ABSTRACT
The inflammasome is a major regulator of inflammation through its activation of procaspase-1, which cleaves prointerleukin-1β (pro-IL-1β) into its mature form. IL-1β is a critical proinflammatory cytokine that dictates the severity of inflammation associated with a wide spectrum of inflammatory diseases. NLRP3 is a key component of the inflammasome complex, and multiple signals and stimuli trigger formation of the NLRP3 inflammasome complex. In the current study, we uncovered a yet unknown mechanism of NLRP3 inflammasome activation by a pathogen-derived factor. We show that the unique bacterial ADP-ribosylating and vacuolating toxin produced by Mycoplasma pneumoniae and designated community-acquired respiratory distress syndrome (CARDS) toxin activates the NLRP3 inflammasome by colocalizing with the NLRP3 inflammasome and catalyzing the ADP-ribosylation of NLRP3. Mutant full-length CARDS toxin lacking ADP-ribosyltransferase (ADPRT) activity and truncated CARDS toxins unable to bind to macrophages and be internalized failed to activate the NLRP3 inflammasome. These studies demonstrate that CARDS toxin-mediated ADP-ribosylation constitutes an important posttranslational modification of NLRP3, that ADPRT activity of CARDS toxin is essential for NLRP3 inflammasome activation, and that posttranslational ADPRT-mediated modification of the inflammasome is a newly discovered mechanism for inflammasome activation with subsequent release of IL-1β and associated pathologies.
IMPORTANCE
Inflammation is a fundamental innate immune response to environmental factors, including infections. The inflammasome represents a multiprotein complex that regulates inflammation via its ability to activate specific proinflammatory cytokines, resulting in an effective host protective response. However, excessive release of proinflammatory cytokines can occur following infection that skews the host response to “hyperinflammation” with exaggerated tissue damage. Mycoplasma pneumoniae, a common bacterial airway pathogen, possesses a unique protein toxin with ADP-ribosyltransferase and vacuolating properties capable of reproducing the robust inflammation and cytopathology associated with mycoplasma infection. Here, we show that the toxin uniquely activates the NLRP3 inflammasome by colocalizing with and ADP-ribosylating NLRP3, possibly leading to “hyperinflammation” and thus uncovering a novel target for therapeutic intervention.
INTRODUCTION
The NLRP3 inflammasome plays a critical role in inflammation through activation of procaspase-1, which cleaves prointerleukin-1β (pro-IL-1β) into its mature form (1–9). As a pyrogenic cytokine, IL-1β amplifies proinflammatory responses during infection with various pathogens (viruses, bacteria, fungi, and parasites) (1–9). IL-1β produced from infected cells acts via an autocrine/paracrine mechanism to activate NF-κB/mitogen-activated protein (MAP) kinase-dependent proinflammatory cytokines and chemokines in order to establish an effective immune response for combating infection. IL-1β can also act as a “double-edged sword,” since inflammasome activation (and subsequent IL-1β release) following infection can skew the proinflammatory response to “hyperinflammation,” which culminates in enhanced tissue damage and exaggerated infection-associated pathologies. Apart from pathogens, inflammasome activation triggered by exogenous allergens contributes to lung remodeling and the development of chronic airway diseases, like asthma and chronic obstructive pulmonary disease (COPD) (10–12). Thus, inflammasome-dependent IL-1β production is a key regulator of host response to exogenous factors (pathogens, allergens, etc.) and to disease outcomes.
Macrophages play important roles in regulating the immune response during infection (13). IL-1β release from macrophages requires three steps: (i) pro-IL-1β gene expression and synthesis of immature pro-IL-1β protein, (ii) pro-IL-1β cleavage by active caspase-1 to generate the mature form of IL-1β, and (iii) mature IL-1β secretion into the extracellular environment (1–9). The generation of mature IL-1β requires cytoplasmic assembly and activation of inflammasomes (1–9, 14, 15). The multiprotein NLR (nucleotide binding oligomerization domain-like receptor) inflammasome complex is comprised of caspase-1, NLR proteins, and adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain). The most well-characterized NLR inflammasome complex is comprised of NLR protein NLRP3 (NOD-like receptor family, pyrin domain containing 3; also known as NALP3 and cryopyrin), ASC, and caspase-1. NLRP3-ASC oligomerization results in caspase-1 recruitment to the complex, after which caspase-1 undergoes autocatalytic processing to generate enzymatically active caspase-1, which is involved in cleaving the precursor pro-IL-1β into its mature secreted form.
Macrophages need two signals to activate the inflammasome. The first signal (signal 1) is required for expression of pro-IL-1β and inflammasome components (e.g., NLRP3). Signal 1 is initiated by the stimulation of pattern recognition receptors (PRRs) (e.g., Toll-like receptors [TLRs] and NOD-like receptors like Nod2) via pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). Once adequate amounts of pro-IL-1β protein accumulate in the cytoplasm, the second signal (signal 2) is required for inflammasome complex assembly and subsequent caspase-1 activation for cleaving pro-IL-1β into its mature form.
Pathogens stimulate signal 2 formation via several “indirect” mechanisms including the production of intracellular reactive oxygen species (ROS), potassium efflux due to pore formation by bacterial toxins, lysosomal disintegration leading to leakage of cathepsin B in the cytosol, production of DAMPs (e.g., ATP), etc. (1–9, 14, 15). So far, there has been no report of a pathogen-encoded factor(s) that interacts directly with the inflammasome complex to trigger its activation. In addition, posttranslational modification of inflammasome components by pathogen-derived molecules (or microbial virulence determinants) has not yet been reported.
In the current study, we uncovered a yet unknown mechanism by which pathogen-encoded factors can activate the inflammasome. We show that a unique bacterial ADP-ribosylating and vacuolating toxin produced by Mycoplasma pneumoniae and designated community-acquired respiratory distress syndrome (CARDS) toxin (16) interacts with the NLRP3 inflammasome complex to trigger its activation. Earlier, we reported that CARDS toxin alone is capable of inducing a robust inflammatory response and cytopathology that reproduces the infectious process (16, 17). Further, IL-1β is among the spectrum of proinflammatory cytokines that exhibit increased expression in mice following exposure to CARDS toxin (17). Mechanistically, we observed that full-length (FL) enzymatically active CARDS toxin directly catalyzes the ADP-ribosylation of the NLRP3 protein, while mutant CARDS toxin lacking ADP-ribosyltransferase (ADPRT) activity or mutant toxin unable to bind and be internalized failed to activate the NLRP3 inflammasome. Thus, our studies provide new insights relative to delineating mechanisms of inflammasome activation by pathogen-derived factors. Specifically, we show the following: (i) the interaction of pathogen-associated factor (i.e., CARDS toxin) with the inflammasome and (ii) the posttranslational modification (i.e., ADP-ribosylation) of the inflammasome component NLRP3 by M. pneumoniae-encoded CARDS toxin. In addition, we identified ADP-ribosylation as a unique posttranslational modification that facilitates inflammasome activation. Thus, it is plausible that during infection-associated and non-infection-associated inflammatory diseases, both pathogen-derived and cellular ADP-ribosylating factors modulate inflammasome function.
RESULTS
CARDS toxin activates NLRP3 inflammasome.
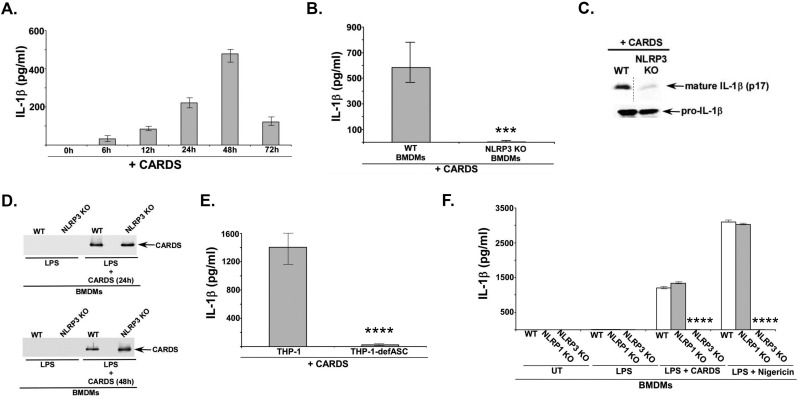
In order to investigate whether CARDS toxin acts as a second signal to activate the inflammasome, we examined the ability of CARDS toxin to release IL-1β from mouse primary bone marrow-derived macrophages (BMDMs) first primed with lipopolysaccharide (LPS) for 4 h (first signal) and then treated with either desalting buffer or CARDS toxin for 0 to 72 h. At each time point, medium supernatants were analyzed by IL-1β-specific enzyme-linked immunosorbent assay (ELISA). We observed activation of the inflammasome by CARDS toxin, since IL-1β production was significantly increased in CARDS toxin-treated cells (Fig. 1A). Further, our studies indicated that optimal IL-1β production by CARDS toxin could be achieved at 48 h after toxin treatment (Fig. 1A).
FIG 1 .
CARDS toxin activates NLRP3 inflammasome. (A) ELISA for IL-1β in medium supernatants of bone marrow-derived macrophages (BMDMs) treated with LPS (100 ng/ml) for 4 h, followed by vehicle buffer (50 mM Tris-HCl [pH 8.0] plus 5% glycerol) or CARDS toxin (+ CARDS) (700 pmol, 0 h to 72 h). The values shown represent the means ± standard deviations (error bars) from three independent experiments. The IL-1β data represent LPS-CARDS toxin-treated cells minus LPS-vehicle-treated cells. (B) ELISA for IL-1β in medium supernatants of wild-type (WT) and NLRP3 knockout (KO) BMDMs treated with LPS (100 ng/ml) for 4 h, followed by CARDS toxin (700 pmol, 48 h). The values represent the means ± standard deviations from three independent experiments performed in triplicate. The P values were calculated using Student’s t test and indicated as follows: ***, P < 0.001. (C) (Top panel) Western blot of mature IL-1β (p17) in the medium supernatants of WT and NLRP3 KO BMDM treated as indicated above. (Bottom panel) Western blot of pro-IL-1β in the cell lysates of WT and NLRP3 KO BMDMs treated as indicated above. (D) WT and NLRP3 KO BMDMs were treated with LPS (100 ng/ml) for 4 h, followed by either 24-h or 48-h treatment of cells with 700 pmol of CARDS toxin. Cell lysates were subjected to Western blot analysis with anti-CARDS toxin antibody. (E) ELISA for IL-1β in the medium supernatants of WT (THP-1) and ASC-deficient (THP-1-defASC) human THP-1 macrophage cell lines treated with 700 pmol of CARDS toxin for 48 h. Values represent the means ± standard deviations from three independent experiments performed in triplicate. The P values were calculated using a Student’s t test and indicated as follows: ****, P < 0.0001. (F) ELISA for IL-1β in medium supernatants of WT, NLRP3 KO, and NLRP1 KO BMDMs treated with LPS (100 ng/ml) for 4 h, followed by treatment of cells with either CARDS toxin (700 pmol, 48 h) or nigericin (15 µM, 30 min) (positive control for NLRP3 activation). Values represent the means ± standard deviations from three independent experiments performed in triplicate. The P values were calculated using ANOVA and indicated as follows: ****, P < 0.0001.
Since CARDS toxin can function as an activator of the inflammasome, we next investigated the nature of CARDS toxin-mediated inflammasome activation. For these studies, we treated wild-type (WT) and NLRP3 knockout (KO) BMDMs with CARDS toxin. CARDS toxin-mediated IL-1β release occurred via the NLRP3 inflammasome, consistent with the drastic loss of IL-1β production in NLRP3 KO cells (Fig. 1B). Diminished IL-1β secretion from NLRP3 KO BMDMs was due to reduced processing of pro-IL-1β to mature IL-1β (the latter is released from cells) based upon Western blot analysis of medium supernatant. For example, we detected markedly decreased mature forms of IL-1β (i.e., 17-kDa mature IL-1β or p17) in CARDS toxin-treated NLRP3 KO cells compared to WT BMDMs (Fig. 1C). However, the loss of mature IL-1β release in NLRP3 KO cells was not due to reduced levels of pro-IL-1β protein (Fig. 1C). Moreover, expression of procaspase-1 was similar in WT and NLRP3 KO cells (data not shown). Also, the loss of IL-1β release from NLRP3 KO cells was not due to differences in the concentrations of CARDS toxin protein, as we detected similar levels of CARDS toxin in WT and NLRP3 KO BMDM total cell lysates at both 24-h and 48-h time points (Fig. 1D).
Since NLRP3 requires adaptor protein ASC for inflammasome assembly and activation, we examined whether CARDS toxin-mediated NLRP3 inflammasome activation was dependent upon ASC. For these studies, we used wild-type and ASC-deficient human macrophage THP-1 cell lines. Treatment of these cells with CARDS toxin revealed that CARDS toxin triggered IL-1β release from WT THP-1 cells, which was drastically abrogated in ASC-deficient THP-1 cells (Fig. 1E). These results demonstrated a requirement of the NLRP3/ASC inflammasome complex for CARDS toxin-mediated IL-1β release. Furthermore, CARDS toxin-mediated IL-1β release from WT THP-1 human macrophages verified the observed results with murine BMDMs. Interestingly, IL-1β release by CARDS toxin specifically required the NLRP3 inflammasome, since CARDS toxin-mediated IL-1β release was observed in cells devoid of the NLRP1 inflammasome (i.e., NLRP1 KO BMDMs), another activator of IL-1β release (Fig. 1F). Thus, our study illustrated the ability of CARDS toxin to selectively activate the NLRP3 inflammasome, leading to IL-1β release.
ADPRT activity of internalized CARDS toxin is required for inflammasome activation and IL-1β release.
Since CARDS toxin possessed NLRP3 inflammasome-activating function (Fig. 1), we investigated the specific property of CARDS toxin essential for such activity. Previous studies have shown that CARDS toxin possesses both ADPRT and vacuolating activities (16, 18). Moreover, the addition of CARDS toxin to mammalian cells results in receptor-mediated binding and internalization (19–21). Therefore, in order to assess whether ADPRT, binding, and internalization activities of CARDS toxin were prerequisite for its inflammasome-activating function, we utilized full-length (FL) CARDS toxin, FL mutant CARDS toxin where the predicted catalytic (i.e., ADPRT activity) glutamate at position 132 was replaced by alanine (E132A), and CARDS toxin truncations designated CARDS249 (has amino acids 1 to 249), which lacks the C-terminal amino acids essential for cellular binding and internalization (16, 18), and 266CARDS (has amino acids 266 to 591), which lacks the essential N-terminal ADPRT amino acid motifs (Fig. 2A) but retains the vacuolating activity. It is important to note that both mutant FL CARDS E132A and truncated 266CARDS proteins exhibit binding and uptake properties comparable to those of WT FL CARDS toxin (18) (data not shown).
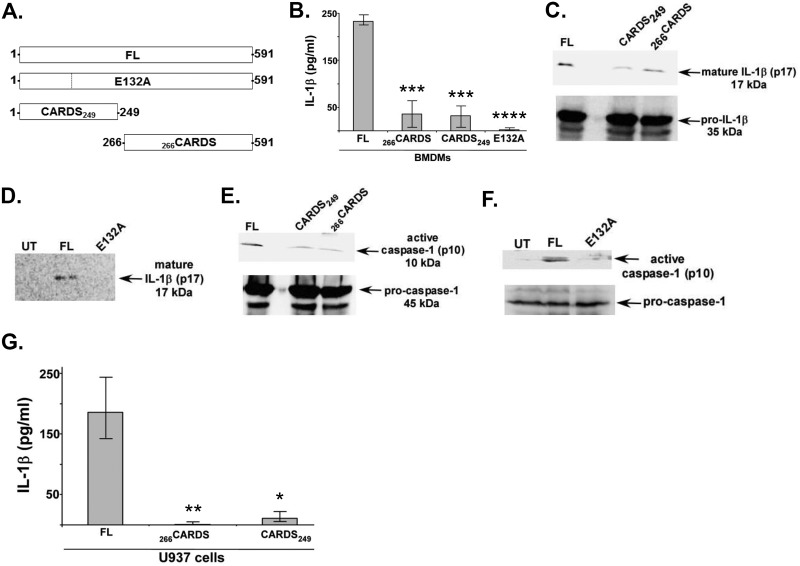
FIG 2 .
Wild-type FL CARDS toxin is required for inflammasome activation. (A) Schematic representations of FL CARDS toxin and its derivatives. FL CARDS toxin is comprised of 591 amino acids. The catalytic glutamic acid (E132) is modified to alanine to abolish ADPRT activity (FL mutant toxin E132A). The ADPRT motif containing amino acids 1 to 249 (CARDS249) or the cell binding region comprised of amino acids 266 to 591 (266CARDS) are expressed separately. (B) ELISA for IL-1β in medium supernatants of mouse WT BMDMs treated with LPS (100 ng/ml) for 4 h, followed by 24-h treatment of cells with 700 pmol of either FL CARDS toxin, CARDS E132A, CARDS249, or 266CARDS. Values represent the means ± standard deviations from three independent experiments performed in triplicate. The P values were calculated using ANOVA and indicated as follows: ***, P < 0.001; ****, P < 0.0001. (C) (Top panel) Western blot for mature IL-1β (p17) in the medium supernatants of BMDMs treated with FL CARDS toxin or CARDS toxin truncations (CARDS249 or 266CARDS) as described above for panel B. (Bottom panel) Western blot of cell lysates for pro-IL-1β showing equivalent amounts of precursor. (D) Western blot for mature IL-1β (p17) in medium supernatants of untreated (UT) and BMDMs treated with FL CARDS toxin or CARDS E132A. (E) (Top panel) Western blot for caspase-1 p10 subunit in the medium supernatants of BMDMs treated with FL CARDS toxin or CARDS249 or 266CARDS as described above for panel B. (Bottom panel) Western blot of cell lysate for procaspase-1 showing equivalent amounts of precursor. (F) Western blot for caspase-1 p10 subunit in the medium supernatants of untreated (UT) and BMDMs treated with FL CARDS toxin or CARDS E132A. (G) ELISA for IL-1β in medium supernatants of human U937 cells treated with LPS (1 µg/ml) for 4 h, followed by treatment of cells with 700 pmol of FL CARDS toxin or truncated CARDS249 or 266CARDS toxins. The values shown represent the means ± standard deviations from three independent experiments performed in triplicate. The P values were calculated using ANOVA and indicated as follows: *, P < 0.05; **, P < 0.01.
In order to elucidate the specific requirement for ADPRT-dependent inflammasome-activating function of CARDS toxin, we treated WT BMDMs with FL CARDS toxin, mutant CARDS E132A toxin, and CARDS toxin truncations CARDS249 and 266CARDS. As shown in Fig. 2B, we observed a significant loss of IL-1β release following treatment of BMDMs with FL mutant CARDS E132A protein and truncated CARDS249 and 266CARDS toxins, reinforcing the essential role of ADP-ribosylation in NLRP3 inflammasome activation. Further, the reduction in IL-1β release occurred due to diminished maturation of IL-1β, which is demonstrated in Western blot profiles showing decreased levels of mature IL-1β (p17) in CARDS249 and 266CARDS truncations (Fig. 2C) and in cells treated with CARDS E132A toxin relative to cells treated with WT FL CARDS toxin (Fig. 2D).
In order to monitor caspase-1 activation, we performed Western blotting and compared the gel pattern of procaspase-1 with that of the active p10 subunit of caspase-1. Detection of the p10 domain (p10 is cleaved only after caspase-1 activation) denotes the conversion between procaspase-1 to enzymatically active mature caspase-1. We observed markedly reduced caspase-1 activation by CARDS E132A, CARDS249, and 266CARDS (Fig. 2E and F). Furthermore, the results with BMDMs were validated in human macrophages, since treatment of U937 cells (human macrophage cell line) with WT FL CARDS toxin versus CARDS249 or 266CARDS revealed strikingly reduced IL-1β release compared to WT FL CARDS toxin (Fig. 2G). These results reinforce the essential role of CARDS toxin ADPRT activity in inflammasome activation, since both CARDS E132A and truncated 266CARDS, which lacks the N-terminal amino acid ADPRT motifs, are capable of binding and internalization but fail to activate the inflammasome. Predictably, truncated CARDS249, which contains the ADPRT domain but lacks the domain required for target cell binding and internalization, also cannot activate the inflammasome.
CARDS toxin induces NLRP3 inflammasome complex formation and associates with the inflammasome speck in BMDM cells.
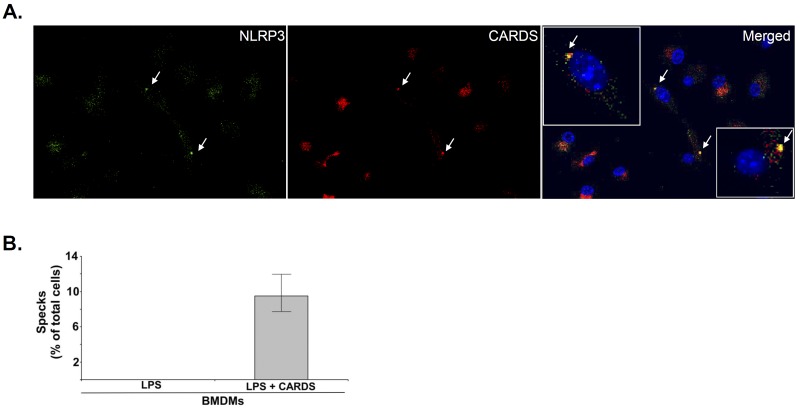
An essential feature of inflammasome activation is the formation of the NLRP3 inflammasome complex (represented by “speck”). Therefore, we monitored mCherry-tagged CARDS (mCherry-CARDS) toxin-induced speck formation in macrophages upon NLRP3 inflammasome activation. In CARDS toxin-treated BMDMs only, we observed the reorganization of endogenous cytoplasmic NLRP3 into single punctate structures at 24 h (Fig. 3A, green). These data are in agreement with the increase in IL-1β production in CARDS toxin-treated macrophages (Fig. 2). In addition, mCherry-CARDS toxin was distributed throughout the cytoplasm (red) and also accumulated around the perinuclear region. Importantly, mCherry-CARDS toxin colocalized with cytoplasmic NLRP3 and formed prominent puncta (specks) (Fig. 3A, merged image; yellow). To further quantify the extent of speck formation in CARDS toxin-treated BMDMs, we analyzed the percentage of cells (compared to the total number of cells) with NLRP3-CARDS toxin specks. Approximately 10% of the total number of CARDS toxin-treated cells exhibited NLRP3-CARDS toxin specks (Fig. 3B), which is consistent with previous reports using the well-established robust NLRP3 inflammasome inducer, nigericin. For example, nigericin treatment of BMDM cells resulted in speck formation in approximately 15% of cells relative to the total number of cells (22). Taken together, these data confirm that CARDS toxin not only triggers formation of speck but also associates with the NLRP3 inflammasome complex in the process of inflammasome activation.
FIG 3 .
Colocalization of CARDS toxin with NLRP3. (A) Coimmunofluorescence analysis of CARDS toxin (red) and NLRP3 (green) in mouse BMDMs. WT BMDMs were treated with LPS (100 ng/ml) for 4 h, followed by mCherry-tagged CARDS toxin (70 pmol) for 24 h. Cells were stained with mouse monoclonal antibody reactive against NLRP3 (1:300) and counterstained with goat anti-mouse IgG conjugated with FITC (green). Nuclei were stained with DAPI (blue). The merged image (yellow) shows colocalization of CARDS toxin with the NLRP3 inflammasome speck (white arrows). The boxed inserts in the merged panel show magnified views of speck-containing cells demonstrating both colocalization and distinct patterns of CARDS toxin and NLRP3 staining. (B) The number of specks in CARDS toxin-treated WT BMDMs was quantified and expressed as the percentage of specks per cell number as described in Materials and Methods. The percentage of cells with specks relative to the total number of cells is shown.
Interaction of NLRP3 with CARDS toxin.
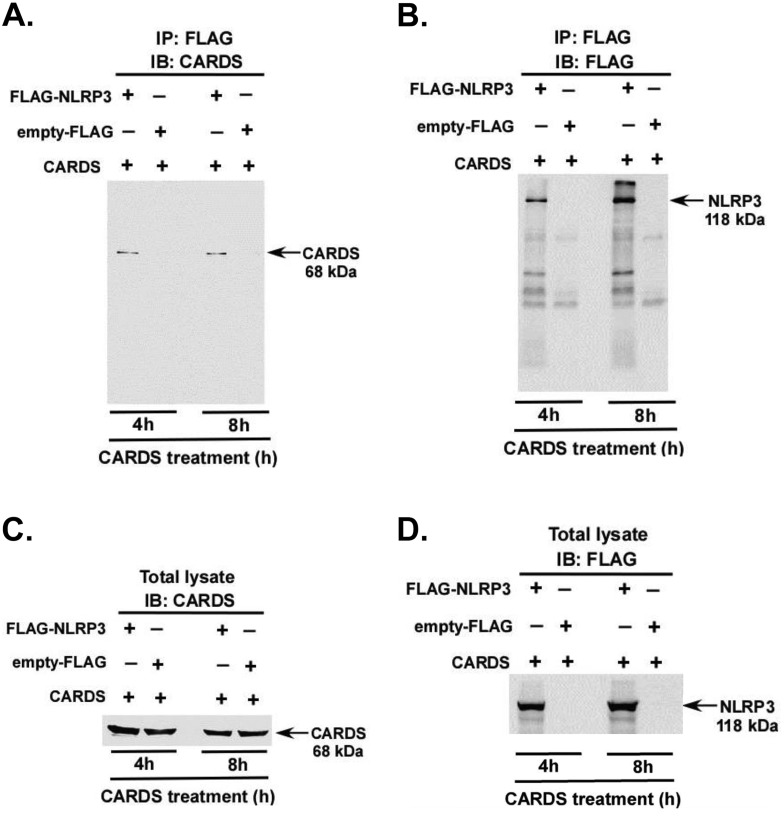
In order to further examine the interaction of CARDS toxin with NLRP3, we performed coimmunoprecipitation assays. For these studies, HEK 293 cells expressing either FLAG-tagged NLRP3 (FLAG-NLRP3) or empty FLAG were treated with WT FL CARDS toxin for 4 h and 8 h. Cell lysates were subjected to immunoprecipitation (IP) with FLAG antibody and immunoblotted (IB) with anti-CARDS toxin antibody. CARDS toxin interacted with NLRP3 at 4 h and 8 h posttreatment (Fig. 4A); no interaction of CARDS toxin with NLRP3 was detected at 0 h (data not shown). The presence of NLRP3 in the coimmunoprecipitated samples was confirmed by immunoblotting with FLAG antibody (Fig. 4B). Similar levels of intracellular NLRP3 and CARDS toxin proteins in the test samples were confirmed by immunoblotting total cell lysates with anti-FLAG and anti-CARDS toxin antibodies (Fig. 4C and D). Thus, these studies demonstrate the close association between CARDS toxin and NLRP3.
FIG 4 .
Interaction of CARDS toxin with NLRP3. (A) HEK 293 cells expressing either FLAG-NLRP3 (+) or empty FLAG (+) were treated with CARDS toxin (350 pmol) for 4 h and 8 h. Cell lysates were immunoprecipitated with anti-FLAG antibody (IP: FLAG), followed by immunoblotting with anti-CARDS toxin antibody (IB: CARDS). (B) As in panel A, except that immunoprecipitated samples were immunoblotted with anti-FLAG antibody. (C) Total lysates collected from CARDS toxin-treated HEK 293 cells expressing either FLAG-NLRP3 or empty FLAG were immunoblotted with anti-CARDS toxin antibody. (D) As in panel C, except that total cell lysates were immunoblotted with anti-FLAG antibody.
ADP-ribosylation of NLRP3 by CARDS toxin.
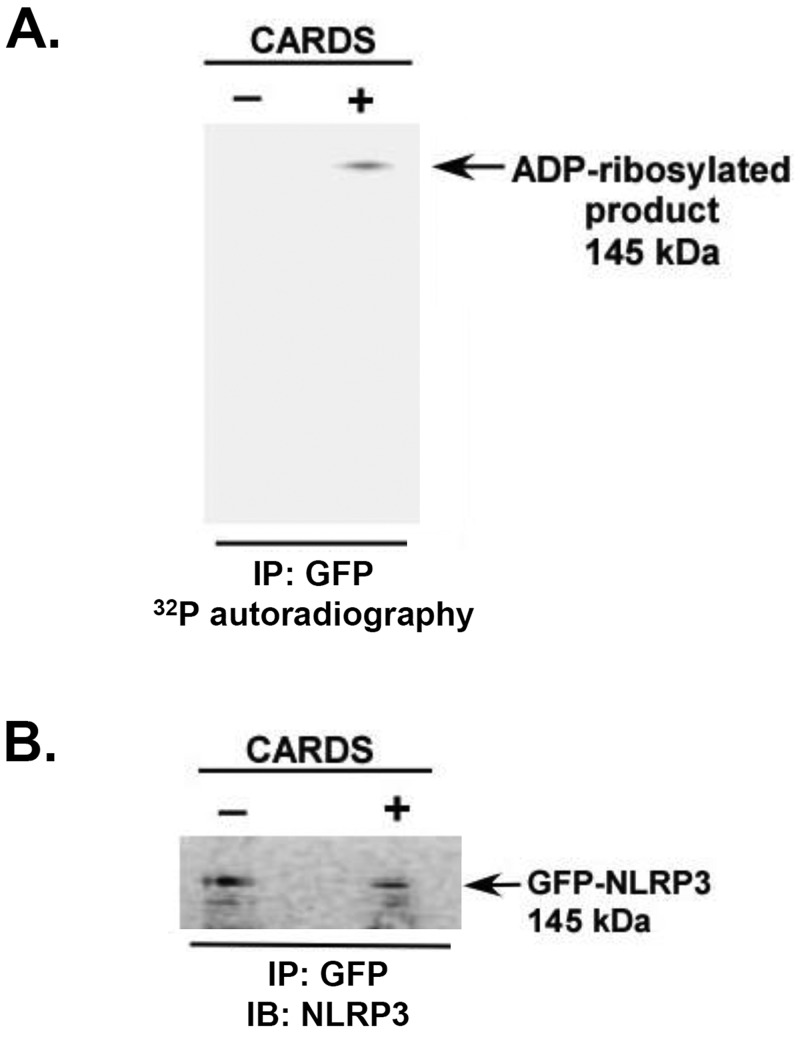
As described above, FL CARDS toxin coimmunoprecipitates with NLRP3, and its ADPRT activity appears essential for inflammasome activation. Therefore, we speculated that CARDS toxin directly ADP-ribosylates NLRP3. Such posttranslational modification of NLRP3 would represent a novel mechanism by which CARDS toxin mediates inflammasome-activating function. Thus, we performed in vitro ADP-ribosylation assays with WT FL CARDS toxin and green fluorescent protein (GFP)-tagged NLRP3 (GFP-NLRP3) expressed in HEK 293 cells. Cell lysates were treated with and without CARDS toxin (140 pmol) in the presence of 32P-NAD, radioimmunoprecipitated with anti-GFP antibody, resolved on Nu-PAGE, transferred to nitrocellulose membranes, and exposed to X-ray films. Autoradiograms demonstrated that NLRP3 was ADP-ribosylated in the presence of CARDS toxin but not in its absence (Fig. 5A). To further confirm NLRP3 ADP-ribosylation, the nitrocellulose membrane exposed to X-ray film was treated with anti-NLRP3 antibody. As shown in Fig. 5B, the 32P-labeled band observed in Fig. 5A corresponds to the NLRP3 band detected by Western blotting of the same blot with anti-NLRP3 antibody. Since ADP-ribosylating activity of CARDS toxin is required for inflammasome activity, this finding further indicates that ADP-ribosylation of NLRP3 by CARDS toxin precedes toxin-mediated inflammasome activation and subsequent IL-1β release.
FIG 5 .
ADP-ribosylation of NLRP3 by CARDS toxin. (A) Cell lysates prepared from HEK 293 cells expressing GFP-NLRP3 were incubated with CARDS toxin (+) (140 pmol) or without CARDS toxin (−) in the presence of 32P-NAD. Then, radiolabeled lysates were immunoprecipitated with anti-GFP antibodies, resolved by Nu-PAGE, transferred to nitrocellulose membranes, and exposed to X-ray films. (B) The same membrane exposed to X-ray film (as shown in panel A) was immunoblotted with anti-NLRP3 antibody to visualize the NLRP3 (i.e., GFP-NLRP3) protein band.
DISCUSSION
Inflammasome-mediated IL-1β release constitutes an important host defense mechanism against pathogens (1–9, 14, 15). However, aberrant inflammasome activation can accompany infection-associated disease leading to “hyperinflammation” and exaggerated histopathology and tissue damage. Inflammasomes (and IL-1β) have also been implicated in the exacerbation, development, and progression of chronic diseases like asthma, COPD, cancer, atherosclerosis, and diabetes (1–12). As mentioned earlier, pathogen-originating factors activate the inflammasome primarily via three mechanisms: (i) change in intracellular ion gradient (e.g., potassium efflux, calcium influx); (ii) generation of reactive oxygen species (ROS); and (iii) lysosomal leakage, leading to release of cathepsins into the cytoplasm. These indirect mechanisms are triggered by a wide variety of pathogen-associated factors. However, until now there has been no report of pathogen-associated factors “functionally” interacting with the inflammasome complex (and its components) during activation. In the current study, we unexpectedly observed the interaction between M. pneumoniae CARDS toxin and the NLRP3 inflammasome, resulting in NLRP3 ADP-ribosylation. Such a posttranslational modification correlated with inflammasome activation and the subsequent release of mature IL-1β. Thus, our studies reveal inflammasome activation by CARDS toxin through its ADPRT enzymatic activity on NLRP3. Furthermore, we identified ADP-ribosylation as a yet unknown posttranslational modification that mediates inflammasome activation.
The NLRP3 inflammasome represents a well-studied inflammasome complex that is activated by various bacteria. Also, pathogen activation of NLRC4, AIM2, and NLRP1 inflammasomes leads to IL-1β production. In these examples of inflammasome activation by bacteria, indirect mechanisms have been implicated, such as potassium efflux via bacterial pore-forming toxins (e.g., listeriolysin O of Listeria monocytogenes, pneumolysin of Streptococcus pneumoniae, and streptolysin O of Streptococcus pyogenes) (23–25). Cytotoxins and hemolysins secreted by Aeromonas veronii and Vibrio cholerae can also activate the NLRP3 inflammasome via potassium efflux and lysosomal destabilization-independent mechanisms (26–28). Intracellular bacteria (Mycobacterium marinum and Mycobacterium tuberculosis) activate NLRP3 inflammasome via the Esx-1 (type VIII) secretion system (29, 30). Chlamydia trachomatis, Chlamydia pneumoniae, Klebsiella pneumoniae, Porphyromonas gingivalis, and Neisseria gonorrhoeae also activate the NLRP3 inflammasome following potassium influx and cathepsin B leakage from lysosomes via unknown bacterial factor(s) (31–35). The NLRC4 inflammasome is activated by Salmonella, Yersinia, Legionella, Pseudomonas, Shigella, and A. veronii using bacterial flagellin-dependent and -independent mechanisms (e.g., rod protein of Pseudomonas) (26, 36–43). The AIM2 inflammasome is activated by Francisella tularensis (44, 45). The NLRP1 inflammasome can be activated by Bacillus anthracis lethal toxin via an unknown mechanism (46, 47). We have shown that CARDS toxin activates the NLRP3 inflammasome via ADP-ribosylation of NLRP3, and it is possible that the toxin can also activate a diverse range of inflammatory pathways, leading to alternate routes of inflammation and robust allergy-like pulmonary responses reported by us in mice (48).
The relationship between M. pneumoniae, CARDS toxin, and acute and chronic airway diseases continues to be reinforced and extended in animal models and humans. We have reported that CARDS toxin-mediated lung remodeling can lead to allergic airway inflammation and pulmonary dysfunction (48). Thus, CARDS toxin may represent a critical factor that initiates and exacerbates asthma and other chronic airway diseases in individuals acutely or persistently infected with M. pneumoniae (49). As CARDS toxin is released into the bronchoalveolar lavage (BAL) fluid from M. pneumoniae-infected cells and tissues, CARDS toxin can then trigger inflammatory responses via autocrine/paracrine mechanisms. The impact of CARDS toxin as an “inflammation-promoting” soluble factor could be substantial, as CARDS toxin can directly launch allergic responses in the airway to promote the asthma phenotype, including mucous metaplasia, airway hyperreactivity, eosinophilia, remodeling, and a dynamic peribronchial and perivascular lymphocytic infiltration that persists long after the primary infection and intoxication events (48). Our studies suggest that one mechanism involves the ability of CARDS toxin to activate the inflammasome in macrophages (alveolar macrophages and exudate macrophages in the alveolar space), which culminates in IL-1β production in the airway and the development of inflammation-dependent airway pathologies, such as asthma (symptomatic or asymptomatic; acute and chronic). In fact, there is evidence for the role of the inflammasome and IL-1β in the development and progression of asthma as follows. (i) Serum IL-1β levels are significantly higher in asthmatic patients than in control subjects (50). (ii) Higher levels of IL-1β were detected in sputum samples from symptomatic asthma patients than in asymptomatic asthmatic patients (51). (iii) Enhanced caspase-1 activity and higher IL-1β levels were observed in the airways of ovalbumin (OVA)-challenged sensitized mice (52). (iv) Dampened pulmonary eosinophilic inflammation and hyperplasia were observed in mice lacking the IL-1β receptor (IL-1R) following allergic challenge (53, 54). (v) OVA-challenged IL-1α/β KO mice displayed reduced airway hypersensitivity (55). (vi) Inhibition of the IL-1β receptor in mice (by IL-1R antagonist) diminished airway hypersensitivity and peribronchial inflammation due to reduced pulmonary infiltration of eosinophils and neutrophils (56). (vii) NLRP3 single nucleotide polymorphisms (SNPs) were associated with higher susceptibility to aspirin-induced asthma (57). However, recent studies have yielded contradictory results regarding the role of NLRP3 in the development of asthma in the murine model. By utilizing NLRP3 KO mice, one group showed the absolute requirement of NLRP3 for the development of OVA-induced asthma in mice (58), while the other group failed to observe any difference in WT versus KO mice (59). Further studies are required to establish the role of NLRP3 in the murine asthma model. Nevertheless, since CARDS toxin by itself initiates an allergic response, it is plausible that NLRP3 and IL-1β play direct roles in contributing to such hyperinflammatory responses. Indeed, high levels of IL-1β were detected in BAL fluid from CARDS toxin-treated mice and baboons (17). In the future, we will further dissect the role of NLRP3 (and other inflammasomes) and IL-1β in the development of allergic responses following CARDS toxin administration.
Another novel aspect of our studies deals with the identification of ADP-ribosylation as a “functional” posttranslational modification for inflammasome activation. To date, only two posttranslational modifications have been attributed to the regulation of inflammasome activity. (i) Phosphorylation of NLRC4 and ASC is required for inflammasome activation (60, 61). (ii) Ubiquitination (and deubiquitination) modulates NLRP3 inflammasome activity and degradation (62). In that regard, our studies have uncovered a role for unique posttranslational modification (i.e., ADP-ribosylation) in the regulation of the NLRP3 inflammasome. In addition, we show that this posttranslational event is mediated by a pathogen-derived virulence factor, i.e., CARDS toxin, rather than a host cellular factor. It is plausible that ADP-ribosylation represents a general mechanism by which inflammasomes are regulated. Pathogen-associated or cellular ADP-ribosylating factors could be involved in this process. How does ADP-ribosylation activate the inflammasome? ADP-ribosylation of NLRP3 could alter the conformation (folding) of NLRP3, leading to several possible scenarios. (i) ADP-ribosylation of NLRP3 may lead to its dissociation from its inhibitory subunit SGT1 and HSP-90. (ii) ADP-ribosylated NLRP3 could interact much more efficiently with itself and ASC to assemble the homo-hetero-oligomeric inflammasome complex. (iii) ADP-ribosylated NLRP3 as part of the inflammasome complex could recruit an additional cellular factor(s) involved in inflammasome activation. Also, CARDS toxin ADP-ribosylates several other host proteins (sizes ranging from 25 kDa to 53 kDa), and their direct or indirect role in inflammasome activation is being examined.
Thus, the unique biological, biochemical, and immunological properties of CARDS toxin, along with its ability to reproduce the cytopathology and inflammatory processes associated with M. pneumoniae infection, suggest that understanding the mechanisms by which the inflammasome is activated via CARDS toxin should lead to therapeutic interventions and improved health in many individuals who suffer from acute and chronic airway and extrapulmonary pathologies linked to infections.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney HEK 293 (American Type Culture Collection [ATCC]) cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 µg/ml streptomycin. U937 (ATCC) cells and THP-1 and ASC-deficient THP-1 (THP-1-defASC) cells (InvivoGen) were maintained in RPMI 1640 medium supplemented with 10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 50 µM 2-mercaptoethanol. U937 cell differentiation was achieved by culturing 5 × 105 cells in complete medium containing 50 nM phorbol 12-myristate 13-acetate (PMA) for 24 h in 12-well cell culture plates. After 24 h, PMA-containing medium was replaced with fresh complete medium, and cells were maintained for 48 h before treatments. Bone marrow-derived macrophages (BMDMs) were obtained from the femurs and tibias of wild-type (WT) and NLRP3 knockout (KO) C57BL/6 mice and cultured for 6 to 8 days as described earlier (15, 63, 64). NLRP3 KO mice were obtained from Jenny Ting (University of North Carolina, Chapel Hill, NC), and NLRP1 KO mice were obtained from Jackson Laboratory. Cells were plated in 12-well cell culture plates containing RPMI 1640 medium, 10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF). 293 and U937 cells were obtained from American Type Culture Collection, Manassas, VA.
Generation of WT FL, mutant FL CARDS E132A, and truncated CARDS toxin derivatives.
Full-length (FL) community-acquired respiratory distress syndrome (CARDS) toxin E132A mutant and CARDS249 (contains amino acids 1 to 249) plasmids were derived as described previously (16, 18). The carboxy CARDS toxin truncated derivative 266CARDS (contains amino acids 266 to 591) and mCherry-tagged FL CARDS toxin were generated using TGA-corrected FL CARDS toxin as the template. Appropriate gene fragments were amplified and cloned into pET19b (Novagen), transformed into competent Escherichia coli BL21(DE3) as described before (16, 18), and verified by complete DNA sequencing of individual plasmids (Department of Microbiology and Immunology Nucleic Acids Core Facility, University of Texas Health Science Center at San Antonio). Expression and purification of recombinant FL, mCherry-tagged CARDS toxin, CARDS E132A, CARDS249, and 266CARDS proteins were achieved as described before (16, 18). All recombinant proteins were desalted in 50 mM Tris-HCl buffer (pH 8.0) plus 5% glycerol using PD-10 columns, and protein purity was assessed by SDS-PAGE. Endotoxin was measured with the Limulus amebocyte lysate system (Associates of Cape Cod, East Falmouth, MA) according to the manufacturer’s directions.
Treatments.
Macrophages were treated with lipopolysaccharide LPS-EB (InvivoGen) for 4 h (BMDMs with 100 ng/ml and U937 with 1 µg/ml). Subsequently, cells were incubated with 15 µM nigericin (Sigma) for 30 min or 700 pmol of FL or truncated versions of CARDS toxin for either 24 h or 48 h. Medium supernatants were used to measure interleukin-1β (IL-1β) by human- and mouse-specific enzyme-linked immunosorbent assay (ELISA) kits (eBioscience).
Immunofluorescence and quantification of speck formation.
For monitoring immunofluorescence, 1 × 105 BMDMs were seeded onto 12-mm glass coverslips (squares no. 1.5; Fisher) and cultured for 48 h. BMDMs were treated with LPS (100 ng/ml) for 4 h, followed by the addition of mCherry-tagged CARDS toxin (70 pmol). At 16 h or 24 h after toxin treatment, BMDMs were fixed with 10% stock formaldehyde in phosphate-buffered saline (PBS) for 1 h at room temperature, treated with 50 mM glycine for 1 h, and permeabilized with 1% Triton X-100 in PBS for 1 h. BMDMs were blocked with 1% BSA in PBS for 1 h, probed with mouse anti-NLRP3 monoclonal antibody (MAb) (1:300) (Adipogen) for 1 h, and washed 5 times (10 min per wash) with PBS. BMDMs were probed with goat anti-mouse fluorescein isothiocyanate (FITC) antibody (1 mg/ml; ProSci Inc.) for 30 min, washed 5 times, and finally mounted onto glass slides using 5 µl SlowFade Gold antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen) and clear nail polish. Slides were analyzed using an Olympus FluoView FV1000 confocal multiphoton spectral laser scanning microscope.
To quantify the magnitude of speck formation, the percentage of cells that contained a speck was determined. Cells from 10 different fields (average of 80 cells/field) were counted based on DAPI-stained nuclei for each of two different experiments. Images were analyzed using ImageJ (http://rsb.info.nih.gov). The data are expressed as the percentage of cells with specks per number of cells per field.
Western blotting.
Cell lysates and medium supernatants collected from BMDMs that were treated with LPS and CARDS toxin (FL and truncations) were subjected to Western blotting analysis with rabbit anti-mouse caspase-1 p10 antibody (1:500) (Santa Cruz) and goat anti-mouse IL-1β p17 antibody (1:2,000) (R&D System). Cell lysates from the immunoprecipitation experiment (as described below) were used for Western blotting with anti-CARDS toxin (1:5,000) (65) and anti-FLAG (1:2,000) (Clontech) antibodies. The membranes were then probed with respective horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse antibodies (1:5,000) (Jackson Immunoresearch).
Immunoprecipitation.
HEK 293 cell monolayers at 80% confluence in 12-well cell culture plates were transfected with Lipofectamine 2000 (Invitrogen) with expression plasmid encoding FLAG-tagged human NLRP3 (0.1 µg/well). At 24 h posttransfection, cells were treated with CARDS toxin (350 pmol) for 4 h and 8 h. Cell lysates were harvested using 1% Triton X-100 in PBS with 5 mM Tris (pH 7.4) and complete EDTA-free protease inhibitor cocktail (Roche). Lysates were sonicated 3 times for 5 s each at 4°C and centrifuged at 13,000 rpm for 10 min. Clear lysate supernatants were transferred to new tubes and mixed with 50-µl portions of mouse anti-FLAG agarose (Clontech) for 12 h at 4°C. Samples were centrifuged at 1,000 rpm for 1 min, and pellets were washed 4 times with PBS containing 5 mM Tris (pH 7.4) and protease inhibitor. For each sample, protein was eluted from the final pellet with 100 mM glycine (pH 2.9). Eluted proteins were precipitated with 20% trichloroacetic acid (TCA) overnight at 4°C. The precipitated protein samples were pelleted by centrifugation at 13,000 rpm for 10 min, washed twice with ice-cold acetone, resuspended in SDS dissolving buffer, and separated on a 12% SDS-polyacrylamide gel. Protein samples were transferred to 0.2-µm nitrocellulose membranes (Bio-Rad) for Western blot analysis with anti-CARDS toxin antibody (1:5,000) (65).
ADP-ribosylation assay.
HEK 293 cells were transfected with Lipofectamine 2000 at 80% confluence in 12-well cell culture plates with expression plasmid encoding GFP-tagged human NLRP3. At 24 h posttransfection, the cells were harvested and suspended in 20 mM Tris (pH 7.5) and EDTA-free protease inhibitor cocktail (Sigma). The cells were sonicated 3 times for 15 s with 45-s intervals at 4°C. Lysates were then centrifuged at 3,000 rpm for 5 min, and the supernatants were transferred to new tubes. Cell-free extracts were incubated with and without 140 pmol of CARDS toxin for 30 min with 32P-NAD as described before (16). Radiolabeled proteins were incubated with green fluorescent protein (GFP) antibodies (final concentration, 0.2 µg/ml) for 1 h at room temperature and then incubated with protein G beads for 1 h at room temperature with gentle rotation. Samples were centrifuged at 3,000 rpm for 1 min, and the pellets were washed 3 times with PBS and dissolved in SDS-PAGE sample buffer and heated for 3 min at 100°C. Aliquots were subjected to SDS-PAGE using standard protocols. After electrophoresis, gels were transferred to nitrocellulose membranes, dried, and exposed to X-ray film for 1 to 7 days. For detection of NLRP3, nitrocellulose membranes were immunoblotted with mouse anti-GFP (1:1,000) (Santa Cruz) or rabbit anti-NLRP3 (1:1,000) antibodies (Santa Cruz) followed by HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (1:5,000) (Jackson Immunoresearch).
Statistical analysis.
For all statistical analyses, Microsoft XL and GraphPad Prism version 5.00 for Windows were used. One-way analysis of variance (ANOVA) was used to identify differences between three or more groups. Differences between two groups were analyzed using two-tailed Student’s t test. Data are expressed as means ± standard deviations (SDs) relative to the number of assays indicated. A comparison was considered statistically significant if the P value was <0.05.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants U19AI070412 (to J.B.B.) and AI083387 (to S.B.) and a grant from the Kleberg Foundation (to J.B.B.). J.A.S. was supported by NIH training grant 5T32AI007271.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We thank Brandon Guin and Lavanya Pandranki for technical assistance and Rose Garza for assembling the manuscript.
Footnotes
Citation Bose S, Segovia JA, Somarajan SR, Chang T, Kannan TR, Baseman JB. 2014. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio 5(6):e02186-14. doi:10.1128/mBio.02186-14.
REFERENCES
- 1. Davis BK, Wen H, Ting JP. 2011. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29:707–735. 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowling JK, O’Neill LA. 2012. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 47:424–443. 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 3. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241–247. 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin C, Flavell RA. 2010. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 30:628–631. 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 5. Kanneganti TD. 2010. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10:688–698. 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koizumi Y, Toma C, Higa N, Nohara T, Nakasone N, Suzuki T. 2012. Inflammasome activation via intracellular NLRs triggered by bacterial infection. Cell. Microbiol. 14:149–154. 10.1111/j.1462-5822.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- 7. Lamkanfi M, Dixit VM. 2009. The inflammasomes. PLoS Pathog. 5:e1000510. 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rathinam VA, Vanaja SK, Fitzgerald KA. 2012. Regulation of inflammasome signaling. Nat. Immunol. 13:333–342. 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–832. 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 10. Birrell MA, Eltom S. 2011. The role of the NLRP3 inflammasome in the pathogenesis of airway disease. Pharmacol. Ther. 130:364–370. 10.1016/j.pharmthera.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 11. dos Santos G, Kutuzov MA, Ridge KM. 2012. The inflammasome in lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 303:L627–L633. 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krause K, Metz M, Makris M, Zuberbier T, Maurer M. 2012. The role of interleukin-1 in allergy-related disorders. Curr. Opin. Allergy Clin. Immunol. 12:477–484. 10.1097/ACI.0b013e3283574d0c. [DOI] [PubMed] [Google Scholar]
- 13. Gordon SB, Read RC. 2002. Macrophage defences against respiratory tract infections. Br. Med. Bull. 61:45–61. 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 14. Lamkanfi M, Dixit VM. 2011. Modulation of inflammasome pathways by bacterial and viral pathogens. J. Immunol. 187:597–602. 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 15. Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. 2012. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 7:e29695. 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kannan TR, Baseman JB. 2006. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 103:6724–6729. 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, Kannan TR, Baseman JB, Dube PH. 2009. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One 4:e7562. 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kannan TR, Krishnan M, Ramasamy K, Becker A, Pakhomova ON, Hart PJ, Baseman JB. 2014. Functional mapping of community-acquired respiratory distress syndrome (CARDS) toxin of Mycoplasma pneumoniae defines regions with ADP-ribosyltransferase, vacuolating and receptor-binding activities. Mol. Microbiol. 93:568–581. 10.1111/mmi.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kannan TR, Provenzano D, Wright JR, Baseman JB. 2005. Identification and characterization of human surfactant protein A binding protein of Mycoplasma pneumoniae. Infect. Immun. 73:2828–2834. 10.1128/IAI.73.5.2828-2834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnan M, Kannan TR, Baseman JB. 2013. Mycoplasma pneumoniae CARDS toxin is internalized via clathrin-mediated endocytosis. PLoS One 8:e62706. 10.1371/journal.pone.0062706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Somarajan SR, Al-Asadi F, Ramasamy K, Pandranki L, Baseman JB, Kannan TR. 2014. Annexin A2 mediates Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin binding to eukaryotic cells. mBio 5(4):e014974-14. 10.1128/mBio.01497-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. 2013. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J. Biol. Chem. 288:2721–2733. 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harder J, Franchi L, Muñoz-Planillo R, Park JH, Reimer T, Núñez G. 2009. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 183:5823–5829. 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 25. McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Pétrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6:e1001191. 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCoy AJ, Koizumi Y, Higa N, Suzuki T. 2010. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J. Immunol. 185:7077–7084. 10.4049/jimmunol.1002165. [DOI] [PubMed] [Google Scholar]
- 27. McCoy AJ, Koizumi Y, Toma C, Higa N, Dixit V, Taniguchi S, Tschopp J, Suzuki T. 2010. Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur. J. Immunol. 40:2797–2803. 10.1002/eji.201040490. [DOI] [PubMed] [Google Scholar]
- 28. Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Núñez G, Suzuki T. 2010. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J. Immunol. 184:5287–5297. 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 29. Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, Sun M, Diehl L, Brown EJ. 2010. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6:e1000895. 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. 2010. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 12:1046–1063. 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 31. Abdul-Sater AA, Saïd-Sadier N, Padilla EV, Ojcius DM. 2010. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 12:652–661. 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460–6469. 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. 2010. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J. Immunol. 184:5743–5754. 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang MT, Taxman DJ, Holley-Guthrie EA, Moore CB, Willingham SB, Madden V, Parsons RK, Featherstone GL, Arnold RR, O’Connor BP, Ting JP. 2009. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J. Immunol. 182:2395–2404. 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 35. Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. 2009. NLRP3 (NALP3, cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 183:2008–2015. 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7:376–387. 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7:576–582. 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 38. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 39. Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7:569–575. 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 40. Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203:1093–1104. 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204:3235–3245. 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3:e111. 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7:318–325. 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 44. Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11:385–393. 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U. S. A. 107:9771–9776. 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boyden ED, Dietrich WF. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240–244. 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 47. Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. 2008. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. U. S. A. 105:7803–7808. 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Medina JL, Coalson JJ, Brooks EG, Winter VT, Chaparro A, Principe MF, Kannan TR, Baseman JB, Dube PH. 2012. Mycoplasma pneumoniae CARDS toxin induces pulmonary eosinophilic and lymphocytic inflammation. Am. J. Respir. Cell Mol. Biol. 46:815–822. 10.1165/rcmb.2011-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peters J, Singh H, Brooks EG, Diaz J, Kannan TR, Coalson JJ, Baseman JG, Cagle M, Baseman JB. 2011. Persistence of community-acquired respiratory distress syndrome toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest 140:401–407. 10.1378/chest.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas SS, Chhabra SK. 2003. A study on the serum levels of interleukin-1beta in bronchial asthma. J. Indian Med. Assoc. 101:286. [PubMed] [Google Scholar]
- 51. Konno S, Gonokami Y, Kurokawa M, Kawazu K, Asano K, Okamoto K, Adachi M. 1996. Cytokine concentrations in sputum of asthmatic patients. Int. Arch. Allergy Immunol. 109:73–78. 10.1159/000237234. [DOI] [PubMed] [Google Scholar]
- 52. Underwood SL, Haddad E-B, Birrell MA, McCluskie K, Pecoraro M, Dabrowski D, Webber SE, Foster ML, Belvisi MG. 2002. Functional characterization and biomarker identification in the Brown Norway model of allergic airway inflammation. Br. J. Pharmacol. 137:263–275. 10.1038/sj.bjp.0704865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Broide DH, Campbell K, Gifford T, Sriramarao P. 2000. Inhibition of eosinophilic inflammation in allergen-challenged, IL-1 receptor type 1-deficient mice is associated with reduced eosinophil rolling and adhesion on vascular endothelium. Blood 95:263–269. [PubMed] [Google Scholar]
- 54. Schmitz N, Kurrer M, Kopf M. 2003. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur. J. Immunol. 33:991–1000. 10.1002/eji.200323801. [DOI] [PubMed] [Google Scholar]
- 55. Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, Homma I, Sudo K, Horai R, Asano M, Iwakura Y. 2003. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int. Immunol. 15:483–490. 10.1093/intimm/dxg054. [DOI] [PubMed] [Google Scholar]
- 56. Wang CC, Fu CL, Yang YH, Lo YC, Wang LC, Chuang YH, Chang DM, Chiang BL. 2006. Adenovirus expressing interleukin-1 receptor antagonist alleviates allergic airway inflammation in a murine model of asthma. Gene Ther. 13:1414–1421. 10.1038/sj.gt.3302798. [DOI] [PubMed] [Google Scholar]
- 57. Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, Hirota T, Harada M, Sakashita M, Suzuki Y, Shimojo N, Kohno Y, Fujita K, Miyatake A, Doi S, Enomoto T, Taniguchi M, Higashi N, Nakamura Y, Tamari M. 2009. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J. Allergy Clin. Immunol. 124:779–785.e6. 10.1016/j.jaci.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 58. Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, Quesniaux V, Ryffel B, Togbe D. 2011. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy 66:1047–1057. 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 59. Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. 2012. Analysis of NLRP3 in the development of allergic airway disease in mice. J. Immunol. 188:2884–2893. 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. 2013. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14:1247–1255. 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Kömüves L, Cupp JE, Arnott D, Monack D, Dixit VM. 2012. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490:539–542. 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 62. Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. 2012. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13:255–263. 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. 2009. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10:1073–1080. 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsai SY, Segovia JA, Chang TH, Morris IR, Berton MT, Tessier PA, Tardif MR, Cesaro A, Bose S. 2014. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 10:e1003848. 10.1371/journal.ppat.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kannan TR, Musatovova O, Balasubramanian S, Cagle M, Jordan JL, Krunkosky TM, Davis A, Hardy RD, Baseman JB. 2010. Mycoplasma pneumoniae community acquired respiratory distress syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol. Microbiol. 76:1127–1141. 10.1111/j.1365-2958.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]