial nucleus.
Prior to entering this study, monkeys were screened for health problems and magnetic resonance imaging (MRI) was used to screen for occult brain damage. Monkeys were maintained first at YNPRC and then at Boston University Medical Center (BUMC). Both facilities are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and all procedures were approved by the Institutional Animal Care and Use Committee at YNPRC and at BUMC and followed National Institutes of Health (NIH) guidelines. All monkeys were behaviorally tested on a variety of cognitive tasks to assess learning, memory, and executive function as described elsewhere (Herndon et al., 1997; Moore et al., 2005). At the end of the study all animals were perfused as described below and subsequently received necropsies that established the absence of occult disease processes that could affect the brain or behavior.
Tissue collection
After being deeply anesthetized with sodium pentobarbital (25 mg/kg to effect), monkeys were killed by exsanguination during transcardial perfusion-fixation of the brain. Thirty animals were perfused with 4 L of 4% paraformaldehyde, nine with 4 L of Krebs-Heinsleit buffer followed by 8 L of 4% paraformaldehyde, and two with 4 L of a solution containing 1% paraformaldehyde and 1.25% glutaraldehyde, all at pH 7.4 and with the aldehydes in 0.1M phosphate buffer. Tissue staining was equivalent in all types of perfusion. Following perfusion, brains were blocked in situ in the coronal stereotactic plane. One blocked hemisphere, containing the full extent of the hypothalamus, was cryoprotected in a phosphate buffer solution containing 20% glycerol and 2% dimethyl sulfoxide (DMSO) for a minimum of 3 days and then quickly frozen in −70°C isopentane and stored at −80°C until processed (Rosene et al., 1986). Frozen sections were cut on a sliding microtome, in the coronal plane into interrupted series of sections spaced at 300-μm intervals with eight series of 30-μm thick sections and 1 series of 60-μm thick sections. For the purposes of the current study, sections from the 60-μm series were mounted on gelatin-subbed slides, allowed to dry, stained with thionin, and coverslipped with Permount.
Area definitions
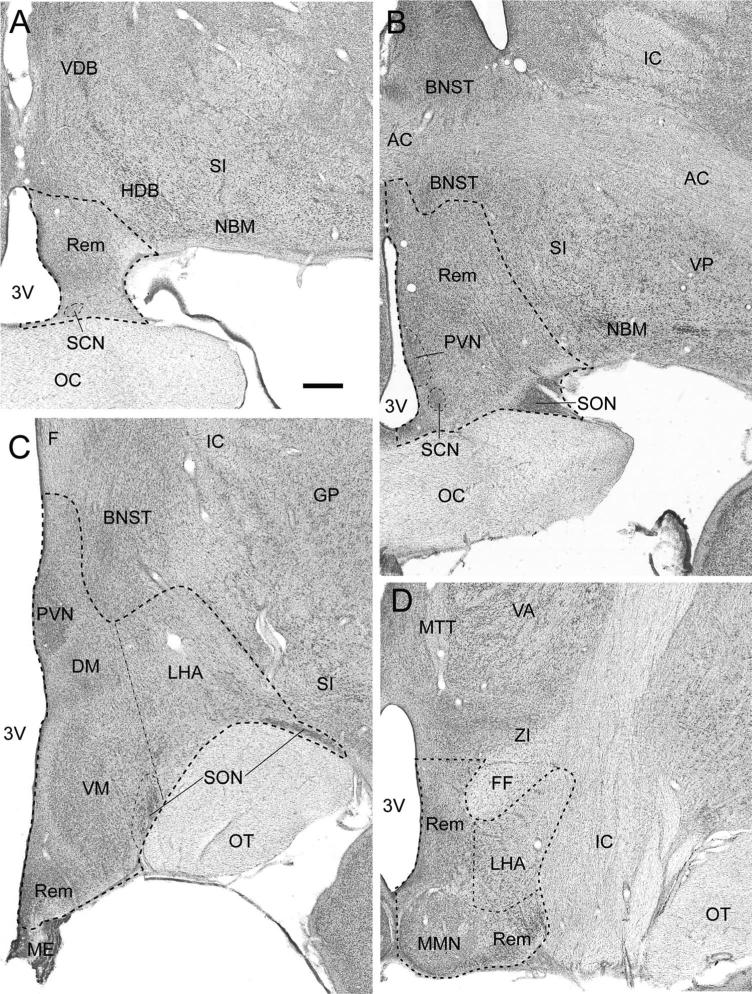
The hypothalamus has traditionally been divided into four areas in the rostral-caudal direction (preoptic, anterior, tuberal, and posterior) (Nauta and Haymaker, 1969; Saper, 1990) and three zones in the medial-lateral direction (periventricular, medial, lateral) (Crosby and Wood-burne, 1940). For the purposes of this study, the Paxinos et al. (2000) atlas of the monkey brain was followed with supplemental help from other atlases and anatomical descriptions (Crouch, 1934; Atlas Dal, 1937; Olszewski, 1952; Snider and Lee, 1961; Nauta and Haymaker, 1969; Rempel-Clower and Barbas, 1998). The boundaries of the hypothalamus and the specific nuclei addressed in this study are illustrated in Figure 1 and briefly summarized as follows.
Figure 1.
Sequential photomicrographs of thionin-stained coronal sections through the monkey hypothalamus at preoptic (A), anterior (B), tuberal (C), and posterior (D) levels. Outlines delineate the unilateral hypothalamic border and selected reference spaces utilized for stereologic counts of neurons and glia. DM, dorsomedial hypothalamic nucleus; LHA, lateral hypothalamic area; MMN, medial mammillary nucleus; PVN, paraventricular nucleus; Rem, unclassified hypothalamic remainder; SCN, suprachiasmatic nucleus; SON, supraoptic nucleus; VM, ventromedial hypothalamic nucleus. Surrounding neuroanatomical landmarks: 3V, third ventricle; AC, anterior commissure; BNST, bed nucleus of the stria terminalis; F, fornix; FF, fields of Forel; GP, globus pallidus; HDB, horizontal limb of the diagonal band of Broca; IC, internal capsule; ME, median eminence; MTT, mammillothalamic tract; NBM, nucleus basalis of Meynert; OC, optic chiasm; OT, optic tract; SI, substantia inominata; STN, subthalamic nucleus; VA, ventral anterior thalamic nucleus; VDB, verticle limb of the diagonal band of Broca; VP, ventral pallidum; ZI, zona incerta. Scale bar = 1 mm in A (applies to all).
The preoptic area is located dorsal to the optic chiasm just caudal to the vascular organ of the lamina terminalis (Fig. 1A). It is bordered dorsally by the vertical limb of the diagonal band and laterally by the horizontal limb of the diagonal band and the basal nucleus of Meynert. The pre-optic area is continuous caudally with the anterior hypothalamic area (Fig. 1B) that has ventral and lateral boundaries similar to the preoptic area but is bounded dorsally by both the anterior commissure and the bed nucleus of the stria terminalis. The tuberal region (Fig. 1C) is found caudal to the optic chiasm and above the tuber cinereum, which attaches to the hypophysis via the infundibular stalk. The tuberal region is bounded laterally by the internal capsule, globus pallidus, and substantia innominata and dorsally by the bed nucleus of the stria terminalis and the ventral thalamic nuclei. The posterior hypothalamic area begins with the appearance of the mammillary complex (Fig. 1D) and is bounded laterally by the internal capsule and subthalamic nucleus and dorsally by the zona incerta.
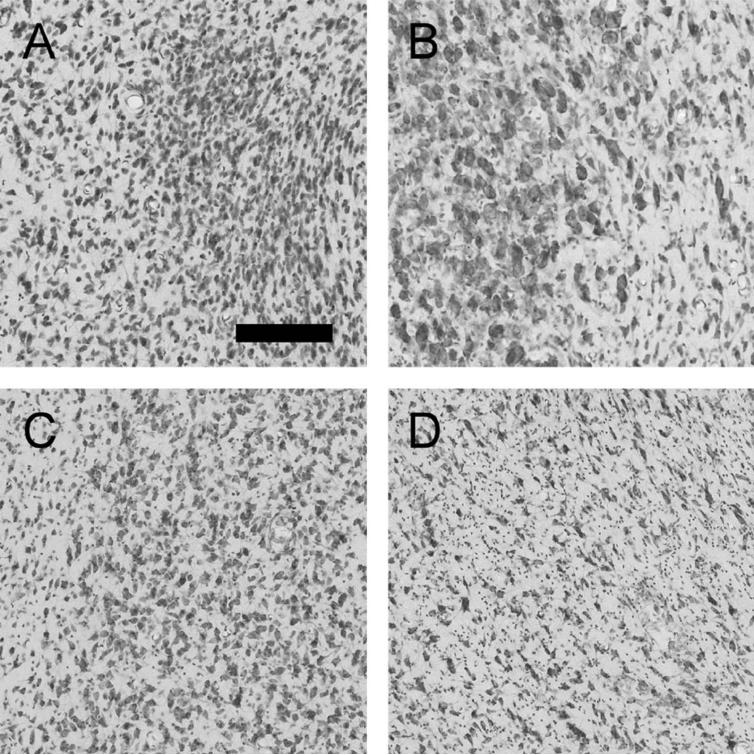
In order to perform volume measurements and cell counts, the entire hypothalamus was identified and outlined in every section. In addition, seven specific nuclei of interest (reference spaces) were identified and outlined. As identified in Figure 1, the seven reference spaces were the suprachiasmatic nucleus (SCN), the paraventricular nucleus (PVN), the supraoptic nucleus (SON), the dorsomedial nucleus (DM), the ventromedial nucleus (VM), the mammillary nucleus (MMN), and the lateral hypothalamic area (LHA). In addition, the remainder of the hypothalamus (marked “Rem” in Fig. 1) was also assessed and together with the seven nuclei was defined as the entirety of the hypothalamus. This allowed for the calculation of total unilateral hypothalamic volume, neuron number, and glial number. Figure 2 provides a higher-powered view of four reference spaces (SCN, PVN, VM, LHA) demonstrating the distinct cytoarchitecture of these areas and showing how they differ from surrounding regions.
Figure 2.
Photomicrographs demonstrating the cytoarchitecture of the SCN, suprachiasmatic nucleus (A), PVN, paraventricular nucleus (B), VM, ventromedial nucleus (C), and LHA, lateral hypothalamic area (D). The SCN consists of small, densely packed neurons and glia as seen on the right side of A, and is distinct from the surrounding hypothalamus as seen on the left side of A. The PVN consists of interspersed small and large neurons (the parvo- and magnocellular components) and glia as shown on the left side of B; it is distinguished from the surrounding hypothalamus that has decreased packing density and lack large neurons as shown on the right side of B. The VM consists of intermediate sized neurons and glia (right side of C), which are more densely packed than the surrounding hypothalamus (left edge of C). The LHA consists of relatively loosely packed small to intermediate sized neurons and glia (entirety of D). Scale bar = 200 μm in A (applies to all).
Equipment
Stereological analysis was done with a Nikon E600 microscope fitted with an ASI MS-2000 motorized stage and stage encoder for z-axis measurements, as well as an Optronics analog video camera. The stereology plug-in of Bioquant Nova 6.75.10 (Nashville, TN) was used with a high-resolution color monitor to perform all outlines, counts, and measurements. Reference spaces were outlined using a 2× objective and cell counts were per formed using a 100× oil-immersion objective.
Cell counts
Cells were counted using the unbiased 3D counting scheme of the optical fractionator (West et al., 1991; West, 1993a) and volumes were assessed using the Cavalieri estimator of Gundersen and Jensen (1987). All sections spanning the hypothalamus (male: 28 ± 1 sections; female: 26 ± 2 sections) from the series cut at 60-μm thickness were examined under low power of a light microscope and a list was made of the hypothalamic areas present in each section. Some reference spaces were omitted in particular animals due to either missing sections or section damage (see “Areas NOT Available to Count” in Table 1). Pilot studies were conducted to determine the sampling scheme needed to satisfy stereological principles for each reference space. Based on these studies, the number of sections, grid dimensions, and disector or “counting box” dimensions needed to produce 200+ cell counts was determined and references spaces measured and cells counted in either every section in the series, or every other or every third, resulting in sampling spaced at 300, 600, or 900 μm depending on their size as shown in Table 2. All disectors were 60 × 60 μm in the horizontal plane.
TABLE 2.
Stereology Parameters for All Reference Spaces
| Reference space | Section Interval | Neuron grid Dimensions (μm) | Glia grid dimensions (μm) | Disector Height (μm) | Mean sections counted (male) | Mean sections counted (female) |
|---|---|---|---|---|---|---|
| SCN | 1/10 | 100 × 100 | 141 × 141 | 10 | 5 ± 1 | 4± 1 |
| SON | 1/10 | 300 × 300 | 424 × 424 | 10 | 11 ± 2 | 11± 2 |
| PVN | 1/20 | 300 × 300 | 424 × 424 | 10 | 6 ± 1 | 5± 1 |
| VM | 1/10 | 400 × 400 | 566 × 566 | 10 | 6± 1 | 5± 1 |
| DM | 1/10 | 350 × 350 | 495 × 495 | 10 | 6± 1 | 5± 1 |
| MMN | 1/10 | 450 × 450 | 636 × 636 | 10 | 6± 1 | 6± 1 |
| LHA | 1/30 | 700 × 700 | 990 × 990 | 10 | 7 ± 1 | 6± 1 |
| Rem | 1/30 | 1000 × 1000 | 1414 × 1414 | 10 | 9± 1 | 9± 1 |
SCN: Suprachiasmatic Nucleus; SON: Supraoptic Nucleus; PVN: Paraventricular Nucleus; VM: Ventromedial Nucleus; DM: Dorsomedial Nucleus; MMN: Medial Mammillary Nucleus; LHA: Lateral Hypothalamic Area; Rem: Remaining hypothalamus not included in above areas.
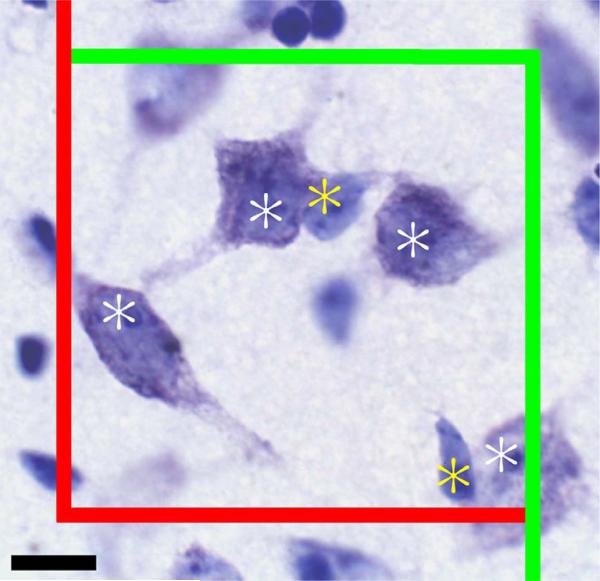
After the reference spaces were outlined with the 2× objective, the Bioquant program randomly placed a grid of known dimensions over each reference space. The program then placed disector boxes of known dimensions at all grid intersections falling within the reference space. Figure 3 shows an example disector box with the inclusion (green) and exclusion (red) planes as well as counted glia (yellow asterisk) and neurons (white asterisk). Neurons were counted in all disectors, while glia, due to their greater number, were counted in 50% of the disectors. Disectors for glial counts were arranged on alternating diagonals, a checkerboard pattern. The counting object used for neurons was the nucleolus. No neurons with greater than one nucleolus were observed. The counting object for glia was the nucleus. No attempt was made to distinguish between glial types since specific stains were not used.
Figure 3.
Representative counting frame in the VM (ventromedial nucleus). Counting frame dimensions are 60 × 60 μ. Counting objects (nucleolus for neurons and nucleus for glia) falling on the green borders were counted, those falling on the red borders were not. Objects that would be counted at the displayed plane of focus are marked with a white asterisk for neurons and a yellow asterisk for glia. Scale bar = 10 μ.
Guard volumes were used to eliminate potential bias due to lost caps from the cutting process (Hedreen, 1998). First, with the 100× objective, the section thickness at the disector location was measured, allowing the program to place the 10-μm deep disector box 1 μm below the top of the section, creating a 1 μ guard volume. The z-axis dimension of all disectors was 10 μ. Since shrinkage of frozen sections cut at an instrument setting of 60 μ results in a final postprocessing section thickness of 15–23 μm, guard volumes at the bottom of the section varied from 3–11 μ. Next, the upper z-axis plane was identified and objects intersecting this were not counted. Then the focus was moved down through the disector and counting objects were marked as they came into focus except those contacting either of the side exclusion planes (right and lower border as viewed on monitor) were not counted, while those contacting the other two sides (left and upper border as viewed on monitor) or the lower z-axis plane when it was reached were counted. This counting scheme ensures that counts are unbiased since objects cannot be double-counted. The neuron and/or glia count were recorded for that disector and the stage moved automatically to the next disector until all disectors were examined within the reference space.
In order to calculate the total cell number estimate from those counted, the following formula was used:
Where ΣQ− was the number of cells counted, ssf was the fraction of sections counted, asf was the disector area divided by the grid area, and tsf was the disector height divided by the average mounted tissue thickness. The average coefficient of error (Gundersen et al., 1999) for each reference space is reported in Tables 3–5. Neuron and glia numbers reported for individual areas in this study are unilateral. Cell estimates reported for the total unilateral hypothalamus were reached by summing estimates of all areas counted. Animals in which one or more reference spaces were unavailable for counting were not included in the total unilateral hypothalamus dataset, but were included in analyses of the specific reference spaces that were examined.
TABLE 3.
Mean Estimated Neuron Number in All Reference Spaces With Relation to Age
| Area | Neurons | SD | n | CE | r (age) | P (age) |
|---|---|---|---|---|---|---|
| MALE | ||||||
| SCN | 11,854 | 3,032 | 19 | 0.04 | –0.16 | 0.521 |
| SON | 45,732 | 12,402 | 22 | 0.06 | 0.40 | 0.065 |
| PVN | 111,018* | 27,098 | 21 | 0.06 | 0.58 | 0.006* |
| VM | 174,964 | 35,493 | 21 | 0.05 | 0.05 | 0.843 |
| DM | 71,481 | 21,642 | 21 | 0.06 | 0.32 | 0.164 |
| MMN | 110,058 | 28,393 | 21 | 0.07 | –0.08 | 0.743 |
| LHA | 498,487 | 64,275 | 23 | 0.08 | –0.11 | 0.614 |
| Remainder | 2,064,296 | 475,146 | 21 | 0.06 | 0.01 | 0.983 |
| Total unilateral hypothalamus | 3,044,802 | 547,852 | 17 | 0.06 | 0.09 | 0.723 |
| FEMALE | ||||||
| SCN | 12,435 | 5,207 | 17 | 0.05 | –0.46 | 0.061 |
| SON | 41,835 | 7,995 | 18 | 0.06 | 0.00 | 0.991 |
| PVN | 117,755 | 24,045 | 18 | 0.05 | –0.04 | 0.872 |
| VM | 181,762 | 35,774 | 18 | 0.04 | 0.06 | 0.816 |
| DM | 60,879 | 12,747 | 18 | 0.07 | –0.15 | 0.553 |
| MMN | 99,964 | 23,131 | 16 | 0.07 | –0.03 | 0.912 |
| LHA | 519,841 | 75,209 | 17 | 0.08 | 0.01 | 0.958 |
| Remainder | 2,125,898 | 321,289 | 17 | 0.05 | 0.24 | 0.360 |
| Total unilateral hypothalamus | 3,142,319 | 410,162 | 16 | 0.06 | 0.20 | 0.442 |
The male paraventricular nucleus (PVN) was the only area to display an age-related change in neuron number.
P < 0.05; SD: standard deviation; CE: coefficient of error for neuron estimate; n: number of subjects; r: value of Pearson's Product-Moment Correlation; P: corresponding P value; SCN: suprachiasmatic nucleus; SON; supraoptic nucleus; VM: ventromedial nucleus; DM: dorsomedial nucleus; MMN: medial mammillary nucleus; LHA: lateral hypothalamic area.
TABLE 5.
Mean Cavalieri Principle Estimated Volume of All Reference Spaces With Relation to Age
| Area | Vol (mm3) | SD (mm3) | n | CE | r (age) | P (age) |
|---|---|---|---|---|---|---|
| MALE | ||||||
| SCN | 0.185 | 0.043 | 19 | 0.08 | –0.04 | 0.887 |
| SON | 2.144 | 0.543 | 22 | 0.04 | 0.40 | 0.064 |
| PVN | 2.767 | 0.662 | 21 | 0.08 | 0.39 | 0.078 |
| VM | 3.936 | 0.673 | 21 | 0.06 | 0.13 | 0.566 |
| DM | 1.825 | 0.472 | 21 | 0.07 | 0.28 | 0.224 |
| MMN | 3.107 | 0.582 | 21 | 0.05 | –0.17 | 0.461 |
| LHA | 29.988 | 6.714 | 23 | 0.06 | –0.06 | 0.775 |
| Remainder | 56.186 | 6.744 | 21 | 0.06 | 0.31 | 0.170 |
| Total unilateral hypothalamus | 98.825 | 12.982 | 17 | 0.06 | 0.15 | 0.552 |
| FEMALE | ||||||
| SCN | 0.146 | 0.040 | 17 | 0.10 | –0.49 | 0.046 |
| SON | 1.833 | 0.397 | 18 | 0.03 | 0.20 | 0.433 |
| PVN | 2.652 | 0.490 | 18 | 0.07 | 0.32 | 0.203 |
| VM | 3.507 | 0.653 | 18 | 0.07 | 0.18 | 0.475 |
| DM | 1.321 | 0.330 | 18 | 0.09 | –0.10 | 0.684 |
| MMN | 2.514 | 0.345 | 16 | 0.06 | –0.12 | 0.669 |
| LHA | 26.227 | 3.278 | 17 | 0.06 | –0.11 | 0.683 |
| Remainder | 53.153 | 7.765 | 17 | 0.06 | –0.24 | 0.356 |
| Total unilateral hypothalamus | 89.920 | 10.750 | 16 | 0.07 | –0.30 | 0.211 |
No region of interest in either sex displayed an age-related change in volume. SD: standard deviation; n: number of subjects; CE: mean coefficient of error for Cavalieri estimator volumes of reference spaces; r: value of Pearson's Product-Moment Correlation; P: corresponding P value; SCN: suprachiasmatic nucleus; SON; supraoptic nucleus; VM: ventromedial nucleus; DM: dorsomedial nucleus; MMN: medial mammillary nucleus; LHA: lateral hypothalamic area.
Volume
Volumes of each reference space were estimated using a version of the Cavalieri Principle, as described in Gundersen and Jensen (1987):
Where T = distance between sections (mm); ΣP = sum of points that fall within the reference area on each section; and a(p) = area per point (mm3). The number of points was determined by summing the total number of disectors counted within each reference space. The area per point is equivalent to the area of the grid (i.e., 100 × 100 μ for SCN).
Statistics
In general we took a 2-fold approach to analyzing the data in this study. First, overall comparisons/correlations were conducted to look for omnibus differences/relationships among the variables: age, sex, total neuron number, total glia number, total volume. Since these were few in number and relatively unrelated to each other a P-value of 0.05 was used with these analyses as a cutoff for statistical significance. These omnibus assessments were then followed with more specific hypothesis-driven analyses. These specific correlation tests were protected from multiple comparisons using the Sidak-Bonferroni method, which showed that a P-value of 0.006 was required for significance. All correlations were run using Data Desk 6.2 (Ithaca, NY; 2004). Analysis of covariance (ANCOVA) and multivariate analysis of covariance (MANCOVA) analyses with sex as the between-groups variable and age controlled as a covariable were conducted in SPSS 13.0.0 for Mac OS X (Chicago, IL). Normalization of neuron and glia counts to either brain weight or hypothalamic volume did not change the findings of the present study; therefore, raw data are reported.
RESULTS
Neuron number
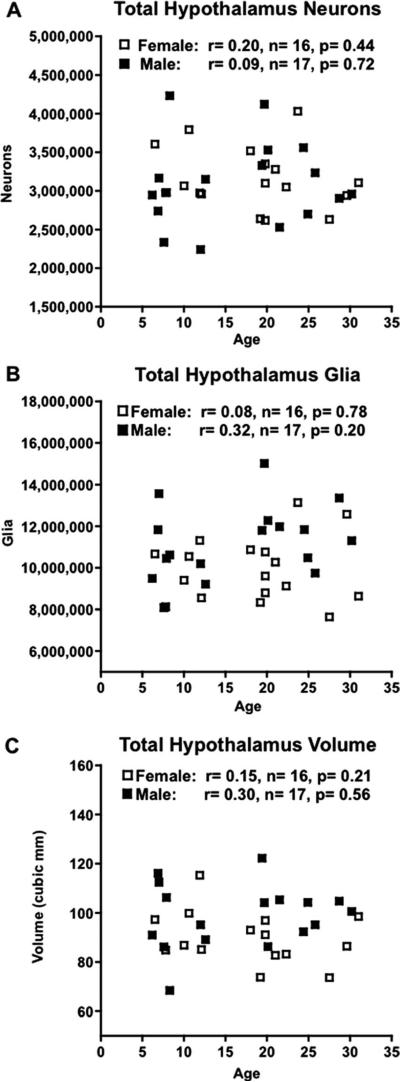
Neuron counts from the eight references spaces— seven specific nuclei, as well as the remainder of the hypothalamus of one hemisphere—were added together to provide an estimate of total unilateral hypothalamic neuron number, shown in Figure 4A for males (3.04 × 106 ± 0.13 × 106) and females (3.14 × 106 ± 0.10 × 106). Neither male nor female hypothalami displayed a significant correlation between total neuron number and age (r = 0.09 with P = 0.72, r = 0.20 with P = 0.44, respectively).
Figure 4.
Graphs showing morphometric parameters studied versus age in 16 female (□) and 17 male (■) rhesus monkeys with Pearson's Product-Moment correlations (r values) and corresponding P-values. A: Design-based stereologic estimate of total unilateral hypothalamic neuron number in thionin-stained sections showed no significant age-related change in either sex. B: Design-based stereologic estimate of total uni-lateral hypothalamic glia number in thionin-stained sections showed no age-related change in either sex. C: Cavalieri estimate of total unilateral hypothalamic volume displayed no age-related change in either sex.
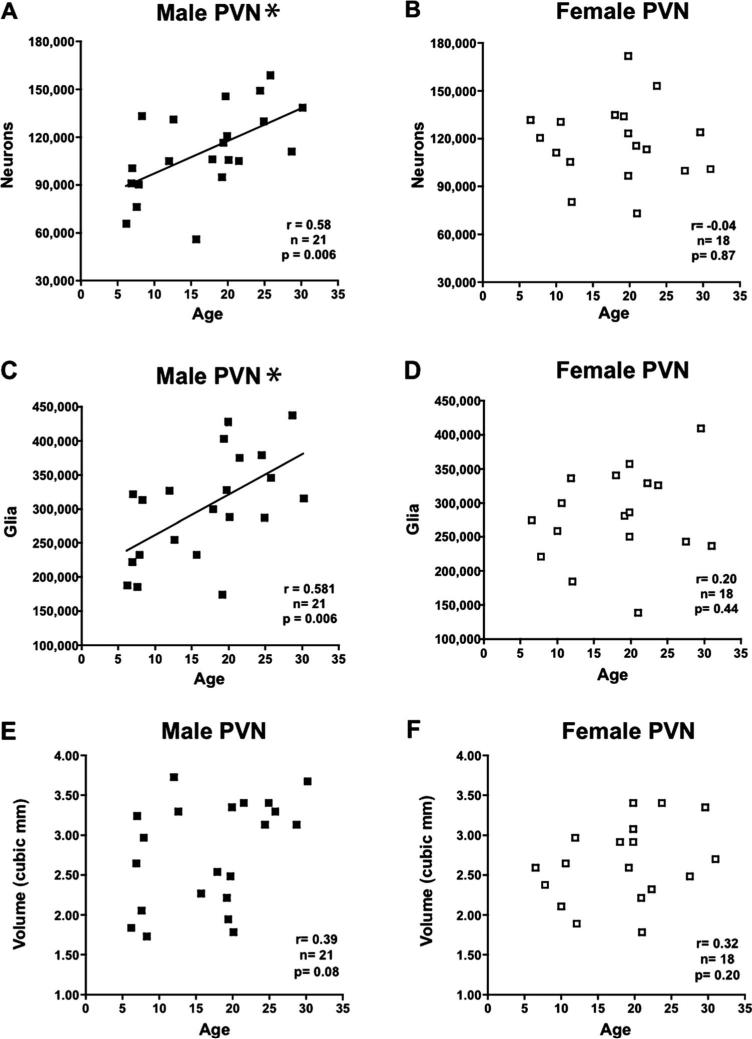
When neuron number was analyzed in males and females for each individual area using correlation, there was again no significant relationship with age in the SCN, SON, VM, DM, MMN, LHA, or Remainder as shown in Table 3. However, the PVN of male monkeys (Fig. 5A) displayed a significantly higher neuron number in aged animals (P = 0.006), while females (Fig. 5B) showed no change (P = 0.872).
Figure 5.
Graphs showing design-based stereologic estimates of cell number and Cavalieri volume estimates versus age in the paraventricular nucleus (PVN) in 18 female (□) and 23 male (■) rhesus monkeys with Pearson Product Moment correlations (r values) and corresponding P-values. A: Estimate of PVN neuron number displayed a significant increase with age in males. B: Estimate of PVN neuron number showed no change with age in females. C: Estimate of PVN glia number displayed a significant increase with age in males. D: Estimate of PVN glia number showed no change with age in females. E: Estimate of PVN volume displayed a trend toward increasing with age in males. F: Estimate of PVN volume displayed no change with age in females. *P < 0.05.
Glia number
As with the neurons, glia were also counted in eight hypothalamic reference spaces within a single hemisphere (Table 4). These numbers were then summed to estimate the total unilateral number of glia. Similar to neuron numbers, the total hypothalamic glial number showed no significant correlations with age in either male or female animals (r = 0.32 with P = 0.20 and r = 0.08 with P = 0.78, respectively) (Fig. 4B).
TABLE 4.
Mean Estimated Glia Number in All Reference Spaces With Relation to Age
| Area | Glia | SD | CE | n | r (age) | P (age) |
|---|---|---|---|---|---|---|
| MALE | ||||||
| SCN | 19,883 | 6,212 | 0.05 | 19 | 0.12 | 0.633 |
| SON | 242,662 | 84,811 | 0.04 | 22 | 0.34 | 0.119 |
| PVN | 301,848* | 77,842 | 0.06 | 21 | 0.58 | 0.006* |
| VM | 335,596 | 76,054 | 0.05 | 21 | 0.13 | 0.589 |
| DM | 208,645 | 60,330 | 0.06 | 21 | 0.36 | 0.107 |
| MMN | 390,250 | 122,283 | 0.06 | 21 | –0.19 | 0.422 |
| LHA | 4,228,518 | 829,059 | 0.05 | 23 | –0.12 | 0.595 |
| Remainder | 5,715,799 | 1,101,554 | 0.05 | 21 | 0.37 | 0.104 |
| Total unilateral hypothalamus | 11,251,254 | 1,747,304 | 0.05 | 17 | 0.32 | 0.196 |
| FEMALE | ||||||
| SCN | 15,518 | 5,139 | 0.06 | 17 | –0.22 | 0.405 |
| SON | 222,044 | 69,645 | 0.04 | 18 | 0.10 | 0.708 |
| PVN | 279,011 | 65,212 | 0.05 | 18 | 0.20 | 0.412 |
| VM | 303,609 | 73,957 | 0.05 | 18 | 0.11 | 0.673 |
| DM | 155,263 | 40,562 | 0.06 | 18 | –0.06 | 0.822 |
| MMN | 336,937 | 44,386 | 0.06 | 16 | –0.05 | 0.869 |
| LHA | 3,200,325 | 549,710 | 0.05 | 17 | 0.40 | 0.114 |
| Remainder | 5,408,954 | 1,053,287 | 0.05 | 17 | –0.11 | 0.672 |
| Total unilateral hypothalamus | 9,849,165 | 1,592,430 | 0.05 | 16 | 0.08 | 0.777 |
The male paraventricular nucleus (PVN) was the only area to display an age-related change in glia number.
P < 0.05; SD: standard deviation; CE: coefficient of error for glia estimate; n: number of subjects; r: value of Pearson's Product-Moment Correlation; P: corresponding P value; SCN: suprachiasmatic nucleus; SON; supraoptic nucleus; VM: ventromedial nucleus; DM: dorsomedial nucleus; MMN: medial mammillary nucleus; LHA: lateral hypothalamic area.
When glia number was analyzed in males and females for each individual area, there was no significant correlation with age for the SCN, SON, VM, DM, MMN, LHA, or Remainder in either sex (Table 4). However, for the PVN of male monkeys there was a significantly higher glia number in aged animals (P = 0.006) (Fig. 5C), while the female PVN displayed no change in glia number with age (P = 0.41) (Fig. 5D).
Volume
The volumes for each of the eight hypothalamic areas as estimated by the Cavalieri method were added together to give a total unilateral hypothalamic volume. The mean total volume in males was 98.83 ± 12.98 mm3, while in females it was 89.92 ± 10.75 mm3 (Table 5). There was no significant correlation with age for total uni-lateral hypothalamic volume in either male (r = 0.15, P = 0.56) or female (r = 0.30, P = 0.21) animals (Fig. 4C). Analysis of the volumes of individual references spaces in both males and females showed no significant correlation with age. It is worth noting that despite the increase in the numbers of neurons and glia in the PVN of male monkeys described above, there was no age-related change in volume (P = 0.078, Fig. 5E).
Male/female comparisons
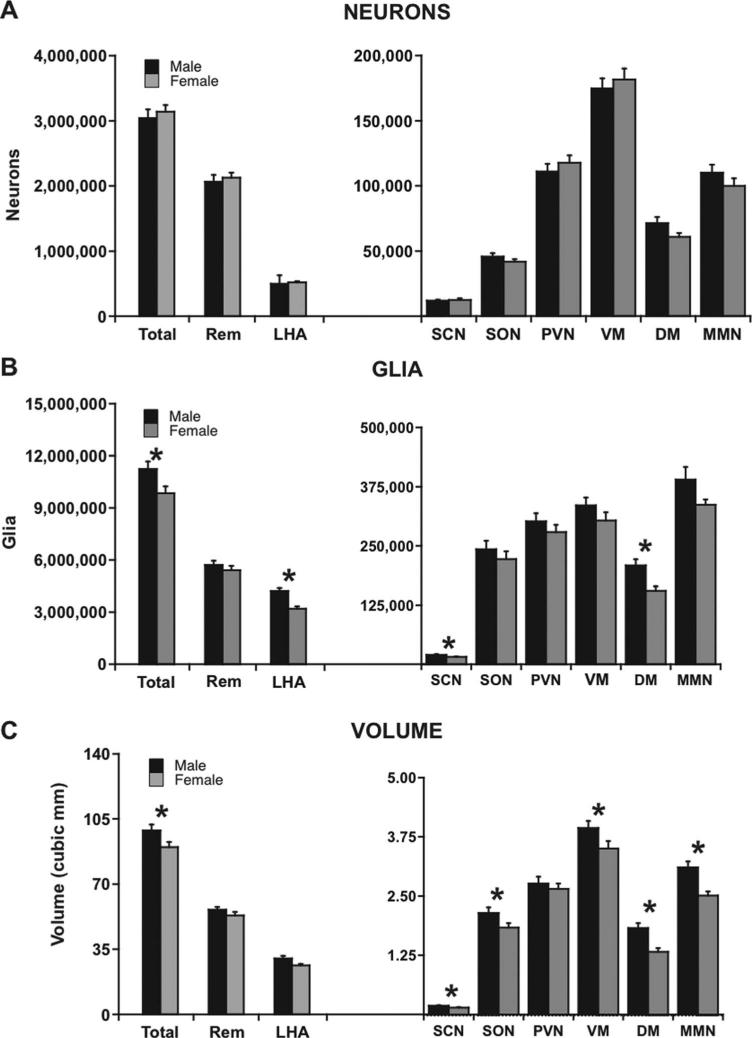
Since most parameters in the areas examined do not change with age, it is reasonable to compare the pooled means of the males versus those of the females to consider whether there are any differences due to sex. As shown in Figure 6A, there was no significant male-female difference for total hypothalamic neuron number (P = 0.558). Given that no sex difference was observed in total neuron number by ANCOVA, statistics for individual nuclei were not examined (Fig. 6A).
Figure 6.
Graphs showing male-female comparisons of design-based stereologic estimates of cell number and Cavalieri volume estimates in the total unilateral hypothalamus as well as the eight specific reference spaces studied in thionin-stained sections. Comparisons of total unilateral measurements were ANCOVAs, while comparisons of individual reference spaces were done by MANCOVA. Error bars show standard error of the mean. A: There was no overall male-female difference in neuron number; therefore, individual reference spaces were not examined for male-female differences. B: Males had significantly more hypothalamic glia than females overall (P = 0.017) as well as in the SCN (P = 0.019), DM (P = 0.002), and LHA (P = 0.0001). C: Males had a significantly larger hypothalamus than females overall (P = 0.05), as well as in the SCN (P = 0.011), SON (P = 0.027), VM (P = 0.036), DM (P = 0.001), and MMN (P = 0.008). DM, dorsomedial nucleus; LHA, lateral hypothalamic area; MMN, medial mammillary nucleus; PVN, paraventricular nucleus; Rem, hypothalamic remainder; SCN, suprachiasmatic nucleus; SON, supraoptic nucleus; Total, total unilateral hypothalamus; VM, ventromedial nucleus. *P < 0.05.
Unlike neuron number, total unilateral glia number was significantly higher in males than in females, with P = 0.017 (Fig. 6B, Table 4). Analyses of individual nuclei by MANCOVA showed significantly more glia in the male SCN, DM, and LHA than in the corresponding female nuclei (Fig. 6B).
The greatest number of male-female differences was observed in hypothalamic volume comparisons, where total hypothalamic volume and the volume of five of the eight areas measured were larger in males compared to females. As a whole, the male monkey hypothalamus was significantly larger than that of females (P = 0.050; Fig. 6C). Examination of specific nuclei through MANCOVA revealed that the male SCN, SON, VM, DM, and MMN were all significantly larger in male monkeys than female nuclei (Fig. 6C). There were no volume differences observed between the male and female PVN, LHA, or Remainder.
DISCUSSION
In this study we present the first quantitative investigation of the monkey hypothalamus in both male and female rhesus monkeys utilizing unbiased stereologic methods to assess the question of whether there is neuron loss across the adult lifespan. In summary, the monkey hypothalamus displays no overall age-related loss of neurons or of glia in either males or females nor is there any loss of neurons in the seven specific areas examined or in the remainder (Rem) of the hypothalamus. Additionally, there was no age-related loss of volume, either overall or in any of the specific nuclei examined. However, there was a surprising age-related increase in the number of neurons and glia that was unique to the PVN of males. In contrast to the stability of neuron and glia numbers and volume with age, when comparing males with females, the male has both a larger hypothalamus and a greater number of glia than the female. However, there was no difference between males and females in the number of neurons either overall or in any of the eight references spaces examined. Overall, hypothalamic integrity appears to be maintained over age in both male and female animals with the exception of the unexpected increase in the number of neurons and glia in the PVN of males.
Technical considerations
The current study utilized the stereologic principles of the optical fractionator (West et al., 1991) for both neuron and glia counts and the Cavalieri Principle (Gundersen et al., 1987, 1999) for reference volume measurements. These unbiased methods are considered by many to be superior to earlier assumption-based or model-based methods for morphometric measurements in neurobio-logic systems (Mayhew and Gundersen, 1996; Long et al., 1999; Schmitz and Hof, 2005). The practical differences in these counting methods are demonstrated by the fact that prior to the use of stereology, it appeared that there was a generalized loss of neurons in the aging brain. Since the advent of stereologic techniques, it has become apparent that while its impossible to rule out some instances of limited and localized neuronal losses with age, there is no evidence that age-related neuron loss is a global phenomenon (Peters et al., 1998b).
The total hypothalamus was measured as well as seven specific reference spaces within it. Nevertheless, certain identifiable nuclei were not examined separately for one of three reasons. First, some areas are quite small and were therefore only available on 1–2 sections in most subjects. These included areas like the lateral mammillary nucleus, the tuberomammillary nucleus, and the sexually dimorphic nuclei of the anterior and preoptic areas. This few sections does not allow implementation of unbiased stereology methods, so these areas were included in the Remainder. Second, the arcuate nucleus was not counted as a distinct reference space due to its location at the ventral surface of the hypothalamus, where it spreads across the midline and was often asymmetrically distributed after bisection of the hemispheres. While loss of tissue due to asymmetric blocking accounted for <1% of the total hypothalamic volume, it was often greater than 10% of the arcuate nucleus, making it impossible to accurately quantify this area, so it too was included in the Remainder. Third, areas like the medial and lateral preoptic nuclei, anterior nucleus, and posterior nucleus were not separately counted as their borders could not be reliably and consistently identified in the Nissl-stained sections. Hence, these three areas were included in the Remainder.
Total hypothalamus: neurons, glia, volume, and sex
We are unaware of any literature reporting counts of neurons or glia, or measurement of volume of the total hypothalamus in any species. The glia:neuron ratio for the hypothalamus as a whole was 3.69:1 in males and 3.13:1 in females. Widely varying glia:neuron ratios have been reported in the literature, but none for the monkey hypothalamus. In the parvocellular portion of the thalamic lateral geniculate nucleus, the glia:neuron ratio is about 0.33:1, while in the magnocellular portion it is about 0.37:1 (Selemon and Begovic, 2007). The ratio in frontal cortex of primates, including humans, varies from 0.46:1 to 1.65:1 (Sherwood et al., 2006). Finally, in the only stereologic study of a hypothalamic area that measured glia and neurons separately the ratio varied from 5:1 to 8:1 in the MMN of aging male humans (Begega et al., 1999b).
While many studies have been published comparing specific regions and nuclei within the hypothalamus between the sexes and describing sexual dimorphisms in different nuclei including the VM (Matsumoto and Arai, 1983, 1986; Byne, 1998; Leal et al., 1998; Madiera et al., 2001), we are unaware of any reports comparing the overall hypothalamus between the sexes with regard to either numbers of neurons or glia or total hypothalamic volume. In this study, when comparing the male and female hypothalamus independent of age, we found no difference in total neuron number, but did find significantly more glia and a greater volume in the male hypothalamus.
Specific nuclei and areas
The seven specific regions assessed in this study of the normal aging rhesus monkey have not been systematically studied but a variety of reports illuminate aging and sex differences in individual structures.
Suprachiasmatic nucleus (SCN)
Early studies using a variety of methods failed to detect age-related changes (Roozendaal et al., 1987; Chee et al., 1988; Woods et al., 1993). Several studies examining the SCN in humans have produced conflicting results. Swaab et al. (1985) reported a decline in SCN volume and total cell number (neurons plus glia) as did Goudsmit et al. (1990), while Hoffman et al. (1988) found no change in SCN cell number with age. Only one previous study has used stereological methods to investigate SCN neuron numbers in relation to age. In the rat, Madeira et al. (1995) performed a stereological analysis of the rat SCN to assess “total cell number” (neurons plus astrocytes), as well as to estimate volume, and reported no age-related changes but did find a trend toward decrease in SCN volume with age in female rats (P = 0.065). In the present study, only the female SCN suggested an age-related neuron loss, but it did not reach criteria for significance. Similarly, volume of the female SCN also trended toward decrease with age but did not reach criteria for significance. However, numbers of glia cells were unchanged with age in the female SCN. Given the parallel trend toward SCN volume loss in aged female rats and monkeys, and the fact that Madeira et al. (1995) did not separate neuron and astrocyte counts, a future study with a greater number of subjects may find a subtle age-related decline in the female SCN volume and/or neuron number.
While earlier nonstereological studies in the rat did report that the male SCN was larger (Gorski et al., 1978; Robinson et al., 1986), similar nonstereologic studies in humans found no difference in SCN volume, neuron number, or glia number between males and females (Swaab et al., 1985; Hofman et al., 1988) but did report a more spherical nucleus in the male (Swaab et al., 1985; Hofman et al., 1988). In the rat, the stereological study of Madeira et al. (1995) found no effect of sex on total cell number (neurons plus astrocytes) or volume of the SCN. The present study found the female SCN to be significantly smaller than the male (Fig. 6C) but there were no differences in neuron or glial numbers.
Supraoptic nucleus (SON)
One early nonstereologic study reported both stable neuron numbers with age in males and in females but increased SON volume in aged females (Hsu and Peng, 1978; Peng and Hsu, 1982) while another reported a 15– 21% loss of SON neurons with age (Remacha and Camarero, 1988). One study in humans, ranging from 10–93 years old, found no change in SON neuron number (Goudsmit et al., 1990), while a second study of the same age-range (10–93 years) found an increased SON cell number (neurons plus glia), suggesting an increase in glial number with age (Hofman et al., 1990). Unlike the SCN, there appear to be no stereologic studies of the SON in any species. In the present study we report no significant change in SON neuron number, glial number, or volume with age in either male or female monkeys. It is interesting to note, however, that both neuron number and reference space volume in male monkey SON displayed a trend toward an increase with age, with P = 0.065 and P = 0.064, respectively.
In humans, both Hofman et al. (1990) and Goudsmit et al. (1990) reported no sexual dimorphisms in the human SON with regard to volume or cell number. In the current study, a comparison of males and females found no sex difference in SON glial number, but a significantly larger SON volume in males (P = 0.027). The larger male SON volume may reflect the trend toward increased neuron number with age in the SON of males. The significant correlation between male SON neuron number and volume (r = 0.665, P = 0.0005) supports this hypothesis.
Paraventricular nucleus (PVN)
Nonstereologic studies in the rat (Peng and Hsu, 1982; Sartin and Lamperti, 1985) and in the mouse (Sturrock, 1992) reported no change in PVN neuron number with age in males. Similar studies in humans found no significant change in neuron count, total cell count (neurons plus glia), cell density, or volume with age (Hofman et al., 1988; Goudsmit et al., 1990).
Male-female comparisons in the present study yielded no sex differences in glia number or volume of the PVN, which is consistent with human studies (Hofman et al., 1988; Goudsmit et al., 1990). In contrast, in the present study the PVN was the only area in which we found a significant change in neuron number with age, but instead of a loss, there was a significant increase that was limited to males (P = 0.006). The male PVN was also the only area with a significant age-related increase in glia number (P = 0.006). Finally, the male PVN dem onstrated a trend toward increasing volume with age (P = 0.078). This result is plausible given our findings of significant age-related increases in both neuron and glia number in this nucleus.
The PVN contains both magnocellular and parvocellular neurons. Since we counted all neurons regardless of size, a claim cannot be made as to which neuronal type is augmented, but the literature provides some clues. A study that counted the number of corticotropin-releasing hormone (CRH)-positive neurons, which are part of the parvocellular PVN, in the human hypothalamus, reported an increased number of immunopositive neurons with age (Raadsheer et al., 1994). Conversely, the magnocellular portion of the nucleus consists of vasopressin and oxytocin-producing neurons. Van der Woude et al. (1995) reported an increase in vasopressinergic neurons with age in humans. Since the immunostained neuron number was not expressed in terms of total neuron number in the above studies, it is impossible to know if the increased immunostaining is due to new gene expression in already present neurons or entirely new neurons. It is possible that neurogenesis is occurring in the nucleus, as has been observed in the porcine vasopressin and oxytocin-containing nucleus (Rankin et al., 2003).
Dorsomedial nucleus (DM)
There are no stereologic studies and only a few nonstereologic studies of DM; these are in male rats and reported no differences in neuron density, neuron number, or area volume with age (Peng and Hsu, 1982; Sartin and Lamperti, 1985). In the present study we found no indications of change in neuron number, glial number, or DM volume with age in either male or female monkeys. However, we found that the male DM had a greater number of glia (P = 0.002) and a larger volume (P = 0.001) than that observed in the female. To our knowledge, this sexual dimorphism has not previously been reported for DM in any species.
Ventromedial nucleus (VM)
In a stereology-based study in the rat, Madeira et al. (2001) found no neuron loss with age but did report an increase in female VM volume that is consistent with the nonstereological study (Hsu and Peng, 1978). Madeira et al. (2001) also found that while the male VM was significantly larger than the female, there was no difference in neuron number between the sexes. This is consistent with earlier, nonstereologic investigations that reported a significantly larger VM volume in the male than in the female rat (Matsumoto and Arai, 1983). Another nonstereologic study reported no age-related changes in the VM of the male rat neuron number or volume (Peng and Hsu, 1982), while Sabel and Stein (1981) reported a decrease in VM neuron number in aged male rats. In the present study we found no age-related changes in neuron number, glial number, or volume in either male or female monkeys. However, in agreement with Madeira et al. (2001) findings in the rat, we did find that male monkeys had a significantly larger VM volume than females (P = 0.036) without a significant sexual dimorphism in glial or neuron number, suggesting a larger neuropil in males.
Medial mammillary nucleus (MMN)
One stereologic study in rodents (Begega et al., 1999a) found no change in neuron or glia number but did report a significant decrease in volume. In the human, Begega et al. (1999b) found no change in any of these parameters. In terms of sexual dimorphisms, in the rat, Lopez et al. (1994) utilized stereology and reported a greater number of glia and larger volume in the male MMN than the female, but found no difference in neuron number. In the present study we found no age-related change in MMN neuron number, glia number, or volume in male or female subjects. When comparing the male MMN to the female's, there was no sex difference in neuron or glia number; however, the male MMN was significantly larger than that observed in the female (P = 0.008).
Lateral hypothalamic area (LHA)
There are no quantitative studies of the LHA that address age, sex, or basic measures of neuron or glial number or area volume. In our study of the monkey, we found no age-related change in neuron number, glia number, or LHA volume in either sex. However, in comparing males to females, the number of glia was significantly higher in the LHA of males (P = 0.0001), even though there was no sex difference in LHA volume or neuron number.
SUMMARY
The monkey hypothalamus does not display evidence of significant reductions in neuron or glia number over the adult lifespan, confirming that like multiple areas of the cerebral cortex and limbic system (see Peters et al., 1998b, and Introduction for review) normal aging of the monkey brain does not lead to a loss of neurons. In fact, the only age-related change in the hypothalamus was found in the male PVN, where there was a statistically significant increase in the number of neurons and glia. In contrast to the stability of cell numbers with age, there were several statistically significant differences between male and female, where the male hypothalamus was significantly larger overall and had significantly more glia in total. Interestingly, while male-female differences were found in most of the areas examined (SCN, SON, VM, DM, LHA, MMN), there was no uniform pattern affecting a single parameter between the sexes.
In conclusion, considering the relatively large sample size studied (n = 41) and the number of subdivisions examined in both male and female rhesus monkeys from young adults to the most aged, it seems unlikely that there is any major loss of neurons in the rhesus monkey hypothalamus with age. Hence, age-related alterations in homeostatic functions such as alterations in circadian rhythms are likely to reflect altered physiological functions rather than simple cell loss.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH); Grant numbers: P01-AG000001, P51-RR000165, R37-AG17609, and R01-MH69686.
LITERATURE CITED
- Atlas Dal WR. Topography of the brainstem of the rhesus monkey with special reference to diencephalon. J Comp Neurol. 1937;66:263–289. [Google Scholar]
- Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol. 2008;19:1296–1303. doi: 10.1111/j.1540-8167.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- Begega A, Cuesta M, Rubio S, Santin LJ, Arias JL. Age-related changes of the nucleoloar organizer regions without neuron loss in medial mamillary bodies (hypothalamus) in old rats. Mech Ageing Dev. 1999a;108:113–125. doi: 10.1016/s0047-6374(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Begega A, Cuesta M, Santin LJ, Rubio S, Astudillo A, Arias JL. Unbiased estimation of the total number of nervous cells and volume of medial mammillary nucleus in humans. Exp Gerontol. 1999b;34:771–782. doi: 10.1016/s0531-5565(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Byne W. The medial preoptic and anterior hypothalamic regions of the rhesus monkey: cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–350. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Chee CA, Roozendaal B, Swaab DF, Goudsmit E, Mirmiran M. Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging. 1988;9:307–312. doi: 10.1016/s0197-4580(88)80070-8. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Woodburne RT. The comparative anatomy of the preoptic area and the hypothalamus. A Res Nerv Ment Dis, Proc. 1940;20:52–169. [Google Scholar]
- Crouch RL. The nuclear configuration of the hypothalamus and the subthalamus of Macacus rhesus. J Comp Neurol. 1934;59:431–449. [Google Scholar]
- Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–577. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- Dyke B, Gage TB, Mamelka PM, Goy RW, Stone WH. A demographic analysis of the Wisconsin Regional Primate Center rhesus colony, 1962–1982. Am J Primatol. 1986;10:257–269. doi: 10.1002/ajp.1350100306. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neuron atrophy during aging: programmed or sporadic? Trends Neurosci. 1993;16:104–110. doi: 10.1016/0166-2236(93)90134-8. [DOI] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte ET, Locascio JJ, Lipsitz LA, Hyman BT. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobio Aging. 1997;18:549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Hofman MA, Fliers E, Swaab DF. The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer's disease. Neurobiol Aging. 1990;11:529–536. doi: 10.1016/0197-4580(90)90114-f. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology — reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hedreen JC. Lost caps in histological counting methods. Anat Rec. 1998;250:366–372. doi: 10.1002/(SICI)1097-0185(199803)250:3<366::AID-AR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morpho-metric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J Anat. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Goudsmit E, Purba JS, Swaab DF. Morpho-metric analysis of the supraoptic nucleus in the human brain. J Anat. 1990;172:259–270. [PMC free article] [PubMed] [Google Scholar]
- Hsu HK, Peng MT. Hypothalamic neuron number of old female rats. Gerontology. 1978;24:434–440. doi: 10.1159/000212283. [DOI] [PubMed] [Google Scholar]
- Huang CC, Sandroni P, Sletten DM, Weigand SD, Low PA. Effect of age on adrenergic and vagal baroreflex sensitivity in normal subjects. Muscle Nerve. 2007;36:637–642. doi: 10.1002/mus.20853. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippo-campal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Korbo L, Pakkenberg B, Ladefoged O, Gundersen HJ, Arlien-Soborg P, Pakkenberg H. An efficient method for estimating the total number of neurons in rat brain cortex. J Neurosci Methods. 1990;31:93–100. doi: 10.1016/0165-0270(90)90153-7. [DOI] [PubMed] [Google Scholar]
- Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: effects of age and sex. J Comp Neurol. 1998;401:65–88. doi: 10.1002/(sici)1096-9861(19981109)401:1<65::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Long JM, Mouton PR, Jucker M, Ingram DK. What counts in brain aging? Design-based stereological analysis of cell number. J Gerontol A Biol Sci Med Sci. 1999;54:B407–417. doi: 10.1093/gerona/54.10.b407. [DOI] [PubMed] [Google Scholar]
- Lopez L, Brana M, Burgos P, Arias JL. Structural dimorphism in the mammillary bodies of the rat. Neurosci Lett. 1994;176:197–200. doi: 10.1016/0304-3940(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJ. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: an unbiased stereological study. J Comp Neurol. 1995;361:585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Ruela C, Paula-Barbosa MM. Differential effects of the aging process on the morphology of the hypothalamic ventromedial nucleus of male and female rats. Neurosci Lett. 2001;314:73–76. doi: 10.1016/s0304-3940(01)02294-7. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Lei DL, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective cell loss in the hypothalamus of patients suffering from depression. J Neuropath Exp Neurol. 2005;64:224–229. doi: 10.1093/jnen/64.3.224. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus of the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188(Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin Card Sorting Test. J Neurosci Methods. 2005;146:165–173. doi: 10.1016/j.jneumeth.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morton A. A quantitative analysis of the normal neuron population of the hypothalamic magnocellular nuclei in man and of their projections to the neurohypophysis. J Comp Neurol. 1969;136:143–157. doi: 10.1002/cne.901360203. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Mizuno M, Katakami H, Gore AC, Terasawa E. Aging-related changes in in vivo release of growth hormone-releasing hormone and somatostatin from the stalk-median eminence in female rhesus monkeys (Macaca mulatta). J Clin Endocrinol Metab. 2003;88:827–833. doi: 10.1210/jc.2002-021568. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Haymaker W. Hypothalamic nuclei and fiber connections. In: Haymaker W, Andersen E, Nauta WJH, editors. The hypothalamus. Thomas; Springfield, IL: 1969. pp. 136–209. [Google Scholar]
- Olszewski J. The thalamus of the Macaca mulatta. S. Karger; New York: 1952. [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150(Pt 1):1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotactic coordinates. Academic Press; San Diego: 2000. [Google Scholar]
- Peng MT, Hsu HK. No neuron loss from hypothalamic nuclei of old male rats. Gerontology. 1982;28:19–22. doi: 10.1159/000212507. [DOI] [PubMed] [Google Scholar]
- Peng MT, Lee LR. Regional differences of neuron loss of rat brain in old age. Gerontology. 1979;25:205–211. doi: 10.1159/000212341. [DOI] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on Area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;6:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998a;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998b;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Oorschot DE, Verwer RW, Tilders FJ, Swaab DF. Age-related increase in the total number of corticotropin-releasing hormone neurons in the human paraventricular nucleus in controls and Alzheimer's disease: comparison of the disector with an unfolding method. J Comp Neurol. 1994;339:447–457. doi: 10.1002/cne.903390311. [DOI] [PubMed] [Google Scholar]
- Rankin SL, Partlow GD, McCurdy RD, Giles ED, Fisher KR. Postnatal neurogenesis in the vasopressin and oxytocin-containg nucleus of the pig hypothalamus. Brain Res. 2003;971:189–196. doi: 10.1016/s0006-8993(03)02350-3. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacha MLB, Camarero MAL. Quantitative and morphometric parameters determining the development of the supraoptic nucleus in the albino-rat. Acta Anat (Basel) 1988;133:111–117. doi: 10.1159/000146625. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Fox TO, Dikkes P, Pearlstein RA. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3D computer reconstructions and morphometrics. Brain Res. 1986;371:380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen-sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34:1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Sabel BA, Stein DG. Extensive loss of subcortical neurons in the aging rat brain. Exp Neurol. 1981;73:507–516. doi: 10.1016/0014-4886(81)90284-3. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamus. In: Paxinos G, editor. The human nervous system. Academic Press; San Diego: 1990. pp. 389–413. [Google Scholar]
- Sartin JL, Lamperti AA. Neuron numbers in hypothalamic nuclei of young, middle-aged and aged male rats. Experientia. 1985;41:109–111. doi: 10.1007/BF02005902. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta). Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuro-science. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007;151:1–10. doi: 10.1016/j.psychres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratio in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Snider RS, Lee JC. A stereotaxic atlas of the monkey brain (Macaca mulatta) University of Chicago Press; Chicago: 1961. [Google Scholar]
- Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age. 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. Stability of neuron number in the ageing mouse paraventricular nucleus. Ann Anat. 1992;174:337–340. doi: 10.1016/s0940-9602(11)80301-8. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Touitou Y. Effects of ageing on endocrine and neuroendocrine rhythms in humans. Horm Res. 1995;43:12–19. doi: 10.1159/000184231. [DOI] [PubMed] [Google Scholar]
- Van der Woude PF, Goudsmit E, Wierda M, Purba JS, Hofman MA, Bogte H, Swaab DF. No vasopressin cell loss in the human hypothalamus in aging and Alzheimer's disease. Neurobiol Aging. 1995;16:11–18. doi: 10.1016/0197-4580(95)80003-a. [DOI] [PubMed] [Google Scholar]
- Vogels OJ, Broere CA, Nieuwenhuys R. Neuronal hyper-trophy in the human supraoptic and paraventricular nucleus in aging and Alzheimer's disease. Neurosci Lett. 1990;109:62–67. doi: 10.1016/0304-3940(90)90538-k. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993a;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993b;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereo-logical estimation of the total number of neurons in the-subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Age-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab. 2002;87:5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- Woods WH, Powell EW, Andrews A, Ford CW., Jr Light and electron microscopic analysis of two divisions of the suprachiasmatic nucleus in the young and aged rat. Anat Rec. 1993;237:71–88. doi: 10.1002/ar.1092370108. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Masuda K, Quasarano C, Rosene DL, Killiany RJ, Wang S. Aging of intrinsic circadian rhythms and sleep in a diurnal non-human primate, Macaca mulatta. J Biol Rhythms. 2011;26:149–59. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]