Abstract
Elevated levels of metallothionein (MT) found in rapidly growing tissues such as neonatal liver and various types of human tumors have suggested a role for MT in cell proliferation. To further explore this possibility we investigated the concentration of MT in human colonic cancer (HT-29) cells at different stages of proliferation by means of immunocytochemistry and competitive binding. MT is increased in subconfluent proliferating cells relative to growth-inhibited confluent cells, much as it is in growing tissues. Cycling cells synchronized with compactin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, revealed an oscillation of cytoplasmic MT that reached a maximum in successive late G1 phases and at the G1/S transition. Individual phase of the cell cycle were assessed by [3H]thymidine incorporation and by immunofluorescence employing an antibody that detects a nuclear antigen associated with proliferation. An enzyme-linked immunosorbent assay was used to quantify the relative amounts of MT in homogenate supernatants of HT-29 cells. A 2- to 3-fold increase in MT in actively proliferating cells and the regulation of the protein during the mitotic cell cycle point to a physiological role for MT in cellular proliferation and suggest that it may also serve as a proliferation marker.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Weser U. Partial purification, characterization and translation in vitro of rat liver metallothionein messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):841–852. doi: 10.1042/bj1750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Gallant K. R., Cherian M. G. Regulation of the ontogeny of rat liver metallothionein mRNA by zinc. Eur J Biochem. 1987 Aug 3;166(3):527–531. doi: 10.1111/j.1432-1033.1987.tb13545.x. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Huet Y. M., Lehman L. D., Dey S. K. Metallothionein gene regulation in the preimplantation rabbit blastocyst. Development. 1987 Jul;100(3):463–469. doi: 10.1242/dev.100.3.463. [DOI] [PubMed] [Google Scholar]
- Andrews G. K. Regulation of metallothionein gene expression. Prog Food Nutr Sci. 1990;14(2-3):193–258. [PubMed] [Google Scholar]
- Bakka A., Samarawickrama G. P., Webb M. Metabolism of zinc and copper in the neonate: effect of cadmium administration during late gestation in the rat on the zinc and copper metabolism of the newborn. Chem Biol Interact. 1981 Mar 1;34(2):161–171. doi: 10.1016/0009-2797(81)90128-9. [DOI] [PubMed] [Google Scholar]
- Brady F. O., Webb M. Metabolism of zinc and copper in the neonate. (Zinc, copper)-thionein in the developing rat kidney and testis. J Biol Chem. 1981 Apr 25;256(8):3931–3935. [PubMed] [Google Scholar]
- Bremner I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1987;52:81–107. doi: 10.1007/978-3-0348-6784-9_5. [DOI] [PubMed] [Google Scholar]
- Brown R. E., Jarvis K. L., Hyland K. J. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989 Jul;180(1):136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Chen M. L., Failla M. L. Degradation of zinc-metallothionein in monolayer cultures of rat hepatocytes. Proc Soc Exp Biol Med. 1989 Jun;191(2):130–138. doi: 10.3181/00379727-191-42898. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Huang P. C., Klaassen C. D., Liu Y. P., Longfellow D. G., Waalkes M. P. National Cancer Institute workshop on the possible roles of metallothionein in carcinogenesis. Cancer Res. 1993 Feb 15;53(4):922–925. [PubMed] [Google Scholar]
- Chesters J. K., Boyne R. Nature of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp Cell Res. 1991 Feb;192(2):631–634. doi: 10.1016/0014-4827(91)90085-9. [DOI] [PubMed] [Google Scholar]
- Chesters J. K., Petrie L., Vint H. Specificity and timing of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp Cell Res. 1989 Oct;184(2):499–508. doi: 10.1016/0014-4827(89)90347-9. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Czupryn M., Brown W. E., Vallee B. L. Zinc rapidly induces a metal response element-binding factor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10395–10399. doi: 10.1073/pnas.89.21.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L. Effects of various trace metals on the binding of cadmium to rat hepatic metallothionein determined by the Cd/hemoglobin affinity assay. Toxicol Appl Pharmacol. 1985 Mar 30;78(1):158–162. doi: 10.1016/0041-008x(85)90315-1. [DOI] [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Olson K. A., Rybak S. M. A monoclonal antibody to human angiogenin. Inhibition of ribonucleolytic and angiogenic activities and localization of the antigenic epitope. Biochemistry. 1994 May 10;33(18):5421–5427. doi: 10.1021/bi00184a010. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Varshney U., Jahroudi N., Foster R., Shworak N. W. Structure and expression of the human metallothionein genes. Experientia Suppl. 1987;52:361–372. doi: 10.1007/978-3-0348-6784-9_34. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Grider A., Kao K. J., Klein P. A., Cousins R. J. Enzyme-linked immunosorbent assay for human metallothionein: correlation of induction with infection. J Lab Clin Med. 1989 Feb;113(2):221–228. [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hartmann H. J., Weser U. Copper-thionein from fetal bovine liver. Biochim Biophys Acta. 1977 Mar 28;491(1):211–222. doi: 10.1016/0005-2795(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hsu S. C., Qi M., DeFranco D. B. Cell cycle regulation of glucocorticoid receptor function. EMBO J. 1992 Sep;11(9):3457–3468. doi: 10.1002/j.1460-2075.1992.tb05425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Metallothionein gene expression is regulated by serum factors and activators of protein kinase C. Mol Cell Biol. 1987 Apr;7(4):1358–1363. doi: 10.1128/mcb.7.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakóbisiak M., Bruno S., Skierski J. S., Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3628–3632. doi: 10.1073/pnas.88.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Slater E. P., Herschman H. R. Regulation of metallothionein synthesis in HeLa cells by heavy metals and glucocorticoids. J Cell Physiol. 1981 Jan;106(1):63–74. doi: 10.1002/jcp.1041060108. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K., Sandoval L., Band V., Pardee A. B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991 Jul 1;51(13):3602–3609. [PubMed] [Google Scholar]
- Krauter B., Nagel W., Hartmann H. J., Weser U. Copper-thionein in melanoma. Biochim Biophys Acta. 1989 Oct 9;1013(3):212–217. doi: 10.1016/0167-4889(89)90137-7. [DOI] [PubMed] [Google Scholar]
- Krezoski S. K., Villalobos J., Shaw C. F., 3rd, Petering D. H. Kinetic lability of zinc bound to metallothionein in Ehrlich cells. Biochem J. 1988 Oct 15;255(2):483–491. [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- Leibbrandt M. E., Khokha R., Koropatnick J. Antisense down-regulation of metallothionein in a human monocytic cell line alters adherence, invasion, and the respiratory burst. Cell Growth Differ. 1994 Jan;5(1):17–25. [PubMed] [Google Scholar]
- Leyshon-Sørland K., Mørkrid L., Rugstad H. E. Metallothionein: a protein conferring resistance in vitro to tumor necrosis factor. Cancer Res. 1993 Oct 15;53(20):4874–4880. [PubMed] [Google Scholar]
- Masters B. A., Kelly E. J., Quaife C. J., Brinster R. L., Palmiter R. D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Heintz N. Transcriptional regulation in the eukaryotic cell cycle. Trends Biochem Sci. 1991 Nov;16(11):430–435. doi: 10.1016/0968-0004(91)90170-z. [DOI] [PubMed] [Google Scholar]
- Michalska A. E., Choo K. H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., McGown A. T., Crowther D., Mander A., Fox B. W. Metallothionein levels in ovarian tumours before and after chemotherapy. Br J Cancer. 1991 May;63(5):711–714. doi: 10.1038/bjc.1991.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Mumberg D., Lucibello F. C. Signals and genes in the control of cell-cycle progression. Biochim Biophys Acta. 1993 Aug 23;1155(2):151–179. doi: 10.1016/0304-419x(93)90003-u. [DOI] [PubMed] [Google Scholar]
- Nagel W., Hartmann H. J., Weser U. Monoclonal antibodies to monomeric rat liver metallothionein-I: the immunoreactivity of lysine residues in metallothionein. Immunol Lett. 1990 Dec;26(3):291–295. doi: 10.1016/0165-2478(90)90162-j. [DOI] [PubMed] [Google Scholar]
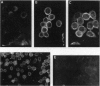
- Nartey N. O., Banerjee D., Cherian M. G. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of fetal human liver and kidney and its changes during development. Pathology. 1987 Jul;19(3):233–238. doi: 10.3109/00313028709066555. [DOI] [PubMed] [Google Scholar]
- Nemer M., Travaglini E. C., Rondinelli E., D'Alonzo J. Developmental regulation, induction, and embryonic tissue specificity of sea urchin metallothionein gene expression. Dev Biol. 1984 Apr;102(2):471–482. doi: 10.1016/0012-1606(84)90212-4. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Koga M. Purification and characterization of zinc-binding protein from the liver of the partially hepatectomized rat. Biochem J. 1979 Dec 1;183(3):683–690. doi: 10.1042/bj1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panemangalore M., Banerjee D., Onosaka S., Cherian M. G. Changes in the intracellular accumulation and distribution of metallothionein in rat liver and kidney during postnatal development. Dev Biol. 1983 May;97(1):95–102. doi: 10.1016/0012-1606(83)90067-2. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
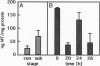
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Price-Haughey J., Gedamu L. Heavy metal induced protein synthesis in fish cell lines. Experientia Suppl. 1987;52:465–469. doi: 10.1007/978-3-0348-6784-9_46. [DOI] [PubMed] [Google Scholar]
- ROBINSON J. H., SMITH J. A. Density-dependent regulation of proliferation rate in cultured, androgen-responsive, tumour cells. J Cell Physiol. 1976 Sep;89(1):111–122. doi: 10.1002/jcp.1040890111. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Tanaka S., Cuddy M. Cell cycle analysis of p26-BCL-2 protein levels in proliferating lymphoma and leukemia cell lines. Cancer Res. 1992 May 15;52(10):2802–2805. [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Soncin F., Shapiro R., Fett J. W. A cell-surface proteoglycan mediates human adenocarcinoma HT-29 cell adhesion to human angiogenin. J Biol Chem. 1994 Mar 25;269(12):8999–9005. [PubMed] [Google Scholar]
- Steinebach O. M., Wolterbeek B. T. Metallothionein biodegradation in rat hepatoma cells: a compartmental analysis aided 35S-radiotracer study. Biochim Biophys Acta. 1992 Apr 22;1116(2):155–165. doi: 10.1016/0304-4165(92)90112-8. [DOI] [PubMed] [Google Scholar]
- Steinebach O. M., Wolterbeek H. T. Determination of zinc-65, copper-64 and sulphur-35 labelled rat hepatoma tissue culture metallothioneins by high-performance liquid chromatography with on-line radioactivity detection. J Chromatogr. 1993 Sep 22;619(2):199–214. doi: 10.1016/0378-4347(93)80109-h. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Seagrave J. Inducibility of metallothionein throughout the cell cycle. Mol Cell Biol. 1984 Oct;4(10):2243–2245. doi: 10.1128/mcb.4.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama C., Suzuki J. S., Hemelraad J., Nishimura N., Nishimura H. Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology. 1993 Nov;18(5):1193–1201. [PubMed] [Google Scholar]
- Tsujikawa K., Imai T., Kakutani M., Kayamori Y., Mimura T., Otaki N., Kimura M., Fukuyama R., Shimizu N. Localization of metallothionein in nuclei of growing primary cultured adult rat hepatocytes. FEBS Lett. 1991 Jun 3;283(2):239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H. J., van Driel R., Beck J. L., van Dierendonck J. H., Brakenhoff G. J., Ramaekers F. C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J Cell Sci. 1989 Apr;92(Pt 4):531–540. doi: 10.1242/jcs.92.4.531. [DOI] [PubMed] [Google Scholar]
- Wick M., Bürger C., Brüsselbach S., Lucibello F. C., Müller R. Identification of serum-inducible genes: different patterns of gene regulation during G0-->S and G1-->S progression. J Cell Sci. 1994 Jan;107(Pt 1):227–239. doi: 10.1242/jcs.107.1.227. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Su S., Gedamu L. Basal and inducible expression of metallothionein and heat shock protein 70 genes in bovine articular chondrocytes. Exp Cell Res. 1993 Oct;208(2):371–377. doi: 10.1006/excr.1993.1258. [DOI] [PubMed] [Google Scholar]
- Zeng J., Heuchel R., Schaffner W., Kägi J. H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991 Feb 25;279(2):310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]
- Zeng J., Vallee B. L., Kägi J. H. Zinc transfer from transcription factor IIIA fingers to thionein clusters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9984–9988. doi: 10.1073/pnas.88.22.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Nuclear distribution of the Ki-67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res. 1989 Jun 1;49(11):2999–3006. [PubMed] [Google Scholar]