Abstract
The brain reward circuitry plays a key role in emotional and motivational behaviors, and its dysfunction underlies neuropsychiatric disorders such as schizophrenia, depression and drug addiction. Here, we characterized the neuronal activity pattern induced by acute amphetamine administration and during drug-seeking behavior in the zebrafish, and demonstrate the existence of conserved underlying brain circuitry. Combining quantitative analyses of cfos expression with neuronal subtype-specific markers at single-cell resolution, we show that acute d-amphetamine administration leads to both increased neuronal activation and the recruitment of neurons in the medial (Dm) and the lateral (Dl) domains of the adult zebrafish pallium, which contain homologous structures to the mammalian amygdala and hippocampus, respectively. Calbindin-positive and glutamatergic neurons are recruited in Dm, and glutamatergic and γ-aminobutyric acid (GABAergic) neurons in Dl. The drug-activated neurons in Dm and Dl are born at juvenile stage rather than in the embryo or during adulthood. Furthermore, the same territory in Dm is activated during both drug-seeking approach and light avoidance behavior, while these behaviors do not elicit activation in Dl. These data identify the pallial territories involved in acute psychostimulant response and reward formation in the adult zebrafish. They further suggest an evolutionarily conserved function of amygdala-like structures in positive emotions and motivated behavior in zebrafish and mammals.
Keywords: addiction, adult neurogenesis, amphetamine, amygdala, cfos, reward
Introduction
Animals exhibit an astonishing diversity of behaviors, but also a common set of adaptive responses and processes. It is therefore not surprising that basic neural systems mediating core motivational and emotional processes have been evolutionary conserved – with emotions being defined as common central states of neural circuits that assign positive or negative values to a stimulus or experience and give rise to behavioral and/or physiological responses (Kalueff et al., 2012; Kittilsen, 2013; Anderson & Adolphs, 2014). From this point of view, the brain can be considered as a modular system that can increase in complexity by building on and interconnecting to preexisting building blocks (Kelley, 2004). One such evolutionary conserved key element of the brain is the reward system. Drugs of abuse, for example amphetamine, strongly activate the reward system and can trigger strong emotional and motivational responses in most, if not all, vertebrates (Robbins et al., 2008; Sesack & Grace, 2010; O'Connell & Hofmann, 2011).
The zebrafish (Danio rerio) has been used to identify and characterize evolutionarily conserved neural systems (Mueller & Wullimann, 2009), and is ideally suited for a genetic and molecular analysis of emotional and motivational behaviors, including the response to drugs of addiction (Norton & Bally-Cuif, 2010; Guo et al., 2012; Klee et al., 2012). In mammals, the amygdala and the hippocampus are part of the mesocorticolimbic reward circuitry. The amygdala plays a pivotal role in mediating negative but also positive emotions, and in conveying value of motivational signals (Paton et al., 2006; Murray, 2007; Morrison & Salzman, 2010; Johansen et al., 2011). It is strongly activated by drugs of abuse and drug-associated cues (Brown et al., 1992; Mead et al., 1999; Buffalari & See, 2010), and plays a role in goal-directed behavior (Parkes & Balleine, 2013; Rhodes & Murray, 2013). The amygdala is reciprocally connected to the hippocampus (Pitkänen et al., 2000; Kishi et al., 2006), which strongly contributes to spatial learning and memory (Scoville & Milner, 1957; Milner, 1972). Molecular genetic, developmental, hodological and behavioral data suggest that the medial (Dm) and the lateral zone of the dorsal telencephalon (Dl) of ray-finned fish likely contain the structures homologous to the mammalian amygdala and hippocampus, respectively (Rodríguez et al., 2002; Portavella et al., 2004; Northcutt, 2006; Martín et al., 2011; Mueller et al., 2011). A precise functional mapping of these territories, however, is still largely missing.
As a first cellular approach towards establishing the neuroanatomical correlates of reward and motivation-based behavior in zebrafish, we used here the immediate-early gene (IEG) cfos (Clayton, 2000; Lau et al., 2011) to identify the telencephalic regions activated by amphetamine and during drug-induced place preference behavior. We show that Dm (encompassing amygdala-like areas) and Dl (encompassing hippocampus-like areas) are activated by amphetamine, and Dm, but not Dl, during drug-seeking behavior. Our analysis supports the functional conservation of at least two pallial components of the brain reward system in zebrafish, an important emerging model for the molecular genetic dissection of the neural networks underlying emotional and motivational behaviors.
Materials and methods
Fish strain and handling
Wild-type zebrafish (Danio rerio) of the AB strain were used in all experiments. Adult zebrafish were maintained and bred following standard procedures (Westerfield, 2000), and in accordance with the institute guidelines for animal welfare.
Molecular cloning of cfos
The primers 5′-ATGATGTTTACCAGCCTTAACGC-3′ and 5′-TCAAAGAGTGAGGAGGGTTGG-3′ were used to amplify cfos from a cDNA library constructed from an adult AB wild-type brain, and it was cloned into the StrataClone PCR Cloning Vector pSC-A (Agilent Technologies, Santa Clara, CA, USA). The retrieved 1050-base pair cfos sequence includes the whole coding region without the 5′- or 3′-untranslated region. Sequencing revealed more than 99% sequence identity with the published zebrafish cfos reference sequence (Accession Number NM_205569; Gene ID 394198).
Acute amphetamine and saline injections
Adult zebrafish (4–6 months old; 300–400 mg weight) were first injected intraperitoneally with 110 mm sodium chloride (63.8 mg/kg). This first injection proved necessary to calibrate the system in order to avoid unspecific pain and fear-induced cfos expression (not shown). Two hours after this first injection (a time interval in which cfos mRNA expression is returned to basal levels; Clayton, 2000), the fish were split into a control and an amphetamine group (four to six fish per group). The control group was again injected with 110 mm sodium chloride; the amphetamine group was injected with 32.61 mm of d-amphetamine hemisulfate salt (60 mg/kg; Sigma-Aldrich A5880) in 110 mm sodium chloride. Thirty minutes after this second injection (at the peak of cfos mRNA expression; Clayton, 2000), the fish from the control and amphetamine group were killed immediately by putting them in ice-cold phosphate-buffered saline (PBS), their brains were then dissected and analysed for cfos expression.
Conditioned place preference (CPP) behavior assay
The CPP test was performed as previously described (Ninkovic & Bally-Cuif, 2006; Ninkovic et al., 2006; Webb et al., 2009), with the following modifications: (i) the two ‘black spots’ on the initially non-preferred compartment were omitted; and (ii) the fish were habituated for 3 days (instead of 2 days) to the test environment and their initial place preference was measured on the fourth day using the ZebraLab software (Viewpoint, Lyon, France). Similarly, control fish exhibiting a non-oriented behavior were also habituated to the experimental setup for 3 days and their behavior was tracked on the fourth day. To elicit or monitor a drug-seeking approach or a light-avoidance behavior, fish were repeatedly injected with amphetamine or saline, respectively. Following conditioning, amphetamine-treated fish exhibited a change in place preference of 71.83 ± 5.95%, now spending 76.91 ± 4.94% (n = 6) of their time in the initially non-preferred compartment; as expected, saline-injected fish did not change place preference (change of −0.04 ± 4.05%), spending 78.24 ± 4.80% (n = 6) of their time in the preferred compartment. Control fish were housed in tanks of the same size but with no color cues, and therefore spent equal amounts of their time on either side of the tank (side 1 : 51.5 ± 2.9%; side 2 : 48.5 ± 2.9%). The final behavior testing, which measured place preference after conditioning, was not preceded by amphetamine or saline injection. All the fish were killed 30 min after monitoring, and their brains were dissected and analysed for cfos expression.
5′-Bromo-2′-deoxyuridine (BrdU) labeling
Adult (4–6 months old) or juvenile (1 month old) fish were placed for 6 h in tank water containing 1 mm BrdU (Sigma-Aldrich B5002). Population density was controlled (8–10 adult and 15–20 juvenile fish per 500 mL) and the fish were kept in the dark during the application of the chemical.
In situ hybridization and immunohistochemistry
In situ hybridization and immunohistochemistry were performed essentially as previously described (Adolf et al., 2006; Brend & Holley, 2009; Lauter et al., 2011), but with modifications (see below) permitting highly sensitive multicolor stainings at single-cell resolution. The following probes were used – cfos; vglut2.2 (courtesy of Rebecca Schmidt, Karlsruhe, Germany); and gad65/67 (Higashijima et al., 2004). In brief, adult brains were dissected and fixed in 4% paraformaldehyde in PBS overnight at 4 °C. The samples were then dehydrated and incubated with 2% H2O2 for 35 min to block endogenous peroxidase activity. After rehydration, the brains were embedded in a bovine serum albumin (Sigma-Aldrich A3912) gelatin type A (Sigma-Aldrich G1819) mixture (Levin, 2004) that contained 6.47% formaldehyde and 0.075% glutaraldehyde. This embedding procedure allowed us to carry out the hybridization of the RNA probes on adult brain sections rather than on whole-mount brains [as it is commonly done in fluorescent in situ hybridizations (FISH) in the zebrafish], which, in our experience, greatly increases the sensitivity and signal-to-noise ratio. Subsequently, 50-μm serial sections were cut with a Leica VT1000S microtome. The sections were washed in PBS containing 0.1% Tween-20 (PBST), incubated for 30 min with 10 μg/μL Proteinase K, washed in PBST, refixed for 20 min at room temperature (RT) with 4% paraformaldehyde, washed in PBST, and prehybridized for at least 1 h at 65 °C. Digoxigenin (DIG)-, fluorescein (FLUO)- and dinitrophenol (DNP)-labeled anti-sense RNA probes were hybridized overnight at 65 °C with a concentration of 1 ng/μL as previously described (Lauter et al., 2011). The sections were sequentially incubated with horseradish peroxidase-conjugated anti-FLUO (1 : 500; Roche, Basel, Switzerland), anti-DIG (1 : 500; Roche) and anti-DNP (1 : 500; PerkinElmer, Waltham, MA, USA) antibodies. The RNA probes were sequentially revealed with custom-made FLUO (FITC; 1 : 200; http://www.xenbase.org/other/static/methods/FISH.jsp), tetramethylrhodamine (TAMRA; 1 : 250) and cyanine 5 (Cy5; 1 : 100; PerkinElmer) conjugated tyramide in PBST containing 2% (v/v) dextran sulfate (Sigma-Aldrich D8906) and 0.003% (v/v) H2O2 for 35 min at RT.
Immunohistochemistry was performed after in situ hybridization. To reveal BrdU, the sections were incubated with 2 m HCl in PBST at 37 °C for 30 min. For other primary antibodies, no pre-treatment was applied. Free-floating sections were washed in PBST, blocked in 10% (v/v) normal goat serum (NGS) in PBST for 1 h at RT and incubated in NGS/PBST at 4 °C overnight with the primary antibodies. Next, sections were washed in PBST, blocked in NGS/PBST for 30 min at RT and incubated in PBST at 4 °C overnight with the secondary antibodies. The sections were washed in PBST, the nuclei counterstained for 10 min at RT with 4’,6-diamidino-2-phenylindole (DAPI; 1 μg/μL) and mounted on microscope slides in Vectashield (Vector Laboratories H-100, Burlingame, CA, USA).
Primary antibodies were rat anti-BrdU (1 : 250; Abcam, Cambridge, UK), rabbit anti-calbindin D28k (1 : 500; Swant, Marly, Switzerland), rabbit anti-cfos (1 : 2000; sc-52; Santa Cruz Biotechnology, Dallas, TX, USA), human anti-HuC/D (1 : 2000; courtesy of Bernard Zalc, Salpêtrière Hospital, Paris, France), mouse anti-parvalbumin (1 : 500; Merck Millipore, Billerica, MA, USA). Goat antibodies coupled to AlexaFluor dyes (488, 546 or 647; 1 : 1000; Molecular Probes, Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies.
Image acquisition, analysis and cell counting
Images were acquired with a Zeiss LSM 700 confocal microscope using a 20× air (numerical aperture 0.8) or 40× oil-immersion (numerical aperture 1.3) objective with 405, 488, 555 and 639 nm lasers. Images were collected at a size of 512 × 512 pixels and automatically stitched upon acquisition using the ‘Tilescan’ mode of the zeiss zen software. Confocal data were processed and analysed with volocity 6.3 (PerkinElmer) software. Cell counting and intensity measurement of single-labeled cells was performed on 42-μm Z-stacks with 2-μm Z-intervals (21 optical sections), and an X and Y pixel size of 0.391 μm from 50-μm serial sections using volocity 6.3 (PerkinElmer) software. Colocalization analysis of double-labeled cells was performed manually using volocity's 6.3 (PerkinElmer) ‘point tool’. Intensity measurements of cfos expression were normalized using the mean intensity of cfos expression per cell of saline-injected control fish. Cell counting and intensity measurements were performed within regions of interest (ROIs) that correspond to the Dm and Dl and the subpallium. Dm, Dl and the subpallium were defined by calbindin and parvalbumin expression and/or DAPI staining. cfos-positive and DAPI cells were counted in a ROI measuring 390 μm (width) × 200 μm (height) in Dm, and 200 μm (width) × 390 μm (height) in both Dl and the subpallium (Fig.3E and F). Data from the coexpression analysis of cfos together with the neuronal markers calbindin, vglut2.2 and gad65/67 were obtained from a ROI measuring 580 μm (width) × 200 μm (height) in Dm, and 200 μm (width) × 390 μm (height) in both Dl and the subpallium (Figs4 and 5). In the BrdU time course analyses of juvenile- and adult-born neurons, distance measurements in micrometers were performed within a ROI of 580 μm (width) × 135 μm (height) and 200 μm (width) × 270 μm (height) for Dm and Dl, respectively (Fig.6). cfos-positive cells in fish exhibiting a non-oriented or oriented behavior were counted within a ROI measuring 580 μm (width) × 200 μm (height) in Dm, and 200 μm (width) × 390 μm (height) in both Dl and the subpallium (Fig.8).
Figure 3.
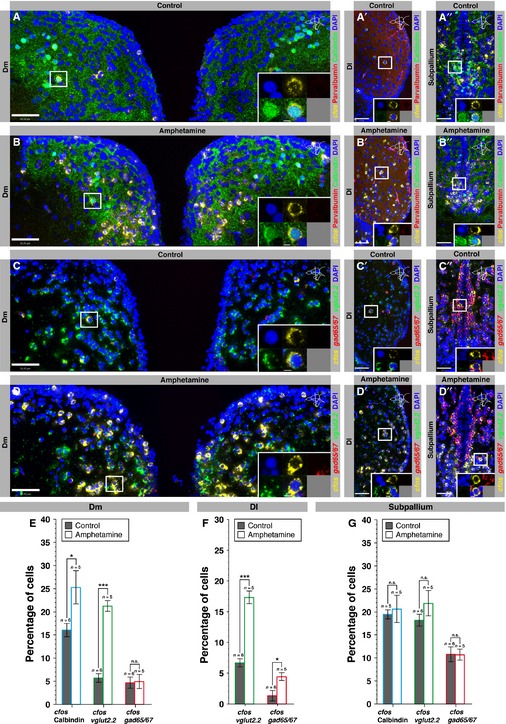
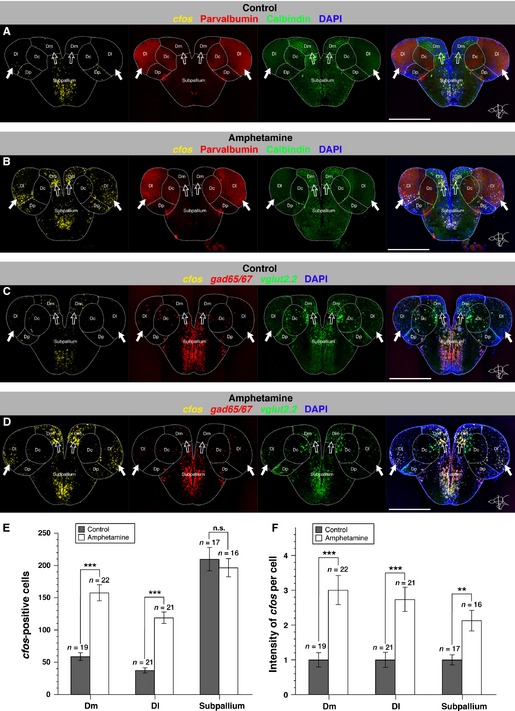
Dm and Dl nuclei are activated by an acute d-amphetamine treatment. (A and B) Single optical sections of the brain of a saline-injected control fish (A) and an amphetamine-injected fish (B) depicting cfos expression (FISH, yellow) together with parvalbumin and calbindin (immunohistochemistry, red and green, respectively), and DAPI (blue) staining. (C and D) Single optical sections of the brain of a saline-injected control fish (C) and an amphetamine-injected fish (D) depicting cfos expression (FISH, yellow), vglut2.2 (FISH, green) and gad65/67 (FISH, red) expression together with DAPI staining. Note the increase in cfos expression in Dm and Dl areas (arrows) in amphetamine- compared with saline-injected control fish. Brain regions are outlined by white dots. Dc, central zone of the dorsal telencephalon; Dl, lateral zone of the dorsal telencephalon; Dm, medial zone of the dorsal telencephalon; Dp, posterior zone of the dorsal telencephalon. Scale bar – 500 μm. (E) Number of cfos-positive cells per section in Dm, Dl and the subpallium for control (black bars) and amphetamine (white bars) fish. (F) Quantification of the intensity of cfos expression per cell for control (black bars) and amphetamine (white bars) fish. Error bars depict SEM. n is the number of analysed brains. ***P < 0.001; **P < 0.01; n.s., not significant (unpaired two-tailed Student's t-test).
Figure 4.

Calbindin, glutamatergic and GABAergic neurons in Dm and Dl are recruited by an acute amphetamine treatment. (A–B′′) Single optical sections of Dm (A, B), Dl (A′, B′) and the subpallium (A′′, B′′) of a saline-injected control fish (A–A′′) and an amphetamine-injected fish (B–B′′) showing cfos expression (FISH, yellow) together with a double immunostaining for calbindin (green) and parvalbumin (red), counterstained with DAPI (blue). The insets depict the cells that are boxed in the main frames at a higher magnification. (C–D′′) Single optical sections of Dm (C, D), Dl (C′, D′) and the subpallium (C′′, D′′) of a saline-injected control fish (C–C′′) and an amphetamine-injected fish (D–D′′) showing, in triple FISH, cfos (yellow), vglut2.2 (green) and gad65/67 (red) expression, counterstained with DAPI (blue). Scale bars – 50 μm (main frames); 5 μm (insets). (E) Proportion of calbindin-, vglut2.2- and gad65/67-positive cells that also express cfos in Dm in control (black bars) and amphetamine (white bars) fish. (F) Proportion of vglut2.2- and gad65/67-positive cells that also express cfos in Dl in control (black bars) and amphetamine (white bars) fish. (G) Proportion of calbindin-, vglut2.2- and gad65/67-positive cells that also express cfos in the subpallium in control (black bars) and amphetamine (white bars) fish. Error bars depict SEM. n is the number of analysed brains. ***P < 0.001; *P < 0.05; n.s., not significant (unpaired two-tailed Student's t-test).
Figure 5.
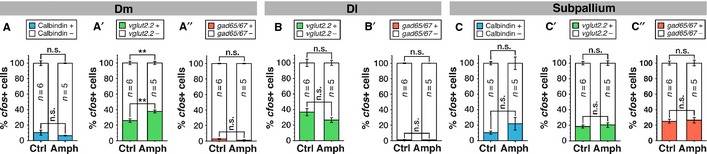
Preferential neuronal recruitment in Dm of the vglut2.2-positive neuronal pool after acute amphetamine administration. (A–A′′) Percentage of cfos-positive cells in Dm that are (A) calbindin-positive (blue bars) or calbindin-negative (white bars), (A′) vglut2.2-positive (green bars) or vglut2.2-negative (white bars), or (A′′) gad65/67-positive (red bars) or gad65/67-negative (white bars) in control and amphetamine fish. (B–B′′) Percentage of cfos-positive cells in Dl that are (B) vlgut2.2-positive (green bars) or vglut2.2-negative (white bars), or (B′) gad65/67-positive (red bars) or gad65/67-negative (white bars) in control and amphetamine fish. (C–C′′) Percentage of cfos-positive cells in the subpallium that are (C) calbindin-positive (blue bars) or calbindin-negative (white bars), (C′) vlgut2.2-positive (green bars) or vglut2.2-negative (white bars), or (C′′) gad65/67-positive (red bars) or gad65/67-negative (white bars) in control and amphetamine fish. Error bars depict SEM. n is the number of analysed brains. **P < 0.01; n.s., not significant (unpaired two-tailed Student's t-test).
Figure 6.
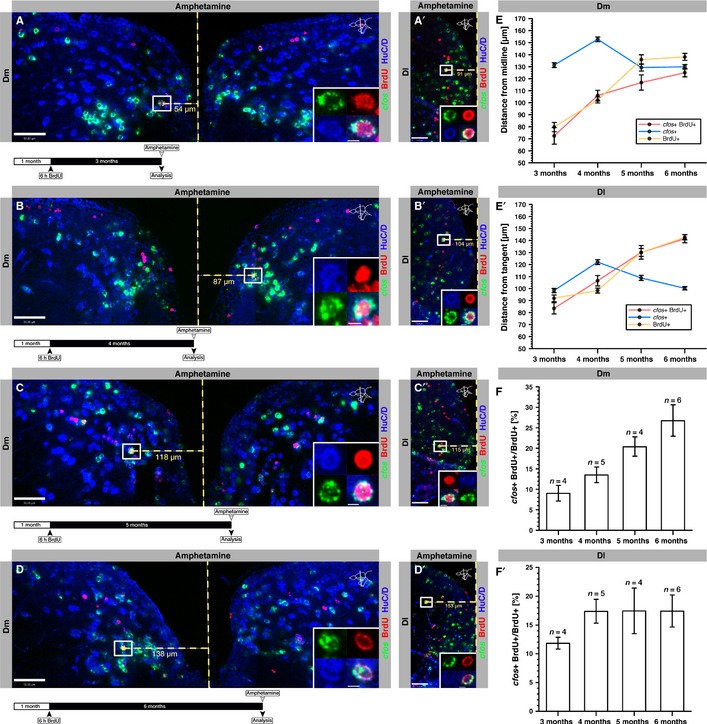
Juvenile-born neurons respond to acute amphetamine treatment. (A–D′) Single optical sections of Dm (A–D) and Dl (A′–D′) of fish labeled with BrdU at 1 month and analysed after 3 (A, A′), 4 (B, B′), 5 (C, C′) and 6 (D, D′) months within 30 min following acute amphetamine administration. A vertical dashed yellow line represents the midline of Dm (A) or the tangent of Dl (A′), used to measure the distance of BrdU-labeled neurons from the VZ. Length measurements in micrometers are shown for the cell boxed in the main frame beneath the horizontal dashed yellow measurement line. The insets depict the boxed and measured cells at a higher magnification. A schematic design of the experiment is depicted on the lower left beneath each confocal image. Scale bars – 50 μm (main frames); 5 μm (insets). (E) Quantification of the distance of cfos-positive (blue line), BrdU-positive (yellow line) and cfos/BrdU double-positive cells (red line) from the VZ of Dm after the indicated time points. (E′) Quantification of the distance of cfos-positive (blue line), BrdU-positive (yellow line) and cfos/BrdU double-positive cells (red line) from the VZ of Dl after the indicated time points. (F, F′) Proportion of BrdU-positive neurons expressing cfos in Dm (F) and Dl (F′) at the indicated time points following an administration of BrdU to 1-month-old fish (for absolute values, see Fig.7). Error bars depict SEM. n is the number of analysed brains. (F) one-way anovaF3,15 = 7.25, P = 0.0004 post-test for linear trend between 3 and 6 months, highly significant; (F′) one-way anovaF3,15 = 0.95, P = 0.1830 post-test for linear trend between 3 and 6 months.
Figure 8.
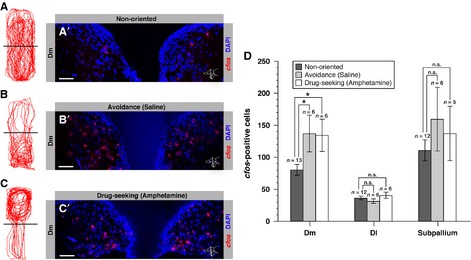
Dm is activated in both negative and positive emotional contexts. (A–A′) Swimming trajectory of a fish placed in a neutral tank (non-oriented behavior) (A) and its corresponding neuronal activation pattern in Dm analysed via cfos expression (A′). (B–B′) Swimming trajectory of a saline-injected fish placed in a visually cued tank (bright – top; dark – bottom) and following an unconditioned light avoidance behavior (B) and its corresponding neuronal activation pattern in Dm analysed via cfos expression (B′). (C–C′) Swimming trajectory of an amphetamine-conditioned fish that exhibits a drug-seeking approach behavior (C) and its corresponding neuronal activation pattern in Dm analysed via cfos expression (C′). The amphetamine-conditioned fish was placed in the same visually cued tank as in (B) (bright – top; dark – bottom), but after pairing amphetamine with the initially non-preferred bright compartment. (D) Number of cfos-positive cells per section in Dm, Dl and the subpallium in control fish that exhibit non-oriented behavior (black bars), an unconditioned avoidance (gray bars) or a drug-seeking behavior (white bars). Error bars depict SEM. n is the number of analysed brains. *P < 0.05; n.s., not significant (unpaired two-tailed Student's t-test).
Statistics
All the quantitative data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were performed using unpaired two-tailed Student's t-tests (Figs3E and F, 4E–G, 5A–C′′, and 8D) or a one-way anova followed by a post-test for linear trend (Figs6E and F′, and 7A and B) with Prism 6.0 (GraphPad, La Jolla, CA, USA) software. Differences were considered significant for P < 0.05. Graphs were created with Aabel 3.0.6 (Gigawiz, Oklahoma City, OK, USA) and Illustrator 15.0.2 (Adobe, San Jose, CA, USA).
Figure 7.
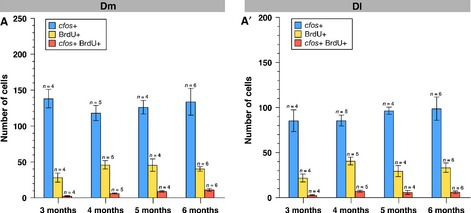
Time-dependent analysis of the juvenile-labeled BrdU-positive cells and activated neurons following amphetamine treatment. Number of BrdU-positive, cfos-positive and BrdU/cfos-double-positive neurons in Dm (A) and Dl (A′) at the indicated time points following an administration of BrdU to 1-month-old fish. Error bars depict SEM. n is the number of analysed brains.
Results
To identify and characterize the territories involved in reward processing in the zebrafish telencephalon, adult fish were injected with the psychostimulant d-amphetamine. Acute administration of d-amphetamine induces a net increase of biogenic amines, notably dopamine, at the synapse, directly activating primary reward centers (Sulzer et al., 2005; Sulzer, 2011). Upon chronic administration in zebrafish (Ninkovic & Bally-Cuif, 2006; Ninkovic et al., 2006; Webb et al., 2009), as in mammals, it triggers a dose-dependent conditioned response measurable in a place preference assay, which fades when the drug-place association is decreased. Neuronal activation in amphetamine- vs. saline-injected control fish was revealed using FISH for the IEG cfos, the expression of which reliably highlights neuronal recruitment and plasticity (Knapska et al., 2007). Within the telencephalon, the most striking changes in cfos induction were observed at an antero-posterior level slightly rostral to the anterior commissure (Fig.1) located between cross-sections 85 and 92 according to Wullimann's neuroanatomical atlas (Wullimann et al., 1996). The main telencephalic subdivisions are all present at this level, and are readily identified by a specific DAPI, calbindin D28k and parvalbumin staining pattern (Mueller et al., 2011, and see below).
Figure 1.
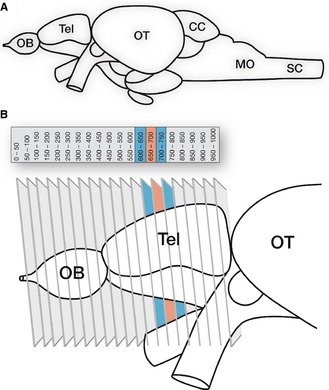
Schematic overview of the zebrafish brain depicting the ROI. (A) Whole brain. (B) Telencephalon and sectioning scheme. Twenty 50-μm-thick coronal sections were cut from the telencephalon (total antero-posterior coverage – approx. 1 mm). We focused on an area located just rostral to the anterior commissure (red and blue sections). CC, corpus cerebelli; MO, medulla oblongata; OB, olfactory bulb; OT, optic tectum; SC, spinal cord; Tel, telencephalon..
Adapted and modified from Wullimann et al. (1996) and Wullimann & Mueller (2004)
Dm and Dl nuclei are activated by acute d-amphetamine administration
To identify the most reliable readout for neuronal activation, we first compared the patterns of cfos mRNA and cFos protein expression in double-staining experiments in adult fish that were killed within 30 min following brief handling or injection sessions. Combining in situ hybridization and immunohistochemistry on single brain sections revealed only partly overlapping expression patterns (Fig.2). Notably, a number of cells could be observed that were positive for cFos protein but negative for cfos mRNA, suggesting protein stability beyond 30 min. We thus chose to monitor cfos mRNA as a trustworthy readout of cfos induction, and developed a novel protocol for highly sensitive multicolor FISH at single-cell resolution on adult zebrafish brain sections (J.W. von Trotha, in preparation; see Materials and methods).
Figure 2.
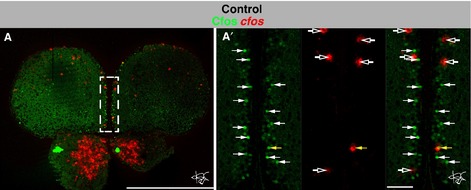
Non-identical cFos protein and cfos mRNA expression in a saline-injected control fish. (A–A′) Single optical section at an anterior level of the brain of a saline-injected control fish depicting cFos protein (immunohistochemistry, green) and cfos mRNA (FISH, red). The dashed white box in (A) outlines the region magnified in (A′). Note that a much larger number of cells are positive for cFos protein (solid white arrows) than for cfos mRNA (open white arrows), and that only one cell expresses both cFos protein and cfos mRNA (solid yellow arrow). Scale bars – 500 μm (A); 50 μm (A′).
We observed a strong increase in cfos expression following acute d-amphetamine injection in Dm and Dl, hosting the territories homologous to the mammalian amygdala and hippocampus, respectively (Fig.3A–D). Notably, in Dl, cfos-expressing cells were more evenly distributed than in Dm, in which we observed a dense activation pattern in the ventromedial region (Figs3B and D, and 4B and B′, and D and D′). In Dm, saline-injected control fish expressed cfos in 58.74 ± 5.97 SEM cells (n = 19 brains), whereas amphetamine-injected fish expressed cfos in 157.60 ± 12.41 SEM cells (n = 22 brains), corresponding to a 2.7-fold increase in the number of activated cells upon drug administration (unpaired two-tailed t-test t39 = 6.83, P < 0.0001; Fig.3E). In Dl, we found 37.36 ± 3.99 SEM and 119.0 ± 8.88 SEM cfos-positive cells in control (n = 21 brains) and amphetamine (n = 21 brains) fish, respectively, which corresponds to a 3.2-fold increase (unpaired two-tailed t-test t40 = 8.39, P < 0.0001; Fig.3E). In the subpallium, however, we observed similar numbers of cfos-positive cells following saline or amphetamine injection [respectively, 209.8 ± 18.12 SEM (n = 17 brains) and 196.6 ± 14.04 SEM (n = 16 brains); unpaired two-tailed t-test t31 = 0.58, P = 0.5723; Fig.3E], arguing for a drug-specific increase of cfos expression in Dm and Dl rather than a general effect of amphetamine injection. We further verified that there was no difference in the total number of DAPI-stained cells in Dm, Dl and the subpallium in amphetamine and control fish (data not shown). In addition to the increase in the number of cfos-positive cells following amphetamine injection, the intensity of cfos expression per cell may also be modified. To assess this point, we measured the intensity of FISH staining on confocal images. We observed a 3.0-fold ± 0.42 SEM and 2.74-fold ± 0.35 SEM increase in the intensity of cfos expression per cell in Dm (unpaired two-tailed t-test t39 = 4.12, P = 0.0002) and Dl (unpaired two-tailed t-test t40 = 4.22, P = 0.0001), respectively (Fig.3F). In the subpallium, we could also observe a 2.13-fold ± 0.35 SEM increase in the intensity of cfos expression per cell in amphetamine-injected fish (unpaired two-tailed t-test t31 = 3.48, P = 0.0015; Fig.3F). Together, these results show that amphetamine specifically activates Dm (amygdala) and Dl (hippocampus) areas in the adult zebrafish pallium, both by recruiting neurons and by increasing neuronal activation or plasticity. The latter process, also observed in the subpallium, may partially contribute to the neuronal response to amphetamine.
Calbindin and glutamatergic neurons in Dm are activated by acute d-amphetamine administration
To further characterize the drug-activated cells in Dm and Dl, we made use of the neuronal subtype markers calcium-binding protein (CBP) calbindin D28k, vglut2.2, a marker of glutamatergic neurons, and gad65/67, a marker of γ-aminobutyric acid (GABA)ergic neurons. Expression of these markers was analysed together with cfos in triple-fluorescent labeling experiments monitored under confocal microscopy. In the telencephalon, calbindin is mostly expressed in Dm and the subpallium (Figs3A and B, and 4A, B, A′′ and B′′). Its expression is complementary to parvalbumin, another CBP, which labels Dl and the central zone of the dorsal telencephalon (Dc; Grandel et al., 2006; Mueller et al., 2011; Figs3A and B, and 4A′ and B′). vglut2.2 is strongly expressed throughout the pallium but only weakly in the subpallium, whereas gad65/67 is strongly expressed in the subpallium, although scattered expression can also be seen in the pallium (Mueller & Guo, 2009; Figs3C and D, and 4C – D′′).
We found that, in amphetamine-injected fish, 25.31 ± 3.59% SEM (n = 5 brains) of the calbindin-positive cells in Dm also expressed cfos, which represents a significant, 1.6-fold increase compared with control fish, where only 16.06 ± 1.43% SEM (n = 6 brains) of the calbindin-positive cells expressed cfos (unpaired two-tailed t-test t9 = 2.56, P = 0.0306; Fig.4E). The number of calbindin-positive cells in amphetamine and control brains, expectedly, was unchanged (data not shown). In Dm, we further observed a strongly significant, 3.73-fold increase in the proportion of vglut2.2 neurons expressing cfos upon amphetamine treatment (unpaired two-tailed t-test t9 = 10.65, P < 0.0001), whereas the proportion of gad65/67-positive neurons expressing cfos remained unchanged in amphetamine (n = 5 brains) vs. control fish (n = 6 brains; unpaired two-tailed t-test t9 = 0.12, P = 0.9045; Fig.4E). The mean number of cells expressing vglut2.2 or gad65/67 in the amphetamine and the control sample was about the same (data not shown). Together, these results indicate that, among the neuronal subtypes tested, acute amphetamine administration recruits neurons within the calbindin-positive and glutamatergic population(s) in Dm.
To determine whether this cell recruitment in Dm preferentially targets a specific neuronal subtype, we determined the percentage of cfos-positive cells that also expressed calbindin, vglut2.2 or gad65/67 in amphetamine compared with saline conditions. We found that the proportion of calbindin- or gad65/67-positive neurons was unchanged within the cfos-expressing population after amphetamine treatment, but observed a significant increase in the proportion of vglut2.2-positive neurons (unpaired two-tailed t-test t9 = 3.88, P = 0.0038; Fig.5A – A′′). Thus, upon acute amphetamine administration, the pool of active neurons in Dm enlarges while maintaining a constant proportion of calbindin and GABAergic subtypes but disproportionally increasing its recruitment of glutamatergic neurons.
As a control for the specificity of our analysis, we quantified the expression of cfos among the different neuronal subtypes in the subpallium. Because this territory primarily responds to acute amphetamine treatment by increasing the intensity of cfos expression while maintaining a stable number of cfos-positive cells (Fig.3E and F), we did not expect changes in the subtype-specific expression of cfos. Accordingly, we did not observe a difference in the proportion of cfos-positive neurons within the calbindin-, vglut2.2- or gad65/67-positive populations between amphetamine (n = 5 brains) and control brains (n = 5 brains; Fig.4G). Likewise, the distribution of cfos positivity between the different neuronal subtypes analysed remained unchanged (Fig.5C – C′′).
Glutamatergic and GABAergic neurons in Dl are activated by acute d-amphetamine administration
Neuronal recruitment within the glutamatergic population was similar in Dl to that observed in Dm, with a strongly significant, 2.59-fold increase in the proportion of vglut2.2-positive neurons expressing cfos in fish that received an injection of the psychostimulant (unpaired two-tailed t-test t9 = 9.14, P < 0.0001; Fig.4F). The GABAergic population, however, appeared more robustly recruited than in Dm, with the proportion of gad65/67-positive neurons expressing cfos enhanced by 3.32-fold (unpaired two-tailed t-test t9 = 2.83, P = 0.0198; Fig.4F). There was no difference in the overall number of vglut2.2 and gad65/67 cell numbers between amphetamine and control fish (data not shown). In contrast to Dm, there was no preferential activation of glutamatergic or GABAergic neurons in Dl after acute amphetamine administration (Fig.5B and B′). We conclude that, in Dl, both glutamatergic and GABAergic neurons are activated by amphetamine, with a proportionally similar recruitment within each population.
Juvenile- rather than adult-born neurons respond to acute d-amphetamine in the adult zebrafish
The zebrafish is an excellent system to investigate the function of adult-born neurons, as adult neurogenesis is much more widespread in the zebrafish than in the mammalian brain (Adolf et al., 2006; Grandel et al., 2006; Zupanc & Sîrbulescu, 2011; Kizil et al., 2012; Schmidt et al., 2013). In the adult pallium, neuronal progenitor cells are aligned along the superficially located ventricle. They deposit neurons in the parenchyma in a concentric manner and with little cell mixing as the brain grows (Chapouton et al., 2007; Kaslin et al., 2008; Schmidt et al., 2013). Because newborn neurons have been shown more plastic in the adult rodent hippocampus and olfactory bulb (Schmidt-Hieber et al., 2004; Ge et al., 2007; Nissant et al., 2009; Alonso et al., 2012; Gu et al., 2013), we expected that adult-born neurons would be preferentially recruited when the reward system is stimulated during adulthood.
To test whether adult-born neurons in Dm and Dl respond to acute d-amphetamine administration, 4-month-old fish were given a 6-h BrdU pulse to label proliferating progenitors, and the response of BrdU-labeled neurons to amphetamine was analysed after a 2-month chase. This duration is sufficient for the functional integration of adult-born neurons in the zebrafish pallium (Rothenaigner et al., 2011). Even after this relatively long time interval, however, we found that the BrdU-labeled neurons remained relatively close to the ventricular zone (VZ; about 5–10 and 15–20 μm away in Dm and Dl, respectively) and failed to reach the amphetamine-responsive areas identified above. Accordingly, they were mostly cfos-negative (data not shown). This suggested that most of the neurons that respond to amphetamine in Dm and Dl in a middle-aged adult (5–7 months old) are born at juvenile stage rather than at adulthood, although we cannot generally exclude the possibility that some adult-born neurons may at much later stages also participate in the amphetamine response. To determine whether and at what age juvenile-born neurons respond to amphetamine, 1-month-old fish were given a 6-h BrdU pulse, and their brains were subsequently analysed after 3, 4, 5 and 6 months of chase (Fig.6A–D). We found that, on average, cfos-expressing cells were located 135.8 ± 5.66 μm SEM (n = 2259 cells) away from the VZ in Dm, and 107.4 ± 5.39 μm SEM away in Dl (n = 2983 cells), and that both of these distances remained fairly constant over time (Fig.6E and E′, blue lines). In contrast, as expected, the BrdU-labeled neurons dislocated away from the VZ with increasing time intervals, thereby progressively moving into a field of high cfos expression (Fig.6A–E′, yellow lines). For example, after a 3-month chase, BrdU-positive neurons in Dm were found 79.73 ± 3.84 μm SEM (n = 83 cells) away from the VZ, and at 6 months they were located at 138.09 ± 2.93 μm SEM (n = 297 cells; Fig.6A, D and E). Accordingly, the proportion of BrdU-positive neurons that also expressed cfos increased from 9.05 ± 1.93% SEM after 3 months to 26.78 ± 3.84% SEM after 6 months (one-way anovaF3,15 = 7.25, P = 0.0004 post-test for linear trend; Figs6F and 7A). In Dl, BrdU-positive neurons were found at 91.90 ± 2.75 μm SEM (n = 107 cells) and 142.65 ± 2.11 μm SEM (n = 381 cells) from the VZ after 3 and 6 months, respectively (Fig.6A′, D′ and E′). Thus, the repositioning from superficial towards deeper brain structures occurred slightly faster for newborn neurons in Dl than in Dm and, together with a more proximate field of cfos expression, resulted in more and younger drug-activated BrdU/cfos double-positive neurons in Dl compared with Dm (Fig.6E and E′). These results were confirmed when directly quantifying the proportion of BrdU-positive neurons expressing cfos – in contrast to the continuous increase seen in Dm, in Dl a maximal proportion of juvenile-born neurons responding to amphetamine was already attained when these neurons were 4 months old (4 months – 17.38 ± 2.07% SEM of cfos+ BrdU+ cells; 6 months – 17.42 ± 2.78% SEM of cfos+ BrdU+ cells; one-way anovaF3,15 = 0.95, P = 0.1830 post-test for linear trend; Figs6F′ and 7A′). Together, our results suggest that, in the zebrafish pallium, juvenile rather than adult-born neurons respond to amphetamine, and that the neurons born in Dl participate in this response at an earlier age than those born in Dm.
Dm is activated during drug-seeking following conditioning
In ray-finned fish, such as the zebrafish, Dm, but not Dl, was recently shown to be involved in light avoidance (Lau et al., 2011) and taste aversion behavior (Martín et al., 2011). Both studies suggest that Dm plays a similar role to the mammalian amygdala in the negative emotional and motivational context of fear-related behaviors (Johansen et al., 2011). Our results showing a strong activation of Dm in response to acute d-amphetamine administration suggested that Dm may also mediate reward-stimulated behavior, and therefore prompted us to examine whether it may also play a role in motivated behavior. This behavior can be investigated in the zebrafish via a CPP test (Ninkovic & Bally-Cuif, 2006; Webb et al., 2009; Mathur et al., 2011). We used a biased CPP test where adult zebrafish are placed in a tank showing two visually distinct compartments (dark vs. bright), chosen such that fish exhibit an initial preference for one compartment. Thus, saline-injected fish exhibit an avoidance behavior for the initially non-preferred compartment (bright) and do not change their place preference over time (Fig.8B). In contrast, after a repeated pairing of a drug such as amphetamine with the initially non-preferred compartment, fish exhibit a drug-seeking approach behavior and reverse their place preference from the initially preferred (dark) to the initially non-preferred (bright) compartment (Fig.8C; for details, see Materials and methods).
To analyse the involvement of Dm neurons in CPP behavior, we therefore compared cfos expression in amphetamine-injected fish with control fish placed in a neutral tank that did thus not exhibit a directed behavior (Fig.8A). The number of cfos-positive cells in Dm in fish involved in drug-seeking behavior was 134.30 ± 25.28 SEM (n = 6 brains), compared with 80.38 ± 8.51 SEM (n = 13 brains) in control fish, representing a 1.7-fold increase (unpaired two-tailed t-test t17 = 2.58, P = 0.0194; Fig.8A′, C′ and D). To compare the activated Dm area with that involved in unconditioned place preference driven by light avoidance, the same analysis was conducted in fish injected with saline and placed in the visually cued experimental tank (Fig.8B). We observed 1.7 times more cfos-positive neurons in Dm in saline fish (137.0 ± 28.56 SEM cfos-positive cells, n = 6 brains; unpaired two-tailed t-test t17 = 2.50, P = 0.0229) compared with control fish (Fig.8A′, B′ and D), mimicking in location and fold increase the cfos response attained in drug-seeking fish. In contrast, we did not observe a difference in the number of cfos-positive cells in Dl or the subpallium between fish that exhibited drug-seeking or avoidance behavior and control fish (Fig.8D).
These results suggest that Dm is similarly activated during motivated behavior (drug-seeking approach) and unconditioned light avoidance behavior. They also highlight commonalities (Dm) and differences (Dl) in the neuronal activity pattern observed following acute amphetamine injection and during drug-seeking behavior. These findings are summarized in Fig.9.
Figure 9.
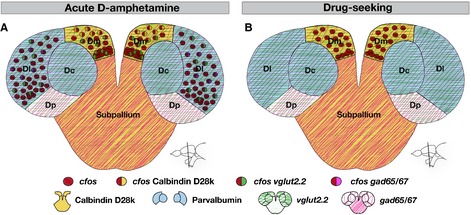
Schematic visualization of the telencephalic regions in the zebrafish activated by acute amphetamine treatment and during drug-seeking or light avoidance behavior. (A) Neuronal activation visualized by cfos expression (red dots) after amphetamine injection. Coexpression of cfos together with neuronal markers in their respective expression domains is color-coded. (B) Neuronal activation visualized by cfos expression (red dots) during drug-seeking behavior.
Discussion
We have used acute injections of the psychostimulant d-amphetamine, together with a quantitative analysis of cfos expression and markers of neuronal subtypes, to identify and characterize conserved elements of the brain reward circuitry in the zebrafish telencephalon. This led to the identification of drug-activated territories in Dm and Dl, containing areas homologous to the mammalian amygdala and hippocampus, respectively. We further demonstrated that Dm is similarly recruited in amphetamine-conditioned motivational behavior. Our results suggest an evolutionary conserved function of the amygdala in encoding values and motivational signals, and locate the relevant domain in the zebrafish adult brain to the ventral part of Dm.
Validity of the cfos FISH approach as a read-out of neuronal recruitment or plasticity
cfos expression has been used as an immediate marker of neuronal recruitment and plasticity in a number of brain territories, including the amygdala (Knapska et al., 2007). Compared with other IEGs, its expression is primarily neuronal and tends to be extinguished upon repeated encounter of the same stimulus, such that it better reveals novel neuronal activation/plasticity events (Knapska et al., 2007). Indeed, we noted that a first episode of fish handling or intra-peritoneal injection led to multiple sites of cfos induction, which were virtually abolished already after the second handling/injection episode (not shown). We used this property here to buffer the background effects of our amphetamine/saline administration procedure using a pre-handling/injection session, before measuring the specific drug-associated changes in cfos expression.
Most studies aiming to provide spatial mapping of neuronal activation relied on the immunodetection of cFos protein (Brown et al., 1992; Mead et al., 1999; Miller & Marshall, 2005; Rademacher et al., 2006) This is largely for practical reasons, as immunostaining allows single-cell resolution and can be easily combined with the co-detection of several other antigens, for example for neuronal subtypes. cFos protein, however, exhibits longer stability than cfos mRNA (approximately 2 h vs. 30 min, respectively), which likely resulted in the lasting cFos patterns that we observed (Fig.2). The new FISH technique that we developed for this study has multiple advantages in this respect – it combines highly sensitive detection of the dynamic cfos mRNA expression pattern at single-cell resolution and a quantitative analysis, together with the possible co-detection of other cell type-specific mRNAs or antigens in multicolor stainings. As shown in this study, this new mapping technique proved invaluable for the precise spatiotemporal dissection of the neural circuits underlying drug response and motivated behavior.
Evolutionary conserved function of amygdala nuclei in the processing of emotions
The mammalian amygdala is a heterogeneous collection of nuclei involved in the formation of stimulus-value associations and the storage of the emotional aspects of memories. In mammals, the amygdala is pivotal for the processing of both negative and positive emotions (Phelps & LeDoux, 2005; Murray, 2007; Johansen et al., 2011). It is activated by drugs of abuse or merely the presentation of drug-associated cues, as distinctive neuronal populations assign either a positive or a negative value to a motivational signal (Brown et al., 1992; Carelli et al., 2003; Paton et al., 2006). More recently, it has been shown that the amygdala also integrates spatial and motivational information (Peck et al., 2013). A number of studies specifically implicated the basolateral amygdala (BLA) in these properties, both for positive and aversive learning (Baxter & Murray, 2002; Paton et al., 2006; Shabel & Janak, 2009; Johansen et al., 2011; Tye et al., 2011). The BLA is also involved in processing unconditioned emotional states such as those driving ethological avoidance, for example the avoidance of predators or bright, highly visible places in the wild (reviewed in Knapska et al., 2007). Although amygdala nuclei have been considered homologous between mammals, birds, reptiles and some amphibians, based on comparison of developmental and anatomical data, such similarities are less obvious within the teleost brain (Moreno & González, 2007). Recent studies based on cfos detection implicated the Dm subdivision of the zebrafish telencephalon in the innate anxiety-like response displayed in a dark-light box (Lau et al., 2011), and we confirm these results in the present work. Further, Dm lesions were shown to impair the retention of an active avoidance learning response in goldfish (Portavella et al., 2004; Martín et al., 2011). Together, these observations suggest that Dm hosts equivalents to the amygdala nuclei driving avoidance strategies and avoidance learning. Importantly, our results now extend this morpho-functional conservation to the activation by drugs of abuse and to the processing of rewarded learning and positive emotions. They also further circumscribe the relevant amygdala territory to a ventral nucleus of Dm. From our data, it is tempting to speculate that the functional similarities between the BLA and ventral Dm may further extend to goal-directed behavior, adding new evidence for the homology of the two structures. The strict operational definition of goal-directed behavior involves both contingency degradation and instrumental devaluation (reviewed in Mannella et al., 2013). In the CPP test, lowering the association between amphetamine and the initially non-preferred compartment after conditioning decreases the drug-seeking response (Ninkovic & Bally-Cuif, 2006), verifying contingency degradation.
The functional conservation of a BLA-like domain within Dm that we propose here is complemented by neuroanatomical similarities. In agreement with its pallial location and expression of pallial identity markers (reviewed in Wullimann & Mueller, 2004), most neurons in Dm express vglut2.2. Only a small fraction expresses gad65/67. Similarly, the mammalian BLA is predominantly composed of glutamatergic neurons (80%), with only a minority of GABAergic interneurons (20%; McDonald & Mascagni, 2001; Marek et al., 2013). Both neurotransmitters are involved in BLA functions in mammals, including motivated learning (reviewed in Knapska et al., 2007). Although we have not tested their functional implication in zebrafish, the recruitment of vglut2.2-positive neurons in response to amphetamine in Dm is in agreement with a conserved involvement of glutamatergic signaling in the reception or processing of rewarding cues. Although we did observe an increase in the proportion of gad65/67-positive neurons expressing cfos upon amphetamine administration in Dl, this was not the case in Dm, which may indicate a differential response of GABAergic neurons to amphetamine treatment between the two areas. Finally, neuronal recruitment by amphetamine administration preferentially targets the glutamatergic subtype whereas the proportion of cfos-positive GABAergic neurons remains constant, suggesting that this recruitment occurs at the expense of other neurons, possibly producing neuropeptides. These neurons remain to be identified.
The GABAergic and glutamatergic neuronal phenotypes of the mammalian BLA can be subcategorized based on their expression of various CBPs, including parvalbumin, calbindin, calretinin and the calcium-sensitive enzyme calcium/calmodulin-dependent kinase II (Pitkänen & Kemppainen, 2002). Although we have not analysed co-expression in detail, the striking similarity in glutamatergic and GABAergic composition between the BLA and Dm does not extend to the expression of parvalbumin and calbindin. Both CBPs are expressed in the BLA and the hippocampus (Kemppainen & Pitkänen, 2000; McDonald & Mascagni, 2001; Pitkänen & Kemppainen, 2002; Jinno & Kosaka, 2006). In contrast, in the zebrafish telencephalon their expression is largely mutually exclusive – Parvalbumin is expressed in Dl, in which calbindin-positive neurons are only few, if any, but is excluded from Dm, where calbindin-positive neurons are numerous (Mueller et al., 2011; and this study). Although it has been shown that calbindin expression can be affected by amphetamine (Gonçalves et al., 2010), our study is, to the best of our knowledge, the first to report a specific activation of calbindin neurons in an amygdala-like region. Finally, it was shown in primates that distinct populations of amygdala neurons encode positive or negative motivational signals (Paton et al., 2006); whether this is also the case in the zebrafish remains to be determined.
Mapping reward circuitry components in the adult zebrafish brain
Compared with the amygdala, the precise function of the hippocampus in addiction is much less characterized (Robbins et al., 2008; Ricoy & Martinez, 2009; Koob & Volkow, 2010). Thus, it remains to be determined whether the specific activation of glutamatergic and GABAergic neurons that we observed in Dl of the zebrafish pallium also has counterparts in mammals. However, the hippocampus and the amygdala interact substantially (White & McDonald, 1993; Packard et al., 1994; McIntyre et al., 2002; McDonald & Hong, 2013) and this cross-talk is a crucial process in drug addiction, as drugs of abuse simultaneously convey their rewarding properties on both the emotional and memory systems (Phelps, 2004; Robbins et al., 2008; Wells et al., 2011). Although we showed that both Dm and Dl are activated by acute amphetamine injections, only Dm, but not Dl, was activated during drug-seeking behavior. This observation highlights that the direct targets of acute amphetamine administration and motivational processes involve partly distinct neuroanatomical correlates. In addition, it suggests that, following conditioning, CPP does not primarily rely on the processing of spatial information but is triggered instead through stimulus–response associations. Specifically, hippocampal circuits are likely to assess the salience of stimuli based on their novelty (reviewed in Mannella et al., 2013). Hence, hippocampal activation would be expected to vanish after the reiterated association of a constant dose of amphetamine with the same visual cue.
Another key component of the mammalian mesocorticolimbic reward circuitry is the nucleus accumbens (NAc; Koob & Volkow, 2010). A striatal homolog of the mammalian NAc in teleosts remains yet to be identified, even if conserved expression of molecular markers has suggested that the zebrafish subpallium is composed of striatal-, pallidal- and septal-like subdivisions (Ganz et al., 2012). Although we observed the highest number of cfos-expressing cells in amphetamine-injected fish in the subpallium, the number of cells recruited after amphetamine treatment was not increased as compared with saline-injected control fish. However, the intensity of cfos expression per cell in response to amphetamine was about twofold higher, suggesting that neurons in the subpallium may also participate in the drug response, in addition to those activated in Dm and Dl.
Adult neurogenesis and amphetamine response
The vast neurogenic and regenerative potential of the adult zebrafish brain combined with a molecular genetic amenability and behavioral analyses make this system a prime candidate to decipher the role of adult neurogenesis in drug addiction and emotional and motivational behaviors (Norton & Bally-Cuif, 2010; Zupanc & Sîrbulescu, 2011; Guo et al., 2012; Kizil et al., 2012; Schmidt et al., 2013). Our study, however, did not reveal a strong response of adult-born neurons to amphetamine; instead, it suggests that most drug-activated neurons both in Dm and Dl of a middle-age adult are born at the juvenile rather than the adult stage. Our BrdU analysis in adults allowed tracing only a subset and not all adult-born neurons, and it was restricted to a 2-month tracing interval. Nevertheless, together with our comprehensive juvenile analysis that showed a recruitment of 4.5- and 6-month-old neurons in Dl and Dm, respectively, this suggests that the neuronal response is mediated mostly by early- rather than late-born neurons firmly integrated into their specific networks (Rothenaigner et al., 2011). This may be surprising in two ways. First, adult-born neurons exhibit enhanced synaptic plasticity and contribute to learning and memory processes in rodents (Schmidt-Hieber et al., 2004; Ge et al., 2007; Deng et al., 2010), which would make them prime candidates for recruitment in a behavioral process conditionally induced in the adult. Second, it has been shown that adult hippocampal neurogenesis is affected by drugs of abuse in rodents (Eisch et al., 2000; Eisch & Harburg, 2006; Mandyam et al., 2008), and that impaired neurogenesis increases drug-seeking behavior and the risk to drug relapse (Noonan et al., 2010; Mandyam & Koob, 2012; Recinto et al., 2012; Canales, 2013). Along these lines, we previously documented a link between the abnormal regulation of expression of genes involved in adult neurogenesis and the absence of amphetamine-induced CPP in the zebrafish mutant no addiction (Webb et al., 2009). When tools are available to target specific subsets of the pallial and subpallial germinal zones, it will be important to assess the long-term effects of adult neurogenesis manipulations on CPP behavior.
Conclusions
Taken together, our data highlight for the first time that the reward circuitry in zebrafish involves Dm, a territory that shares developmental origin, gene expression and neuronal connections with the mammalian BLA. Like the mammalian BLA, Dm is activated both upon acute administration of the rewarding drug amphetamine and following conditioning during drug-seeking behavior. These findings suggest an evolutionary conserved function of the amygdala in the processing of positive emotions and induction of motivated behavior.
Conflict of interests
The authors declare that no competing interests exist.
Acknowledgments
The authors thank all members of the Zen group for their scientific input along this project, W.H.J. Norton for discussions and help with molecular biology, P. Affaticati for discussions and help with FISHs, M. Gutiérrez for testing various antibodies, M. Chaminade for teaching the CPP assay, R. Schmidt for providing the vglut2.2 construct, and S. Bedu for expert fish care. Work in the L. B.-C. laboratory was funded by the EU project ZF-Health (FP7/2010-2015 grant agreement no. 242048), the ANR (grants ANR-08-CEX-08-000-01 and ANR-2012-BSV4-0004-01), the Ecole des Neurosciences de Paris, the FRM (FRP ‘Equipe’ DEQ20120323692) and the European Research Council (AdG 322936).
Glossary
- BLA
basolateral amygdala
- BrdU
5′-bromo-2′-deoxyuridine
- CBP
calcium-binding protein
- CPP
conditioned place preference
- DAPI
4′,6-diamidino-2-phenylindole
- DIG
digoxigenin
- Dl
lateral zone of the dorsal telencephalic area
- Dm
medial zone of the dorsal telencephalic area
- DNP
dinitrophenol
- FISH
fluorescent in situ hybridization
- FLUO
fluorescein
- GABA
γ-aminobutyric acid
- IEG
immediate-early gene
- NAc
nucleus accumbens
- NGS
normal goat serum
- PBS
phosphate-buffered saline
- PBST
PBS containing 0.1% Tween
- ROI
region of interest
- RT
room temperature
- VZ
ventricular zone
References
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhäuser B, Strähle U, Götz M. Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Wagner S, Bardy C, Gabellec M-M, Torquet N. Lledo P-M. Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG. Murray EA. The amygdala and reward. Nat. Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Brend T. Holley SA. Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J. Vis. Exp. 2009;25:1–3. doi: 10.3791/1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS. Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J. Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM. See RE. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Curr. Top. Behav. Neurosci. 2010;3:73–99. doi: 10.1007/7854_2009_18. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Deficient plasticity in the hippocampus and the spiral of addiction: focus on adult neurogenesis. Curr. Top. Behav. Neurosci. 2013;15:293–312. doi: 10.1007/7854_2012_230. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG. Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J. Neurosci. 2003;23:8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R. Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. BioEssays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiol. Learn. Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB. Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ. Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW. Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M. Brand M. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 2012;520:633–655. doi: 10.1002/cne.22757. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang C-H, Hsu K-S, Ming G-L. Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO. Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur. J. Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I. Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Gu Y, Janoschka S. Ge S. Neurogenesis and hippocampal plasticity in adult brain. Curr. Top. Behav. Neurosci. 2013;15:31–48. doi: 10.1007/7854_2012_217. [DOI] [PubMed] [Google Scholar]
- Guo S, Wagle M. Mathur P. Toward molecular genetic dissection of neural circuits for emotional and motivational behaviors. Dev. Neurobiol. 2012;72:358–365. doi: 10.1002/dneu.20927. [DOI] [PubMed] [Google Scholar]
- Higashijima S-I, Mandel G. Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J. Comp. Neurol. 2004;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- Jinno S. Kosaka T. Cellular architecture of the mouse hippocampus: a quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci. Res. 2006;56:229–245. doi: 10.1016/j.neures.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE. LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Kyzar EJ, Cachat J, Gebhardt M, Landsman S, Robinson K, Maximino C, Herculano AM, Jesuthasan S, Wisenden B, Bally-Cuif L, Lange M, Vernier P, Norton W, Tierney K, Tropepe V, Neuhauss SCF ZNRC; The International Zebrafish Neuroscience Research Consortium. Time to recognize zebrafish “affective” behavior. Behaviour. 2012;149:1019–1036. [Google Scholar]
- Kaslin J, Ganz J. Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. T. Roy. Soc. B. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kemppainen S. Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J. Comp. Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S. Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J. Comp. Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Kittilsen S. Functional aspects of emotions in fish. Behav. Process. 2013;100:153–159. doi: 10.1016/j.beproc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V. Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Klee EW, Schneider H, Clark KJ, Cousin MA, Ebbert JO, Hooten WM, Karpyak VM, Warner DO. Ekker SC. Zebrafish: a model for the study of addiction genetics. Hum. Genet. 2012;131:977–1008. doi: 10.1007/s00439-011-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T. Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol. Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Koob GF. Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BYB, Mathur P, Gould GG. Guo S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA. 2011;108:2581–2586. doi: 10.1073/pnas.1018275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter G, Söll I. Hauptmann G. Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Dev. 2011;6:10. doi: 10.1186/1749-8104-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. A novel immunohistochemical method for evaluation of antibody specificity and detection of labile targets in biological tissue. J. Biochem. Bioph. Meth. 2004;58:85–96. doi: 10.1016/s0165-022x(03)00149-0. [DOI] [PubMed] [Google Scholar]
- Mandyam CD. Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN. Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol. Psychiat. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella F, Gurney K. Baldassarre G. The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front. Behav. Neurosci. 2013;7:135. doi: 10.3389/fnbeh.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW. Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J. Physiol. (Lond.) 2013;591:2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín I, Gómez A, Salas C, Puerto A. Rodríguez F. Dorsomedial pallium lesions impair taste aversion learning in goldfish. Neurobiol. Learn. Mem. 2011;96:297–305. doi: 10.1016/j.nlm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Mathur P, Lau B. Guo S. Conditioned place preference behavior in zebrafish. Nat. Protoc. 2011;6:338–345. doi: 10.1038/nprot.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ. Hong NS. How does a specific learning and memory system in the mammalian brain gain control of behaviour? Hippocampus. 2013;23:1084–1102. doi: 10.1002/hipo.22177. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Pal SN, Marriott LK. Gold PE. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J. Neurosci. 2002;22:1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Vasilaki A, Spyraki C, Duka T. Stephens DN. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur. J. Neurosci. 1999;11:4089–4098. doi: 10.1046/j.1460-9568.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- Miller CA. Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur. J. Neurosci. 2005;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin. Neur. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Moreno N. González A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J. Anat. 2007;211:151–163. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE. Salzman CD. Re-valuing the amygdala. Curr. Opin. Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T. Guo S. The distribution of GAD67-mRNA in the adult zebrafish (teleost) forebrain reveals a prosomeric pattern and suggests previously unidentified homologies to tetrapods. J. Comp. Neurol. 2009;516:553–568. doi: 10.1002/cne.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T. Wullimann MF. An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav. Evolut. 2009;74:30–42. doi: 10.1159/000229011. [DOI] [PubMed] [Google Scholar]
- Mueller T, Dong Z, Berberoglu MA. Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei) Brain Res. 2011;1381:95–105. doi: 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn. Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ninkovic J. Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–274. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Folchert A, Makhankov YV, Neuhauss SCF, Sillaber I, Straehle U. Bally-Cuif L. Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D-amphetamine in zebrafish. J. Neurobiol. 2006;66:463–475. doi: 10.1002/neu.20231. [DOI] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K. Lledo P-M. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat. Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC. Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- Norton W. Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA. Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L. McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc. Natl. Acad. Sci. USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL. Balleine BW. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J. Neurosci. 2013;33:8753–8763. doi: 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE. Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CJ, Lau B. Salzman CD. The primate amygdala combines information about space and value. Nat. Neurosci. 2013;16:340–348. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Kemppainen S. Comparison of the distribution of calcium-binding proteins and intrinsic connectivity in the lateral nucleus of the rat, monkey, and human amygdala. Pharmacol. Biochem. Be. 2002;71:369–377. doi: 10.1016/s0091-3057(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N. Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. NY Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B. Salas C. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 2004;24:2335–2342. doi: 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC. Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur. J. Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant ARH, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O. Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SEV. Murray EA. Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques. J. Neurosci. 2013;33:3380–3389. doi: 10.1523/JNEUROSCI.4374-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoy UM. Martinez JL. Local hippocampal methamphetamine-induced reinforcement. Front. Behav. Neurosci. 2009;3:47. doi: 10.3389/neuro.08.047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD. Everitt BJ. Drug addiction and the memory systems of the brain. Ann. NY Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C. Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 2002;22:2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenaigner I, Krecsmarik M, Hayes JA, Bahn B, Lepier A, Fortin G, Götz M, Jagasia R. Bally-Cuif L. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development. 2011;138:1459–1469. doi: 10.1242/dev.058156. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Strähle U. Scholpp S. Neurogenesis in zebrafish – from embryo to adult. Neural Dev. 2013;8:3. doi: 10.1186/1749-8104-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P. Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scoville WB. Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosur. Ps. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR. Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ. Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc. Natl. Acad. Sci. USA. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW. Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C. Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KJ, Norton WH, Trümbach D, Meijer AH, Ninkovic J, Topp S, Heck D, Marr C, Wurst W, Theis FJ, Spaink HP. Bally-Cuif L. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009;10:R81. doi: 10.1186/gb-2009-10-7-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM. Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn. Memory. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4. Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- White NM. McDonald RJ. Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav. Brain Res. 1993;55:269–281. doi: 10.1016/0166-4328(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Wullimann MF. Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J. Comp. Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B. Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Berlin: Birkhäuser; 1996. [Google Scholar]
- Zupanc GKH. Sîrbulescu RF. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur. J. Neurosci. 2011;34:917–929. doi: 10.1111/j.1460-9568.2011.07854.x. [DOI] [PubMed] [Google Scholar]


