Abstract
Background
Few clinical trials in chronic idiopathic constipation (CIC) patients have evaluated abdominal symptom severity and whether CIC patients with abdominal symptoms respond similarly to patients with limited abdominal symptoms.
Aims
To examine abdominal symptom severity and relationships between symptoms and global measures at baseline; compare linaclotide's effect on symptoms in subpopulations with more or less abdominal pain; and assess relationships between symptom improvement and global measures in these two subpopulations.
Methods
In two phase 3 trials, patients meeting modified Rome II CIC criteria were assigned to linaclotide 145 μg, 290 μg, or placebo once daily. Patients rated abdominal and bowel symptoms daily during 2-week pre-treatment and 12-week treatment periods. Linaclotide's effect on symptoms and global measures [constipation severity, health-related quality of life (HRQOL), treatment satisfaction] and their inter-relationships were assessed in post hoc analyses of abdominal pain subpopulations.
Results
Of 1271 CIC patients, 23%, 32%, and 43% reported moderate-to-severe abdominal pain, discomfort, and bloating, respectively, during baseline. In more-severe abdominal pain patients, abdominal symptoms were more strongly correlated than bowel symptoms with global measures, but in less-severe abdominal pain patients, abdominal and bowel symptoms were similarly correlated with global measures, at baseline and post-treatment. Linaclotide significantly improved all symptoms and global measures in both subpopulations.
Conclusions
When abdominal pain is present in CIC, abdominal and not bowel symptoms may drive patient assessments of constipation severity, HRQOL, and treatment satisfaction. Linaclotide (145 μg and 290 μg) is an effective treatment for both abdominal and bowel symptoms, even in CIC patients with more severe abdominal pain at baseline. (Clinicaltrials.gov: NCT00765882, NCT00730015).
Introduction
Chronic idiopathic constipation (CIC) is a functional bowel disorder affecting an estimated 12–19% of the United States (US) population1 with similar prevalence rates reported in Europe and other developed countries.2,3 CIC is generally characterised by infrequent stools, hard stools, straining, and unsuccessful defection.4 In addition to these physical symptoms, CIC is also detrimental to health-related quality of life (HRQOL)5–8 and work productivity,6 and is a burden on the healthcare system.9–12
In clinical studies, CIC is usually defined by the Rome criteria, which diagnose CIC based on patient recall of bowel symptoms, including number of bowel movements (BMs) per week and the percentages of BMs that include hard stool, straining, sensation of incomplete evacuation, manual facilitation, and sensation of blockage.13 Although CIC is defined by these bowel symptoms, many individuals also experience abdominal symptoms including pain, discomfort, and bloating.14–18 Abdominal pain in conjunction with constipation, however, is commonly considered the hallmark of irritable bowel syndrome with constipation (IBS–C).19,20 Indeed, the Rome criteria for IBS include abdominal pain or discomfort that is relieved by BMs or whose onset corresponds with change in stool frequency or form.21 The Rome criteria for CIC, in contrast, exclude patients who meet IBS criteria, but do not otherwise exclude individuals with abdominal pain per se or other abdominal symptoms. Consequently, there are patients who meet the Rome criteria for CIC, who do not meet IBS criteria, yet still have abdominal pain.15–17,22–26
While the Rome criteria are valuable as a standard set of rules to apply to clinical studies, their current CIC and IBS-C classifications are less useful in the clinic. In practice, a patient with constipation can alternately fulfil the criteria for CIC or IBS-C over time,27 and, in some patients, distinguishing between CIC and IBS-C at any given timepoint may be difficult.28,29 These and other recent findings17,24,25,27,28,30 support a view of CIC and IBS-C not as distinct entities but as overlapping disorders that lie on a spectrum of abdominal pain. This lack of diagnostic distinction may not be problematic for clinicians because current treatment approaches are largely the same for both disorders.22,31 Constipation, whether diagnosed as CIC or IBS-C, is a heterogeneous disorder, and each patient seeks treatment for their specific set of bowel and abdominal symptoms. Abdominal pain, when combined with the symptoms of chronic constipation, can have added impact on health status, healthcare-seeking behaviour, HRQOL, and perception of severity.16,26,32
Linaclotide, a guanylate cyclase-C agonist, is approved in the US, Canada, and Mexico for the treatment of CIC (145 μg) and IBS-C (290 μg) in adults. In two phase 3 clinical trials in CIC, linaclotide (145 μg and 290 μg) improved bowel symptoms, abdominal discomfort and abdominal bloating.33 In two phase 3 clinical trials in IBS-C, linaclotide (290 μg only) improved bowel and abdominal symptoms, including abdominal pain.34,35
The objectives of these post hoc analyses of the pooled, Phase 3 CIC population were: (i) to examine the severity of abdominal symptoms and assess their relationships with bowel symptoms and global measures at baseline, (ii) to compare the effects of linaclotide on abdominal and bowel symptoms in subpopulations of patients with more severe or less severe abdominal pain at baseline and (iii) to assess the relationships between symptom improvement and global measures in these two subpopulations.
Methods
Trial design
Detailed study methods and results for the two phase 3 clinical trials (Trials 303 and 01; Clinicaltrials.gov numbers NCT00730015 and NCT00765882) have been published.33 In brief, these randomised, multicenter (US/Canada), double-blind, placebo-controlled, parallel-group trials included 2-week baseline periods followed by randomisation of patients in equal proportions to placebo, linaclotide 145 μg, or linaclotide 290 μg once daily for 12 weeks. The trials were identical except Trial 303 included a 4-week randomised-withdrawal period following the treatment period. The trials were designed, conducted, and reported in accordance with the Good Clinical Practice guidelines, and protocols and procedures were approved by Institutional Review Boards. All patients gave written informed consent before participation.
Trial patients
Male and female patients ≥18 years were eligible for enrolment if they met modified Rome II CIC criteria36 and did not meet Rome II IBS-C criteria as determined by the investigator's assessment of a patient's recall of their medical history. Patients were randomised into the treatment period if, during the 2-week baseline period, they reported ≤6 spontaneous BMs (SBM = a BM occurring in the absence of laxative, enema, or suppository use during the preceding 24 h) per week and <3 complete SBMs (CSBM = SBMs accompanied by patient self-reporting a feeling of complete evacuation) per week.
Symptom assessments
Patients reported bowel habits and abdominal symptom severity ratings daily to an interactive voice response system (IVRS). Bowel habits included SBMs, CSBMs, stool consistency [Bristol Stool Form Scale (BSFS),37 1–7 range; higher scores indicate more liquid stool and lower scores indicate harder stool], and severity of straining (5-point ordinal scale: 1 = not at all to 5 = an extreme amount). Abdominal symptoms (pain, discomfort, bloating) were measured on a 5-point ordinal scale (1 = none to 5 = very severe).
Global measures
Patients rated constipation severity weekly via the IVRS (5-point ordinal scale, 1 = none to 5 = very severe) by answering the question: ‘On average, how would you rate your constipation severity during the past 7 days?’ The Patient Assessment of Constipation Quality of Life Questionnaire (PAC–QOL) was assessed at baseline and week 12. This self-administered questionnaire assesses the effects of constipation on 4 HRQOL dimensions (physical discomfort, psychosocial discomfort, worries/concerns, satisfaction) and yields an overall score (0–4), where higher scores represent poorer HRQOL.38 Patients also reported treatment satisfaction (5-point ordinal scale; 1 = not at all satisfied to 5 = very satisfied) at all trial visits after randomisation by answering the question: ‘Overall, how satisfied are you with the study medication's ability to relieve your constipation symptoms?’.
Safety assessments
At each study visit, patients were asked an open-ended question regarding adverse events (AEs). Patients reported AEs by recalling events since their prior visit. Investigators recorded patient-reported AEs and judged each event for severity and relationship with blinded trial medication. Other safety evaluations included physical examinations, electrocardiogram recordings, vital-sign measurements, and standard clinical laboratory tests. These results have been published.33
Statistical methods
All statistical analyses were post hoc and were based on pooled results from the two phase 3 CIC trials. The safety population included patients who received ≥1 dose of trial medication (linaclotide 145 μg, linaclotide 290 μg, or placebo) during the treatment period. The intent-to-treat (ITT) population included patients in the safety population who had ≥1 post-randomisation assessment of the daily IVRS information that determined whether an SBM was a CSBM. The ITT population was divided into two subpopulations based on mean abdominal pain scores during the 2-week baseline period. A none-to-mild subpopulation included patients with baseline mean abdominal pain scores <3.0 on the 5-point ordinal scale, and a moderate-to-severe subpopulation with mean scores ≥3.0.
Pearson product-moment correlation coefficients (r) were calculated to describe linear relationships between variables (symptoms, constipation severity, PAC-QOL scores, treatment satisfaction). Absolute values of r are presented.
Week 12 changes from baseline in abdominal and bowel symptoms were analysed in the subpopulations using an ancova model with treatment group, geographical region, and study as factors and the baseline value as covariate. Change-from-baseline means are least squares means from the ancova model. If patients discontinued or had missing weekly data, a last-observation-carried-forward (LOCF) method was applied. Week 12 per cent changes from baseline were also analysed. The change from baseline results are presented because per cent change from baseline data are less reliable due to low and zero baseline values, which result in extreme numbers and missing data, respectively. Both change and per cent change yielded the same patterns of treatment response and correlations.
Results
The pooled ITT population included 1271 CIC patients randomised to receive once daily placebo (n = 423), linaclotide 145 μg (n = 430), or linaclotide 290 μg (n = 418).
Baseline
During the 2-week baseline period, 91%, 96%, and 97% of the 1271 patients reported experiencing some level of abdominal pain, discomfort, and bloating, respectively (Table1). During baseline, 23%, 32%, and 43% reported moderate-to–severe (mean ≥3 on the 5-point ordinal scale) abdominal pain, discomfort, and bloating, respectively; 36%, 47%, and 60% reported no abdominal pain-free, discomfort-free, or bloating-free days (Table1).
Table 1.
Baseline abdominal symptoms (ITT population, N = 1271)
| Abdominal pain* | |
| Mean abdominal pain score (s.d.) | 2.2 (0.89) |
| Patients reporting mean baseline abdominal pain score of | |
| 1.0 to <3 (none to mild) n (%) | 982 (77.3) |
| 3.0 to ≤5 (moderate to very severe) n (%) | 289 (22.7) |
| Patients reporting no abdominal pain†n (%) | 114 (9.0) |
| Patients reporting no pain-free days‡ n (%) | 454 (35.7) |
| Abdominal discomfort* | |
| Mean abdominal discomfort score (s.d.) | 2.5 (0.84) |
| Patients reporting mean baseline abdominal discomfort score of | |
| 1.0 to <3 (none to mild) n (%) | 861 (67.7) |
| 3.0 to ≤5 (moderate to very severe) n (%) | 410 (32.3) |
| Patients reporting no abdominal discomfort† n (%) | 52 (4.1) |
| Patients reporting no discomfort-free days‡ n (%) | 598 (47.1) |
| Abdominal bloating* | |
| Mean abdominal bloating score (s.d.) | 2.8 (0.88) |
| Patients reporting mean baseline abdominal bloating score of | |
| 1.0 to <3 (none to mild) n (%) | 719 (56.6) |
| 3.0 to ≤5 (moderate to very severe) n (%) | 552 (43.4) |
| Patients reporting no abdominal bloating† n (%) | 39 (3.1) |
| Patients reporting no bloating-free days‡ n (%) | 756 (59.5) |
s.d., standard deviation.
5-point ordinal scale (1 = none, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe).
Per cent of patients reporting a score = 1 (none) each day of the 2-week baseline period.
Per cent of patients with no abdominal pain/discomfort/bloating daily scores = 1 (none) during baseline.
There was considerable overlap in the moderate-to-severe abdominal symptoms (Figure1). Nearly all patients with moderate-to-severe abdominal pain also reported moderate-to-severe abdominal discomfort and bloating. Baseline abdominal symptoms were also highly correlated with each other (pairwise Pearson r = 0.71–0.89). In contrast, abdominal pain was only weakly correlated with CSBM rate, SBM rate, and BSFS (r = 0.21, 0.17, 0.10, respectively), but was moderately correlated with straining (r = 0.41).
Figure 1.
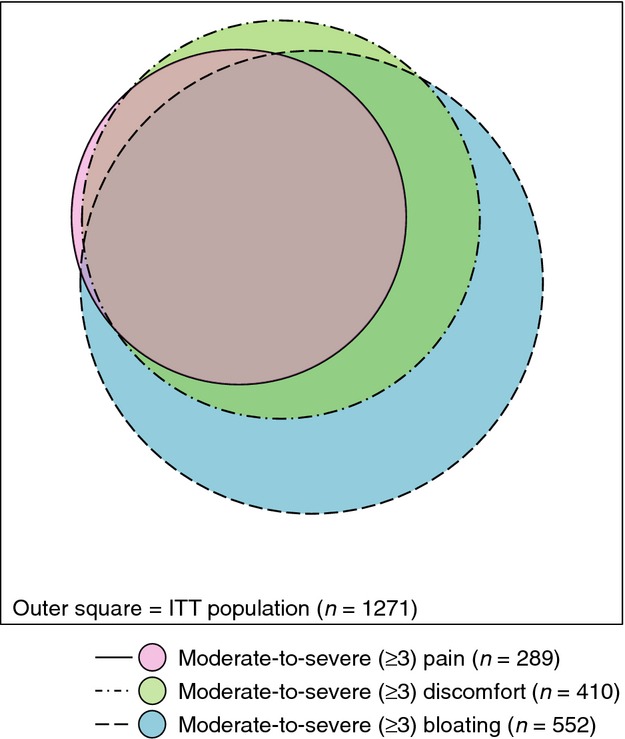
Subpopulations of patients with moderate-to-severe abdominal symptoms (ITT population). Venn diagram depicting the relative sizes and overlaps of the subpopulations of patients with moderate-to-severe (≥3) abdominal pain, abdominal discomfort, and abdominal bloating at baseline in the ITT population.
Abdominal pain subpopulations
Baseline
Demographical characteristics were similar between the moderate-to-severe (n = 289) and none-to-mild (n = 982) abdominal pain subpopulations (Table S1). At baseline, the moderate-to-severe subpopulation had a worse mean constipation severity score (3.8 vs. 3.1) and a worse mean PAC-QOL overall score (2.6 vs. 1.9) compared with the none-to-mild subpopulation (Table S1). In the moderate-to-severe abdominal pain subpopulation, constipation severity was more strongly correlated with abdominal symptoms (r = 0.53–0.60) than with bowel symptoms, including straining (r = 0.07–0.38) (Figure2a). In contrast, in the none-to-mild abdominal pain subpopulation, constipation severity was more strongly correlated with straining (r = 0.43) than with abdominal symptoms (r = 0.19–0.36) or with other bowel symptoms (r = 0.16–0.30) (Figure2a).
Figure 2.
Correlations of baseline global measures with baseline abdominal and bowel symptoms. (a) Correlations of constipation severity score at baseline with abdominal and bowel symptoms at baseline. Pearson correlation coefficients, r, absolute values shown; *P < 0.05. (b) Correlations of PAC-QOL overall score at baseline with abdominal and bowel symptoms at baseline. Pearson correlation coefficients, r, absolute values shown; *P < .05.
As with constipation severity, the PAC-QOL overall score in the moderate-to-severe abdominal pain subpopulation was more strongly correlated with abdominal symptoms (r = 0.39–0.45) than with bowel symptoms, including straining (r = 0.14–0.33) (Figure2b). Unlike constipation severity, the PAC-QOL overall score in the none-to-mild abdominal pain subpopulation was also more strongly correlated with abdominal symptoms (r = 0.33–0.49) than with bowel symptoms (r = 0.06–0.23) (Figure2B).
Treatment outcomes
For both subpopulations, changes from baseline in abdominal pain, discomfort and bloating scores were significantly improved vs. placebo at both dose levels of linaclotide (P < 0.05) (Table2). The changes from baseline in the moderate-to-severe abdominal pain subpopulation were greater than in the none-to-mild subpopulation. Similarly, for both subpopulations, changes from baseline in CSBM rate, SBM rate, BSFS score and severity of straining as well as the changes from baseline in constipation severity and PAC-QOL overall scores were significantly improved vs. placebo in all linaclotide-treated patients, at both dose levels (P < 0.05), and changes were generally greater in the moderate-to-severe abdominal pain subpopulation than in the none-to-mild subpopulation (Table2). These patterns were also found in per cent change from baseline analyses. There were no dose-level-related trends in changes in abdominal symptoms, bowel symptoms, or global measures.
Table 2.
Week 12 change from baseline in abdominal symptoms, bowel symptoms and global measures in the abdominal pain subpopulations
| Change from baseline | None-to-mild abdominal pain subpopulation | Moderate-to-severe abdominal pain subpopulation | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 330) | LIN 145 μg (n = 329) | LIN 290 μg (n = 323) | Placebo (n = 93) | LIN 145 μg (n = 101) | LIN 290 μg (n = 95) | |
| LS mean | LS mean | LS mean | LS mean | LS mean | LS Mean | |
| Abdominal symptoms | ||||||
| Pain† | −0.17 | −0.29* | −0.31* | −0.56 | −1.13*** | −1.03** |
| Discomfort† | −0.23 | −0.37* | −0.37* | −0.63 | −1.07** | −1.02* |
| Bloating† | −0.15 | −0.31* | −0.32* | −0.62 | −0.97* | −0.96* |
| Bowel symptoms | ||||||
| CSBM rate | 0.50 | 1.88*** | 2.04*** | 0.85 | 2.05* | 1.92* |
| SBM rate | 0.63 | 2.63*** | 2.48*** | 1.20 | 3.04* | 3.76* |
| BSFS score‡ | 0.42 | 1.61*** | 1.64*** | 0.68 | 2.11*** | 1.80*** |
| Straining§ | −0.52 | −1.01*** | −1.09*** | −0.86 | −1.83*** | −1.51** |
| Global measures | ||||||
| Constipation severity† | −0.16 | −0.79*** | −0.78*** | −0.69 | −1.40*** | −1.31*** |
| PAC-QOL overall score¶ | −0.36 | −0.75*** | −0.78*** | −1.05 | −1.52*** | −1.45* |
BSFS, Bristol Stool Form Scale; CSBM, complete spontaneous bowel movement; LIN, linaclotide; LS, least squares (mean); PAC-QOL, Patient Assessment of Constipation Quality of Life Questionnaire; SBM, spontaneous bowel movement.
Week 12 (LOCF) change from baseline least squares means presented.
For all parameters, changes from baseline were significant vs. placebo at both dose levels of linaclotide (P < .0001) for the ITT population.
P < 0.05,
P ≤ 0.001,
P ≤ 0.0001 for linaclotide vs. placebo; P values obtained from ancova model with study, treatment group, and geographical region as factors and baseline value as a covariate.
5-point ordinal scale (1 = none, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe).
Bristol Stool Form Scale (1 = separate hard lumps like nuts to 7 = watery, no solid pieces)
5-point ordinal scale (1 = not at all, 2 = a little bit, 3 = a moderate amount, 4 = a great deal, 5 = an extreme amount).
PAC-QOL overall score from 0 to 4, where a higher score represents poorer quality of life.
Within the none-to-mild and moderate-to-severe abdominal pain subpopulations, both the linaclotide-145 μg and linaclotide-290 μg dose groups demonstrated similar degrees of improvement over placebo in abdominal pain during the treatment period (Figure3). This separation from placebo began at week 1 and was sustained throughout the treatment period.
Figure 3.

Weekly mean change from baseline in abdominal pain. (a) Mean change from baseline in abdominal pain (LOCF) by week during the 12-week treatment period, by dose group in patients who had none-to-mild abdominal pain at baseline. Abdominal pain measured on a 5-point ordinal scale (1 = none to 5 = very severe). (b) Mean change from baseline in abdominal pain (LOCF) by week during the 12-week treatment period, by dose group in patients who had moderate-to-severe abdominal pain at baseline. Abdominal pain measured on a 5-point ordinal scale (1 = none to 5 = very severe).
In the moderate-to-severe abdominal pain subpopulation, constipation severity at week 12 was more strongly correlated with changes in abdominal symptoms (r = 0.65–0.67) than with changes in bowel symptoms (r = 0.30–0.49) (Figure4a). Similarly, PAC-QOL overall score and treatment satisfaction at week 12 were both more strongly correlated with changes in abdominal symptoms than with changes in bowel symptoms (Figure4b,c). In contrast, in the none-to-mild abdominal pain subpopulation, the correlations of constipation severity with changes in abdominal symptoms (r = 0.32–0.42) were similar to those of constipation severity with changes in bowel symptoms (r = 0.36–0.45) (Figure4a). These patterns were also observed for week 12 PAC-QOL overall score (Figure4b) and week 12 treatment satisfaction (Figure4c).
Figure 4.

Correlation of global measures after treatment with improvement in abdominal and bowel symptoms. (a) Correlation of constipation severity (week 12 LOCF) with improvement in abdominal and bowel symptoms (baseline to week 12 LOCF). Pearson correlation coefficients, r, absolute values shown; *P < 0.05. (b) Correlation of PAC-QOL overall score (week 12) with improvement in abdominal and bowel symptoms (baseline to week 12 LOCF). Pearson correlation coefficients, r, absolute values shown; *P < 0.05. (c) Correlation of treatment satisfaction (week 12 LOCF) with improvement in abdominal and bowel symptoms (baseline to week 12 LOCF). Pearsoncorrelation coefficients, r, absolute values shown; *P < 0.05.
Overall AE rates were similar between the two abdominal pain subpopulations (Table S2). The moderate-to-severe abdominal pain subpopulation had lower rates of diarrhoea for both dose groups (12%, 11%, and 4% for 145 μg, 290 μg, and placebo respectively) compared with the none to mild subpopulation (17%, 15%, and 5% for 145 μg, 290 μg, and placebo, respectively).
Discussion
These post hoc analyses demonstrate the importance of abdominal pain, discomfort and bloating in this population of CIC patients. While it may not be surprising that CIC patients have abdominal discomfort and bloating, it is notable that abdominal pain is also common. Over 90% of patients in these Phase 3 clinical trials reported at least some abdominal pain during the 2-week baseline period, and nearly a quarter averaged at least moderate abdominal pain. Although abdominal pain might be expected to strongly correlate with BM frequency and stool consistency, this study found only weak correlations, perhaps because this analysis used the 2-week average of the daily assessments and therefore did not account for day-to-day variability in these symptoms. A previous study showed that severity of abdominal pain and bloating cumulatively increased with consecutive days without a BM in patients with IBS-C.39
The significance of abdominal symptoms in CIC patients is demonstrated by the correlations between abdominal symptoms and ratings of constipation severity. Those correlations were stronger in patients who had at least moderate baseline abdominal pain compared with patients who had mild or no abdominal pain. In addition, in those patients with at least moderate abdominal pain, their ratings of constipation severity and PAC-QOL overall score were more highly associated with their abdominal symptoms than with the bowel symptoms that define their CIC. This result is consistent with the PAC-QOL validation study, which found no significant associations between this score and the number of complete evacuations per week, but did find significant associations with abdominal pain and patients’ severity ratings.38 That abdominal symptoms are highly associated with global measures when abdominal pain is present is also consistent with a large survey study using Rome II criteria that found that CIC patients with abdominal pain had poorer health, poorer HRQOL and more somatic symptoms than CIC patients without pain.15 A recent study demonstrated that CIC patients with pain were more likely to regard themselves as constipated, report more symptoms of constipation, use laxatives, and seek healthcare.26
Linaclotide significantly improved all abdominal and bowel symptoms versus placebo in CIC patients with at least moderate abdominal pain as well as in CIC patients with mild or no abdominal pain. Interestingly, regardless of abdominal symptom severity, linaclotide at the lower dose of 145 μg was as effective in treating those symptoms, including abdominal pain, as linaclotide at the higher dose of 290 μg; there were no dose-related trends. The finding that both 145 μg and 290 μg are similarly effective in treating CIC symptoms is noteworthy because linaclotide is approved in the US, Canada and Mexico at 145 μg for the treatment of CIC in adults and at 290 μg for the treatment of IBS-C in adults. However, when the 145 μg and 290 μg doses were evaluated in a phase 2b study in patients who met modified Rome II criteria for IBS-C, the 290 μg dose was generally more effective than the 145 μg dose, especially at relieving abdominal pain in IBS-C patients with more severe abdominal pain at baseline.40 Although the 290 μg dose was studied in both indications in phase 3, the CIC trials enrolled patients meeting modified Rome II criteria for functional constipation33 while the IBS-C trials enrolled patients meeting modified Rome II criteria for IBS with constipation.35,41 These inherent differences in the trial populations, plus differences in the scales used to measure abdominal symptoms across the trials, prevent formal comparison of the phase 3 CIC and IBS-C trial results.
In this study, improvements from baseline for all symptoms were greater in patients who had at least moderate abdominal pain at baseline. In these more-severe abdominal pain patients, improvements in abdominal symptoms were not only highly correlated with treatment satisfaction, constipation severity and PAC-QOL but were also more strongly correlated with these global measures than were improvements in bowel symptoms. This finding was not true for patients who had mild or no pain at baseline. In those patients, improvements in abdominal and bowel symptoms were similarly and more moderately linked to the global measures. Linaclotide was well tolerated in both abdominal pain subpopulations. The lower rates of diarrhoea in patients who had at least moderate abdominal pain at baseline may be because these patients also had worse constipation severity at baseline.
Some patients with CIC, like patients with IBS-C, may present with abdominal symptoms, including abdominal pain, in addition to bowel symptoms.16,20,27,28,42,43 It is possible that patients with at least moderate abdominal pain have increased visceral sensitivity, similar to some patients with IBS,44 but confirmatory studies are needed. Although the population of patients in this study met the Rome II CIC definition by history, most reported at least some abdominal pain during the 2-week baseline and nearly a quarter averaged at least moderate abdominal pain during this period. Our findings also suggest that when patients with CIC have abdominal pain, it is important to relieve their abdominal symptoms because they are more strongly associated with both constipation severity and HRQOL than are bowel symptoms.
There are potential limitations to these findings. First, the population in these trials is likely skewed towards the more severe end of the CIC spectrum because patients enrolling in a clinical trial may be more likely to have moderate-severe symptoms. In addition, inclusion in these trials was limited to patients who had ≤6 SBMs/week and <3 CSBMs/week during the 2-week baseline period. These criteria likely excluded CIC patients with milder bowel symptoms. Thus, the rate and severity of abdominal pain in this CIC population may not reflect the rate/severity of abdominal pain in a broader population of CIC patients. Second, the patients in this study were enrolled based on Rome II CIC criteria, including the exclusion of IBS. These criteria rely on patient recall of abdominal pain or discomfort and its association with bowel patterns over the past 12 months, as well as on an investigator's judgment of a patient's description. Due to the subjective nature of this diagnosis and the significant overlap of symptoms, it is possible that different clinicians might have diagnosed some patients as IBS-C rather than CIC. The findings of this study, however, are unaffected by the Rome designation because these analyses were based on actual baseline symptom ratings in a population of patients with CIC. Thus, these findings are relevant for clinicians who treat patients who have chronic constipation with concurrent abdominal symptoms and apply whether the clinician regards that patient as a CIC or an IBS-C patient.
In summary, these analyses underscore the heterogeneity of chronic constipation as a bowel disorder, regardless of whether it is defined as CIC or IBS by the Rome criteria. When present, abdominal pain is an important facet of chronic constipation. In patients with more-severe abdominal pain, their abdominal symptoms and not their bowel symptoms appear to drive assessment of constipation severity and HRQOL. Consequently, it makes sense that relief of abdominal symptoms may also drive their perception of treatment satisfaction and improve their HRQOL. Thus, evaluation of abdominal pain in patients with chronic constipation is important for determining the best treatment for the management of the condition. These post hoc analyses suggest that linaclotide is an effective treatment for both abdominal and bowel symptoms, even in those patients with more severe abdominal pain, and the 145 μg dose appears as effective as the 290 μg dose in patients with CIC.
Authorship
Guarantor of article: Jeffrey Johnston.
Author contributions: Anthony Lembo and Jennifer Chickering developed the initial draft of the manuscript. Lin Chang, Anthony Lembo and Jennifer Chickering participated in the planning of analyses, assisted in the interpretation of data and provided critical revision for important intellectual content. Xinming Hao provided statistical design and interpretation of analyses, as well as critical revision of the manuscript. Jeffrey Johnston, Bernard Lavins, and Steven Shiff designed the trials. Jeffrey Johnston, Bernard Lavins, Caroline Kurtz, Mark Currie, Steven Shiff and Xinwei Jia assisted in the interpretation of data and critical revision of the manuscript for important intellectual content. All authors had access to the study data and reviewed and approved the final version of the manuscript.
Acknowledgments
Declarations of personal interests: Lin Chang has served as an advisory board member and consultant for Ironwood Pharmaceuticals, Forest Laboratories, Salix Pharmaceuticals, Takeda, Enterra Health and Purdue Pharma. She has received research grants from Ironwood Pharmaceuticals. Anthony Lembo has served as an advisory board member and consultant for Ironwood Pharmaceuticals, Forest Laboratories, Salix Pharmaceuticals and Prometheus Labs. He has received a research grant from Prometheus Labs. Bernard Lavins, Xinming Hao, Jennifer Chickering, Mark Currie, Caroline Kurtz and Jeffrey Johnston are employees of Ironwood Pharmaceuticals and own stock/stock options in Ironwood Pharmaceuticals. Steven Shiff and Xinwei Jia are employees of Forest Laboratories and own stock/stock options in Forest Laboratories.
Declaration of funding interests: These trials were funded by Forest Research Institute and Ironwood Pharmaceuticals.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Demographical characteristics and baseline global measures.
Table S2. Adverse events in the abdominal pain subpopulations.
References
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- Suares NC, Ford AC. Diagnosis and treatment of irritable bowel syndrome. Discov Med. 2011;11:425–33. [PubMed] [Google Scholar]
- Peppas G, Alexiou VG, Mourtzoukou E, Falagas ME. Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroenterol. 2008;8:5. doi: 10.1186/1471-230X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–8. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- Wald A, Scarpignato C, Kamm MA, et al. The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther. 2007;26:227–36. doi: 10.1111/j.1365-2036.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56:2688–95. doi: 10.1007/s10620-011-1639-5. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–93. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938–49. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136:741–54. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Cai Q, Buono JL, Spalding WM, et al. Healthcare costs among patients with chronic constipation: a retrospective claims analysis in a commercially insured population. J Med Econ. 2013;17:148–58. doi: 10.3111/13696998.2013.860375. [DOI] [PubMed] [Google Scholar]
- Sethi S, Mikami S, Leclair J, et al. Inpatient burden of constipation in the United States: an analysis of national trends in the United States from 1997 to 2010. Am J Gastroenterol. 2014;109:250–6. doi: 10.1038/ajg.2013.423. [DOI] [PubMed] [Google Scholar]
- Nyrop KA, Palsson OS, Levy RL, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–48. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Pemberton JH, Locke GR. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–38. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha AE, Locke GR, Zinsmeister AR, et al. Differences between painless and painful constipation among community women. Am J Gastroenterol. 2006;101:604–12. doi: 10.1111/j.1572-0241.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Morris C, Hu Y, et al. Further characterization of painful constipation (PC): clinical features over one year and comparison with IBS. J Clin Gastroenterol. 2008;42:1080–8. doi: 10.1097/mcg.0b013e31815146f9. [DOI] [PubMed] [Google Scholar]
- Mearin F, Lacy BE. Diagnostic criteria in IBS: useful or not? Neurogastroenterol Motil. 2012;24:791–801. doi: 10.1111/j.1365-2982.2012.01992.x. [DOI] [PubMed] [Google Scholar]
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. doi: 10.1111/j.1365-2036.2006.03238.x. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Dorn SD, Lembo A, Pressman A. American gastroenterological association medical position statement on constipation. Gastroenterology. 2013;144:211–7. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Pare P. The approach to diagnosis and treatment of chronic constipation: suggestions for a general practitioner. Can J Gastroenterol. 2011;25:36–40. doi: 10.1155/2011/368189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl. 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frissora CL. Diagnosis, treatment, and management of irritable bowel syndrome with constipation and chronic constipation. Med Gen Med. 2005;7:71. [PubMed] [Google Scholar]
- Bassotti G, Carlani E, Baldón M, Gullà N, Morozzi B, Villanacci V. Painful constipation: a neglected entity? Rev Esp Enferm Dig. 2011;103:25–8. doi: 10.4321/s1130-01082011000100005. [DOI] [PubMed] [Google Scholar]
- Shekhar C, Monaghan PJ, Morris J, et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749–57. doi: 10.1053/j.gastro.2013.07.014. quiz e13-4. [DOI] [PubMed] [Google Scholar]
- Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39:312–21. doi: 10.1111/apt.12573. [DOI] [PubMed] [Google Scholar]
- Rey E, Balboa A, Mearin F. Chronic constipation, irritable bowel syndrome with constipation and constipation with pain/discomfort: similarities and differences. Am J Gastroenterol. 2014;109:876–84. doi: 10.1038/ajg.2014.18. [DOI] [PubMed] [Google Scholar]
- Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228–34. doi: 10.1038/ajg.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(Suppl. 1):S5–21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- Talley NJ. Differentiating functional constipation from constipation-predominant irritable bowel syndrome: management implications. Rev Gastroenterol Disord. 2005;5:1–9. [PubMed] [Google Scholar]
- Frissora CL, Koch KL. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Curr Gastroenterol Rep. 2005;7:264–71. doi: 10.1007/s11894-005-0018-9. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Weiser K, De LR. The treatment of irritable bowel syndrome. Therap Adv Gastroenterol. 2009;2:221–38. doi: 10.1177/1756283X09104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536–43. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–36. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–25. doi: 10.1038/ajg.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–12. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Functional bowel disorders and functional abdominal pain. In: Drossman DA, editor. ROME II: The Functional Gastrointestinal Disorders. Degnon Associates: McLean, Virginia, USA; 2000. pp. 360–91. [Google Scholar]
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540–51. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- Palsson OS, Baggish JS, Turner MJ, Whitehead WE. IBS patients show frequent fluctuations between loose/watery and hard/lumpy stools: implications for treatment. Am J Gastroenterol. 2012;107:286–95. doi: 10.1038/ajg.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Kurtz CB, Macdougall JE, et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome. Gastroenterology. 2010;139:1877–86. doi: 10.1053/j.gastro.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Rao S. Phase 3 clinical trial of linaclotide in patients with IBS-C demonstrates significant improvements in abdominal pain and bowel symptoms. Neurogastroenterol Motil. 2011;23:2. [Google Scholar]
- Locke GR, Zinsmeister AR, Fett SL, Melton LJ, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- Basilisco G, Coletta M. Chronic constipation: a critical review. Dig Liver Dis. 2013;45:886–93. doi: 10.1016/j.dld.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Posserud I, Syrous A, Lindström L, Tack J, Abrahamsson H, Simrén M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–23. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographical characteristics and baseline global measures.
Table S2. Adverse events in the abdominal pain subpopulations.



