Summary
Anthropogenic stress on natural systems, particularly the fragmentation of landscapes and the extirpation of predators from food webs, has intensified the need to regulate abundance of wildlife populations with management. Controlling population growth using fertility control has been considered for almost four decades, but nearly all research has focused on understanding effects of fertility control agents on individual animals. Questions about the efficacy of fertility control as a way to control populations remain largely unanswered.
Collateral consequences of contraception can produce unexpected changes in birth rates, survival, immigration and emigration that may reduce the effectiveness of regulating animal abundance. The magnitude and frequency of such effects vary with species‐specific social and reproductive systems, as well as connectivity of populations. Developing models that incorporate static demographic parameters from populations not controlled by contraception may bias predictions of fertility control efficacy.
Many population‐level studies demonstrate that changes in survival and immigration induced by fertility control can compensate for the reduction in births caused by contraception. The most successful cases of regulating populations using fertility control come from applications of contraceptives to small, closed populations of gregarious and easily accessed species.
Fertility control can result in artificial selection pressures on the population and may lead to long‐term unintentional genetic consequences. The magnitude of such selection is dependent on individual heritability and behavioural traits, as well as environmental variation.
Synthesis and applications. Understanding species' life‐history strategies, biology, behavioural ecology and ecological context is critical to developing realistic expectations of regulating populations using fertility control. Before time, effort and funding are invested in wildlife contraception, managers may need to consider the possibility that many species and populations can compensate for reduction in fecundity, and this could minimize any reduction in population growth rate.
Keywords: behaviour, demography, ecological process, fertility control, fitness, immunocontraception, population dynamics, population ecology, wildlife contraception, wildlife management
Short abstract
Upland land use is associated with curlew declines, with predation a likely mechanism, and this may apply to other breeding waders. The removal of isolated woodland plantations from otherwise unafforested landscapes may help reduce predation pressure across a range of systems including moorland. However, direct predator control may also be important to conserve ground‐nesting birds in these landscapes, for example, where moorland management and forestry coexist as major land uses. Predator control may also mitigate climate change effects by enhancing wader productivity, particularly where climate effects coincide with changing land use. Emerging land uses in open landscapes, including native woodland restoration and wind farms, require careful siting to minimize further impacts on open‐area breeding birds.
Controlling reproductive capacity of wildlife
Humans have been attempting to control the abundance of animals for over 13,000 years (Diamond 2002). Although regulating the herd size of domestic animals has been a feature of human economies for millennia, people have also sought to regulate populations of wild animals using hunting and culling techniques. Recent societal trends have motivated wildlife managers to seek nonlethal strategies of regulating births using fertility control (Hobbs, Bowden & Baker 2000; Porton 2005). This trend may be attributed to an increasingly mutualistic societal view arising from abundance of wildlife in suburban and urban areas (Teel & Manfredo 2009). In such circumstances, perception of wildlife species may focus less on their role as a natural resource and more on how they are part of our social environment (Leong 2010). This may lead humans to be more aware of, and concerned about, the welfare of these species. The balance of human perception and wildlife abundance is precarious because as highly adaptive species increase in density, wildlife–human conflicts increase, and society can then be confounded with the dichotomy of desire for wildlife protection and relief from wildlife conflict (Knuth et al. 2001; Lauber & Knuth 2004; Lauber et al. 2007; Hadidian 2009).
The practice of using fertility control to manage reproduction in wildlife emerged over 40 years ago (Asa & Porton 2005). Wildlife managers have been attracted to fertility control as a means to regulate overabundant wildlife when animals threaten people's lives, livelihoods or property; when they cause declines in more desirable species; and when their densities are high enough to increase disease transmission or disrupt ecosystem function (Caughley 1981; Hone 2007). The vast majority of empirical knowledge about wildlife fertility control comes from individual‐level studies of drug safety and efficacy (Garrott 1995; Kirkpatrick, Lyda & Frank 2011). As a consequence, we know relatively little about how fertility control influences population ecology. It remains uncertain whether findings of research on the efficacy of fertility control at the individual level can allow inferences to populations. There may be compensation in vital rates that allow individuals to maintain fitness, thus offsetting the effects of fertility control agents. If such compensation occurs, then it may be infeasible to control some wildlife populations using fertility control.
Types of fertility control applied to wildlife include products that disturb normal reproductive hormone cascades or interfere with conception, such as immunocontraceptive vaccines, pharmaceuticals, hormone derivatives, agonists, antagonists, mechanical devices and surgical techniques (Asa 2005). All of these methods may prevent births, but they also may induce unintended changes to behaviour and physiology (Nettles 1997; Gray & Cameron 2010). It is not surprising that hormonal derivatives such as melengestrol and levonorgestrel affect behaviour (Gray & Cameron 2010), but it is surprisingly uncertain how variable such behavioural changes can be, even within similar taxa. Stump‐tailed macaque Macaca arctoides females, for example, can become markedly more aggressive towards conspecifics when treated with a synthetic progestin (Linn & Steklis 1990), but hamadryas baboon Papio hamadryas females can become more passive when treated with a similar progestin (Portugal & Asa 1995).
Any fertility control application that changes the reproductive capacity of an individual has the potential to induce individual behavioural changes that can alter family group structure, influence interspecific and intraspecific interactions and ultimately shape population dynamics in unforeseen ways. Fertility control in free‐roaming wildlife populations has been associated with changes in immigration (Ramsey 2005; Merrill, Cooch & Curtis 2006), decreased group fidelity (Nuñez et al. 2009; Madosky et al. 2010), increased survival (Caughley, Pech & Grice 1992; Kirkpatrick & Turner 2007; Williams et al. 2007), altered reproductive behaviour (Nuñez, Adelman & Rubenstein 2010; Ransom, Cade & Hobbs 2010) and shifted phenology (Ransom, Hobbs & Bruemmer 2013). Understanding and predicting these sometimes subtle and incremental shifts is difficult, and the challenge of separating the influences of multiple population growth controls can be daunting (Sibly & Hone 2002). To exacerbate these problems, the longitudinal studies needed to detect and quantify long‐term population‐level effects of fertility control treatments are expensive and time‐consuming.
Insights into population‐level effects of fertility control have largely been accomplished through efforts that simulate population dynamics (Caughley, Pech & Grice 1992; Hone 1992; Hobbs, Bowden & Baker 2000; Davis & Pech 2002; Merrill, Cooch & Curtis 2006). Simulation models can be useful tools for screening management alternatives; however, widespread behavioural and demographic changes induced by fertility control may subvert the underlying assumptions of demographic parameters in models that fail to consider these changes. The assumptions of many fertility control population models could be wrong if the vital rates informing them are based on the wealth of a priori ecological knowledge about species and systems before fertility control was applied.
Here, we synthesize findings from studies on population‐level effects of wildlife fertility control and compare some of those outcomes with those from individual‐level studies. We specifically investigate the demographic and behavioural components from wildlife fertility control studies, seeking to understand feedbacks that might be influencing population‐level outcomes of fertility control strategies. We attempt to identify some of the underlying processes to help inform modelling efforts and future design for empirical population‐level fertility control studies, and ultimately try to determine whether, in fact, nature can overcome control efforts at a scale that makes fertility control ineffective as a wildlife management tool.
The feedback dilemma
Considering animal behaviour in broad ecological and conservation contexts is critical to understanding population ecology (Sutherland 1998; Buchholz 2007); yet, a lack of rigorously quantified behaviour data often inhibits our ability to assess the magnitude of its importance. At the individual level, wildlife contraceptive studies have illuminated a wide array of behavioural changes attributed to fertility control applications (Nettles 1997; Gray & Cameron 2010). Studies on captive animals incorporate more experimental control than is usually possible with free‐ranging wildlife, but the artificial environments inherent in captive studies often preclude the insights towards ecological feedbacks that studies of free‐ranging wildlife contribute. Consideration of results from both types of studies is needed to understand the potential population‐level effects of fertility control acting through birth, survival, immigration and emigration. Feedbacks on population growth may be negative or positive, and the nature of feedback direction may vary with the ecology of the species in question.
Fertility control is designed to reduce the probability that an individual gives birth, so it should be expected that fecundity of treated animals will be less than that of untreated animals; however, indirect effects make the broader influence of treatment on birth rates less clear. Unintentionally protracted contraception has been attributed to physiological changes in ovarian function after fertility control treatments (Kirkpatrick et al. 1995; McShea et al. 1997; Nettles 1997; Powell & Monfort 2001; Stoops et al. 2006). Such residual infertility was observed in feral horses Equus caballus and Przewalski horses E. ferus przewalskii repeatedly treated with porcine zona pellucida (PZP) and was further complicated by shifts in the seasonal birth pulse when fertility resumed (Nuñez 2009; Feh 2012; Ransom, Hobbs & Bruemmer 2013). Increased frequency of reproductive behaviour has been observed in females treated with PZP vaccines and has the potential to focus attention of breeding males away from fertile females and towards the attractive, but sterile, females (Shumake & Wilhelm 1995; McShea et al. 1997; Heilmann et al. 1998; Nuñez, Adelman & Rubenstein 2010; Ransom, Cade & Hobbs 2010). Similarly, both elk Cervus elaphus and mule deer Odocoileus hemionus males demonstrated increased reproductive behaviour towards females treated with gonadotropin‐releasing hormone vaccine (GnRH) or leuprolide (a GnRH agonist) after the untreated cohort became pregnant (Baker et al. 2002, 2004; Conner et al. 2007; Powers et al. 2011). All of these studies represent a potential mechanism for decreased fitness in males and could result in a higher net contraceptive effect at the population level (Ransom 2012).
In contrast, fecundity of untreated female mice Mus domesticus increased when fertility‐controlled females were present, attenuating the contraceptive effect (Chambers, Singleton & Hinds 1999). Many species have been reported exhibiting a compensatory reproduction response when a subset of the population has been perturbed, such as might be the case with large‐scale fertility control application (Swenson 1985; Kirkpatrick & Turner 1991; Boyce, Sinclair & White 1999; Williams 1999). How such effects originate through changes in social networks is unknown. Likewise, the effects of fertility control on biochemical and olfactory communication are virtually unknown, but have been implicated in disrupting intraspecific interactions in a primate (Crawford, Boulet & Drea 2010) and could lead to greater uncertainty about fecundity at the population level.
Fidelity to family groups has decreased in conjunction with fertility control applications (Fayrer‐Hosken et al. 2000; Nuñez et al. 2009; Madosky et al. 2010), and time spent with conspecifics or potential mates has also changed (Bertrand et al. 1996; Poiani et al. 2002; Harrenstien et al. 2004). Territoriality increased for pikas Ochotona curzoniae, and the increase was related to decreased litter size (Liu et al. 2012). These types of changes can affect time budgets such that basic maintenance behaviours of feeding and resting decrease, resulting in lower body condition in animals receiving contraception (see Ji, Clout & Sarre 2000; for example). Similarly, female elk treated with leuprolide had comparable maintenance behaviours as untreated elk, but retained less body fat than untreated, pregnant females, possibly due to decreased anabolic hormone exposure during their infertile year (Conner et al. 2007).
Increased or decreased agonism between conspecifics has also been attributed to some contraceptive applications (Linn & Steklis 1990; Portugal & Asa 1995; Penfold, Patton & Jochle 2005; Snape, Hinds & Miller 2011). These behaviours can alter dominance hierarchies and thus access to resources (Dublin 1983; Schulte et al. 2000; Creel 2001). More directly, some deer treated with hormonal applications lost body condition or stopped eating altogether (Bell & Peterle 1975; White, Warren & Fayrer‐Hosken 1994). Low body condition arising from any of these processes can enhance the fertility control effect by changing the ability of individuals to meet the nutritional requirements needed to support pregnancy and lactation (Cook et al. 2004; Rhind 2004).
Apart from the many potential direct and indirect effects of fertility control on births, some applications may also influence survival. Body condition and longevity can increase when the costs associated with reproduction decrease. For example, feral horses treated with a PZP vaccine had higher survival probability than untreated controls (Turner & Kirkpatrick 2002; Ransom 2012). Similarly, survival was higher among fertility‐controlled rabbits Oryctolagus cuniculus (Twigg et al. 2000; Williams et al. 2007), coyotes Canis latrans (Bromley & Gese 2001) and foxes Vulpes vulpes (Saunders et al. 2002). These results show that reductions in recruitment caused by fertility control can be offset at least in part by enhanced survival of females, thereby attenuating the effect of fertility control on population growth rate.
Feedbacks amplifying the effect of fertility control can also occur. Disease transmission was 28% higher in populations of possums Trichosurus vulpecula with sterile females, illustrating the potential for decreased survival in fertility‐controlled populations (Caley & Ramsey 2001). Phenological shifts in birth patterns among females that formerly received contraception may also have implications for survival of both females and their neonates if births occur asynchronously with forage resources (Ransom, Hobbs & Bruemmer 2013).
Perhaps, the most challenging impediment to predicting population‐level effects of fertility control occurs when populations are open to immigration and emigration, processes that can be accelerated by feedbacks from the application of fertility control. Male possums, for example, increased in frequency at sites where females received contraception (Ji, Clout & Sarre 2000), and in one study, the rate of immigration equalled the rate of reduction in births attributed to fertility control (Ramsey 2005). A similar feedback was also observed in deer (Merrill, Cooch & Curtis 2006). Home ranges of fertility‐controlled female rats Rattus argentiventer, koalas Phascolarctos cinereus and white‐tailed deer were larger than those of fertile females (Jacob, Matulessy & Sudarmaji, 2004; Gilman et al. 2010; Hynes et al. 2011). This was explained by an absence of range‐limiting dependent young, and in deer, this larger home range was positively correlated with increased mortality rates (Gilman et al. 2010).
For nearly all of the collateral effects discussed here, there are almost as many cases where no collateral effects were detected when a different fertility control method was used or a different species was studied (Gray & Cameron 2010: Fig. 2). Such variation in individual and species responses emphasizes the need for careful consideration of species biology, reproductive system, physiology, behavioural ecology, population biology and ecological context when considering fertility control management for a population.
Population‐level effects of fertility control
Scientific progress towards understanding population‐level effects of fertility control has been slow despite an articulate call for population‐level research of wildlife contraception nearly two decades ago (Garrott 1995). We reviewed 479 papers in the scientific literature on wildlife fertility control from 1980 to 2011 and found that 90% focused on individual‐level effects (Fig. 1). Half of the papers were published in the last decade, reflecting an increasing interest in wildlife fertility control. There was an increasing trend in population‐level studies, but still only 10% of the papers reviewed addressed population‐level effects.
Figure 1.
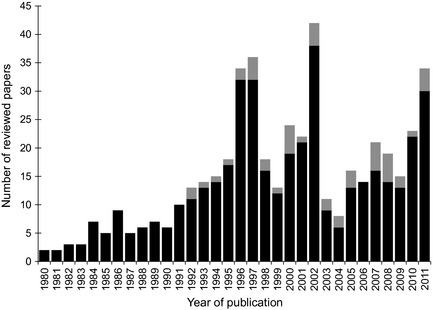
Trends in publication (n = 479 papers) for research on fertility control in wild and feral fauna from 1980 to 2011. Proportion of each bar in grey depicts investigations that included empirical or simulated population‐level effects.
Only 6·5% of all papers we reviewed considered behavioural or indirect demographic effects in population‐level assessment of fertility control (Table 1), and only half of those quantitatively considered such effects in modelling population dynamics with fertility control (Table 2). This is surprising given that such a large number of individual‐level studies describe the potential for indirect effects to influence fertility control efficacy at the population level (Nettles 1997; Gray & Cameron 2010). The number of studies that empirically tested fertility control management for achieving long‐term reduction in population size was too small for us to conduct a meaningful quantitative meta‐analysis. It is worth noting, however, that the studies evaluating fertility control in open populations of promiscuous breeders did not sufficiently reduce population size for long time horizons (Twigg et al. 2000; Ramsey 2005; Jacob, Singleton & Hinds 2008), and a study of an open population of deer had mixed results (Merrill, Cooch & Curtis 2006). Efforts to manage relatively small, closed populations of feral horses, deer, elephants and feral cats using fertility control were much more successful (Rudolph, Porter & Underwood 2000; Delsink et al. 2006; Rutberg & Naugle 2007; Kirkpatrick & Turner 2008; Mendes‐de‐Almeida et al. 2011; Ransom 2012).
Table 1.
Number of reviewed scientific publications from 1980 to 2011 that included population‐level effects of fertility control on wild or feral animals in theoretical, simulation or empirical contexts and the number of those publications that considered behavioural or indirect demographic feedbacks on population‐level effectiveness of the application
| Fauna type | Type of study | Feedback on fertility control efficacy | ||||
|---|---|---|---|---|---|---|
| Theoretical | Simulation | Empirical | Negative | Positive | Not considered | |
| Ungulate | 1 | 10 | 6 | 4 | 0 | 13 |
| Rodent | 2 | 2 | 6 | 6 | 1 | 3 |
| Marsupial | 0 | 1 | 3 | 4 | 0 | 0 |
| Pachyderm | 2 | 2 | 1 | 2 | 0 | 3 |
| Carnivore | 0 | 3 | 1 | 0 | 0 | 4 |
| Fish | 1 | 0 | 0 | 1 | 0 | 0 |
| Non‐specific | 4 | 3 | 0 | 4 | 0 | 3 |
| Total | 10 | 21 | 17 | 21 | 1 | 26 |
Table 2.
Population‐level effects of fertility control in simulated (S) and empirical (E) studies that explicitly incorporated demographic feedbacks into analyses
| Source | Taxa | Data | Individual treatment efficacy | Percent of population treated | Population effect | Feedback | Mechanism |
|---|---|---|---|---|---|---|---|
| Caughley, Pech & Grice (1992) | Gregarious, stratified by dominance structure | S | Non‐specific: 100% | 0–100% in social groups of up to 5 females | If female loses dominance at treatment, productivity increases | Negative | Increased births from subordinate females |
| Hone (1992) | a: long‐lived, low fecundity b: short‐lived, high fecundity | S | Non‐specific: 100% | 20–100% of original fecundity of females | Curvilinear response: for b, 80% fertility reduction ≈ 50% abundance reduction | Negative | Increased survival |
| Seagle & Close (1996) | White‐tailed deer (Odocoileus virginianus) | S | Non‐specific: 100% | 0–100% of females | No reduction at <50% of females treated in open population | Negative | Increased immigration |
| Barlow, Kean & Briggs (1997) | a: monogamous and, b: polygamous | S | Non‐specific: 100% | 0–100% of females and/or 0–100% of males | Greatest effect in (a) with both sexes treated; less effect in (b) | Negative | Increased survival; increased births in b |
| Twigg et al. (2000); Williams et al. (2007) | European rabbit (Oryctolagus cuniculus) | E | Tubal ligation: 100% | 0%, 40%, 60%, 80% of females | After 4 years, no difference in population size between treatments | Negative | Increased survival, increased immigration, decreased emigration |
| Singleton et al. (2002) | House mouse (Mus domesticus) | E, S | Tubal ligation & ovariectomy: 100% | 67% of females | 75% density reduction after 11 weeks, but compensation by 20–24 weeks | Negative | Increased births per female |
| Davis & Pech (2002) | Based on red fox (Vulpes vulpes) | S | Non‐specific: 100% | 0–100% of females | Absent density dependence, density increased with up to ~50% treatment | Negative | Increased survival |
| Merrill, Cooch & Curtis (2006) | White‐tailed deer (Odocoileus virginianus) | S | Non‐specific: 100% | 30%, 45%, 60% of females | No population reduction if movement rates were >25% of observed and treatment was <45% | Negative | Increased immigration |
| Ramsey (2005) | Brushtail possum (Trichosurus vulpecula) | E | Tubal ligation: 100% | 0%, 50%, 80% of females | 60% recruitment for 50% treated/74% for 80% treated: no population effect | Negative | Increased immigration, increased survival |
| Kirkpatrick & Turner (2008) | Feral horse (Equus caballus) | E | PZP: 0–8% birth rate in females | 42–76% of females | Population decreased 22·8% in 11 years | Negative | Increased survival |
| Jacob, Singleton & Hinds (2008) | Ricefield rat (Rattus argentiventer) | E | Tubal ligation, progesterone: 100% | Up to 76% of females | No population effect in open population | Negative | Increased births, increased survival |
| Budke & Slater (2009) | Feral cat (Felis catus), closed population | S | Surgical or contraception: 100% | 0–30% of females | 91% of adult females need to be sterile to stop population growth | Negative | Increased survival |
A broad consensus among both empirical and simulated studies is that in most free‐roaming wildlife populations, a majority of the individuals in one or both sexes must be infertile to realize meaningful reduction in population growth. Reduction in abundance can require aggressively maintained treatment for long periods of time (Hobbs, Bowden & Baker 2000). This can mean that >50% of females must be treated in closed populations of long‐lived, low‐fecundity species to achieve moderate reduction in growth (Hone 1992; Hobbs, Bowden & Baker 2000), and perhaps, >90% of females must be treated in open populations where immigration can compensate for birth reductions (Merrill, Cooch & Curtis 2006). Controlling populations of short‐lived, high‐fecundity animals is even more daunting because the high recruitment rates require fertility control treatments to be applied frequently (Singleton et al. 2002; Williams et al. 2007; Jacob, Singleton & Hinds 2008). In some applications, the lag time between treatment and effect on birth rates can result in too many new fertile females being recruited into the population for treatment strategies to be efficiently applied, even in long‐lived, low‐fecundity species (Nielsen, Porter & Underwood 1997; Whyte, van Aarde & Pimm 1998). Achieving population goals using fertility control for highly promiscuous breeders may not be feasible (Caughley, Pech & Grice 1992).
Some simulations have concluded that the response of a population to fertility control is nonlinear, and if survival increases as fecundity declines, then the survival is more influential on population growth than births (Hone 1992; Sinclair 1997; Hobbs, Bowden & Baker 2000; Merrill, Cooch & Curtis 2003). The logical conclusion to this is that fertility control is generally more effective at maintaining populations than actually reducing them, and thus, effective use of fertility control to maintain a population at reduced abundance may require culling to get the population to the desired level within a manageable time frame (Hone 1992; Barlow, Kean & Briggs 1997; Hobbs, Bowden & Baker 2000). Perhaps, most alarming is that many of these simulations assume 100% sterility, which is generally only achieved using surgical applications. Physically treating such large numbers of animals may be prohibitive due to the labour, time and expense necessary for capture, handling and application.
In contrast to these discouraging results, there is some evidence that fertility control can reduce and maintain populations at desired targets for abundance. Female feral horses have a long lifespan and can potentially produce offspring annually for up to 20 years of their life. A fertility control study at Assateague Island, the USA, reported zero population growth in only 2 years, with 42–76% of adult female feral horses treated annually with PZP (Kirkpatrick & Turner 2008). Population decline began 8 years after treatment began, and by year 11, the population had decreased nearly 23%. This sharply contrasts most simulation predictions, but may be explained by the indirect effects of treatment, the population parameters, life‐history strategies of the species and the continual accessibility to individuals for treatment. This example reflects a relatively small, closed population of a polygynous species with strong social hierarchies. Thus, the lack of immigration and potentially reduced fecundity in subordinate females may act to supplement the effects of fertility control at the population level. Residual effects of treatment and decreased birth rate among untreated females contributed to a 33% difference in expected and realized births in another study of PZP in feral horses (Ransom 2012). Survival, however, appears to have increased in older females in both studies, which partially offsets the effects of fertility control. This complex system of feedbacks demands a deeper understanding of the influences acting on vital rates in populations proposed for control, and before we categorically dismiss the use of fertility control to functionally reduce populations, we must carefully consider how those feedbacks are operating.
Implications towards fitness
The genetic consequences of controlling any individual's fertility could have long‐term effects on population fitness. Such artificial selection can theoretically occur in two ways. First, by purposeful or inadvertent non‐random selection of animals targeted for contraception, managers may prevent reproduction in wildlife in ways that change gene flow in a population. This would have genetic effects similar to those that may arise from traditional means of wildlife management, such as non‐random lethal removal of individuals from the gene pool. The second and far more uncertain mechanism is that inferred by the animal itself. Behavioural decisions unique to individuals shape their fitness, especially among males (Smith & Blumstein 2008). Such may be the case when polygynous males divert reproductive behaviour or when agonism increases or decreases in response to fertility control in the population. The age of individuals also interacts with demographic parameters of the population, as well as environmental variation, to influence fitness (Gaillard et al. 2000).
Individual physiology introduces a special consideration in the case of immunocontraceptives, and some authors have questioned the soundness of using a wild animal's immune system to select for the ability to reproduce (Nettles 1997; Cooper & Herbert 2001; Cooper 2004; Cooper & Larsen 2006). Immunocontraceptives are typically <100% effective at preventing pregnancy because they impose their effects through stimulation of the immune system. Depending on the magnitude of heritability, it is possible to select for decreased immune function and likely decreased fitness in relatively few generations. For example, if 10% of females in a population fail to mount a significant antibody response to an immunocontraceptive vaccine, and that phenotype has 80% heritability, then approximately 20% of female progeny will likewise not respond (Cooper & Larsen 2006). Thus, the trait has doubled in a single generation, assuming all females within the population were vaccinated. This has the potential to lead to immune incompetence and resistance to immunocontraception in the population. If immune responses responsible for contraception are mediated through the same or similar genetic pathways as those responsible for responding to pathogens and disease states, it is possible and even likely to select for decreased population fitness.
Alternatively, if a large proportion of the variation in contraceptive response is attributable to the environment, then even intense selection will have little effect on the phenotype of future generations (Magiafoglou et al. 2003). Difficulties in estimating heritability and finding reliable, accurate and sensitive indicators of changes in phenotype of immune function, given multigene effects on the system, pose significant challenges to resolve this question. However, there is evidence from studies with mice, chickens and pigs that strongly suggest that antibody production, delayed type hypersensitivity and phagocytic activity are heritable traits (Mouton, Sant'Anna & Biozzi 1988; Sarker et al. 1999; Wilkie & Mallard 1999; Pinard‐van der Laan 2002). Significant changes in both humoral and cell‐mediated immune responses can be achieved in as few as three generations in controlled environments (Sarker et al. 1999). Genetic variability in phenotype can change in response to environmental conditions (Hoffman 1999). This emphasizes the need for both laboratory and field studies of immunocontraceptives when investigating variance in immune response.
The proportion of the population targeted for fertility control will also influence selection pressure applied by non‐response. From a practical standpoint, managers are likely to treat as many animals as possible when beginning a fertility control programme, particularly in extensively managed populations that are above management objectives. This will maximize non‐response selection pressure (Garrott 1995). Targeting for contraception may be more discriminating in intensively managed feral horse herds by selecting only a proportion of the population and ensuring that each female contributes an offspring to the population prior to contraception (Nuñez, Adelman & Rubenstein 2010). This strategy would result in decreased selection for non‐response. Additionally, immigration and emigration will affect gene flow in the population and dilute the selection pressure. Treatment application intensity, non‐response rate, change in fitness and migration will all influence the strength of artificial selection. This is one aspect of fertility control that wildlife managers have little, if any, valid data with which to make an informed decision. A cross‐disciplinary approach involving immunologists, reproductive scientists, population or conservation geneticists and wildlife biologists may begin to answer these questions.
Conclusion
Study of individual effects of fertility control applications is an important step towards technology development of potential tools for managing wildlife, but understanding their ecological effects is a critical prerequisite for using them. Natural systems and processes are strained under anthropogenic pressures and ever‐fragmenting landscapes, and resources to address these challenges are often lacking. Actions such as population‐level fertility control demand that practitioners weigh not only the biological and ecological costs, but also the economic, political and regulatory costs involved for implementation.
Definitive generalizations about the biological and ecological conclusions of fertility control across taxa and biomes remain elusive. Primary empirical studies are still needed to evaluate population‐level effects of fertility control on large wildlife populations over long time periods. Given the resources necessary to conduct such studies, it is likely that most future insight will arise from adaptive management applications. As such, fertility control as a management tool must be applied in thoughtful, calculated ways to achieve the greatest likelihood of success at reducing or maintaining population numbers. Current evidence suggests that population‐level management of wildlife using fertility control is more likely to be successful if it is applied to populations (i) that are relatively small, (ii) that have little or no opportunity for an influx of new animals, (iii) that are comprised of a species that exhibits a relatively long gestation period and (iv) where a large proportion of individual animals can be accessed and aggressively treated. Fertility control management of non‐native species might be more successful than management of native species because non‐natives have not evolved in the ecosystem of concern, and the system is not dependent upon the species for natural function. For these reasons, alterations to life history, natural selection or behaviour may be also more publically acceptable, as long as they do not affect the conservation of native wildlife species. This hypothesis has not been tested to date. Likewise, applications to wildlife species occupying highly disturbed ecosystems may be effective because there may be disproportionately large influences from anthropogenic sources (i.e. where ecosystem drivers are more dependent on human actions than on natural processes), and the effects of fertility control on natural processes may be relatively minor in comparison.
If the necessary biological and ecological conditions can be met for effective use of fertility control to regulate wildlife populations, it is critical to address regulatory, political and economic concerns through public discourse and debate between agency, academic, political and public stakeholders who value their common wildlife resources for a wide variety of reasons. This includes transparent consideration of laws and regulations governing label and off‐label use of drugs and vaccines; the welfare of animals subjected to capture, handling and treatment; and public acknowledgement that the targeted population effect and the ability of a management tool to reach such goals are not absolute. Rigorous economic analysis of the cost‐effectiveness of fertility control relative to other management options is necessary to economically justify investing in the control actions (sensu Sebastián‐González et al. 2011). This requires quantitative evaluation of the population‐level and ecosystem‐level benefits of the proposed actions.
Robust quantitative tools are necessary for modelling outcomes due to the disparate responses observed across species, populations and control options. Feedbacks from fertility control application vary widely and can produce quite unexpected results. In some cases, unintended changes to births, survival, immigration or emigration could provide a formidable barrier to effective management of populations using fertility control. Scientists, managers and practitioners are thus faced with understanding the often confounding nature of the actions implemented to achieve goals for populations. Fertility control can lead to complex population‐level effects on the target species as well as cascading impacts on ecosystems. Both population‐level and ecosystem‐level impacts can and should be explored using a range of tools including empirical studies and simulation modelling. Expanding the prevailing birth‐centric research theme to more thoughtful data collection and modelling that considers births, deaths, immigration, emigration, gene flow and ecological interactions will improve the quality and utility of fertility control studies.
Acknowledgements
A special thank you to the late Francis Singer for pursuing the fertility control research that ultimately led to this manuscript. Preparation of this manuscript was funded by the U.S. Geological Survey Wildlife Program. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- Asa, C.S. (2005) Types of contraception: the choices Wildlife Contraception (eds Asa C.S. & Porton I.J.), pp. 29–65. Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- Asa, C.S. & Porton, I.J. (2005) The need for wildlife contraception Wildlife Contraception Issues, Methods, and Applications (eds Asa C.S. & Porton I.J.), pp. xxv–xxxii. Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- Baker, D.L. , Wild, M.A. , Conner, M.M. , Ravivarapu, H.B. , Dunn, R.L. & Nett, T.M. (2002) Effects of GnRH agonist (leuprolide) on reproduction and behaviour in female wapiti (Cervus elaphus nelsoni). Reproduction Supplement, 60, 155–167. [PubMed] [Google Scholar]
- Baker, D.L. , Wild, M.A. , Conner, M.M. , Ravivarapu, H.B. , Dunn, R.L. & Nett, T.M. (2004) Gonadotropin‐releasing hormone agonist: a new approach to reversible contraception in female deer. Journal of Wildlife Diseases, 40, 713–724. [DOI] [PubMed] [Google Scholar]
- Barlow, N.D. , Kean, J.M. & Briggs, C.J. (1997) Modelling the relative efficacy of culling and sterilisation for controlling populations. Wildlife Research, 24, 129–141. [Google Scholar]
- Bell, R.L. & Peterle, T.J. (1975) Hormone implants to control reproduction in white‐tailed deer. Wildlife Society Bulletin, 28, 152–156. [Google Scholar]
- Bertrand, M.R. , DeNicola, A.J. , Beissinger, S.R. & Swihart, R.K. (1996) Effects of parturition on home ranges and social affiliations of female white‐tailed deer. Journal of Wildlife Management, 60, 899–909. [Google Scholar]
- Boyce, M.S. , Sinclair, A.R.E. & White, G.C. (1999) Seasonal compensation of predation and harvesting. Oikos, 87, 419–426. [Google Scholar]
- Bromley, C. & Gese, E.M. (2001) Effects of sterilization on territory fidelity and maintenance, pair bonds, and survival rates of free‐ranging coyotes. Canadian Journal of Zoology, 79, 386–392. [Google Scholar]
- Buchholz, R. (2007) Behavioural biology: an effective and relevant conservation tool. Trends in Ecology and Evolution, 22, 401–407. [DOI] [PubMed] [Google Scholar]
- Budke, C.M. & Slater, M.R. (2009) Utilization of matrix population models to assess a 3‐year single treatment nonsurgical contraception program versus surgical sterilization in feral cat populations. Journal of Applied Animal Welfare Science, 12, 277–292. [DOI] [PubMed] [Google Scholar]
- Caley, P. & Ramsey, D. (2001) Estimating disease transmission in wildlife, with emphasis on leptospirosis and bovine tuberculosis in possums, and effects of fertility control. Journal of Applied Ecology, 38, 1362–1370. [Google Scholar]
- Caughley, G. (1981) Overpopulation Problems in Management of Locally Abundant Wild Mammals (eds Jewell P.A., Holt S. & Hart D.), pp. 7–19. Academic Press, New York, New York, USA. [Google Scholar]
- Caughley, G. , Pech, R. & Grice, D. (1992) Effect of fertility control on a population's productivity. Wildlife Research, 19, 623–627. [Google Scholar]
- Chambers, L.K. , Singleton, G.R. & Hinds, L.A. (1999) Fertility control of wild mouse populations: the effects of hormonal competence and an imposed level of sterility. Wildlife Research, 26, 579–591. [Google Scholar]
- Conner, M.M. , Baker, D.L. , Wild, M.A. , Powers, J.G. , Hussain, M.D. , Dunn, R.L. & Nett, T.M. (2007) Fertility control in free‐ranging elk using Gonadotropin‐Releasing Hormone Agonist Leuprolide: effects on reproduction, behavior, and body condition. Journal of Wildlife Management, 71, 2346–2356. [Google Scholar]
- Cook, J.C. , Johnson, B.K. , Cook, R.C. , Riggs, R.A. , Delcurto, T. , Bryant, L.D. & Irwin, L.L. (2004) Effects of summer‐autumn nutrition and parturition date on reproduction and survival of elk. Wildlife Monographs, 155, 1–61. [Google Scholar]
- Cooper, D.W. (2004) Should immunocontraception be used for wildlife population management? Australian Mammalogy, 26, 61–65. [Google Scholar]
- Cooper, D.W. & Herbert, C.A. (2001) Genetics, biotechnology and population management of over‐abundant mammalian wildlife in Australasia. Reproduction, Fertility, and Development, 13, 451–458. [DOI] [PubMed] [Google Scholar]
- Cooper, D.W. & Larsen, E. (2006) Immunocontraception of mammalian wildlife: ecological and immunogenetic issues. Reproduction, 132, 821–828. [DOI] [PubMed] [Google Scholar]
- Crawford, J.C. , Boulet, M. & Drea, C.M. (2010) Smelling wrong: hormonal contraception in lemurs alters critical female odour clues. Proceedings of the Royal Society B, 278, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel, S. (2001) Social dominance and stress hormones. Trends in Ecology & Evolution, 16, 491–497. [Google Scholar]
- Davis, S.A. & Pech, R.P. (2002) Dependence of population response to fertility control on the survival of sterile animals and their role in regulation. Reproduction Supplement, 60, 89–103. [PubMed] [Google Scholar]
- Delsink, A.K. , Van Altena, J.J. , Grobler, D. , Bertschinger, H. , Kirkpatrick, J. & Slotow, R. (2006) Regulation of a small, discrete, African elephant population through immunocontraception on the Makalali conservancy. South African Journal of Science, 102, 403–405. [Google Scholar]
- Diamond, J. (2002) Evolution, consequences and future of plant and animal domestication. Nature, 418, 700–707. [DOI] [PubMed] [Google Scholar]
- Dublin, H.T. (1983) Cooperation and reproductive competition among female African elephants Social Behaviour of Female Vertebrates (ed. Wasser S.K.), pp. 291–313. Academic Press, New York, USA. [Google Scholar]
- Fayrer‐Hosken, R.A. , Grobler, D. , Van Altena, J.J. , Bertschinger, H.J. & Kirkpatrick, J.F. (2000) Immunocontraception of African elephants. Nature, 407, 149. [DOI] [PubMed] [Google Scholar]
- Feh, C. (2012) Delayed reversibility of PZP (porcine zona pellucida) in free‐ranging Przewalski's horse mares. International Wild Equid Conference, pp. 69 University of Veterinary Medicine, Vienna, Austria. [Google Scholar]
- Gaillard, J.‐M. , Festa‐Bianchet, M. , Yoccoz, N.G. , Loison, A. & Toigo, C. (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annual Review of Ecology and Systematics, 31, 367–393. [Google Scholar]
- Garrott, R.A. (1995) Effective management of free‐ranging ungulate populations using contraception. Wildlife Society Bulletin, 23, 445–452. [Google Scholar]
- Gilman, R.T. , Mathews, N.E. , Skinner, B.G. , Julis, V.L. , Frank, E.S. & Paul‐Murphy, J. (2010) Effects of maternal status on the movement and mortality of sterilized female white‐tailed deer. Journal of Wildlife Management, 74, 1484–1491. [Google Scholar]
- Gray, M.E. & Cameron, E.Z. (2010) Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction, 139, 45–55. [DOI] [PubMed] [Google Scholar]
- Hadidian, J. (2009) The socioecology of urban wildlife management Wildlife and Society, the Science of Human Dimensions (eds Manfredo M.J., Vaske J.J., Brown P.J., Decker D.J. & Duke E.A.), pp. 202–213. Island Press, Washington D.C., USA. [Google Scholar]
- Harrenstien, L.A. , Munson, L. , Chassy, L.M. , Liu, I.K.M. & Kirkpatrick, J.F. (2004) Effects of porcine zona pellucida immunocontraceptives in zoo felids. Journal of Zoo and Wildlife Medicine, 35, 271–279. [DOI] [PubMed] [Google Scholar]
- Heilmann, T.J. , Garrott, R.A. , Cadwell, L.L. & Tiller, B.L. (1998) Behavioral response of free‐ranging elk treated with an immunocontraceptive vaccine. Journal of Wildlife Management, 62, 243–250. [Google Scholar]
- Hobbs, N.T. , Bowden, D.C. & Baker, D.L. (2000) Effects of fertility control on populations of ungulates: general, stage‐structured models. Journal of Wildlife Management, 64, 473–491. [Google Scholar]
- Hoffman, A.A. (1999) Heritable variation and evolution under favourable and unfavourable conditions. Trends in Ecology and Evolution, 14, 96–101. [DOI] [PubMed] [Google Scholar]
- Hone, J. (1992) Rate of increase and fertility control. Journal of Applied Ecology, 29, 695–698. [Google Scholar]
- Hone, J. (2007) Wildlife Damage Control. CSIRO Publishing, Victoria, Australia. [Google Scholar]
- Hynes, E.F. , Handasvde, K.A. , Shaw, G. & Renfree, M. (2011) The effects of gestagen implants on the behaviour of free‐ranging female koalas. Applied Animal Behaviour Science, 134, 209–216. [Google Scholar]
- Jacob, J. , Matulessy, J. & Sudarmaji,, (2004) Effects of imposed sterility on movement patterns of female ricefield rats. Journal of Wildlife Management, 68, 1138–1144. [Google Scholar]
- Jacob, J. , Singleton, G.R. & Hinds, L.A. (2008) Fertility control of rodent pests. Wildlife Research, 35, 487–493. [Google Scholar]
- Ji, W. , Clout, M.N. & Sarre, S.D. (2000) Responses of male brushtail possums to sterile females: implications for biological control. Journal of Applied Ecology, 7, 926–934. [Google Scholar]
- Kirkpatrick, J.F. , Lyda, R.O. & Frank, K.M. (2011) Contraceptive vaccines for wildlife: a review. American Journal of Reproductive Immunology, 66, 40–50. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, J.F. & Turner, J.W. Jr (1991) Compensatory reproduction in feral horses. The Journal of Wildlife Management, 55, 649–652. [Google Scholar]
- Kirkpatrick, J.F. & Turner, A. (2007) Immunocontraception and increased longevity in equids. Zoo Biology, 26, 237–244. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, J.F. & Turner, A. (2008) Achieving population goals in a long‐lived wildlife species (Equus caballus) with contraception. Wildlife Research, 35, 513–519. [Google Scholar]
- Kirkpatrick, J.F. , Naugle, R. , Liu, I.K.M. , Bernoco, M. & Turner, J.W. Jr (1995) Effects of seven consecutive years of porcine zona pellucida contraception on ovarian function in feral mares. Biology of Reproduction Monograph Series 1, 6, 411–418. [Google Scholar]
- Knuth, B.A. , Siemer, W.F. , Duda, M.D. , Bissell, S.J. & Decker, D.J. (2001) Wildlife management in suburban environments Human Dimensions of Wildlife Management in North America (eds Decker D.J., Brown T.L. & Siemer W.F.), pp. 219–242. The Wildlife Society, Bethesda, Maryland, USA. [Google Scholar]
- Lauber, T.B. & Knuth, B.A. (2004) Effects of information on attitudes toward suburban deer management. Wildlife Society Bulletin, 32, 322–331. [Google Scholar]
- Lauber, T.B. , Knuth, B.A. , Tantillo, J.A. & Curtis, P.D. (2007) The role of ethical judgements related to wildlife fertility control. Society and Natural Resources, 20, 119–133. [Google Scholar]
- Leong, K.M. (2010) The tragedy of becoming common: landscape change and perceptions of wildlife. Society and Natural Resources, 23, 111–127. [Google Scholar]
- Linn, G.S. & Steklis, H.D. (1990) The effects of depo‐medroxyprogesterone acetate (DMPA) on copulation‐related and agonistic behaviors in an island colony of stumptail macaques (Macaca arctoides). Physiology & Behavior, 47, 403–408. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Qu, J. , Wang, Z. & Wang, Y.‐l., Zhang, Y. & Zhang, Z., (2012) Behavioral mechanisms of male sterilization on plateau pika in Qinghai‐Tibet plateau. Behavioural Processes, 89, 278–285. [DOI] [PubMed] [Google Scholar]
- Madosky, J.M. , Rubenstein, D.I. , Howard, J.J. & Stuska, S. (2010) The effects of immunocontraception on harem fidelity in a feral horse (Equus caballus) population. Applied Animal Behaviour Science, 128, 50–56. [Google Scholar]
- Magiafoglou, A. , Schiffer, M. , Hoffman, A.A. & McKechnie, S.W. (2003) Immunocontraception for population control: will resistance evolve? Immunology and Cell Biology, 81, 152–159. [DOI] [PubMed] [Google Scholar]
- McShea, W.J. , Monfort, S.L. , Hakim, S. , Kirkpatrick, J. & Liu, I. (1997) The effect of immunocontraception on the behavior and reproduction of white‐tailed deer. Journal of Wildlife Management, 61, 560–569. [Google Scholar]
- Mendes‐de‐Almeida, F. , Remy, G.L. , Gershony, L.C. , Rodrigues, D.P. , Chame, M. & Labarthe, N.V. (2011) Reduction of feral cat (Felis catus Linnaeus 1758) colony size following hysterectomy of adult female cats. Journal of Feline Medicine and Surgery, 13, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, J.A. , Cooch, E.G. & Curtis, P.D. (2003) Time to reduction: factors influencing management efficacy in sterilizing overabundant white‐tail deer. Journal of Wildlife Management, 67, 267–279. [Google Scholar]
- Merrill, J.A. , Cooch, E.G. & Curtis, P.D. (2006) Managing an overabundant deer population by sterilization: effects of immigration, stochasticity, and the capture process. Journal of Wildlife Management, 70, 268–277. [Google Scholar]
- Mouton, D. , Sant'Anna, O. & Biozzi, G. (1988) Multigenic control of specific and non‐specific immunity in mice. Livestock Production Science, 20, 277–286. [Google Scholar]
- Nettles, V.F. (1997) Potential consequences and problems with wildlife contraceptives. Reproduction, Fertility, and Development, 9, 137–143. [DOI] [PubMed] [Google Scholar]
- Nielsen, C.K. , Porter, W.F. & Underwood, H.B. (1997) An adaptive management approach to controlling suburban deer. Wildlife Society Bulletin, 25, 470–477. [Google Scholar]
- Nuñez, C.M.V. (2009) Management of wild horses with porcine zona pellucida: history, consequences, and future strategies Horses: Biology, Domestication, and Human Interactions (ed. Leffhalm J.E.). Nova Science Publishers, Hauppauge, New York, USA. [Google Scholar]
- Nuñez, C.M.V. , Adelman, J.S. & Rubenstein, D.I. (2010) Immunocontraception in wild horses (Equus caballus) extends reproductive cycling beyond the normal breeding season. PLoS ONE, 5, e13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez, C.M.V. , Adelman, J.S. , Mason, C. & Rubenstein, D.I. (2009) Immunocontraception decreases group fidelity in a feral horse population during the non‐breeding season. Applied Animal Behaviour Science, 117, 74–83. [Google Scholar]
- Penfold, L.M. , Patton, M.L. & Jochle, W. (2005) Contraceptive agents in aggression control Wildlife Contraception: Issues, Methods, and Applications (eds Asa C.S. & Porton I.J.), pp. 184–194. Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- Pinard‐van der Laan, M.H. (2002) Immune modulation: the genetic approach. Veterinary Immunology and Immunopathology, 87, 199–205. [DOI] [PubMed] [Google Scholar]
- Poiani, A. , Coulson, G. , Salamon, D. , Holland, S. & Nave, C.D. (2002) Fertility control of eastern grey kangaroos: do levonorgestrel implants affect behavior? Journal of Wildlife Management, 66, 59–66. [Google Scholar]
- Porton, I.J. (2005) Ethics of wildlife contraception Wildlife Contraception (eds Asa C.S. & Porton I.J.), pp. 3–16. Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- Portugal, M.M. & Asa, C.S. (1995) Effects of chronic melengestrol acetate contraceptive treatment on perineal tumescence, body weight, and sociosexual behavior of hamadryas baboons (Papio hamadryas). Zoo Biology, 14, 251–259. [Google Scholar]
- Powell, D.M. & Monfort, S.L. (2001) Assessment: effects of porcine zona pellucida immunocontraception on estrous cyclicity in feral horses. Journal of Applied Animal Welfare Science, 4, 271–284. [DOI] [PubMed] [Google Scholar]
- Powers, J.G. , Baker, D.L. , Davis, T.L. , Conner, M.M. , Lothridge, A.H. & Nett, T.M. (2011) Effects of Gonadotropin‐Releasing Hormone immunization on reproductive function and behavior in captive female Rocky Mountain Elk (Cervus elaphus nelsoni). Biology of Reproduction, 85, 1152–1160. [DOI] [PubMed] [Google Scholar]
- Ramsey, D. (2005) Population dynamics of brushtail possums subject to fertility control. Journal of Applied Ecology, 42, 348–360. [Google Scholar]
- Ransom, J.I. (2012) Population Ecology of Feral Horses in an Era of Fertility Control Management. Ph.D. Colorado State University, USA. [Google Scholar]
- Ransom, J.I. , Cade, B.S. & Hobbs, N.T. (2010) Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses. Applied Animal Behaviour Science, 124, 51–60. [Google Scholar]
- Ransom, J.I. , Hobbs, N.T. & Bruemmer, J. (2013) Contraception can lead to trophic asynchrony between birth pulse and resources. PLoS ONE, 8, e54972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind, S.M. (2004) Effects of maternal nutrition on fetal and neonatal reproductive development and function. Animal Reproduction Science, 82–83, 169–181. [DOI] [PubMed] [Google Scholar]
- Rudolph, B.A. , Porter, W.F. & Underwood, H.B. (2000) Evaluating immunocontraception for managing suburban white‐tailed deer in Irondequoit, New York. Journal of Wildlife Management, 64, 463–473. [Google Scholar]
- Rutberg, A.T. & Naugle, R.E. (2007) Population‐level effects of immunocontraception in white‐tailed deer (Odocoileus virginianus). Wildlife Research, 35, 494–501. [Google Scholar]
- Sarker, N. , Tsudzuki, M. , Nishibori, M. & Yamamoto, Y. (1999) Direct and correlated response to divergent selection for serum immunoglobulin M and G levels in chickens. Poultry Science, 78, 1–7. [DOI] [PubMed] [Google Scholar]
- Saunders, G. , McIlroy, J. , Berghout, M. , Kay, B. , Gifford, E. , Perry, R. & Van De Ven, R. (2002) The effects of induced sterility on the territorial behaviour and survival of foxes. Journal of Applied Ecology, 39, 56–66. [Google Scholar]
- Schulte, B.A. , Feldman, E. , Lambert, R. , Oliver, R. & Hess, D.L. (2000) Temporary ovarian inactivity in elephants: relationship to status and time outside. Physiology and Behavior, 71, 123–131. [DOI] [PubMed] [Google Scholar]
- Seagle, S.W. & Close, J.D. (1996) Modeling white‐tailed deer Odocoileus virginianus population control by contraception. Biological Conservation, 76, 87–91. [Google Scholar]
- Sebastián‐González, E. , Sánchez‐Zapata, J.A. , Botella, F. , Figuerola, J. , Hiraldo, F. & Wintle, B.A. (2011) Linking cost efficiency evaluation with population viability analysis to prioritize wetland bird conservation actions. Biological Conservation, 144, 2354–2361. [Google Scholar]
- Shumake, S.A. & Wilhelm, E.S. (1995) Comparisons of effects of four immunocontraceptive treatments on estrous cycle and rutting behavior in captive white‐tailed deer. pp. 9. Denver Wildlife Research Center Product Development Section Progress Report, Denver, Colorado, USA.
- Sibly, R.M. & Hone, J. (2002) Population growth rate and its determinates: an overview. Philosophical Transactions of the Royal Society B, 357, 1153–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, A.E. (1997) Fertility control of mammal pests and the conservation of endangered marsupials. Reproduction, Fertility, and Development, 9, 1–16. [DOI] [PubMed] [Google Scholar]
- Singleton, G.R. , Farroway, L.N. , Chambers, L.K. , Lawson, M.A. , Smith, A.L. & Hinds, L.A. (2002) Ecological basis for fertility control in the house mouse (Mus domesticus) using immunocontraceptive vaccines. Reproduction Supplement, 60, 31–39. [PubMed] [Google Scholar]
- Smith, B.R. & Blumstein, D.T. (2008) Fitness consequences of personality: a meta‐analysis. Behavioral Ecology, 19, 448–455. [Google Scholar]
- Snape, M.A. , Hinds, L.A. & Miller, L.A. (2011) Administration of the GnRH‐targeted immunocontraceptive vaccine ‘GonaConTM’ to the tammar wallaby, Macropus eugenii: side‐effects and welfare implications 8th European Vertebrate Pest Management Conference (eds Jacob J. & Esther A.), pp. 114 Julius Kühn‐Institut, Berlin, Germany. [Google Scholar]
- Stoops, M.A. , Liu, I.K.M. , Shideler, S.E. , Lasley, B.L. , Fayrer‐Hosken, R.A. , Benirschke, K. , Murata, K. , Van Leeuwen, E.M.G. & Anderson, G.B. (2006) Effects of porcine zona pellucida immunisation on ovarian follicular development and endocrine function in domestic ewes (Ovis aries). Reproduction, Fertility, and Development, 18, 667–676. [DOI] [PubMed] [Google Scholar]
- Sutherland, W.J. (1998) The importance of behavioural studies in conservation biology. Animal Behaviour, 56, 801–809. [DOI] [PubMed] [Google Scholar]
- Swenson, J.E. (1985) Compensatory reproduction in an introduced mountain goat population in the Absaroka Mountains, Montana. Journal of Wildlife Management, 49, 837–843. [Google Scholar]
- Teel, T.L. & Manfredo, M.J. (2009) Understanding the diversity of public interests in wildlife conservation. Conservation Biology, 24, 128–139. [DOI] [PubMed] [Google Scholar]
- Turner, A. & Kirkpatrick, J.F. (2002) Effects of immunocontraception on population, longevity and body condition in wild mares (Equus caballus). Journal of Reproduction and Fertility Supplement, 60, 187–195. [PubMed] [Google Scholar]
- Twigg, L.E. , Lowe, T.J. , Martin, G.R. , Wheeler, A.G. , Gray, G.S. , Griffin, S.L. & M., O.R.C., Robinson, D.J. & Hubach, P.H., (2000) Effects of surgically imposed sterility on free‐ranging rabbit populations. Journal of Applied Ecology, 37, 16–39. [Google Scholar]
- White, L.M. , Warren, R.J. & Fayrer‐Hosken, R.A. (1994) Levonorgestrel implants as a contraceptive in captive white‐tailed deer. Journal of Wildlife Diseases, 30, 241–246. [DOI] [PubMed] [Google Scholar]
- Whyte, I. , van Aarde, R. & Pimm, S.L. (1998) Managing the elephants of Kruger National Park. Animal Conservation, 1, 77–83. [Google Scholar]
- Wilkie, B. & Mallard, B. (1999) Selection for high immune response: an alternative approach to animal health maintenance. Veterinary Immunology and Immunopathology, 72, 231–235. [DOI] [PubMed] [Google Scholar]
- Williams, J.S. (1999) Compensatory reproduction and dispersal in an introduced mountain goat population in central Montana. Wildlife Society Bulletin, 27, 1019–1024. [Google Scholar]
- Williams, C.K. , Davey, C.C. , Moore, R.J. , Hinds, L.A. , Silvers, L.E. , Kerr, P.J. et al (2007) Population responses to sterility imposed on female european rabbits. Journal of Applied Ecology, 44, 291–301. [Google Scholar]


