Abstract
The degree to which plant pathogen infestation occurs in a host plant is expected to be strongly influenced by the level of species diversity among neighbouring host and non-host plant species. Since pathogen infestation can negatively affect host plant performance, it can mediate the effects of local biodiversity on ecosystem functioning.
We tested the effects of tree diversity and the proportion of neighbouring host and non-host species with respect to the foliar fungal pathogens of Tilia cordata and Quercus petraea in the Kreinitz tree diversity experiment in Germany. We hypothesized that fungal pathogen richness increases while infestation decreases with increasing local tree diversity. In addition, we tested whether fungal pathogen richness and infestation are dependent on the proportion of host plant species present or on the proportion of particular non-host neighbouring tree species.
Leaves of the two target species were sampled across three consecutive years with visible foliar fungal pathogens on the leaf surface being identified macro- and microscopically. Effects of diversity among neighbouring trees were analysed: (i) for total fungal species richness and fungal infestation on host trees and (ii) for infestation by individual fungal species.
We detected four and five fungal species on T. cordata and Q. petraea, respectively. High local tree diversity reduced (i) total fungal species richness and infestation of T. cordata and fungal infestation of Q. petraea and (ii) infestation by three host-specialized fungal pathogen species. These effects were brought about by local tree diversity and were independent of host species proportion. In general, host species proportion had almost no effect on fungal species richness and infestation. Strong effects associated with the proportion of particular non-host neighbouring tree species on fungal species richness and infestation were, however, recorded.
Synthesis. For the first time, we experimentally demonstrated that for two common forestry tree species, foliar fungal pathogen richness and infestation depend on local biodiversity. Thus, local tree diversity can have positive impacts on ecosystem functioning in managed forests by decreasing the level of fungal pathogen infestation.
Keywords: biodiversity and ecosystem functioning, determinants of plant community diversity and structure, disease dilution effect, fungal pathogen richness and infestation, Kreinitz experiment, local neighbourhood, neighbouring species identity effects, Quercus petraea, Shannon diversity effects, Tilia cordata
Introduction
Anthropogenic global change is the most important driver of declining biodiversity and has caused dramatic and irreversible alterations to the Earth's natural ecosystems (Knops et al. 1999; Chapin et al. 2000; Keesing et al. 2010). The loss of species has been shown to have a negative impact on fundamental ecosystem processes and overall system performance (Naeem et al. 1994; Chapin et al. 2000). Moreover, ecosystem stability is crucially dependent on biodiversity (for the diversity–stability relationship see McCann 2000; Proulx et al. 2010), as greater diversity may buffer ecosystems against inter-annual environmental variation, particularly among phylogenetically distantly related species, due to the asynchronous population dynamics of resident species (Cadotte, Dinnage & Tilman 2012). In addition, a reduction in plant species richness has been found to increase ecosystem vulnerability to invasions (Bouchard et al. 2013; Oakley & Knox 2013), alter herbivore communities (Beizhou et al. 2012) and enhance the spread of fungal plant diseases (Knops et al. 1999; Pautasso, Holdenrieder & Stenlid 2005). For example, Lau et al. (2008) reported a 91% reduction in pathogen infestation of Lespedeza capitata caused by Pythium spp. or Fusarium spp., where the host plant species was grown in mixed compared to monospecific stands. Using an epidemiological approach at the landscape level, Haas et al. (2011) found that the risk of infection by the plant pathogen Phytophthora ramorum was lower in sites with higher species diversity.
There are a number of ways in which pathogens affect host plant species. In infected plants, transpiration and photosynthesis rates are reduced, thus impairing the hosts' growth rates (Hajji, Dreyer & Marçais 2009). This results in a reduction in competitiveness, with negative effects on overall fitness. Pathogen infections also result in increased host mortality. For instance, disease-driven seedling mortality (e.g. by powdery mildew species) in temperate forests was high in the vicinity of conspecific adults and decreased with distance (negative distance effect; Yamazaki, Iwamoto & Seiwa 2009). As an outcome of negative density or distance effects, plant species coexistence can be enhanced if stronger competitors are more severely affected by pathogens than competitively inferior species (Janzen-Connell effects; Bradley, Gilbert & Martiny 2008; Mordecai 2011, 2013). Such pathogen-mediated negative feedbacks have been demonstrated in many recent studies and have been shown to result in an increased productivity of plant species mixtures as compared to monocultures (Maron et al. 2011; Schnitzer et al. 2011; Hendriks et al. 2013).
Although interactions across different trophic levels are considered crucial to our understanding of biodiversity functioning and multidiversity patterns (Duffy et al. 2007; Mordecai 2013), only a few biodiversity–ecosystem functioning (BEF) experiments have hitherto explicitly addressed the role of host richness and identity effects of particular host species caused by host–pathogen interactions (e.g. Roscher et al. 2007; Latz et al. 2012). Assuming that more diverse host communities provide a wider range of resources available at higher trophic levels, a simultaneous increase in host plant and consumer richness would be expected and has indeed been demonstrated for trematode parasites in amphibian hosts (Johnson et al. 2013), for insect and mammal herbivores (Koricheva et al. 2006; Scherber et al. 2010; Castagneyrol & Jactel 2012), but not yet for fungal pathogen richness in forests.
One possible mechanism by which host diversity affects pathogen infestation is host density, as demonstrated by a number of observational and experimental studies on host availability of (sub)tropical and temperate tree species (e.g. Seiwa et al. 2008; Yamazaki, Iwamoto & Seiwa 2009; Bagchi et al. 2010; Benítez et al. 2013). For instance, Mundt, Sackett & Wallace (2011) demonstrated that the epidemic spread of a rust disease was suppressed by declining host frequency in the neighbourhood, and independently of local plant species diversity. In tropical forests, seedling mortality of Sebastiana longicuspis caused by stem pathogens was three times higher in high- compared to low density plots (Bell, Freckleton & Lewis 2006). Similarly, the infection (disease risk) and infestation (disease severity) of foliar pathogens of understorey plant species were found to be inversely related to the relative host proportions (García-Guzmán & Dirzo 2006).
Whether the diversity of producers results in an increase or decrease in herbivore and pathogen species richness or degree of herbivory and pathogen infestation also depends on the degree of host specialization (e.g. Castagneyrol et al. 2014). With increasing host species diversity, species richness and level of infestation by generalized consumers and pathogens are expected to increase while the occurrence of specialized consumers and pathogens is expected to decrease. However, such a relationship has so far only been shown for insect and mammal herbivores (Koricheva et al. 2006; Jactel & Brockerhoff 2007; Sobek et al. 2009; Schuldt et al. 2011). Specialized herbivores are expected to cause less damage with increasing host species diversity because of decreasing host plant densities, which translates into reduced resource concentration (Root 1973; Schuldt et al. 2010; Fig.1a). Such patterns have been shown for several interactions between plants and specialized herbivores (e.g. Otway, Hector & Lawton 2005; Unsicker et al. 2006; Anderson, Sallam & Congdon 2009), and they have recently been summarized in meta-analyses by Castagneyrol et al. (2014). Decreasing damage with increasing host species diversity may also be expected for plant–fungal pathogen relationships, as most fungal pathogens are highly specialized on one single host species (Prell 1996), making them exclusively dependent on the availability of this specific resource.
Fig. 1.
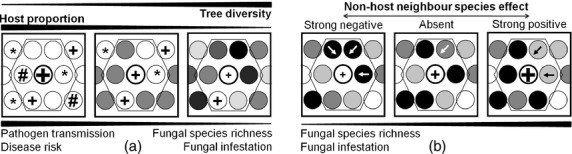
Graphical illustration of how tree diversity among neighbouring trees may affect fungal pathogens (i.e. fungal pathogen species richness and infestation) at the local neighbourhood scale in experimental tree communities of constant individual density. (a) Fungal pathogens of a target individual are expected to decrease with increasing tree species diversity. One reason for these effects may be the reduction in pathogen transmission among neighbouring individuals in communities of high tree diversity due to the larger distances between potential host individuals. Dilution of host individuals implies a reduction in the proportion of host species with increasing tree species diversity. This may lead to a reduction in the overall disease risk of a tree species. (b) The effects of the proportions of local non-host neighbouring species under a given neighbouring trees species richness may either facilitate or impede the fungal pathogen infestation of a target individual. These effects could be caused by the proportion of a particular non-host neighbour species, depending on whether the species changes the environmental conditions in a manner favourable or unfavourable to the pathogen. The first case would be a neighbour-species-mediated facilitation of the pathogens, resulting in an increase in fungal infestation by a particular non-host neighbour species. The latter case represents a neighbour-species-mediated facilitation of the host species, causing a decline in fungal infestation by the particular non-host neighbouring species. Circles represent individuals with differing shades of grey indicating different species. Circles with a thick black outline represent target individuals. Their local neighbourhood comprises all individuals within the hexagon. Symbols in the circles represent pathogens, with different symbols indicating different fungal pathogen species and different size of symbols showing a different degree of fungal pathogen infestation. Circles with arrows indicate that the neighbouring species changes the local environment of a target species.
Reduced resource availability, i.e. a decrease in host proportion, may in turn be the key mechanism affecting how biodiversity reduces the severity of fungal diseases, since higher plant diversity has been shown to result in host dilution effects and, thus, to lower encounter and transmission rates of fungal pathogens among susceptible co-occurring host individuals (García-Guzmán & Dirzo 2006; Keesing, Holt & Ostfeld 2006; Sobek et al. 2009). Such effects have been described from communities in grassland experiments (Mitchell et al. 2002, 2003; Zhu et al. 2005; Roscher et al. 2007), but also from forests differing in tree diversity (Pautasso, Holdenrieder & Stenlid 2005; Vacher et al. 2008; Haas et al. 2011; Hantsch et al. 2013). For instance, in Californian coastal forests, pathogen transmission was reduced and tanoak killing was hampered by lower densities of tanoaks in mixtures with non-host neighbour species (Cobb et al. 2012). In consequence, disease severity has been found to increase with host species availability, with the highest levels occurring in monospecific stands where host plant proportion is greatest (Fig.1a; Root 1973; Otway, Hector & Lawton 2005; Johnson et al. 2012).
In addition, many studies have encountered strong species identity effects, which were often more pronounced than effects of species diversity or host proportion (De Deyn et al. 2004; Sobek et al. 2009; Nadrowski, Wirth & Scherer-Lorenzen 2010). Thus, identity and density of non-host plant species may affect pathogen infection. With regard to pathogen infestation, the presence of individuals of a particular non-host species in the local neighbourhood of a target host individual can either increase or decrease the level of pathogen infestation (Fig.1b). Neighbour-mediated facilitation has also been demonstrated by sowing non-host plant species into grassland diversity experiments, e.g. Mitchell et al. (2002) found that species enrichment resulted in declining pathogen infestation. Such neighbourhood effects have also been mediated through the modification of sun exposure, local microclimate or soil conditions (Bahnweg et al. 2008). Such indirect effects are subsumed in the so-called associational resistance hypothesis (Tahvanainen & Root 1972), which states that a diverse host community reduces pathogen infestation due to a higher structural heterogeneity, which might operate through a higher microclimatic heterogeneity. Microclimatic properties are certainly fundamental for fungal pathogen development, since growth of mycelia, sexual and asexual reproduction and the spread of spores depend on critical temperatures and air humidity (Tainter & Baker 1996; Makowski, Bancal & Vicent 2011; Peñuelas et al. 2012). In the case of trees, microclimatic properties not only depend on topographic variability, but they can be affected by varying canopy sizes and crown structures of neighbouring plant species in a particular neighbourhood composition (Jactel et al. 2009; Calonnec et al. 2013). Hence, neighbouring tree size (e.g. height, basal area or crown size) may be considered as one of the most important traits determining herbivore activity or pathogen infestation of a particular tree individual (Potvin & Dutilleul 2009; Castagneyrol et al. 2013; Kamiya et al. 2014). The existence of size-dependent effects mediated by microclimate (Bourke 1970) necessitates the comparison of plant neighbourhoods that are as evenly structured as possible, e.g. by comparing trees of equal age. To this end, experimental approaches with neighbours of a similar size in a controlled experimental setting are highly advantageous. However, it has to be kept in mind that such experimentally controlled conditions may differ from mature forest ecosystems that exhibit a large variability in local plant neighbourhoods.
The abiotic environment is a covariate that has to be taken into account in the bivariate relationship between pathogens and their host species (Bourke 1970; Warren & Mordecai 2010). On the one hand, favourable conditions, such as temporarily high humidity (Bourke 1970; Laneri et al. 2010) and warm temperatures (Tainter & Baker 1996; Gutknecht, Field & Balser 2012), have been found to drive pathogen development, reproduction and persistence (Warren & Mordecai 2010). On the other hand, climatic extremes (e.g. winter frost, hail, summer drought, storms) may damage plant individuals, thereby facilitating pathogen infection directly (i.e. increased disease spread) or indirectly (Bourke 1970; Thomas, Blank & Hartmann 2002; Bock et al. 2010). In consequence, pathogen species richness and infestation vary with time, depending on the climatic conditions within season and between seasons (Root 1973; Jarosz & Burdon 1992; Lappalainen et al. 1999; Vacher et al. 2008). However, the degree of temporal variation in foliar fungal pathogen species richness and infestation might also depend on host diversity, as some studies hint at increasing temporal ecosystem stability with increasing plant diversity (Tilman 1996; Tilman, Reich & Knops 2006; Eisenhauer et al. 2011). However, a lower temporal variation in fungal pathogen infestation with increasing host diversity has not yet been shown in BEF experiments.
For this study, we used one of the European BEF tree diversity experiments, the Kreinitz experiment in Germany, to analyse the simultaneous influence of tree diversity, host and non-host species proportion, non-host species identity and inter-annual variation in weather conditions on foliar fungal species richness and fungal infestation. We focused on two disease-prone tree species (Quercus petraea and Tilia cordata), both of which belong to genera known for their susceptibility to foliar fungal pathogens (Roslin, Laine & Gripenberg 2007; Desprez-Loustau et al. 2010; Tack, Gripenberg & Roslin 2012). Here, we separately tested the following hypotheses on each of the two tree species at the plant neighbourhood scale: (i) increasing tree diversity increases fungal species richness and decreases fungal infestation; (ii) increasing proportion of host species increases fungal infestation; (iii) the presence and proportion of particular non-host species determine fungal species richness and fungal infestation; and (iv) increasing tree diversity decreases inter-annual variation in fungal species richness and fungal infestation brought about by climatic differences between years.
Materials and methods
Study Site
The tree diversity experiment in Kreinitz was established in 2005 to analyse above- and below-ground links in the relationship between biodiversity and ecosystem functioning. The experiment site is located in Saxony (51°23′10″ N, 13°15′43″ E) and was established on a former arable field in an unshaded area. The plots were situated on flat ground and ranged in elevation between 110 and 120 m a.s.l., with similar aspects and slopes. The soils were weakly acidic (pH 4.6–6.3) and were of a sandy texture. From a species pool of six native tree species (Fagus sylvatica, Fraxinus excelsior, Picea abies, Pinus sylvestris, Quercus petraea and Tilia cordata), 49 communities were established, each differing in species richness. Communities ranged from bare ground (n = 1 plot), monocultures of all species (n = 6), all possible two-species mixtures (n = 15), all possible three-species mixtures (n = 20), all possible five-species mixtures (n = 6) and one-six-species mixture (n = 1) (Fig.2a). Each community was replicated across two blocks, resulting in a total of 98 plots, each with 49 species compositions in either of the blocks. Plot size was 25 m2 (5 m * 5 m), with each plot containing 30 tree individuals of the respective species mixture. Tree species were planted randomly in five rows with a between-row distance of 1 m and a within-row distance of 0.8 m (Fig.2b). Dead trees were replaced during the first 2 years from 2005 to 2007, while site management included mowing and weed removal, using the herbicide Roundup®, during the first 4 years of the experiment.
Fig. 2.
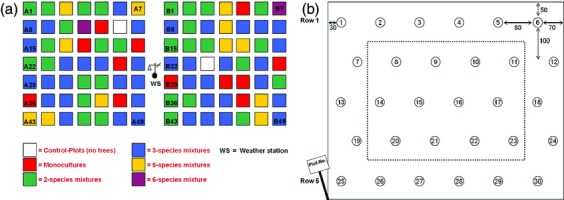
The experimental design of the tree diversity experiment in Kreinitz: (a) Different tree species mixtures (differing colours) were arranged randomly within 49 plots, and identical mixtures were repeated in the two blocks (A and B). (b) The general plot design included 30 tree individuals arranged along five rows. Within plots, individuals of each tree species were randomly distributed. The number of individuals per tree species depended on the tree species mixture but was equal among tree species in each plot. The dotted line shows the core area within the plot.
Study Species and Leaf Sampling
The highly infected tree species Q. petraea and T. cordata were chosen as target species for foliar fungal analysis. Tree individuals were selected randomly in all plots containing at least one of the two study species, with locations being chosen in the core area of each plot (dotted line in the plot, Fig.2b). We focused on the local plant neighbourhood, which comprised the directly adjacent neighbouring trees (Fig.2b; mean species height in 2011: F. sylvatica = 146 cm, F. excelsior = 282 cm, P. sylvestris = 296 cm, P. abies = 307 cm, Q. petraea = 256 cm, T. cordata = 190 cm). Such a neighbourhood approach has been widely applied, particularly in small-scale studies (e.g. Biere & Honders 1998; Bolker 1999; Bucheli & Shykoff 1999). As demonstrated in a preceding study in the BIOTREE experiment (Hantsch et al. 2013), the inclusion of more than the direct adjacent row of neighbours would also have been ecologically meaningful, but such was not feasible given the limited plot size of the Kreinitz experiment, which contained only 30 individuals per plot. Moreover, a plot-based evaluation (such as that employed in the BIOTREE experiment by Hantsch et al. 2014) was not possible, as we only focused on two target species, which did not occur in all plots. The outermost row in all plots was not sampled to ensure that all target trees could potentially have six trees in their local plant neighbourhood. However, in cases where potential core individuals had died (in eight of 1692 cases for T. cordata and five of 1692 cases for Q. petraea), edge individuals were sampled. We sampled a standardized number of individuals from Q. petraea and T. cordata in the following mixtures: six individuals in each of the monocultures, three individuals per species in each of the two-species mixture plots, two individuals per species in each of the three-species mixture plots and one individual in each of the five- and six-species mixture plots. This sampling was repeated over 3 years (2010–2012), resulting in 282 data points (94 tree individuals * 3 years) for each of the two study species. On occasion, strong drought events or disease effects caused target individuals to be devoid of leaves, in which cases we randomly selected alternative target individuals as replacements. Four branches were sampled from each of the total of 564 individuals of both tree species, across three consecutive years. Branches were selected in such a way that they were exposed to opposite directions in the upper and lower parts of the crown. Five leaves per branch were selected at random. Thus, 20 leaves per individual and year were collected and dried at 60 °C for 3 days and then stored in the dark at room temperature.
Macro- and Microscopic Analyses
Using macroscopic and microscopic analyses, all foliar fungal pathogens on the leaf surface were identified over three consecutive years (2010–2012). Using a random subset of 10 of 20 leaves per target individual, we identified taxa of foliar fungal pathogens on the leaf surface to the species level. We applied a proper fungus preparation for light microscopic determination of fungal spore, stroma and/or fruiting body traits and compared microscopic traits with the relevant literature (after Brandenburger 1985; Ellis & Ellis 1997; Braun & Cook 2012). We included only foliar fungal pathogens; endophytic fungi were excluded as they are not visible on the leaf surface and are not detectable by light microscopic techniques. Similarly, epiphytic and saprophytic fungi growing on the leaf surface were excluded due to their non-pathogen character. Depending on the fungal developmental stage, identification was not always possible to the species level. In cases where fructification was missing, a few fungi could only be identified to the genus level (Zygosporium sp. and Ramichloridium sp.) or phylum level (Ascomycota). Pathogen infestation was quantified for all fungal taxa on both the upper and lower leaf surfaces by scanning the surfaces using stereomicroscope analyses. The total percentage of leaf area infested by each fungal pathogen species was visually estimated and rated on a scale with seven damage classes: 0%, 1–5%, 6–10%, 11–25%, 26-50%, 51–75% and 76–100% (Schuldt et al. 2012; Hantsch et al. 2013). Fungal infestation per leaf was calculated by summing the infestations of all fungal species encountered on the respective leaf. Fungal infestation per tree individual was calculated by averaging foliar fungal infestation of all analysed leaves of the individual.
Weather Conditions
The Kreinitz tree diversity experiment was equipped with a meteorological station (see Fig.2a), which was set to record temperature and precipitation at 1-h intervals. According to available climate records, 2010 was the coldest and most humid year (mean temperature = 9.85 °C, precipitation = 881.71 mm) while 2011 and 2012 showed warmer and drier local weather conditions, respectively (mean temperature = 11.65 °C/11.41 °C, precipitation = 657.11 mm/559.47 mm). To test for climatic influence on fungal pathogen species, we used those variables as predictors for fungal species richness and fungal infestation across the different years.
Data Analysis
Both fungal species richness and fungal infestation were related to the predictors tree diversity (calculated as Shannon diversity; Hantsch et al. 2013), host species proportion and non-host species proportion in the vicinity of each target tree. In all linear mixed-effects models, the study ‘year’ (2010, 2011 and 2012) was included as a categorical predictor to test the extent to which fungal species richness and infestation were dependent on the particular climatic conditions. We also included the interaction of the different predictors with the ‘year’. Tree diversity, host species proportion and non-host species proportion were calculated for all six trees in the vicinity of the target tree (excluding the target tree; Fig.1b). Tree diversity was based on the presence/absence information. Host and non-host species proportions were based on the basal areas of neighbouring individuals, which is considered to be a proxy for neighbouring tree fitness (studies including neighbour effects: e.g. Potvin & Dutilleul 2009; Gundale et al. 2012; Castagneyrol et al. 2013). Hence, all calculations were based on presence/absence, proportion and basal area data gathered for each of the neighbouring individuals. As a consequence, Shannon tree diversity varied between 0 (monoculture) and 1.561 (highest diversity), host species proportion between 0.167 (lowest host proportion) and 1 (host monoculture) and non-host species proportion between 0 (absence of non-host species) and 1 (highest proportion of non-host species). Since individuals were planted randomly within plots, the exact composition of the local neighbourhood of each focal tree individual may differ slightly, even in the same tree species mixtures. Consequently, the two blocks were not exact replicates of the specific neighbourhood compositions.
Effects were assessed at two hierarchical levels. At the tree species level, fungal species richness and fungal infestation were analysed as an effect of Shannon tree diversity for each of the two target species, separately. In addition, effects of host species proportions and non-host species proportions were used instead of tree diversity. We did not include Shannon tree diversity, host species proportions and non-host species proportions in these models, as we expected strong multicollinearities among these predictors. Instead, we decided to test the predictors separately and systematically, which allowed for direct comparisons of potential effects. A significant positive host species proportion effect may indicate that tree diversity operates through dilution of the host species, whereas a positive or negative non-host species proportion effect would indicate a shift within microclimatic environmental conditions.
At the fungal species level, fungal infestation was evaluated separately for every fungal taxon using the same models as mentioned earlier. However, we ran separate models for each fungal species. All calculations were carried out using linear mixed-effects models (lme; package nlme; Pinheiro 2013), including block and plot as hierarchical nested random factors and assigning a Gaussian error distribution (R Core Team 2013). Both F-tests based on anovas are provided (as Tables) and estimates (in the Supporting Information). We used the varcomp procedure (package ape) to assess the proportion of total random variation explained by plot. The amount of variation explained by the total model was obtained from regression predicted against observed responses.
Results
In total, we encountered four and five host-specific foliar fungal pathogen species on Tilia cordata and Quercus petraea, respectively. All fungal species were identified as having originated from the phylum Ascomycota. Tilia cordata hosted the most abundant fungal pathogen Passalora microsora (Sacc.) U. Braun, alongside the less abundant pathogen Asteromella tiliae (F. Rudolphi) Butin & Kehr. The two fungal species often covered more than a tenth of the leaf surface, causing an uncountable number of small or single large leaf spots, respectively. The large spots of Asteromella tiliae have an arborescent appearance due to a margin of fine, ramified capillaries. Another unidentified small ascomycete (identification not possible due to lack of fructification) exhibiting dark black-dotted stroma occurred frequently on T. cordata, along with the rare irregular leaf spot fungus Apiognomonia errabunda (Roberge) Höhn. Quercus petraea was host to the most abundant fungal pathogens Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. and Erysiphe hypophylla (Nevod.) U. Braun & Cunningt., both of which are powdery mildew species that often covered more than one-tenth of the leaf surface. In addition, two other less frequent fungal pathogens of the genera Zygosporium Mont. and Ramichloridium Stahel ex de Hoog were identified on Q. petraea. We additionally encountered another unidentifiable, small ascomycete on leaves of Q. petraea, which exhibited dark black-dotted stroma.
Tree Species Level
At the tree species level, fungal species richness and overall fungal infestation on T. cordata significantly decreased with increasing tree diversity among neighbouring species (Fig.3a,b, Table1). Fungal species richness of T. cordata was similar in all 3 years. However, a significant interaction between tree diversity and ‘year’ indicated a stronger decline in fungal species richness with increasing tree diversity in 2012 than in 2011 or 2010 (Fig.3a, Table1, estimates are provided in Table S1 in Supporting Information). In contrast, fungal infestation was generally found to be higher in 2010 than in 2011 or 2012 (Table1 and Table S1). Local neighbourhood tree diversity had no effect on fungal species richness on Q. petraea; however, as with T. cordata, it reduced fungal infestation (Fig.3c,d and Table1). In addition, there were significant differences in fungal species richness and fungal infestation between all 3 years, with generally lower values in 2010 than in the other years (Fig.3c,d, Table1 and Table S1).
Fig. 3.
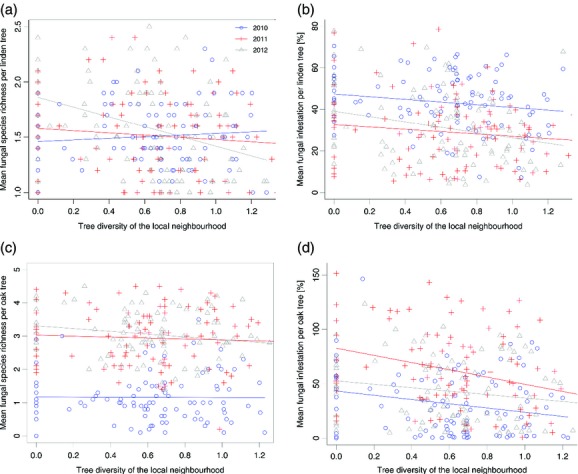
The relationship between tree diversity among neighbouring trees and fungal species richness (a,c) and the overall fungal infestation (b,d) of Tilia cordata (a: R2 = 0.277, P = 0.045; b: R2 = 0.475, P = 0.046) and Quercus petraea (c: R2 = 0.705, P = 0.102; d: R2 = 0.417, P = 0.004) individuals, respectively. Different colours indicate different years (2010–2012). Tree diversity is quantified as Shannon diversity, with zero indicating monocultures. n = 282.
Table 1.
Linear mixed-effects model results at the tree species level. Effect of tree diversity among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012. d.f. = 233. F and P refer to the ratio of explained to random variance and error probability, respectively. Significant results are indicated in bold. Variance of the random factors block and plot, respectively: Fungal species richness T. cordata = 2.9 % and 12.1%, Q. petraea = 2.5% and 25.6%; Fungal infestation T. cordata = < 0.1% and 27.4 %, Q. petraea = < 0.1% and 22.5%
| Response variable | Explanatory variable |
Tilia cordata |
Quercus petraea |
||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Fungal species richness | Intercept | 922.426 | < 0.001 | 417.423 | < 0.001 |
| Tree diversity (TD) | 4.055 | 0.045 | 2.69 | 0.102 | |
| Year | 1.761 | 0.174 | 233.196 | < 0.001 | |
| TD × Year | 7.562 | 0.001 | 0.954 | 0.387 | |
| Fungal infestation | Intercept | 507.3 | < 0.001 | 220.135 | < 0.001 |
| Tree diversity (TD) | 4.03 | 0.046 | 8.389 | 0.004 | |
| Year | 31.429 | < 0.001 | 28.296 | < 0.001 | |
| TD × Year | 1.01 | 0.366 | 1.075 | 0.343 | |
For both target species, there were no significant effects linking host species proportion in the local neighbourhood with fungal species richness or fungal infestation (Table2). However, for T. cordata, fungal species richness showed a significant interaction between host proportion and ‘year’, since fungal species richness increased with increasing host proportion in 2012 but decreased in 2010 and 2011 (Table2, estimates are provided in Table S2).
Table 2.
Linear mixed-effects model results at the tree species level. Effect of tree species proportions among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012. d.f. = 233. F and P refer to the ratio of explained to random variance and error probability, respectively. Significant results are indicated in bold. Conspecific host species proportions are marked in grey
| Proportion of neighbour tree species |
Tilia cordata |
Quercus petraea |
||||||
|---|---|---|---|---|---|---|---|---|
|
Fungal species richness |
Fungal infestation |
Fungal species richness |
Fungal infestation |
|||||
| F | P | F | P | F | P | F | P | |
| Intercept | 797.88 | < 0.001 | 532.47 | < 0.001 | 53.834 | < 0.001 | 203.494 | < 0.001 |
| P.Fs. | 0.636 | 0.426 | 12.395 | 0.001 | 1.101 | 0.295 | 1.133 | 0.288 |
| Year | 1.883 | 0.154 | 29.791 | < 0.001 | 143.74 | < 0.001 | 27.796 | < 0.001 |
| P.Fs. × Year | 0.323 | 0.725 | 2.049 | 0.131 | 0.098 | 0.907 | 0.205 | 0.815 |
| Intercept | 834.44 | < 0.001 | 434.443 | < 0.001 | 350.911 | < 0.001 | 218.018 | < 0.001 |
| P.Fe. | 0.054 | 0.817 | 0.779 | 0.378 | 0.739 | 0.391 | 6.647 | 0.011 |
| Year | 1.867 | 0.157 | 31.483 | < 0.001 | 156.14 | < 0.001 | 29.096 | < 0.001 |
| P.Fe. × Year | 0.849 | 0.429 | 2.169 | 0.117 | 10.085 | < 0.001 | 1.775 | 0.172 |
| Intercept | 780.496 | < 0.001 | 452.118 | < 0.001 | 370.838 | < 0.001 | 308.425 | < 0.001 |
| P.Pa. | 0.456 | 0.5 | 1.184 | 0.277 | 2.148 | 0.144 | 18.071 | < 0.001 |
| Year | 1.808 | 0.166 | 32.153 | < 0.001 | 145.986 | < 0.001 | 28.313 | < 0.001 |
| P.Pa. × Year | 0.027 | 0.973 | 3.235 | 0.041 | 2.219 | 0.111 | 3.055 | 0.049 |
| Intercept | 781.01 | < 0.001 | 545.266 | < 0.001 | 356.56 | < 0.001 | 204.174 | < 0.001 |
| P.Ps. | 0.826 | 0.365 | 11.849 | 0.001 | 0.484 | 0.487 | 0.748 | 0.388 |
| Year | 1.945 | 0.145 | 31.615 | < 0.001 | 147.143 | < 0.001 | 29.219 | < 0.001 |
| P.Ps. × Year | 6.242 | 0.002 | 2.757 | 0.066 | 2.923 | 0.056 | 4.474 | 0.012 |
| Intercept | 793.674 | < 0.001 | 439.613 | < 0.001 | 357.114 | < 0.001 | 200.31 | < 0.001 |
| P.Qp. | 0.087 | 0.769 | 0.018 | 0.893 | 0.193 | 0.661 | 0.382 | 0.537 |
| Year | 1.824 | 0.164 | 31.609 | < 0.001 | 151.181 | < 0.001 | 28.227 | < 0.001 |
| P.Qp. × Year | 1.039 | 0.356 | 2.105 | 0.124 | 6.157 | 0.003 | 1.914 | 0.15 |
| Intercept | 814.632 | < 0.001 | 523.984 | < 0.001 | 356.29 | < 0.001 | 205.925 | < 0.001 |
| P.Tc. | 0.332 | 0.565 | 2.423 | 0.121 | 0.261 | 0.61 | 1.391 | 0.24 |
| Year | 1.867 | 0.157 | 31.746 | < 0.001 | 144.247 | < 0.001 | 28.243 | < 0.001 |
| P.Tc. × Year | 5.864 | 0.003 | 0.962 | 0.384 | 0.234 | 0.792 | 1.065 | 0.346 |
P., Proportion; Fs., Fagus sylvatica; Fe., Fraxinus excelsior; Pa., Picea abies; Ps., Pinus sylvestris; Qp., Quercus petraea; Tc., Tilia cordata.
Among the analyses on the effects of local non-host species proportions, we encountered two significant non-host proportion effects for the fungal infestation on T. cordata. As depicted in Table2, overall fungal infestation on T. cordata increased with an increasing proportion of F. sylvatica and decreased with increasing proportion of neighbouring P. sylvestris individuals (Fig.4a,b, Table2). Furthermore, we detected significant interactions between the non-host species proportion and the ‘year’, which indicates a reduction in fungal infestation on T. cordata with increasing proportion of neighbouring P. abies individuals. However, this was only true for the years 2011 and 2012 (estimates are provided in Table S2). In addition, fungal species richness decreased with increasing proportion of Pinus sylvestris in the years 2011 and 2012, whereas it increased in 2010 (Table2 and Table S2). For Q. petraea, there were two main effects of the proportion of non-host neighbour species on fungal infestation (Table2): a high proportion of neighbouring F. excelsior individuals enhanced fungal infestation (Fig.4c, Table2), while a high proportion of P. abies reduced it (Fig.4d, Table2). These effects were found in all 3 years. In contrast to the patterns observed on T. cordata, there were positive non-host proportion effects on Q. petraea. Moreover, a significant interaction indicated that an increasing proportion of F. excelsior in the local neighbourhood enhanced fungal species richness in 2011 and 2012 but reduced it in 2010 (Table2 and Table S2).
Fig. 4.
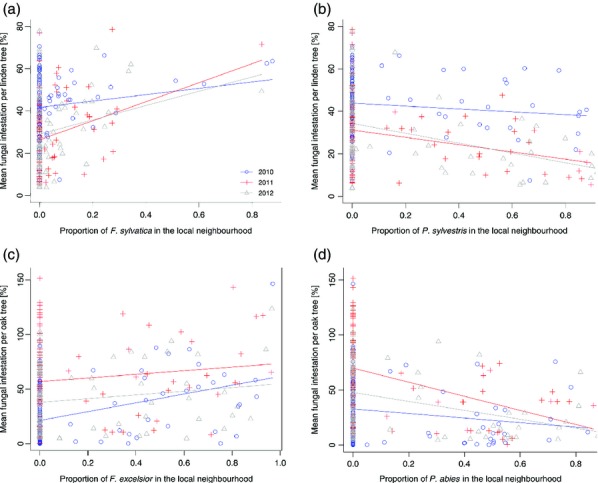
The relationship between non-host neighbour proportion (a: Fagus sylvatica, b: Pinus sylvestris, c: Fraxinus excelsior, d: Picea abies) among neighbouring trees and the overall fungal infestation of Tilia cordata (a: R2 = 0.482, P = 0.001; b: R2 = 0.487, P = 0.001) and Quercus petraea (c: R2 = 0.421, P = 0.011; d: R2 = 0.391, P < 0.001) individuals, respectively. Different colours indicate different years (2010–2012). The non-host proportion was calculated as a proportion of the six neighbouring individuals, with zero indicating the absence of a particular non-host species (e.g. F. sylvatica) among neighbouring trees. n = 282.
Fungal Species Level
At the fungal species level, we analysed tree diversity and host and non-host proportion effects separately for fungal and host tree species. Among the fungal species on T. cordata, P. microsora and the unknown ascomycete showed a marginally significant response to the tree diversity of the local neighbourhood (Table3). Fungal infestation of P. microsora decreased with increasing tree diversity in all study years. Fungal infestation of both P. microsora and the unknown ascomycete species was significantly higher in 2010 than in 2011 and 2012 (estimates provided in Table S3). Of the fungal species that occurred on Q. petraea, the infestation by E. hypophylla was significantly affected by tree diversity in the local neighbourhood (Table3). In addition, fungal infestation of all fungal species on Q. petraea differed significantly between the years, with generally lower infestation occurring in 2010 than in 2011 or 2012 (Table3 and Table S3).
Table 3.
Linear mixed-effects model results at the fungal species level. Effect of tree diversity among neighbouring trees on fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012. d.f. = 233. F and P refer to the ratio of explained to random variance and error probability, respectively. Significant results are indicated in bold. Variance of the random factors block and plot, respectively: T. cordata: Passalora microsora = < 0.1% and 28.9%, Apiognomonia errabunda = 0.18% and < 0.1%, Asteromella tiliae = 1.8% and 15.7%, Species of Ascomycota = < 0.1% and 8.0%; Q. petraea: Erysiphe alphitoides = 3.0% and 11.9%; Erysiphe hypophylla = < 0.1% and 21.9%, Species of Ascomycota = < 0.1% and 19.4%, Zygosporium sp. = < 0.1% and 13.5%, Ramichloridium sp. = 9.5% and 16.2%
| Explanatory variable |
Tilia cordata |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Passalora microsora |
Apiognomonia errabunda |
Asteromella tiliae |
Species of Ascomycota |
|||||
| F | P | F | P | F | P | F | P | |
| Intercept | 444.11 | < 0.001 | 2.407 | 0.122 | 10.695 | 0.001 | 230.307 | < 0.001 |
| Tree diversity (TD) | 3.619 | 0.058 | 0.181 | 0.671 | 0.095 | 0.759 | 5.087 | 0.025 |
| Year | 33.444 | < 0.001 | 0.772 | 0.463 | 2.231 | 0.11 | 0.001 | 0.999 |
| TD × Year | 0.498 | 0.608 | 1.351 | 0.261 | 4.369 | 0.014 | 6.253 | 0.002 |
|
Quercus petraea |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Erysiphe alphitoides |
Erysiphe hypophylla |
Species of Ascomycota |
Zygosporium sp. |
Ramichloridium sp. |
||||||
| F | P | F | P | F | P | F | P | F | P | |
| Intercept | 18.787 | < 0.001 | 188.949 | < 0.001 | 223.521 | < 0.001 | 138.457 | < 0.001 | 51.206 | < 0.001 |
| Tree diversity (TD) | 3.241 | 0.073 | 7.874 | 0.005 | 0.031 | 0.86 | 0.344 | 0.558 | 0.234 | 0.629 |
| Year | 20.807 | < 0.001 | 15.655 | < 0.001 | 71.802 | < 0.001 | 62.608 | < 0.001 | 73.187 | < 0.001 |
| TD × Year | 0.845 | 0.431 | 0.828 | 0.438 | 0.501 | 0.607 | 1.138 | 0.322 | 0.363 | 0.696 |
As with results recorded at the tree species level, there was no evidence that fungal pathogen infestation by any of the species depended on the proportion of either of the target host species in the local neighbourhood (Table4). However, infestation of the unknown ascomycete on T. cordata showed a significant interaction and increased with increasing host proportion in 2011 and 2012, but decreased in 2010 (Table4, estimates are provided in Table S4). Similarly, a significant interaction between host proportion in the local neighbourhood of Q. petraea and ‘year’ indicated an increase in fungal infestation of the unknown ascomycete with increasing host proportion in 2012, with a decrease in 2010 and 2011 (Table4 and Table S4).
Table 4.
Linear mixed-effects model results at the fungal species level. Effect of tree species proportions among neighbouring trees on the fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012. d.f. = 233. F and P refer to the ratio of explained to random variance and error probability, respectively. Significant results are indicated in bold fonts. Conspecific host species proportions are marked in grey
| Proportion of neighbour tree species |
Tilia cordata |
Quercus petraea |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Passalora microsora |
Apiognomonia errabunda |
Asteromella Tiliae |
Species of Ascomycota |
Erysiphe alphitoides |
Erysiphe hypophylla |
Species of Ascomycota |
Zygosporium sp. |
Ramichloridium sp. |
||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Intercept | 471.062 | < 0.001 | 2.131 | 0.146 | 11.447 | 0.001 | 199.975 | < 0.001 | 19.419 | < 0.001 | 174.464 | < 0.001 | 223.801 | < 0.001 | 138.373 | < 0.001 | 46.52 | < 0.001 |
| P.Fs. | 12.287 | 0.001 | 0.451 | 0.503 | 0.529 | 0.468 | 1.269 | 0.261 | 1.004 | 0.318 | 1.156 | 0.283 | 0.435 | 0.51 | 0.655 | 0.419 | 10.79 | 0.001 |
| Year | 31.98 | < 0.001 | 0.728 | 0.484 | 2.055 | 0.131 | 0.006 | 0.994 | 20.171 | < 0.001 | 15.556 | < 0.001 | 71.489 | < 0.001 | 61.392 | < 0.001 | 72.762 | < 0.001 |
| P.Fs. × Year | 2.496 | 0.085 | 0.155 | 0.857 | 0.392 | 0.676 | 0.607 | 0.546 | 0.015 | 0.985 | 0.302 | 0.74 | 0.14 | 0.869 | 0.025 | 0.978 | 0.651 | 0.522 |
| Intercept | 387.624 | < 0.001 | 1.974 | 0.161 | 10.029 | 0.002 | 195.585 | < 0.001 | 17.911 | < 0.001 | 175.636 | < 0.001 | 222.045 | < 0.001 | 140.661 | < 0.001 | 50.255 | < 0.001 |
| P.Fe. | 0.919 | 0.339 | 6.317 | 0.013 | 2.261 | 0.134 | 0.085 | 0.771 | 17.472 | < 0.001 | 1.514 | 0.22 | 1.029 | 0.312 | 0.216 | 0.642 | 5.671 | 0.018 |
| Year | 33.537 | < 0.001 | 0.901 | 0.408 | 1.952 | 0.144 | 0.004 | 0.996 | 21.394 | < 0.001 | 16.13 | < 0.001 | 81.703 | < 0.001 | 63.139 | < 0.001 | 78.376 | < 0.001 |
| P.Fe. × Year | 2.041 | 0.132 | 0.432 | 0.65 | 0.216 | 0.806 | 2.41 | 0.092 | 2.486 | 0.085 | 2.496 | 0.085 | 14.427 | < 0.001 | 2.306 | 0.102 | 4.26 | 0.015 |
| Intercept | 402.833 | < 0.001 | 2.406 | 0.122 | 11.509 | 0.001 | 197.56 | < 0.001 | 20.79 | < 0.001 | 260.258 | < 0.001 | 226.427 | < 0.001 | 143.216 | < 0.001 | 51.483 | < 0.001 |
| P.Pa | 1.242 | 0.266 | 1.236 | 0.267 | 0.603 | 0.438 | 0.409 | 0.523 | 7.306 | 0.007 | 17.622 | < 0.001 | 0.508 | 0.477 | 2.706 | 0.101 | 0.114 | 0.737 |
| Year | 34.292 | < 0.001 | 0.743 | 0.477 | 2.169 | 0.117 | 0.003 | 0.997 | 20.84 | < 0.001 | 15.745 | < 0.001 | 72.803 | < 0.001 | 62.123 | < 0.001 | 74.742 | < 0.001 |
| P.Pa. × Year | 3.115 | 0.046 | 0.302 | 0.74 | 1.182 | 0.309 | 0.78 | 0.46 | 3.185 | 0.043 | 1.836 | 0.162 | 2.377 | 0.095 | 1.048 | 0.353 | 2.932 | 0.055 |
| Intercept | 486.47 | < 0.001 | 2.355 | 0.126 | 10.538 | 0.001 | 202.001 | < 0.001 | 22.84 | < 0.001 | 172.181 | < 0.001 | 226.242 | < 0.001 | 139.689 | < 0.001 | 43.811 | < 0.001 |
| P.Ps. | 11.233 | 0.001 | 0.91 | 0.341 | 0.067 | 0.796 | 1.184 | 0.278 | 1.854 | 0.175 | 0.64 | 0.424 | 0.675 | 0.412 | 0.44 | 0.508 | 9.404 | 0.002 |
| Year | 33.402 | < 0.001 | 0.76 | 0.469 | 2.169 | 0.117 | 0.004 | 0.996 | 21.429 | < 0.001 | 16.093 | < 0.001 | 72.282 | < 0.001 | 61.504 | < 0.001 | 72.595 | < 0.001 |
| P.Ps. × Year | 1.858 | 0.158 | 0.205 | 0.815 | 3.379 | 0.036 | 5.041 | 0.007 | 5.544 | 0.004 | 2.351 | 0.098 | 1.689 | 0.187 | 0.117 | 0.89 | 0.075 | 0.928 |
| Intercept | 391.747 | < 0.001 | 2.224 | 0.137 | 10.203 | 0.002 | 197.244 | < 0.001 | 17.88 | < 0.001 | 180.216 | < 0.001 | 224.271 | < 0.001 | 139.346 | < 0.001 | 51.747 | < 0.001 |
| P.Qp. | 0.023 | 0.879 | 1.24 | 0.267 | 0.741 | 0.39 | 0.253 | 0.615 | 1.341 | 0.248 | 1.774 | 0.184 | 0.237 | 0.627 | 0.572 | 0.45 | 1.028 | 0.312 |
| Year | 33.815 | < 0.001 | 0.739 | 0.479 | 2.079 | 0.127 | 0.004 | 0.996 | 20.77 | < 0.001 | 15.628 | < 0.001 | 74.122 | < 0.001 | 63.134 | < 0.001 | 73.451 | < 0.001 |
| P.Qp. × Year | 2.411 | 0.092 | 2.325 | 0.1 | 0.051 | 0.95 | 0.596 | 0.552 | 0.312 | 0.732 | 2.778 | 0.064 | 3.417 | 0.035 | 1.963 | 0.143 | 1.003 | 0.369 |
| Intercept | 462.652 | < 0.001 | 2.129 | 0.146 | 10.999 | 0.001 | 208.65 | < 0.001 | 23.541 | < 0.001 | 172.538 | < 0.001 | 226.251 | < 0.001 | 141.253 | < 0.001 | 61.14 | < 0.001 |
| P.Tc. | 2.027 | 0.156 | 0.513 | 0.475 | 0.001 | 0.98 | 1.291 | 0.257 | 4.243 | 0.041 | 0.222 | 0.638 | 0.24 | 0.625 | 0.48 | 0.489 | 5.351 | 0.022 |
| Year | 33.662 | < 0.001 | 0.775 | 0.462 | 2.175 | 0.116 | 0.003 | 0.997 | 20.745 | < 0.001 | 15.686 | < 0.001 | 71.578 | < 0.001 | 61.79 | < 0.001 | 74.625 | < 0.001 |
| P.Tc. × Year | 0.488 | 0.615 | 0.194 | 0.824 | 2.785 | 0.064 | 7.86 | 0.001 | 1.307 | 0.273 | 0.601 | 0.549 | 0.198 | 0.821 | 0.507 | 0.603 | 0.011 | 0.989 |
P., Proportion; Fs., Fagus sylvatica; Fe., Fraxinus excelsior; Pa., Picea abies; Ps., Pinus sylvestris; Qp., Quercus petraea; Tc., Tilia cordata.
Furthermore, we encountered several non-host species effects of particular fungi on T. cordata. For instance, fungal infestation of P. microsora on T. cordata increased with increasing proportion of F. sylvatica, but it decreased with increasing proportion of P. sylvestris (Table4). In addition, the fungal infestation of A. errabunda increased with increasing proportion of F. excelsior in the local neighbourhood (Table4). As with T. cordata, we detected non-host species effects of particular fungi on Q. petraea. For instance, infestation of E. alphitoides decreased with increasing proportion of both P. abies and T. cordata in the local neighbourhood, but it increased with increasing proportion of F. excelsior (Table4). In addition to E. alphitoides, fungal infestation of E. hypophylla decreased with increasing proportion of P. abies in the local neighbourhood (Table4). Moreover, the fungal infestation of Ramichloridium sp. increased with increasing proportion of F. sylvatica or F. excelsior, but it decreased with an increase in the proportions of either P. sylvestris or T. cordata (Table4). Many of the effects of non-host proportions showed significant interactions with the growth ‘year’, indicating an increase or decrease in fungal infestation with increasing proportion of non-host tree species in the local neighbourhood, albeit only in particular years (Table4 and Table S4).
Discussion
In this study we demonstrated that fungal pathogen species richness and fungal pathogen infestation of the two common tree species Tilia cordata and Quercus petraea significantly depended on the biodiversity of the neighbouring trees as well as on the conditions of the particular study year. The differences between study years were probably brought about by inter-annual variation in climatic conditions. For T. cordata, an increase in local tree diversity resulted in a decrease in fungal species richness and infestation in general. Moreover, we observed a decrease in the infestation by the main fungal species Passalora microsora and by an unknown ascomycete. However, while increasing local tree diversity decreased both overall fungal infestation and the infestation of one of the main fungal pathogens, Erysiphe hypophylla, it had no effect on fungal species richness in Q. petraea. Local biodiversity effects were mainly dependent on the level of local tree diversity or the proportion of non-host species in the local neighbourhood, but not on the proportion of the host species. As such, local biodiversity effects were not simply due to dilution effects caused by a decline in the proportion of neighbouring host trees.
Tree Diversity Effects
Our first hypothesis, stating that fungal species richness increases with increasing tree diversity, could partly be confirmed. While the absence of a positive correlation between fungal species richness and neighbouring tree diversity corresponds to the results of a study on fungal diseases in French forests reported by Vacher et al. (2008), fungal infestation on both T. cordata and Q. petraea decreased with increasing tree diversity. In addition, we also hypothesized a reduction in fungal infestation of single fungal species, since the majority of biotrophic fungi are known to exhibit a narrow host spectrum (e.g. Takamatsu et al. 2006) and consequently do not benefit from increasing tree diversity. This expectation was confirmed by our results, as we encountered a reduction in fungal infestation of three fungi species with increasing tree diversity. Our findings complement the findings of a study of three powdery mildew species on Quercus and F. sylvatica in the BIOTREE experiment in Kaltenborn by Hantsch et al. (2013). The authors detected a decline in fungal infestation with increasing tree diversity in the local tree neighbourhood for the pathogen species Erysiphe alphitoides and E. hypophylla on oak as well as for Phyllactinia orbicularis on beech. While the current study was similarly based on the Shannon diversity of neighbouring trees, it comprised only six tree individuals in a small-scale experiment. In contrast, the Kaltenborn study investigated 252 tree individuals with several rows of neighbours and much larger plot sizes (i.e. 5760 m2 vs. 25 m2 in the Kreinitz experiment). As such, considering the small plot size and the inclusion of only the adjacent row of neighbours, the current study may have precluded the detection of local tree diversity effects on fungal species richness and infestation of particular fungal pathogen species of Q. petraea. Different fungal species might be affected by tree diversity at different scales, depending on their dispersal ability, spore size or their dependence on the microclimate provided by the local tree neighbourhood. The local microclimate can be affected by canopy structure and architecture of the local tree environment as well as on stand management (Jactel et al. 2009; Lombardero, Alonso-Rodríguez & Roca-Posada 2012; Calonnec et al. 2013; Costes et al. 2013). Hence, any extrapolation of our results to experiments at larger scales or natural forests should be taken with caution. Experimental tree stands and forests differ not only in scale but also in age of tree individuals. Several studies have demonstrated a lower susceptibility to fungal infestation in mature forests than in younger stands (see review by Pautasso, Holdenrieder & Stenlid 2005). One reason for differences in infestation rates on young and adult trees may be the level of constitutive or induced defence mechanisms based on secondary metabolites, which have been found to vary with age (Erbilgin & Colgan 2012). For example, infection success of Apiognomonia errabunda was shown to be dependent on endogenous levels of the constitutive concentrations of phenolic compounds (Bahnweg et al. 2008). In support of this interpretation, microbial phyllosphere communities have been described to change with differences in polyphenol concentration, depending on the extent of tree maturation (Metz et al. 2012; Peñuelas et al. 2012). This underscores the need for long-term experiments, and particularly for forest ecosystems.
The effects of local tree diversity on fungal infestation of the two tree species in our study also lend support to the results obtained from grassland experiments (Mitchell et al. 2003; Roscher et al. 2007) and, moreover, point to associational resistance supposedly caused by modified microclimatic conditions (Tahvanainen & Root 1972). Thus, our study is the first to demonstrate associational resistance to foliar fungal pathogens by tree species in experimental stands at the tree species level. However, we did not find a general associational susceptibility of the host trees to fungal pathogens, although one fungus in our study (Apiognomonia errabunda) may have benefitted from the presence of T. cordata, Q. petraea and Fagus sylvatica, as all three tree species share this pathogen (e.g. Sogonov et al. 2007). Such an associational susceptibility resulting from the presence of heterospecific neighbours has already been reported for increased fungal Armillaria root rot (Gerlach et al. 1997), as well as for increasing disease incidence of Phytophthora ramorum (Maloney et al. 2005). However, most of the literature on associational susceptibility by heterospecific neighbours focuses on herbivory damage, in particular when herbivore densities are high (e.g. White & Whitham 2000; Moravie, Borer & Bacher 2006; Barbosa et al. 2009), while associational resistance has been obtained by studies conducted on specialized herbivores, as described for Quercus robur (Jactel & Brockerhoff 2007; Castagneyrol et al. 2013). In addition to the different studies on herbivores, further studies on foliar fungal pathogens should address associational resistance in forest communities to complement our findings in these small-scale experimental stands.
Host Proportion Effects
Surprisingly, we did not find strong support for our second hypothesis, which stated that an increase in local host proportion of T. cordata and Q. petraea results in an increase in the degree of fungal infestation. However, significant interactions of host proportion with growth ‘year’ revealed that fungal infestation by the unknown ascomycete species increased with host proportion during the driest weather conditions in 2012 while it decreased during the wetter years of 2010 and 2011 (with the exception of ascomycete on T. cordata, which increased in 2011). As such, our results confirm the findings of Chanthorn et al. (2013), who also failed to detect significant effects of host density on the infection rate of a host-specific fungus on Cinnamomum subavenium trees. In addition, only weak host density effects were found for particular virus infections on wild pepper (Pagán et al. 2012). However, as several studies have demonstrated a decline in pathogen infestation or transmission with decreasing host availability (e.g. Bell, Freckleton & Lewis 2006; Mundt, Sackett & Wallace 2011; Cobb et al. 2012), the detected absence of host dilution effects was unexpected. This absence of main host proportion effects in combination with the presence of tree diversity effects on fungal pathogens of T. cordata and Q. petraea points to the existence of a mechanism that operates at a very small scale and does not include dilution effects of the host species (see hypothesis depicted in Fig.1a). Thus, it seems that local biodiversity does not affect the intensity of pathogen infestation through declining resource concentration but rather through modifying the pathogen's local environment. Hence, our results contradict the predictions of the ‘resource concentration hypothesis’, which proposes that specialized organisms are concentrated in habitats where their resource is most abundant (Root 1973; Burdon & Chilvers 1982). Furthermore, our study results regarding the effects of host proportions oppose the findings on density dependency of fungal pathogens presented in the literature. For instance, Mundt, Sackett & Wallace (2011) reported an increasingly epidemic spread of a rust fungus on wheat with increasing host frequency. In addition, for a neotropical tree species, Bell, Freckleton & Lewis (2006) showed a density-dependent higher seedling mortality in dense stands caused by plant pathogens.
Non-Host Neighbour Proportion Effects
In contrast to host species proportion, the proportion of particular non-host neighbouring tree species significantly affected fungal species richness as well as fungal infestation. Thus, our third hypothesis on the existence of density-dependent non-host neighbour identity effects was confirmed on the basis of our results. At the tree and the fungal species levels, an increase in the proportion of both coniferous tree species (Pinus sylvestris and Picea abies) reduced fungal infestation. Although it would be difficult to discern the operating mechanisms, microclimate modified by canopy structure and architecture may be considered the most plausible explanation (Calonnec et al. 2013; Costes et al. 2013). However, the role of microclimate in associational resistance has hitherto not been tested experimentally. As an alternative explanation, and one that has been demonstrated for herbivory (Jactel, Brockerhoff & Duelli 2005; Mauchline et al. 2005; Karban 2007; Barber & Marquis 2011; Casadebaig et al. 2012), different neighbourhoods may affect foliar fungi through changing chemical composition in the infected leaves or simply by shielding target trees from spores. However, based on our results, facilitation by the understorey plants that might serve as alternative hosts or shelter (Jactel et al. 2009) could be excluded as a possible explanation, since understorey vegetation was more or less absent in the experimental plots because of the dense canopy cover. Nevertheless, as different neighbourhoods were found to be either positive for the pathogen or positive for the host species in particular years, they represent a case of unspecific and inconsistent facilitation. Thus, a few non-host tree species effects (interactions) seem to be partially idiosyncratic and only operative in particular years and in particular non-host neighbour combinations. These findings confirm those observed for grassland diversity experiments, where the presence of non-host plant species facilitated the host species (Mitchell et al. 2002). In addition, such host species facilitation might also be shaped by crown architecture of the surrounding non-host neighbours, and in our study particularly by higher vertical structuring and canopy density, both of which may serve as dispersal barriers (Calonnec et al. 2013). This would moreover explain the detected negative effect of the two neighbouring conifer species on fungal infestation, as the conifers were taller than our target species. Similarly, the observed positive non-host neighbourhood effects with increasing proportion of the shorter F. sylvatica individuals increased fungal infestation at the tree as well as at the fungal species levels. Such positive identity effects may indicate tree species-specific fungal pathogen facilitation. Fagus sylvatica may exert such effects because of its lower growth rates, whereas the positive effects of proportion of F. excelsior on foliar pathogen infestation might have been caused by another fungus infection. In addition, the F. excelsior individuals were highly affected by ash dieback symptoms, resulting in early defoliation and increased crown thinning (Kowalski 2006). This may have affected the microclimatic conditions in our study (Bourke 1970) by increasing local air temperatures, which may in turn have increased fungal infestation (Calonnec et al. 2013).
Inter-Annual Variation
In general, we encountered a pronounced variation in fungal species richness and fungal infestation between years. This clearly demonstrates a species-specific dependence of fungal development and life cycle on local weather conditions (Tainter & Baker 1996; Jactel et al. 2009). The effect was found to be different in both target tree species. We detected higher fungal infestation in the colder and more humid year 2010 on T. cordata, whereas fungal infestation on Q. petraea was higher in the warmer and drier years of 2011 and 2012. Confirming our results on Q. petraea, fungal species richness and fungal community composition have been reported to increase in Quercus ilex under dry summer conditions (Peñuelas et al. 2012). Similarly, Lappalainen et al. (1999) found differences in endophyte composition and infestation on Betula species between two consecutive years, with increasing infestation by higher temperature and humidity. Nevertheless, our fourth hypothesis, indicating that the inter-annual variation in fungal species richness and fungal infestation decreased with increasing tree diversity, had to be rejected. As a general pattern, we detected a strong but species-specific dependence of fungal infestation on climatic conditions and tree diversity effects that were only present under certain climatic conditions. Thus, our results do not support the idea of a higher temporal stability in these tree plantations conferred by tree diversity. This, however, is in contrast to the results obtained from grassland ecosystems, where a reduced inter-annual variation with increasing plant species diversity has been reported (Tilman, Reich & Knops 2006; Eisenhauer et al. 2011). However, it should be noted that our results are based on three consecutive years, while a proper test for temporal stability may require a longer observation period.
Conclusions
The results obtained in the present experimental study provide strong evidence for tree diversity effects on fungal infestation on two common trees species at the local scale, with lower fungal infestation occurring in more diverse tree species communities. One of our main findings was that local tree diversity effects were not caused by simple host dilution effects, i.e. by decreasing host proportion, but by more complex interactions between local neighbouring trees. Moreover, we could show that particular non-host species in the vicinity of the target species may impede or facilitate fungal pathogen infestation, depending on the species identity as well as their proportion within the local neighbourhood. However, a direct transfer of the results from this small-scale experiment to the larger scale of planted or natural forests may not be applicable and should be implemented with caution. Nevertheless, as our results apply to younger tree plantations, they may provide valuable information for designing new forest plantations. Mixing tree species at the local neighbour scale has been demonstrated to reduce both pathogen transmission and infection rates. As such, subsequent studies in plantations with differing levels of tree species richness are recommended with a view to revealing whether such differences in fungal infestation translate into positive feedbacks on tree growth and survival.
Acknowledgments
We are grateful to M. Baudis, S. Berger, F. Berthold, D. Eichenberg, A. Hallensleben, M. Hofmann, I. Reichelt, C. Ristok and A. Zeuner for helping with the fieldwork. Thanks also to D. Eichenberg for helpful input to the manuscript. We would like to thank A. Austin, L. Sandhu and P. Thrall and two anonymous referees who very much improved the manuscript with their constructive comments. A Graduate Scholarship from Saxony-Anhalt and a grant from the Scholarship Programme of the German Federal Environmental Foundation (DBU) are highly acknowledged. The research leading to these results has also received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 265171, project FunDivEUROPE. H.B. also acknowledges the support of the German Centre for Integrative Biodiversity Research (iDiv), Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118). The Kreinitz experiment is a cooperative research initiative, and we thank the many people who have assisted with the establishment and maintenance of the experiment and who are too numerous to be listed. In particular, we acknowledge the Departments of Community Ecology, Soil Ecology, Soil Physics and Environmental Microbiology and the team of the Bad Lauchstädt field station of the Helmholtz Centre for Environmental Research – UFZ.
Supporting Information
Table S1Linear mixed effect model results at the tree species level. Effect of tree diversity among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S2. Linear mixed effects model results at the tree species level. Effect of tree species proportions among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S3. Linear mixed effect model results at the fungal species level. Effect of tree diversity among neighbouring trees on fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S4. Linear mixed effect model results at the fungal species level. Effect of tree species proportions among neighbouring trees on the fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
References
- Anderson KL, Sallam N, Congdon BC. The effect of host structure on the distribution and abundance of the island sugarcane planthopper, Eumetopina flaviceps Muir, vector of Ramu stund disease of sugarcane. Virus Research. 2009;141:247–257. doi: 10.1016/j.virusres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Bagchi R, Swinfield T, Gallery RE, Lewis OT, Gripenberg S, Narayan L, Freckleton RP. Testing the Janzen-Connell mechanism: pathogens cause overcompensating density dependence in a tropical tree. Ecology Letters. 2010;13:1262–1269. doi: 10.1111/j.1461-0248.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Bahnweg G, Heller W, Stich S, Knappe C, Betz G, Heerdt C, et al. Beech leaf colonization by the endophyte Apiognomonia errabunda dramatically depends on light exposure and climatic conditions. Plant Biology. 2008;7:659–669. doi: 10.1055/s-2005-872943. [DOI] [PubMed] [Google Scholar]
- Barber NA, Marquis RJ. Light environment and the impacts of foliage quality on herbivorous insect attack and bird predation. Oecologia. 2011;166:401–409. doi: 10.1007/s00442-010-1840-9. [DOI] [PubMed] [Google Scholar]
- Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: having right or wrong neighbors. Annual Review of Ecology, Evolution and Systematic. 2009;40:1–20. [Google Scholar]
- Beizhou S, Jie Z, Wiggins NL, Yuncong Y, Guanbo T, Xusheng S. Intercropping with aromatic plants decreases herbivore abundance, species richness, and shifts arthropod community trophic structure. Environmental Entomology. 2012;41:872–879. [Google Scholar]
- Bell T, Freckleton RP, Lewis OT. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecology Letters. 2006;9:569–574. doi: 10.1111/j.1461-0248.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Benítez M-S, Hersh MH, Vilgalys R, Clark JS. Pathogen regulation of plant diversity via effective specialization. Trends in Ecology & Evolution. 2013;28:705–711. doi: 10.1016/j.tree.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Biere A, Honders SC. Anther smut transmission in Silene latifolia and Silene dioica: impact of host traits, disease frequency, and host density. International Journal of Plant Science. 1998;159:228–235. [Google Scholar]
- Bock CH, Graham JH, Gottwald TR, Cook AZ, Parker PE. Wind speed effects on the quantity of Xanthomonas citri subsp. citri dispersed downwind from canopies of grapefruit trees infected with citrus canker. Plant Disease. 2010;94:725–736. doi: 10.1094/PDIS-94-6-0725. [DOI] [PubMed] [Google Scholar]
- Bolker BM. Analytic models for the patchy spread of plant disease. Bulletin of Mathematical Biology. 1999;61:849–874. doi: 10.1006/bulm.1999.0115. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Beauchamp G, Leighton PA, Lindsay R, Bélanger D, Ogden NH. Does high biodiversity reduce the risk of Lyme disease invasion? Parasites & Vectors. 2013;6:195. doi: 10.1186/1756-3305-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke PMA. Use of weather information in the prediction of plant disease epiphytotics. Annual Review of Phytopathology. 1970;8:345–370. [Google Scholar]
- Bradley DJ, Gilbert GS, Martiny JBH. Pathogens promote plant diversity through a compensatory response. Ecology Letters. 2008;11:461–469. doi: 10.1111/j.1461-0248.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Brandenburger W. Parasitische Pilze an Gefäßpflanzen in Europa. Stuttgart, New York: Gustav Fischer Verlag; 1985. [Google Scholar]
- Braun U, Cook RTA. Taxonomic Manual of the Erysiphales (Powdery Mildews) CBS Biodiversity Series. 2012;11:1–707. [Google Scholar]
- Bucheli E, Shykoff JA. The influence of plant spacing on density-dependent versus frequency-dependent spore transmission of the anther smut Microbotryum violaceum. Oecologia. 1999;119:55–62. doi: 10.1007/s004420050760. [DOI] [PubMed] [Google Scholar]
- Burdon JJ, Chilvers GA. Host density as a factor in plant disease ecology. Annual Review of Phytopathology. 1982;20:143–166. [Google Scholar]
- Cadotte MW, Dinnage R, Tilman D. Phylogenetic diversity promotes ecosystem stability. Ecology. 2012;93:223–233. [Google Scholar]
- Calonnec A, Burie J-B, Langlais M, Guyader S, Saint-Jean S, Sache I, Tivoli B. Impacts of plant growth and architecture on pathogen processes and their consequences for epidemic behaviour. European Journal of Plant Pathology. 2013;135:479–497. [Google Scholar]
- Casadebaig P, Quesnel G, Langlais M, Faivre R. A generic model to simulate air-borne diseases as a function of crop architecture. PLoS ONE. 2012;7:e49406. doi: 10.1371/journal.pone.0049406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagneyrol B, Jactel H. Unraveling plant–animal diversity relationships: a meta-regression analysis. Ecology. 2012;93:2115–2124. doi: 10.1890/11-1300.1. [DOI] [PubMed] [Google Scholar]
- Castagneyrol B, Giffard B, Péré C, Jactel H. Plant apparency, an overlooked driver of associational resistance to insect herbivory. Journal of Ecology. 2013;101:418–429. [Google Scholar]
- Castagneyrol B, Jactel H, Vacher C, Brockerhoff EG, Koricheva J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. Journal of Applied Ecology. 2014;51:134–141. [Google Scholar]
- Chanthorn W, Caughlin T, Dechkla S, Brockelman WY. The relative importance of fungal infection, consepcific density and heterogeneity for seedling survival in a dominant tropical tree. Biotropica. 2013;45:587–593. [Google Scholar]
- Chapin FS, III, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- Cobb RC, Filipe JAN, Meentemeyer RK, Gilligan CA, Rizzo DM. Ecosystem transformation by emerging infectious disease: loss of large tanoak from California forests. Journal of Ecology. 2012;100:712–722. [Google Scholar]
- Costes E, Lauri PE, Simon S, Andrieu B. Plant architecture, its diversity and manipulation in agronomic conditions, in relation with pest and pathogen attacks. European Journal of Plant Pathology. 2013;135:455–470. [Google Scholar]
- De Deyn GB, Raaijmakers CE, van Ruijven J, Berendse F, van der Putten WH. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos. 2004;106:576–586. [Google Scholar]
- Desprez-Loustau M-L, Vitasse Y, Delzon S, Capdevielle X, Marçais B, Kremers A. Are plant pathogen populations adapted for encounter with their host? A case study of phenological synchrony between oak and an obligate fungal parasite along an altitudinal gradient. Journal of Evolutionary Biology. 2010;23:87–97. doi: 10.1111/j.1420-9101.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thébault E, Loreau M. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecology Letters. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Eisenhauer N, Milcu A, Allan E, Nitschke N, Scherber C, Temperton V, Weigelt A, Weisser WW, Scheu S. Impact of above- and below-ground invertebrates on temporal and spatial stability of grassland of different diversity. Journal of Ecology. 2011;99:572–582. [Google Scholar]
- Ellis MB, Ellis JP. Microfungi on Land Plants: An Identification Handbook. 2nd edn. Michigan: Richmond Pub., University of Michigan; 1997. [Google Scholar]
- Erbilgin N, Colgan LJ. Differential effects of plant ontogeny and damage type on phloem and foliage monoterpenes in jack pine (Pinus banksiana. Tree Physiology. 2012;32:946–957. doi: 10.1093/treephys/tps047. [DOI] [PubMed] [Google Scholar]
- García-Guzmán G, Dirzo R. Influence of plant abundance on disease incidence in a Mexican tropical forest. Ecoscience. 2006;13:523–530. [Google Scholar]
- Gerlach JP, Reich PB, Puettman K, Baker T. Species, diversity, and density affects tree seedling mortality from Armillaria root rot. Canadian Journal of Forest Research. 1997;27:1509–1512. [Google Scholar]
- Gundale MJ, Hyodo F, Nilsson M-C, Wardle DA. Nitrogen niches revealed through species and functional group removal in a boreal shrub community. Ecology. 2012;93:1695–1706. doi: 10.1890/11-1877.1. [DOI] [PubMed] [Google Scholar]
- Gutknecht JLM, Field CB, Balser TC. Microbial communities and their responses to simulated global change fluctuate greatly over multiple years. Global Change Biology. 2012;18:2256–2269. [Google Scholar]
- Haas SE, Hooten MB, Rizzo DM, Meentemeyer RK. Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecology Letters. 2011;14:1108–1116. doi: 10.1111/j.1461-0248.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- Hajji M, Dreyer E, Marçais B. Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. European Journal of Plant Pathology. 2009;125:63–72. [Google Scholar]
- Hantsch L, Braun U, Scherer-Lorenzen M, Bruelheide H. Species richness and species identity effects on occurrence of foliar fungal pathogens in a tree diversity experiment. Ecosphere. 2013;4:81. [Google Scholar]
- Hantsch L, Braun U, Haase J, Purschke O, Scherer-Lorenzen M, Bruelheide H. No plant functional diversity effects on foliar fungal pathogens in experimental tree communities. Fungal Diversity. 2014;66:139–151. [Google Scholar]
- Hendriks M, Mommer L, de Caluwe H, Smit-Tiekstra AE, Van der Putten WH, De Kroon H. Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. Journal of Ecology. 2013;101:287–297. [Google Scholar]
- Jactel H, Brockerhoff EG. Tree diversity reduces herbivory by forest insects. Ecology Letters. 2007;10:835–848. doi: 10.1111/j.1461-0248.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- Jactel H, Brockerhoff EG. A test of the biodiversity-stability theory: meta-analysis of tree species diversity eVects on insect pest infestations, and re-examination of responsible factors. In: Schulze E-D, Duelli P, Scherer-Lorenzen M, Körner C, editors. Forest Diversity and Function. Temperate and Boreal Systems. Berlin: Springer; 2005. Ecological studies176 235–262. [Google Scholar]
- Jactel H, Nicoll BC, Branco M, Gonzalez-Olabarria JR, Grodzki W, Långström B, et al. The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science. 2009;66:701. [Google Scholar]
- Jarosz AM, Burdon JJ. Host-pathogen interactions in natural populations of Linum marginale and Melampsora lini. III Influence of pathogen epidemics on host survivorship and flower production. Oecologia. 1992;89:53–61. doi: 10.1007/BF00319015. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, Henderson JS, Paull SH, Richgels KLD, Redmond MD. Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology. 2012;93:56–64. doi: 10.1890/11-0636.1. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. PNAS. 2013;110:16916–16921. doi: 10.1073/pnas.1310557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, O'Dwyer K, Nakagawa S, Poulin R. What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biological Reviews. 2014;89:123–134. doi: 10.1111/brv.12046. [DOI] [PubMed] [Google Scholar]
- Karban R. Associational resistance for mule's ears with sagebrush neighbors. Plant Ecology. 2007;191:295–303. [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecology Letters. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell D, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;486:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, Ritchie ME, Howe KM, Reich PB, Siemann E, Groth J. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecology Letters. 1999;2:286–293. doi: 10.1046/j.1461-0248.1999.00083.x. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Vehvilainen H, Riihimaki J, Ruohomaki K, Kaitaniemi P, Ranta H. Diversification of tree stands as a means to manage pests and diseases in boreal forests: myth or reality? Canadian Journal of Forest Research. 2006;36:324–336. [Google Scholar]
- Kowalski T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. Forest Pathology. 2006;36:264–270. [Google Scholar]
- Laneri K, Bhadra A, Ionides EL, Bouma M, Dhiman RC, Rajpal SY, Pascual M. Forcing versus feedback: epidemic malaria and monsoon rains in northwest India. PLoS Computational Biology. 2010;6:e1000898. doi: 10.1371/journal.pcbi.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen JH, Koricheva J, Helander ML, Haukioja E. Densities of endophytic fungi and performance of leafminers (Lepidoptera: Eriocraniidae) on birch along a pollution gradient. Environmental Pollution. 1999;104:99–105. [Google Scholar]
- Latz E, Eisenhauer N, Rall BC, Allen E, Roscher C, Scheu S, Jousset A. Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities. Journal of Ecology. 2012;100:597–604. [Google Scholar]
- Lau JA, Strengbom J, Stone LR, Reich PB, Tiffin P. Direct and indirect effects of CO2, nitrogen, and community diversity on plant-enemy interactions. Ecology. 2008;89:226–236. doi: 10.1890/07-0423.1. [DOI] [PubMed] [Google Scholar]
- Lombardero MJ, Alonso-Rodríguez M, Roca-Posada EP. Tree insects and pathogens display opposite tendencies to attack native vs. non-native pines. Forest Ecology and Management. 2012;281:121–129. [Google Scholar]
- Makowski D, Bancal R, Vicent A. Estimation of leaf wetness duration requirements of foliar fungal pathogens with uncertain data – an application to Mycosphaerella nawae. Phytopathology. 2011;101:1346–1354. doi: 10.1094/PHYTO-01-11-0024. [DOI] [PubMed] [Google Scholar]
- Maloney PE, Lynch SC, Kane SF, Jensen CE, Rizzo DM. Establishment of an emerging generalist pathogen in redwood forest communities. Journal of Ecology. 2005;93:899–905. [Google Scholar]
- Maron JL, Marler M, Klironomos JN, Cleveland CC. Soil fungal pathogens and the relationship between plant diversity and productivity. Ecology Letters. 2011;14:36–41. doi: 10.1111/j.1461-0248.2010.01547.x. [DOI] [PubMed] [Google Scholar]
- Mauchline ML, Osborne JL, Martin AP, Poppy GM, Powell W. The effects of non-host plant essential oil volatiles on the behaviour of the pollen beetle Meligethes aeneus. Entomologia Experimentalis et Applicata. 2005;114:181–188. [Google Scholar]
- McCann KS. The diversity-stability debate. Nature. 2000;405:228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- Metz MR, Frangioso KM, Wickland AC, Meentemeyer RK, Rizzo DM. An emergent disease causes directional changes in forest species composition in coastal California. Ecosphere. 2012;3:86. [Google Scholar]
- Mitchell CE, Mitchell CA, Tilman D, Groth JV. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology. 2002;83:1713–1726. [Google Scholar]
- Mitchell CE, Reich PB, Tilman D, Groth JV. Effects of elevated CO2, nitrogen deposition, and decreased species diversity in foliar fungal plant disease. Global Change Biology. 2003;9:438–451. [Google Scholar]
- Moravie M-A, Borer M, Bacher S. Neighbourhood of host plants influences oviposition decisions of a stem-boring weevil. Basic and Applied Ecology. 2006;7:545–554. [Google Scholar]
- Mordecai EA. Pathogen impacts on plant communities: unifying theory, concepts, and empirical work. Ecological Monographs. 2011;81:429–441. [Google Scholar]
- Mordecai EA. Consequences of pathogen spillover for cheatgrass-invaded grasslands: coexistence, competitive exclusion, or priority effects. The American Naturalist. 2013;181:737–747. doi: 10.1086/670190. [DOI] [PubMed] [Google Scholar]
- Mundt CC, Sackett KE, Wallace LD. Landscape heterogeneity and disease spread: experimental approaches with a plant pathogen. Ecological Applications. 2011;21:321–328. doi: 10.1890/10-1004.1. [DOI] [PubMed] [Google Scholar]
- Nadrowski K, Wirth C, Scherer-Lorenzen M. Is forest diversity driving ecosystem function and service? Current Opinion in Environmental Sustainability. 2010;2:75–79. [Google Scholar]
- Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM. Declining biodiversity can alter the performance of ecosystems. Nature. 1994;368:734–737. [Google Scholar]
- Oakley CA, Knox JS. Plant species richness increases resistance to invasion by non-resident plant species during grassland restoration. Applied Vegetation Science. 2013;16:21–28. [Google Scholar]
- Otway SJ, Hector A, Lawton JH. Resource dilution effects on specialist insect herbivores in a grassland biodiversity experiment. Journal of Animal Ecology. 2005;74:234–240. [Google Scholar]
- Pagán I, González-Jara P, Moreno-Letelier A, Rodelo-Urrego M, Fraile A, Piñero D, García-Arenal F. Effect of biodiversity changes in disease risk: exploring disease emergence in a plant-virus system. PLoS Pathogen. 2012;8:e1002796. doi: 10.1371/journal.ppat.1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautasso M, Holdenrieder O, Schulze E-D, Stenlid J. Susceptibility to fungal pathogens of forests differing in tree diversity. In: Scherer-Lorenzen M, Körner C, editors. Forest Diversity and Function: Temperate and Boreal Systems. Heidelberg, New York: Springer Berlin; 2005. pp. 263–289. Ecological Studies 176. [Google Scholar]
- Peñuelas J, Rico L, Ogaya R, Jump AS, Terradas J. Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biology. 2012;14:565–575. doi: 10.1111/j.1438-8677.2011.00532.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J. 2013. Linear and Nonlinear Mixed Effects Models . Available at: http://lme4.r-forge.r-project.org/slides/2011-01-11-Madison/6NLMMH.pdf . Last accessed 17 July 2014.
- Potvin C, Dutilleul P. Neighborhood effects and size-asymmetric competition in a tree plantation varying in diversity. Ecology. 2009;90:321–327. doi: 10.1890/08-0353.1. [DOI] [PubMed] [Google Scholar]
- Prell H. Interaktionen von Pflanzen und phytopathogenen Pilzen. Jena, Stuttgart: Gutav Fischer Verlag; 1996. [Google Scholar]
- Proulx R, Wirth C, Voigt W, Weigelt A, Roscher C, Attinger S, et al. Diversity promotes temporal stability across levels of ecosystem organization in experimental grasslands. PLoS ONE. 2010;5:e13382. doi: 10.1371/journal.pone.0013382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2013. Vienna, Austria R Foundation for Statistical Computing URL http://www.R-project.org/
- Root RB. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of Collards (Brassica oleracea. Ecological Monographs. 1973;43:95–124. [Google Scholar]
- Roscher C, Schumacher J, Foitzik O, Schulze ED. Resistance to rust fungi in Lolium perenne depends on within-species variation and performance of the host species in grasslands of different plant diversity. Oecologia. 2007;153:173–183. doi: 10.1007/s00442-007-0713-3. [DOI] [PubMed] [Google Scholar]
- Roslin T, Laine A-L, Gripenberg S. Spatial population structure in an obligate plant pathogen colonizing oak Quercus robur. Functional Ecology. 2007;21:1168–1177. [Google Scholar]
- Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- Schnitzer SA, Klironomos JN, HilleRisLambers J, Kinkel LL, Reich PB, Xiao K, Rillig MC, Sikes BA, Callaway RM, Mangan SA, van Nes EH, Scheffer M. Soil microbes drive the classic plant diversity–productivity pattern. Ecology. 2011;92:296–303. doi: 10.1890/10-0773.1. [DOI] [PubMed] [Google Scholar]
- Schuldt A, Baruffol M, Boehnke M, Bruelheide H, Härdtle W, Lang AC, Nadrowski K, von Oheimb G, Voigt W, Zhou H, Assmann T. Tree diversity promotes insect herbivory in subtropical forests of south-east China. Journal of Ecology. 2010;98:917–926. doi: 10.1111/j.1365-2745.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A, Both S, Bruelheide H, Härdtle W, Schmid B, Zhou H, Assmann T. Predator diversity and abundance provide little support for the enemies hypothesis in forests of high tree diversity. PLoS ONE. 2011;6:8. doi: 10.1371/journal.pone.0022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A, Bruelheide H, Durka W, Eichenberg D, Fischer M, Kröber W, et al. Plant traits affecting herbivory on tree recruits in highly diverse subtropical forests. Ecology Letters. 2012;15:732–739. doi: 10.1111/j.1461-0248.2012.01792.x. [DOI] [PubMed] [Google Scholar]
- Seiwa K, Miwa Y, Sahashi N, Kanno H, Tomita M, Ueno N, Yamazaki M. Pathogen attack and spatial patterns of juvenile mortality and growth in a temperate tree, Prunus grayana. Canadian Journal of Forest Research. 2008;38:2445–2454. [Google Scholar]
- Sobek S, Scherber C, Steffan-Dewenter I, Tscharntke T. Sapling herbivory, invertebrate herbivores and predators across a natural tree diversity gradient in Germany's largest connected deciduous forest. Oecologia. 2009;160:279–288. doi: 10.1007/s00442-009-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, White JF. The type species of Apiognomonia A. veneta, with its Discula anamorph is distinct from A. errabunda. Mycological Research. 2007;3:693–709. doi: 10.1016/j.mycres.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Tack AJM, Gripenberg S, Roslin T. Cross-kingdom interactions matter: fungal-mediated interactions structure an insect community on oak. Ecology Letters. 2012;15:177–185. doi: 10.1111/j.1461-0248.2011.01724.x. [DOI] [PubMed] [Google Scholar]
- Tahvanainen JO, Root RB. The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferaea (Coleoptera: Chrysomelidae) Oecologia. 1972;10:321–346. doi: 10.1007/BF00345736. [DOI] [PubMed] [Google Scholar]
- Tainter FH, Baker FA. Principles of Forest Pathology. New York: John Wiley & Sons; 1996. [Google Scholar]
- Takamatsu S, Bolay A, Limkaisang S, Kom-un S, To-anun C. Identity of a powdery mildew fungus occurring on Paeonia and its relationship with Erysiphe hypophylla on oak. Mycoscience. 2006;47:367–373. [Google Scholar]
- Thomas FM, Blank R, Hartmann G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology. 2002;32:277–307. [Google Scholar]
- Tilman D. Biodiversity: population versus ecosystem stability. Ecology. 1996;77:350–363. [Google Scholar]
- Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- Unsicker SB, Baer N, Kahmen A, Wagner M, Buchmann N, Weisser WW. Invertebrate herbivory along a gradient of plant species diversity in extensively managed grasslands. Oecologia. 2006;150:233–246. doi: 10.1007/s00442-006-0511-3. [DOI] [PubMed] [Google Scholar]
- Vacher C, Vile D, Helion E, Piou D, Desprez-Loustau M-L. Distribution of parasitic fungal species richness: influence of climate versus host species diversity. Diversity and Distributions. 2008;14:786–798. [Google Scholar]
- Warren RJ, Mordecai E. Soil moisture mediated interaction between Polygonatum biflorum and leaf spot disease. Plant Ecology. 2010;209:1–9. [Google Scholar]
- White JA, Whitham TG. Associational susceptibility of cottonwood to a box elder herbivore. Ecology. 2000;81:1795–1803. [Google Scholar]
- Yamazaki M, Iwamoto S, Seiwa K. Distance- and density-dependent seedling mortality caused by several diseases in eight tree species co-occurring in a temperate forest. Plant Ecology. 2009;201:181–196. [Google Scholar]
- Zhu Y-Y, Fang H, Wang YY, Fan JX, Yang SS, Mew TW, Mundt CC. Panicle blast and canopy moisture in rice cultivar mixtures. Phytopathology. 2005;95:433–438. doi: 10.1094/PHYTO-95-0433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1Linear mixed effect model results at the tree species level. Effect of tree diversity among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S2. Linear mixed effects model results at the tree species level. Effect of tree species proportions among neighbouring trees on fungal species richness and fungal infestation for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S3. Linear mixed effect model results at the fungal species level. Effect of tree diversity among neighbouring trees on fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.
Table S4. Linear mixed effect model results at the fungal species level. Effect of tree species proportions among neighbouring trees on the fungal infestation of host-specific fungal species for the tree species Tilia cordata and Quercus petraea across all tree individuals of each tree species (n = 282) within the years 2010–2012.


