Abstract
The Wisteria floribunda agglutinin-positive human Mac-2-binding protein (WFA+-M2BP) was recently shown to be a liver fibrosis glycobiomarker with a unique fibrosis-related glycoalteration. We evaluated the ability of WFA+-M2BP to predict the development of hepatocellular carcinoma (HCC) in patients who were infected with the hepatitis C virus (HCV). A total of 707 patients who had been admitted to our hospital with chronic HCV infection without other potential risk factors were evaluated to determine the ability of WFA+-M2BP to predict the development of HCC; factors evaluated included age, sex, viral load, genotypes, fibrosis stage, aspartate and alanine aminotransferase levels, bilirubin, albumin, platelet count, alpha-fetoprotein (AFP), WFA+-M2BP, and the response to interferon (IFN) therapy. Serum WFA+-M2BP levels were significantly increased according to the progression of liver fibrosis stage (P < 0.001). In each distinctive stage of fibrosis (F0-F1, F2, F3, and F4), the risk of development of HCC was increased according to the elevation of WFA+-M2BP. Multivariate analysis identified age >57 years, F4, AFP >20 ng/mL, WFA+-M2BP ≥4, and WFA+-M2BP 1-4 as well as the response to IFN (no therapy vs. sustained virological response) as independent risk factors for the development of HCC. The time-dependent areas under the receiver operating characteristic curve demonstrated that the WFA+-M2BP assay predicted the development of HCC with higher diagnostic accuracy than AFP. Conclusion: WFA+-M2BP can be applied as a useful surrogate marker for the risk of HCC development, in addition to liver biopsy. (Hepatology 2014;60:1563–1570)
The annual incidence of hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-related cirrhosis ranges from 1% to 7%.1,2 Therefore, reliable methods for the early identification of liver fibrosis progression and compensated liver cirrhosis are an essential part of an efficient surveillance program for the detection of HCC.3
Until recently, liver biopsy was considered the gold standard for assessing the severity of liver fibrosis and cirrhosis.4,5 Although liver biopsy is generally accepted to be a safe procedure, it can cause discomfort and carries a small risk of life-threatening complications.6,7 Recently, an assay for Wisteria floribunda agglutinin-positive human Mac-2-binding protein (WFA+-M2BP) was reported as a novel, noninvasive, and rapid bedside method to assess liver fibrosis.8 M2BP has been shown to have multibranching and sialylated N-glycans. WFA is considered to recognize the GalNAc residue of N-glycans and O-glycans or the clustered LacNAc (Gal-GlcNAc) structure. Currently, we are analyzing the glycan structures of WFA+-M2BP in detail using mass spectrometry–based technology.9 Glycans can reflect the differentiation stage of cells, but not necessarily the level of cellular damage, and therefore they can be very effective markers for chronic disease. In the case of hepatitis, glycans are considered to reflect the progression of fibrosis more specifically than viral load. Several reports have identified M2BP as a potential marker of fibrosis progression in proteome study.10–13 Kuno et al. were the first to report that a rapid, simple glycan-based immunoassay for WFA+-M2BP can quantify fibrosis.8,14
On the other hand, we reported that alpha-fetoprotein (AFP) is a noninvasive predictive marker for the development of HCC in patients infected with HCV, which can be used to complement the information of fibrosis stage.15
In this report, we evaluated the utility of WFA+-M2BP to predict the development of HCC in patients who were infected with HCV.
Patients and Methods
Patients
Between January 1992 and December 2003, 832 patients were determined to be positive for both anti-HCV by a second- or third-generation enzyme-linked immunoadsorbent assay and HCV RNA by polymerase chain reaction (PCR). They underwent liver biopsy guided by ultrasonography at the National Hospital Organization, Nagasaki Medical Center (Ōmura, Japan). Among them, 125 (15.0%) patients were excluded from enrollment in this retrospective analysis for the following reasons: (1) positivity for hepatitis B surface antigen (n = 12); (2) a heavy habitual drinking habit defined by an average daily consumption of >100 g of ethanol (n = 26); (3) autoimmune hepatitis (AIH), primary biliary cirrhosis, or idiopathic portal hypertension (n = 8); (4) positive antinuclear antibody (Ab; defined as titer >320×) without the diagnosis of AIH (n = 8); or (5) a short follow-up period <180 days (n = 71). The remaining 707 patients were analyzed retrospectively for the incidence of HCC.
For all patients in our cohort, a blood sample was taken on the day of the liver biopsy at our hospital. All samples were preceded to separate serum and stored at −20˚C. At the time of blood withdrawal, all patients underwent liver biopsy. Their medical histories had been recorded, along with the results of routine tests for blood cell counts, liver biochemical parameters, and markers for HCV infection at the time of ultrasound (US)-guided liver biopsy and at regular intervals thereafter. Complete blood cell counts and biochemical tests were performed using automated procedures in the clinical pathological laboratories of our hospital.
Staging of Hepatic Fibrosis
Liver biopsies were taken by fine-needle aspiration (16G or 18G sonopsy) guided by US. Liver tissue specimens were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. They were evaluated for the stage of hepatic fibrosis by a pathologist according to the criteria of Desmet et al.16
Measurement of WFA+-M2BP
WFA+-M2BP quantification was measured based on a lectin-Ab sandwich immunoassay using the fully automatic immunoanalyzer, HISCL-2000i (Sysmex Co., Hyogo, Japan).8 The measured values of WFA+-M2BP conjugated to WFA were indexed with the obtained values using the following equation:
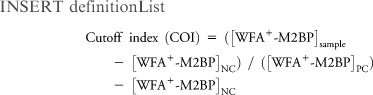 |
where [WFA+-M2BP]sample is the WFA+-M2BP count of serum sample, PC is positive control, and NC is negative control. The positive control was supplied as a calibration solution preliminarily standardized to yield a COI value of 1.0.14
HCV RNA, HCV Core Antigen, and HCV Genotypes
HCV RNA was determined by reverse-transcriptase (RT)-PCR using a commercial kit (Amplicor HCV; Roche Diagnostic Systems, Basel, Switzerland). HCV core antigen was determined using the Lumispot Eiken HCV antigen assay (Eiken Chemicals, Tokyo, Japan). HCV core antigen levels were classified into low and high with a cutoff at 1,000 fmol/mL.17 Genotypes of HCV were determined by RT-PCR with genotype-specific primers (HCV RNA core genotype; Roche Diagnostics, Tokyo, Japan).18
Interferon Therapy
During the observation period, 373 of the 707 (52.8%) patients received interferon (IFN) monotherapy, pegylated (Peg)-IFN monotherapy, or combination therapy with IFN plus ribavirin (RBV) or Peg-IFN plus RBV. Sustained virological response (SVR) was defined as the absence of detectable HCV RNA at the end of 6 months or more of treatment, whereas patients who failed to meet these criteria were judged as having non-SVR. There was no relapse of viremia after 6 months among the SVR patients.
Diagnosis of HCC
Patients were followed up by hematological and biochemical tests at an interval of 1-12 months. Diagnostic imaging by US, computed tomography (CT), and magnetic resonance imaging (MRI) were performed in most patients. HCC was diagnosed by typical vascular patterns on CT, MRI, and angiography or by fine-needle biopsy of space-occupying lesions detected in the liver.
Ethical Considerations
Informed consent was obtained from each patient included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the a priori approval by the institution's human research committee.
Statistical Analysis
Continuous variables (platelet counts, albumin, total bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], AFP, HCV core antigen, and WFA+-M2BP) were dichotomized with respect to the median value or clinically meaningful values in the multivariate analysis. To estimate the cumulative risk of developing HCC, Kaplan-Meier's method and the log-rank test were used. Cox's proportional hazards regression analysis was performed to evaluate risk factors for HCC. Regression analysis was performed to calculate Spearman's rank-correlation coefficient. Kruskal-Wallis' analysis of variance (ANOVA), followed by the Games-Howel's posthoc test, was used to assess whether there were any significant differences in terms of fibrosis stages (F0-F1, F2, F3, and F4). The diagnostic performances of WFA+-M2BP and AFP for censored development of HCC were assessed by using time-dependent receiver operating characteristic (ROC) curves by examining the area under the ROC (AUROC).19 Inclusion of variables was assessed using a step-wise selection method. Cochran-Armitage's test for trend was used in the categorical data analysis to assess for the presence of an association between a variable with two categories and a variable with more than three categories. A P value of 0.05 was considered statistically significant. Data analysis was performed with SPSS statistical software (version 22.0; (SPSS, Inc., Chicago, IL) and JMP 10 (SAS Institute Inc., Cary, NC).
Results
Characteristics at Enrollment
The baseline characteristics of the 707 patients at enrollment are summarized in Table1. Median age was 57.0 years; 120 (17.0%) patients were diagnosed histologically with liver cirrhosis (fibrosis stage F4) and the remaining 587 had chronic hepatitis (fibrosis stage F0, F1, F2, or F3). The median value of AFP was 6 ng/mL. The median value of WFA+-M2BP was 1.9 (range, 0.2-19.2). The average follow-up period was 8.2 years.
Table 1.
Demographic, Clinical, and Virological Characteristics of the 707 Patients Persistently Infected With HCV
| Age, years | 57.0 (19-79) |
| Male, N (%) | 351 (49.6) |
| Observation period, years | 8.2 ± 4.4* |
| IFN therapy | 373 (52.8%) |
| Habitual alcohol intake | 135 (19.1%) |
| Pathological findings | |
| Fibrosis (N) 0-1/2/3/4 | 274/193/120/120 |
| Activity (N) 0-1/2/3 | 199/365/143 |
| Platelet count, ×104/mm3 | 15.6 (3.0-39.1) |
| Albumin, g/dL | 4.2 (2.7-5.3) |
| Bilirubin, mg/dL | 0.7 (0.1-2.5) |
| AST, IU/mL | 53 (11-422) |
| ALT, IU/mL | 82 (1-1,057) |
| AFP, ng/mL | 6 (0.7-510) |
| HCV core antigen ≥1,000 fmol/L (%) | 539 (76.2) |
| HCV genotype, N (%) 1b | 510 (72.1) |
| 2a/2b | 195 (27.6) |
| Unknown | 2 (0.3) |
| WFA+-M2BP | 1.9 (0.2-19.2) |
Values are the medians with ranges in parentheses.
Results are expressed as the mean ± standard deviation.
WFA+-M2BP Value and Fibrosis Stage
The average values (mean ± 1 standard error) for each fibrosis stage were 1.3 ± 0.1 in F0-F1 (n = 274), 2.2 ± 0.1 in F2 (n = 193), 3.3 ± 0.2 in F3 (n = 120), and 5.2 ± 0.3 in F4 (n = 120). The degree of fibrosis was positively correlated with the median value of WFA+-M2BP (P < 0.001) by a nonparametric method (Kruskal-Wallis' one-way ANOVA). Games-Howel's test confirmed that the WFA+-M2BP value increased significantly with increasing stage of liver fibrosis: P < 0.0001 (F0-F1, compared with F2, F3, and F4); P < 0.0001 (F2, compared with F3 and F4); and P < 0.0001 (F3, compared with F4).
We estimated the diagnostic accuracy of WFA+-M2BP for detecting stage F3-F4 disease. The AUROC in the prediction of ≥F3 was 0.815 (range, 0.782-0.842). The desired specificity level of 95% was achieved for a 4.0 threshold, and the sensitivity was 40.0%.
We analyzed the proportions of the patients with different WFA+-M2BP levels stratified by the fibrosis stage (Fig. 1). The proportion of patients with F1 was 125 cases (80.7%) in WFA+-M2BP <1 (n = 155), 101 cases (41.7%) in WFA+-M2BP ≤1 and <2 (n = 242), 42 cases (21.9%) in WFA+-M2BP ≤2 and <4 (n = 192), 6 cases (7.0%) in WFA+-M2BP ≤4 and <8 (n = 86), and 0 cases (0.0%) in WFA+-M2BP ≥8 (n = 32). The proportion of patients with F1 was diminished across increasing quintiles of WFA+-M2BP level (P < 0.0001; Cochran-Armitage's trend test). Conversely, the proportion of patients with F4 was 2 cases (1.3%) in WFA+-M2BP <1 (n = 155), 18 cases (7.4%) in WFA+-M2BP ≤1, and <2 (n = 242), 37 cases (19.3%) in WFA+-M2BP ≤2 and <4 (n = 192), 38 cases (44.2%) in WFA+-M2BP ≤4 and <8 (n = 86), and 25 cases (78.1%) in WFA+-M2BP ≥8 (n = 32). The proportion of patients with F4 was increased with increasing quintiles of WFA+-M2BP level (P < 0.0001; Cochran-Armitage's trend test).
Figure 1.
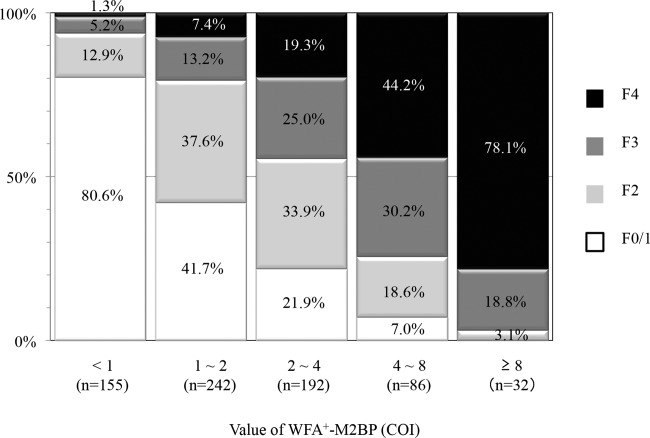
Proportions of patients with different WFA+-M2BP levels stratified by the fibrosis stage. The proportion of patients with F1 was diminished across increasing quintiles of WFA+-M2BP level (P < 0.0001; Cochran-Armitage's trend test), whereas that with F4 was increased (P < 0.0001; Cochran-Armitage's trend test).
Relationship Between the WFA+-M2BP Value and Baseline Biochemical Markers
To determine whether the WFA+-M2BP value was associated with fibrosis stage, age, gender, platelet count, albumin, bilirubin, AST, ALT, AFP, HCV core antigen, HCV genotype, or histological grading, a step-wise multiple linear regression analysis was performed. Our results showed that independent variables, except for ALT, genotype, and histological grading, remained in the final equation (Table2), suggesting that fibrosis stage was most closely associated with serum WFA+-M2BP value (coefficient β, 0.258; P < 0.001).
Table 2.
Step-wise Multiple Linear Regression Model to Identify Significant Independent Factors Affecting Serum WFA+-M2BP Level
| Final Fitted Model | Adjusted R2 | Standardized Coefficient β | P Value |
|---|---|---|---|
| Fibrosis stage | 0.258 | <0.001 | |
| AFP | 0.187 | <0.001 | |
| Albumin | −0.202 | <0.001 | |
| AST (1: <53 IU/L; ≥2: 53 IU/L) | 0.186 | <0.001 | |
| Platelet | 0.501 | −0.147 | <0.001 |
| Sex (1: male; 2: female) | 0.111 | <0.001 | |
| HCV core antigen | −0.098 | <0.001 | |
| Total bilirubin | 0.091 | 0.001 | |
| Age | 0.071 | 0.014 |
Risk Factors for HCC
Cox's regression analysis was performed on several variables, including age, sex, alcohol consumption, IFN therapy during the observation period, biochemical and virological parameters, and serum WFA+-M2BP level. The following factors were identified as posing an increased risk for HCC by the univariate analysis: age; response to IFN therapy (no therapy vs. SVR; P < 0.001); fibrosis stage (F3 and F4 vs. F0-F1; P < 0.001); platelet count (<15 × 104/mm3 vs. ≥15 × 104/mm3; P < 0.001); albumin (<4.2 vs. ≥4.2 g/mL; P < 0.001); AST (<53 vs. ≥53 IU/mL; P < 0.001), ALT (<82 vs. ≥82 IU/mL; P = 0.035), and AFP levels (≥20 and 6-20 vs. <6 ng/mL; P < 0.001); HCV genotype (1b vs. non-1b; P = 0.025); and serum WFA+-M2BP level (≥4 and 1-4 vs. <1; P < 0.001). Multivariate analysis was performed on these factors (Table3) and the following were identified as independent risk factors: fibrosis stage (F4); AFP (≥20 ng/mL); age (≥57 years); response to IFN therapy (no therapy vs. SVR); and WFA+-M2BP (1-4 and ≥4).
Table 3.
Factors Associated With Risk for HCC*
| Features | HR (95% CI) | P Value | |
|---|---|---|---|
| Fibrosis | F0-F1 | 1 | |
| F2 | 0.883 (0.411-1.897) | 0.749 | |
| F3 | 1.347 (0.624-2.906) | 0.448 | |
| F4 | 3.133 (1.536-6.390) | 0.002 | |
| AFP | <6 ng/mL | 1 | |
| 6-20 ng/mL | 1.710 (0.963-3.038) | 0.067 | |
| ≥20 ng/mL | 3.417 (1.807-6.460) | <0.001 | |
| Age | <57 years | 1 | |
| ≥57 years | 2.039 (1.278-3.252) | 0.003 | |
| IFN therapy | No therapy | 1 | |
| Non-SVR | 0.729 (0.467-1.137) | 0.163 | |
| SVR | 0.089 (0.027-0.288) | <0.001 | |
| WFA+-M2BP | <1 | 1 | |
| 1-4 | 5.155 (1.180 − 22.500) | 0.029 | |
| ≥4 | 8.318 (1.784 − 38.791) | 0.007 | |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Determined by multivariate analysis.
Development of HCC
During the follow-up period, HCC developed in 110 (15.6%) patients. Of the 110 patients with HCC, 58 (52.7%) were diagnosed with the disease by histological examination of biopsy-obtained or resected liver specimens. Of these 58 patients, 24 (41.3%) had hypovascular HCC.
Figure 2 shows the relation between Kaplan-Meier's estimates of the cumulative risk of HCC and the different WFA+-M2BP levels at entry. The 10-year cumulative risk of HCC was 1.1% in the patients with WFA+-M2BP <1 at entry, 14.8% among the patients with WFA+-M2BP 1-4, and 54.1% in patients with WFA+-M2BP >4. The incidence rate differed significantly among the three groups (P < 0.001, by the log-rank test), increasing in accord with WFA+-M2BP level.
Figure 2.
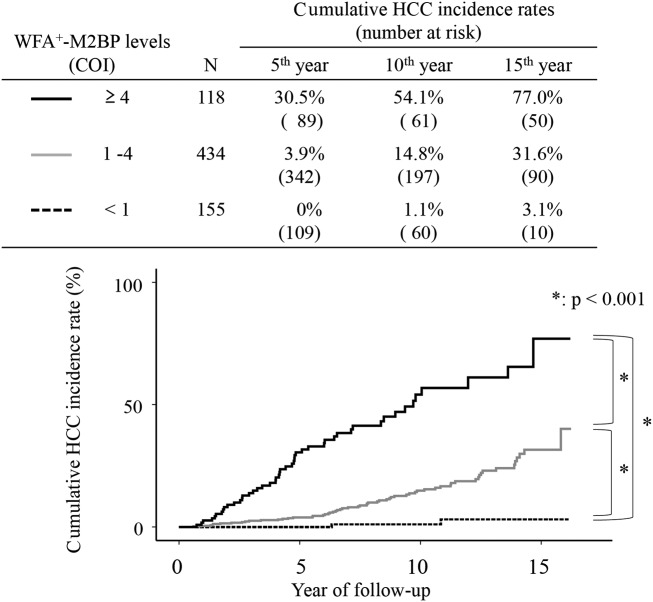
Cumulative incidence of HCC according to WFA+-M2BP level. Cumulative incidences of HCC according to the WFA+-M2BP level were analyzed using Kaplan-Meier's method. Black solid, gray solid, and dotted lines indicate stratified WFA+-M2BP level, ≥4, 1-4, and <1, respectively. Incidence rate differed significantly among the three groups (P < 0.001, by the log-rank test), increasing in accord with WFA+-M2BP level.
Figure 3 shows the relation between the cumulative incidence of HCC and WFA+-M2BP levels, stratified by the fibrosis stage. In patients with fibrosis stage F0-F1, there were significant differences in HCC incidence between those with WFA+-M2BP levels of 1-4 and those with levels of <1 (P < 0.01) and between those with WFA+-M2BP levels of ≥4 and those with levels of <1 (P < 0.01). In patients with fibrosis stage F2-F3, there were significant differences in HCC incidence between those with WFA+-M2BP levels of ≤1 and those with levels of >4 (P < 0.01) and between those with WFA+-M2BP levels of 1-4 and those with levels of >4 (P < 0.001). In patients with fibrosis stage F4, there were significant differences in HCC incidence between those with WFA+-M2BP levels of 1-4 and those with levels of >4 (P < 0.05). As with WFA+-M2BP levels, incidence rates increased with fibrosis stage, and the change in incidence was significant for each fibrosis stage.
Figure 3.
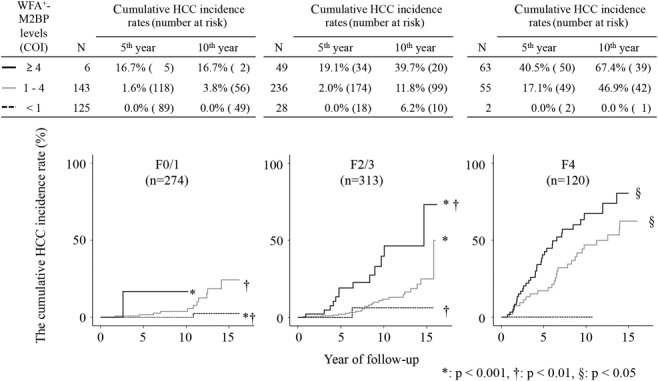
Cumulative incidence of HCC according to WFA+-M2BP levels, stratified by the fibrosis stage. Cumulative incidences of HCC, according to the WFA+-M2BP level, stratified by the fibrosis stage were analyzed using Kaplan-Meier's method. Black solid, gray solid, and dotted lines indicate stratified WFA+-M2BP level, ≥4, 1-4, and <1, respectively. Incidence rates increased in accord with WFA+-M2BP level.
Predictive Accuracy of Cumulative Incidence of HCC Compared With WFA+-M2BP and AFP
AUROC analyses for prediction of the development of HCC at 1, 2, 3, 5, 7, and 10 years (range) were 0.762 (0.553-0.971), 0.792 (0.669-0.915), 0.832 (0.751-0.914), 0.858 (0.805-0.911), 0.821 (0.767-0.876), and 0.800 (0.745-0.855) in WFA+-M2BP and 0.791 (0.684-0.898), 0.790 (0.723-0.857), 0.772 (0.693-0.850), 0.800 (0.741-0.858), 0.796 (0.745-0.848), and 0.821 (0.773-0.868) in AFP, respectively. The WFA+-M2BP assay was superior to AFP for predicting the development of HCC at 3, 5, and 7 years.
Discussion
Liver biopsy has long been considered the gold standard for assessment of hepatic fibrosis,20–23 and the Metavir24 and Desmet et al.16 staging systems are most commonly used. A higher degree of liver fibrosis is known to be the strongest risk factor for hepatocarcinogenesis in hepatitis C patients.1,20 However, it also has its limitations for the staging of fibrosis because of the heterogeneous distribution of fibrosis in the liver,25 and liver biopsy is an invasive procedure with associated morbidity (pain, bleeding, or hemobilia).26 For these reasons, patients are often reluctant to undergo this invasive procedure and instead choose one of several noninvasive methods available for assessing the degree of liver fibrosis.
Nevertheless, in the past, no significant progress was made in the development of noninvasive biomarkers to guide clinical usage. WFA+-M2BP was recently validated as a liver fibrosis glycobiomarker with a fully automated immunoassay.8 In the present study, we assessed the performance of the WFA+-M2BP assay in comparison with liver fibrosis stage and several serum markers, and, based on the results, we estimated whether WFA+-M2BP is a useful predictor of the development of HCC as well as liver biopsy stage.
The first main finding of our study was that there was a significant correlation between the WFA+-M2BP value and the fibrosis stage (Fig. 1). Moreover, step-wise multiple linear regression analysis showed that liver fibrosis stage was most closely associated with serum WFA+-M2BP level. In addition, the degree of necroinflammation had no apparent effect on the WFA+-M2BP value. Based on these results, we proposed a clinical management algorithm using a WFA+-M2BP assay to predict the fibrosis stage. This approach could be used reliably for the first-line pretherapeutic evaluation of fibrosis in HCV-infected patients. On the other hand, the most widely used noninvasive techniques have recently shifted to physical measurements, such as FibroScan,27–30 acoustic radiation force impulse, and real-time strain elastography. FibroScan has the advantages of being rapid and technically simple; however, operator skill affects its diagnostic success rate. Also, stiffness measurements can be difficult to obtain in obese patients and impossible in patients who have ascites. This is regarded as a limitation of transient elastography.27,28 Therefore, we suggest that FibroScan, in conjunction with an assay of serum fibrosis biomarkers, would improve the diagnostic accuracy.
The second main finding of our study was the significant association between the WFA+-M2BP level and the risk of HCC development in hepatitis C patients (Figs. 2 and 3). The diagnostic performance of WFA+-M2BP, based on the AUROC values, was superior to that of AFP for predicting the development of HCC at 3, 5, and 7 years. The WFA+-M2BP value can be used as a noninvasive predictor of HCC development and can be considered a surrogate marker for liver fibrosis. Various risk factors have been reported for HCC development among patients with HCV, including older age,1 male sex,1 heavy alcohol consumption,31 obesity,32 cirrhosis,1,33 lower platelet count,34–36 high serum AFP level,15,36–44 low serum albumin level,31 and high serum ALT and AST level.45–47 Our results were consistent with these findings. Among them, liver fibrosis stage was the strongest prognostic indicator of chronic hepatitis. However, liver biopsy has several disadvantages. In our study, we have shown that the WFA+-M2BP value is also a significant risk factor of HCC development independent of these factors. However, even though WFA+-M2BP can be considered a surrogate marker for liver fibrosis, a distinct advantage of WFA+-M2BP over liver biopsy is its wider dynamic range for the evaluation of liver cirrhosis. In the Metavir and Desmet et al. scoring systems, cirrhosis is represented by a single category (F4). However, the degree of fibrosis may vary widely among patients in this category, and the risk of HCC may not be uniform. In our study, the risk of HCC development increased with increasing WFA+-M2BP level as well as with increasing fibrotic stage. According to the elevation of WFA+-M2BP value, the risk of development of HCC was increased (Fig. 3). In other words, each fibrosis stage can be further stratified with clinical relevance based on the WFA+-M2BP level.
In our study, multivariate analysis identified fibrosis stage, high AFP level, older age, SVR to IFN therapy (no therapy vs. SVR), and high WFA+-M2BP value as independent predictors of HCC development. The stratified WFA+-M2BP value was independently associated with HCC development. These results indicate that the correlation between high WFA+-M2BP and HCC development remains significant, even if HCC develops from a noncirrhotic background. Tateyama et al.15 reported that AFP was a noninvasive predictive marker for the development of HCC in this same cohort; furthermore, not only high AFP levels (≥20 ng/mL), but also slightly elevated AFP levels of between 6 and 20 ng/mL could indicate substantial risks for the development of HCC, complementing the fibrosis stage. Our present study was redesigned by the addition of one parameter (WFA+-M2BP). Multivariate analysis did not identify slightly elevated AFP levels (6-20 ng/mL) as an independent risk factor, but did identify both stratified WFA+-M2BP levels (1-4 and ≥4) as independent risk factors. Also, the time-dependent AUROC analysis suggested that WFA+-M2BP is superior to AFP as a predictor for the development of HCC. These results mean that the WFA+-M2BP level is the most reliable noninvasive predictive marker for the development of HCC in patients infected with HCV.
One of the limitations of the present study is that this cohort of 707 patients was analyzed retrospectively. There is thus need of a future study to prospectively analyze the efficacy of WFA+-M2BP as a predictor of HCC development.
Another limitation is that the hepatocarcinogenesis of the patients who underwent IFN therapy was not evaluated. In this study, among the patients who achieved SVR (n = 139), 3 cases developed HCC during the follow-up period. The WFA+-M2BP titers were 6.4, 5.6, and 1.5, respectively, in the 3 patients. All 3 cases obtained titers higher than 1, and 2 cases obtained titers higher than 4. This result suggests that patients with a high WFA+-M2BP value should be monitored for the development of HCC even after achieving SVR. However, future assessments of the WFA+-M2BP values at IFN administration and at posttreatment will be needed to verify this recommendation.
In conclusion, this study revealed an association between WFA+-M2BP and the risk of HCC development in chronic hepatitis C patients. The results suggested that the WFA+-M2BP assay should not be limited to use as a surrogate for liver biopsy, but rather could be applied as dynamic indicator of the risk of HCC development.
Glossary
- Ab
antibody
- AFP
alpha-fetoprotein
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- AUROC
area under the ROC
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- MRI
magnetic resonance imaging
- Peg-IFN
pegylated IFN
- RBV
ribavirin
- ROC
receiver operating characteristic
- RT-PCR
reverse-transcriptase polymerase chain reaction
- SVR
sustained virological response
- US
ultrasound
- WFA+-M2BP
Wisteria floribunda agglutinin-positive human Mac-2-binding protein
References
- Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl):S62–S71. doi: 10.1053/j.gastro.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology. 2001;33:196–200. doi: 10.1053/jhep.2001.20534. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, et al. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161–S172. doi: 10.1053/jhep.2002.36989. [DOI] [PubMed] [Google Scholar]
- Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36(Suppl):S152–S160. doi: 10.1053/jhep.2002.36381. [DOI] [PubMed] [Google Scholar]
- Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet-doughnut” 272 protein facilitates fibrosis evaluation and therapy assessment in patients 273 with viral hepatitis. Sci. Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu Y, Kuno A, Ito H, Kaji H, Kaneko S, Usui J, et al. IgA nephropathy caused by unusual polymerization of IgA1 with aberrant N-glycosylation in a patient with monoclonal immunoglobulin deposition disease. PloS One. 2014;9:e91079. doi: 10.1371/journal.pone.0091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998;17:1606–1613. doi: 10.1093/emboj/17.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovazzi PA, Trisolini A, Barletta D, Elba S, Manghisi OG, Correale M. Serum 90K/MAC-2BP glycoprotein in patients with liver cirrhosis and hepatocellular carcinoma: a comparison with alpha-fetoprotein. Clin Chem Lab Med. 2005;39:961–965. doi: 10.1515/CCLM.2001.155. [DOI] [PubMed] [Google Scholar]
- Artini M, Natoli C, Tinari N, Costantzo A, Marinelli R, Balsano C, et al. Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J Hepatol. 1996;25:212–217. doi: 10.1016/s0168-8278(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Tilleman K, Deforce D, Colle I, Van Vlierberghe H. The HCV serum proteome: a search for fibrosis protein markers. J Viral Hepat. 2009;16:418–429. doi: 10.1111/j.1365-2893.2009.01083.x. [DOI] [PubMed] [Google Scholar]
- Kuno A, Sato T, Shimazaki H, Unno S, Saitou K, Kiyohara K, et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteomics Clin Appl. 2013;7:642–647. doi: 10.1002/prca.201300010. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Yatsuhashi H, Taura N, Motoyoshi Y, Nagaoka S, Yanagi K, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46:92–100. doi: 10.1007/s00535-010-0293-6. [DOI] [PubMed] [Google Scholar]
- Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- Tanaka E, Ohue C, Aoyagi K, Yamaguchi K, Yagi S, Kiyosawa K, et al. Evaluation of a new enzyme immunoassay for hepatitis C virus (HCV) core antigen with clinical sensitivity approximating that of genomic amplification of HCV RNA. Hepatology. 2000;32:388–393. doi: 10.1053/jhep.2000.9112. [DOI] [PubMed] [Google Scholar]
- Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba K, Orito E, et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35:201–207. doi: 10.1128/jcm.35.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology. 2001;33:196–200. doi: 10.1053/jhep.2001.20534. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, et al. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36(Suppl):S161–S172. doi: 10.1053/jhep.2002.36989. [DOI] [PubMed] [Google Scholar]
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, et al. ARFI, FibroScan®, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281–287. doi: 10.1016/j.jhep.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650–655. [PubMed] [Google Scholar]
- Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C—a study of 527 patients at one establishment. J Viral Hepat. 2000;7:268–275. doi: 10.1046/j.1365-2893.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–136. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz JL, Rosas-Camargo V, Vega-Vega O, Morales-Espinosa D, Mendez-Reguera A, Martinez-Tlahuel JL, et al. Clinical and pathological factors associated with the development of hepatocellular carcinoma in patients with hepatitis virus-related cirrhosis: a long-term follow-up study. Clin Oncol (R Coll Radiol) 2007;19:197–203. doi: 10.1016/j.clon.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–66. [PubMed] [Google Scholar]
- Ganne-Carrie N, Chastang C, Chapel F, Munz C, Pateron D, Sibony M, et al. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology. 1996;23:1112–1118. doi: 10.1002/hep.510230527. [DOI] [PubMed] [Google Scholar]
- Sangiovanni A, Colombo E, Radaelli F, Bortoli A, Bovo G, Casiraghi MA, et al. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol. 2001;96:1575–1580. doi: 10.1111/j.1572-0241.2001.03780.x. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Arase Y, Saitoh S, Kobayashi M, Someya T, Hosaka T, et al. Prediction model of hepatocarcinogenesis for patients with hepatitis C virus-related cirrhosis. Validation with internal and external cohorts. J Hepatol. 2006;44:1089–1097. doi: 10.1016/j.jhep.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Bruce MG, Bruden D, McMahon BJ, Christensen C, Homan C, Sullivan D, et al. Clinical significance of elevated alpha-fetoprotein in Alaskan Native patients with chronic hepatitis C. J Viral Hepat. 2008;15:179–187. doi: 10.1111/j.1365-2893.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Inoue A, Tsukuma H, Oshima A, Yabuuchi T, Nakao M, Matsunaga T, et al. Effectiveness of interferon therapy for reducing the incidence of hepatocellular carcinoma among patients with type C chronic hepatitis. J Epidemiol. 2000;10:234–240. doi: 10.2188/jea.10.234. [DOI] [PubMed] [Google Scholar]
- Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589–595. doi: 10.1002/(sici)1097-0142(19990815)86:4<589::aid-cncr7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Tarao K, Rino Y, Ohkawa S, Tamai S, Miyakawa K, Takakura H, et al. Close association between high serum alanine aminotransferase levels and multicentric hepatocarcinogenesis in patients with hepatitis C virus-associated cirrhosis. Cancer. 2002;94:1787–1795. doi: 10.1002/cncr.10391. [DOI] [PubMed] [Google Scholar]


