Abstract
Paracoccidioidomycosis (PCM) is a systemic fungal disease that is particularly important among individuals living and working in rural areas of endemicity in Latin America. Detection of anti-Paracoccidioides brasiliensis antibodies is of limited value due to false-negative results. Detection of P. brasiliensis-gp43 circulating antigen is a practical approach for a specific diagnosis of the disease. In a previous study we described an inhibition enzyme-linked immunosorbent assay able to detect the 43-kDa P. brasiliensis antigen in sera of 100% of patients with the acute form of PCM and in 95.31 and 100% of patients with the chronic multifocal and unifocal forms of PCM. To investigate its potential application for the follow-up of PCM patients during treatment, antigen levels were monitored at regular intervals for up 8 to 12 months in serum samples from 23 patients. The results showed that treatment with itraconazole resulted in decreasing levels of circulating gp43 that were correlated with the reduction of anti-gp43 antibodies. It was also observed that by the end of 12 months of treatment gp43 levels were <5 μg/ml in all patients.
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by Paracoccidioides brasiliensis, a thermal dimorphic fungus. It is the most prevalent systemic endemic mycosis in many countries in Latin America. Most of the cases have been reported in Brazil, Colombia, and Venezuela. Except for Chile, Guyanas, Surinam, and some Caribbean islands, the disease has been reported from Mexico to Argentina. Nonautochthonous cases have been reported outside the area of endemicity, but all of them had been previously infected in Latin America. Outside the area of endemicity, PCM should be regarded as a disease of travelers who have lived for extended periods of time in such areas. According to McEwen et al. (20), ca. 10 million people may be infected with this fungus, and up to 2% of them may develop the disease. The incidence may increase due to forest destruction and a rise in iatrogenic immunosuppression procedures (35).
The acute or subacute form of PCM affects both genders and chiefly involves the reticuloendothelial system (RES). The chronic form affects mainly adult males, with a predominance of pulmonary and/or mucocutaneous involvement (11). The definitive diagnosis of PCM is based on the visualization of fungal elements in fresh preparations or by histopathology of biological samples, as well as by culturing the fungi from the lesions or from several types of body fluids.
Among the non-culture-based methods for the diagnosis of the disease are several serological methods, including the detection of specific antibodies or antigens. The detection of specific antibodies in serum presents some limitations in terms of sensitivity and specificity and may not be used as the single laboratory criterion to establish a diagnosis of PCM. Unfortunately, there is extensive antigenic cross-reactivity between P. brasiliensis and other fungi, limiting the value of the tests currently being used. In view of the cross-reactivity and variations among the isolates used as sources for antigens production, it is advisable to use more than one test for the diagnosis of PCM. The main PCM diagnostic antigen is the 43-kDa glycoprotein (gp43) (5, 26) that is secreted exocellularly during the infective yeast phase (5, 26, 32) and is recognized by virtually all sera from PCM patients in various tests (2, 5, 23, 33, 34). In routine practice, detection of specific antibodies is mainly used to monitor the evolution of the disease and its response to treatment (24). The most common serological tests used in clinical practice are immunodiffusion (5, 7, 29), immunoenzymatic assays (4, 6, 23), and counterimmunoelectrophoresis (8).
There is no consensus about the optimal duration of antifungal treatment for patients with PCM, but several publications have advocated that detection of circulating specific antibodies is an important parameter to define the time to stop antifungal treatment. However, PCM patients have a strong specific antibody response against gp43 that may persist throughout life. In contrast, some patients, depending on their immune status, are serologically negative at the time of diagnosis, with others showing low levels of specific antibodies for long periods of time. Consequently, sometimes it is very difficult to determine whether these patients are ever cured (11).
Due to these limitations of the antibody detection tests, some researchers have evaluated the performance of assays for the detection of circulating P. brasiliensis gp43 for the diagnosis of PCM. In some invasive fungal diseases, such as aspergillosis, cryptococcosis, and histoplasmosis, the detection of circulating antigens is a useful approach to immunodiagnosis (9, 10, 15, 19, 36) and, in some cases, may also help the clinician to evaluate the clinical response to antifungal therapy. Some tests have been used to detect gp43 circulating antigen with partial success in PCM (12, 13, 14, 16, 17, 21, 22, 30). Recently, we developed a P. brasiliensis-gp43 antigen detection test by using a species-specific murine monoclonal antibody (MAb) in an inhibition enzyme-linked immunosorbent assay (inh-ELISA) system that showed high sensitivity and specificity (18).
We compare here consecutive serological parameters obtained during a 12-month period of evaluation of 23 PCM patients successfully treated with itraconazole (ITZ) and include an analysis of data generated by an inh-ELISA test to monitor gp43 circulating levels and anti-gp43 antibody detection.
MATERIALS AND METHODS
Clinical samples.
We obtained serum samples from a total of 23 patients with active PCM documented by the visualization of characteristic fungal elements by histopathologic and/or direct KOH examinations, isolation by culture, and/or positive serological tests. One serum sample was taken from each patient at the time of diagnosis, and sequential samples were taken during antifungal treatment for 8 to 12 months (at least seven samples per patient). Samples were collected between 2001 and 2002 at the Infectious Diseases Division, University Hospital, Medical School, Federal University of Paraná, Curitiba, Paraná, Brazil. All patients were males and presented with the chronic multifocal form of the disease. The mean age was 47.3 years. Serum samples obtained at the time of diagnosis from patients with other mycologically and/or serologically confirmed mycoses were also evaluated (histoplasmosis [n = 33] and cryptococcosis [n = 20]). Sera (n = 57) from healthy volunteers (blood donors) were included as negative controls.
Antimycotic treatment.
PCM patients were treated with ITZ (Janssen) at 200 mg/day until clinical, radiological, mycological, and serological criteria of cure were reached, such as cessation of all clinical signs and symptoms related to the infection, significant improvement in the chest X-ray pattern of pulmonary lesions, absence of fungi in biological specimens, and negative serology or presence of low titers of specific antibodies (immunodiffusion test).
Fungal isolate, exoantigen preparation, and gp43 purification.
P. brasiliensis B-339 (ATCC 200273) was obtained from the culture collection of the Disciplina de Biologia Celular Da Universidade Federal de São Paulo. The isolate was transformed to the yeast phase, and exoantigen was produced by the method of Camargo et al. (5). Gp43 was purified from this exoantigen as described elsewhere (27). Protein determination was performed by the Bradford method (3).
MAb anti-gp43.
MAb was prepared by Puccia and Travassos (28) and kindly provided by them for use in the present study.
inh-ELISA.
An inh-ELISA test was developed for serum according to the methods of Gómez et al. (14) and Marques da Silva et al. (30). The diluting buffer used in the serum experiments consisted of a pool of normal human serum (NHS) at 1:10 in phosphate-buffered saline-0.05% Tween 20 (PBS-Tween), 20 mM MgCl2, and 1% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.). Purified MAb 17c anti-gp43 was used at 10 μg/ml, and all serum samples were tested at 1:2 in diluting buffer.
Inhibition plate.
An inhibition standard curve was constructed by adding different concentrations of P. brasiliensis gp43 (from 1 ng to 30 μg/ml) to 100 μl of pooled NHS and then adding 100 μl of the standardized concentration of MAb 17c. NHS made up 1:2 in diluting buffer was used as a negative control. All of the standards, samples, and controls were tested in triplicate. Samples were plated onto 96-well flat microtiter plates (Corning Costar) previously blocked by incubation with 200 μl of 5% bovine serum albumin per well made up in PBS-Tween, for 2 h at 37°C. Plates were mixed in a shaker for 30 min at room temperature and then incubated overnight at 4°C.
Reaction plate.
Maxisorp polystyrene plates (Corning Costar) were coated with 500 ng of gp43 in 0.06 M carbonate buffer (pH 9.6) per well (100 μl/well). The plates were left at room temperature for 30 min and then incubated overnight at 4°C. After incubation, the plates were washed three times in PBS-Tween and blocked by incubation with 200 μl of 1% bovine serum albumin in PBS per well for 1 h at 37°C; after three more washes, 100 μl from each well of the inhibition plate (containing a mixture of the MAb-circulating complexes and free MAb) was transferred to the respective wells in the reaction plate and allowed to stand for 2 h at 37°C. After being washed as described above, 100 μl of goat anti-mouse immunoglobulin G-peroxidase (Sigma) was added, and the plates were incubated for 1 h at 37°C. After further washings, the reaction was developed with a solution of o-phenylenediamine (0.5 mg/ml; Sigma) and 0.005% H2O2. The reaction was stopped with 4 N H2SO4 after 8 to 10 min of incubation in the dark. Optical densities were measured at 490 nm with an ELISA reader (Titertek Multiskan EIA reader). The optical density at 492 nm was then plotted on a standard curve constructed from the data derived from MAb titration with NHS containing known quantities of gp43 as described above. The degree of inhibition in MAb binding was shown to be reciprocal to the concentration of circulating antigen in the sample. The cutoff point was established as the receiver operator characteristic (ROC) curve.
Immunodiffusion test.
The immunodiffusion test was performed at the time of diagnosis and monthly during the follow-up of the patients, according to the methodology used in our previous study (5).
Statistical analysis.
Data were analyzed statistically by Stata 7.0 (Windows 98/95/NT version), and specificity, sensitivity, and method efficiency were analyzed by using the ROC curve. Comparisons were made by one-way analysis of variance. Intergroup comparisons were performed by using the Kruskal-Wallis test.
RESULTS
Table 1 shows the characteristics of the 23 PCM patients studied.
TABLE 1.
Characteristics of 23 PCM patients treated with ITZ
| Patient no. and sites of lesionsa | Age (yr) | No. of samples tested | Length of follow-up (mo) | Antigen level (μg/ml) at:
|
||
|---|---|---|---|---|---|---|
| Diag- nosis | First follow-up | End of treatment | ||||
| 1 (L, M, G) | 67 | 8 | 11 | 10.5 | 8.25 | 0 |
| 2 (L, S, G) | 40 | 7 | 9 | 12.75 | 4.36 | 0 |
| 3 (L, M, G) | 51 | 7 | 9 | 7.12 | 2.85 | 0 |
| 4 (L, Of, G) | 56 | 9 | 12 | 30.0 | 30.0 | 2.31 |
| 5 (L, S, M, G) | 31 | 7 | 11 | 14.25 | 4.77 | 0 |
| 6 (L, M, G) | 44 | 7 | 9 | 11.25 | 2.49 | 0 |
| 7 (L, G, Lx) | 44 | 6 | 8 | 14.25 | 3.75 | 3.57 |
| 8 (L, M, G) | 42 | 7 | 9 | 13.5 | 28.5 | 2.57 |
| 9 (L, M, G) | 49 | 7 | 11 | 7.12 | 4.27 | 0 |
| 10 (L, M) | 44 | 7 | 9 | 14.25 | 12.0 | 0 |
| 11 (L, M, G) | 52 | 7 | 10 | 14.25 | 3.07 | 3.21 |
| 12 (L, M, G) | 50 | 6 | 9 | 3.57 | 3.57 | 0 |
| 13 (L, M) | 49 | 6 | 9 | 10.5 | 6.38 | 0 |
| 14 (L, M) | 37 | 5 | 9 | 9.75 | 5.25 | 0 |
| 15 (L, M) | 56 | 6 | 8 | 6.38 | 6.01 | 0 |
| 16 (L, M, G, Lx) | 49 | 6 | 9 | 28.5 | 4.90 | 4.53 |
| 17 (L, M) | 66 | 8 | 10 | 2.49 | 3.75 | 0 |
| 18 (L, M) | 51 | 7 | 10 | 5.64 | 1.56 | 0 |
| 19 (L, Lx) | 40 | 7 | 10 | 14.25 | 5.64 | 0 |
| 20 (L, M) | 37 | 8 | 10 | 16.5 | 6.0 | 0 |
| 21 (L, CNS) | 37 | 6 | 12 | 14.25 | 9.75 | 0 |
| 22 (L, M) | 42 | 8 | 12 | 3.39 | 2.49 | 0 |
| 23 (L, M, G) | 54 | 7 | 12 | 9.75 | 2.13 | 0 |
L, lung; M, mucosa; G, ganglion; Lx, larynx; Of, oropharynx; S, skin; CNS, central nervous system.
Detection of P. brasiliensis antigenemia during follow-up by inh-ELISA.
The standard inhibition curve constructed with known quantities of P. brasiliensis gp43 was similar to that obtained in our previous study (18). This curve was used to determine the concentration of P. brasiliensis gp43 in each sample tested at the time of diagnosis and during treatment. The sensitivity of inh-ELISA ranged from 0.0053 to 30 μg of antigen per ml of serum. The cutoff point was established by the ROC curve, based on the antigen concentration of PCM patients and normal human sera. Antigen concentrations higher than 1.35 μg/ml were considered to be a positive result.
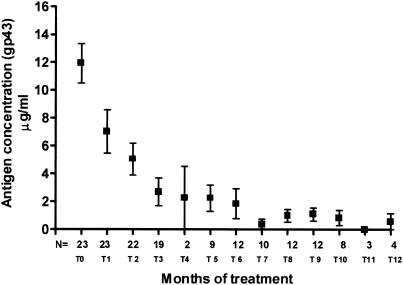
All of the 23 PCM serum samples had levels of circulating gp43 antigen above the cutoff point at the time of diagnosis, with a mean antigen concentration of 11.92 μg/ml (Fig. 1). In the first period analyzed (30 days of antifungal treatment), the mean antigen concentration dropped to 7.23 μg/ml, and decreasing concentrations were detected until the 12th month, when the mean antigen concentration was 0.57 μg/ml. Figure 2 demonstrates the behavior of different patients in relation to gp43 detection compared to anti-gp43 antibody titers during the period of treatment. Sera from patients with histoplasmosis or cryptococcosis and from normal donors showed no detectable antigens with this inh-ELISA methodology (data not shown).
FIG. 1.
Media of circulating antigen concentrations in sera from patients with PCM at the time of diagnosis and during treatment. Error bars indicate the standard deviations.
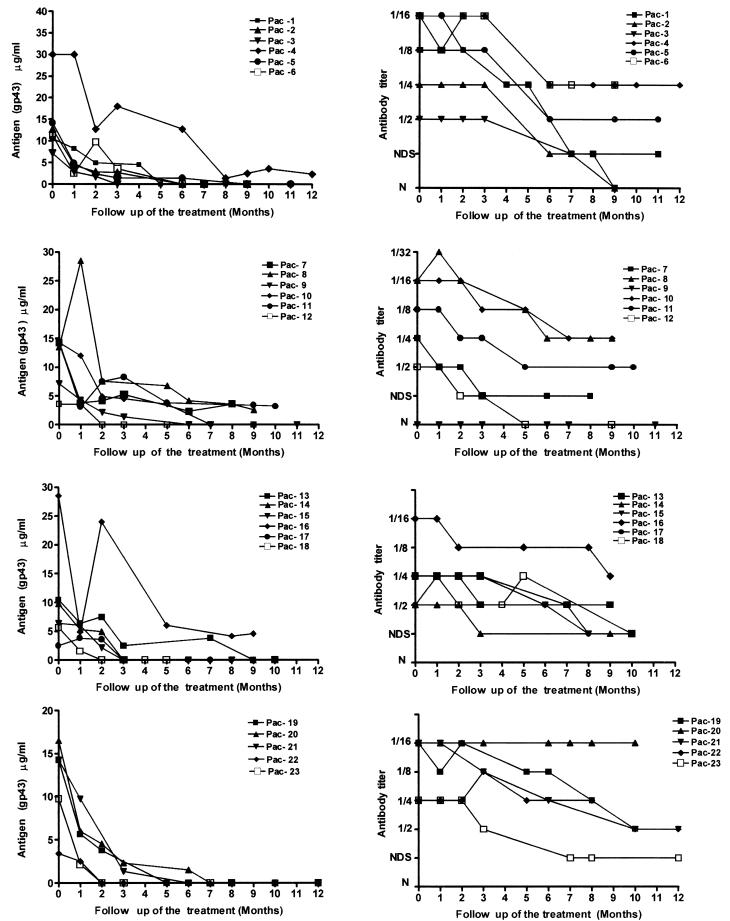
FIG. 2.
Serologic follow-up curves of 23 PCM patients during treatment. Left panels show gp43 antigen concentrations; right panels show anti-gp43 antibody titers.
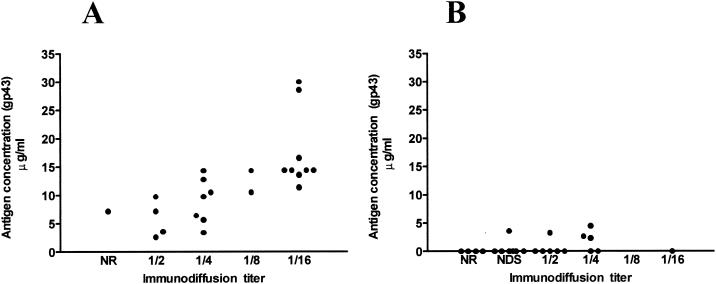
Figure 3 shows the correlation between the levels of antigenemia due to gp43 and anti-gp43 antibodies at the time of diagnosis (Fig. 3A) and at the end of treatment (Fig. 3B). At the time of diagnosis all patients showed detectable gp43 antigen in sera and anti-gp43 antibodies (except for one patient with negative immunodiffusion). At the end of therapy, only 5 (21.73%) patients had detectable gp43 antigen and positive titers by the immunodiffusion test. After the period of antifungal therapy (8 to 12 months), all PCM patients showed clinical improvement, and antigen levels were <5 μg/ml.
FIG. 3.
Correlation between the levels of antigenemia due to gp43 and anti-gp43 antibody titers at the time of diagnosis (A) and at the end of treatment (B). NR, nonreactor; NDS, not diluted sera.
Overall, there was a significant reduction in the levels of circulating gp43 antigen in these patients by week 12 after the initiation of therapy, and this reduction was maintained until the end of the follow-up period. All patients improved clinically and mycologically with azole therapy. The antibody titers determined by immunodiffusion at the time of diagnosis were variable (1:2 to 1:16), and in almost all patients the titers decreased during treatment.
DISCUSSION
One of the main challenges in PCM is to establish when the clinician should stop the antimycotic therapy because there is no consensus about treatment options or about when the patient is really cured. Although healing of apparent lesions may occur within a short time after treatment is initiated, long therapeutic courses are desirable in order to prevent relapses. In this case, the host response must be monitored by indirect methods and, in this scenario, serology provides information about prognosis.
The recent progress and refinement of serologic tests was due in part to the standardization of the preparation of crude or purified antigens, such as the reference Ag7 crude antigen (5) or the gp43 purified antigen (26), making the methods for antibody detection more sensitive and specific. For many years, the follow-up of patients with PCM has been based on the detection of antibody responses against P. brasiliensis crude or purified antigens (4, 23, 24). Thus far, there is no consensus among the different groups about the “gold standard” method for the follow-up of PCM patients.
Recently, in our laboratory, Alves (1) compared three methods (immunodiffusion, ELISA, and capture-ELISA) in order to verify which one was better for the follow-up of PCM patients. In that study, serum samples were tested during the pretreatment period and after 6, 12, and 18 months of treatment. Immunodiffusion and capture-ELISA showed a significant decrease in titer after 6 months in good responder patients. ELISA detected decreasing titers only after 12 months of therapy. None of the methods were able to predict relapse of the disease prior to clinical observation, and increased titers could be observed only later, after the onset of relapse. Even with these limitations, that study indicated that the immunodiffusion test seemed to be best option for the follow-up of PCM patients compared to the other two tests.
However, our experience with the follow-up of patients being treated for PCM has shown that many times the antibody titers obtained by the immunodiffusion test do not correlate with the clinical status of the patient. For example, in some cases, elevated antibody titers (1:64) were observed until the end of treatment when the patients were clinically cured. On the other hand, although for most patients low antibody titers are related to the absence of clinical symptoms (1:2 or 1:4), in some cases low titers are present for months and in concert with clinical symptoms. For these reasons, the use of serology as a criterion of cure in PCM is controversial; instead, we believe that clinical, radiological, mycological, and serological aspects evaluated over a long period of observation must be considered to establish the occurrence of cure.
For diagnostic purposes, tests based on immunodiffusion are generally considered to have high specificity; however, false-negative results may also occur. In a previous study we showed that the lack of reactivity of sera from PCM patients in immunodiffusion tests may be related to the production of low-avidity immunoglobulin G2 antibodies directed against carbohydrate epitopes (25). The sensitivity of immunodiffusion-based tests ranges from 65 to 100% (6, 23), depending on the antigen preparation used. On the other hand, tests with higher sensitivity, such as immunoenzymatic assays, present problems associated with specificity due to cross-reactivity with heterologous sera.
A more rational approach to the diagnosis of PCM may be the detection of P. brasiliensis gp43 antigen in body fluids. gp43 is the dominant antigen and is considered to be a molecular marker of the disease. Usually, almost all PCM sera reacted with gp43 as detected by Western blotting; followed by the 70-kDa antigen, which is also recognized (2).
With respect to the detection of antigen during treatment, Mendes-Giannini et al. (22), by means of Western blot assay and working with pool of PCM sera, observed that gp43 started to disappear from the circulation after 10 months of chemotherapy and was undetectable after 2 years of treatment. No quantitative measurements were made in individual sera. More recently, the same group (31) determined the presence of P. brasiliensis antigens in the urine of patients by an indirect competition enzyme immunoassay (EIA-c) and an immunoblot test for monitoring the response to therapy. By means of EIA-c, the presence of P. brasiliensis antigens could be detected in 75% of the urine samples tested.
One of the main objectives here was to assess the behavior of gp43 antigenemia during the treatment of PCM patients with ITZ until the apparent cure at the end of therapy (i.e., after 8 to 12 months).
PCM is a chronic fungal infection that chiefly involves the RES, as well as the lungs. The definition of cure or good clinical response is sometimes difficult to establish considering the low sensitivity of cultures, the lung sequels, and the limitations related to the evaluation of the infectious status at the RES. As a consequence, there is a consensus that nonculture methods are useful tool to help the clinician to better evaluate the therapy response.
In the present study we evaluated the antigen levels of 23 PCM patients that were successfully treated with ITZ. The definition of a successful response to the treatment was based on the resolution of all signs and symptoms related to the infection, as well as improvement in the radiological findings (data not shown). It is important to highlight that during the 12-month period of clinical follow up, no single patient had any clinical or radiological evidence of relapse.
Although we were not able to evaluate the behavior of the antigen levels during a relapse episode of the fungal infection, clinical improvement of all patients was followed by a decrease of the antigen levels to <5 μg/ml. This finding strongly suggests that gp43 antigenemia may be a useful tool for monitoring the therapeutic response to antifungal treatment.
In this study population, decreasing levels of P. brasiliensis gp43 were detected in patients during ITZ therapy. Of the 23 patients studied, 2 had antigenemia of <5 μg/ml after 2 months of treatment, 15 patients had antigenemia of <5 μg/ml after 3 months, and 5 patients had antigenemia of <5 μg/ml after 6 months of treatment. Only patient 4 presented negative levels of antigenemia at month 8. It was noted that, once decreased, the levels of gp43 in serum remained low until the end of therapy. On the other hand, in most cases, the antibody titers seemed to decrease a little later than this, i.e., after about 6 months of therapy.
Some cases deserve particular attention. For example, patient 20 showed a decreased serum antigenemia after 8 months of treatment, but his anti-gp43 antibody titers remained high (1:16) and did not change throughout the period of treatment. In contrast, patient 9 had undetectable antibodies (as determined by the immunodiffusion test) from the time of diagnosis to month 11 of treatment but showed a substantial decrease in antigenemia levels during treatment.
In conclusion, our results indicate that the detection and quantitation of the immunodominant 43-kDa P. brasiliensis antigen in sera by inh-ELISA is a sensitive method to be used for monitoring patients with PCM under treatment. New studies evaluating PCM patients undergoing treatment with other antifungal drugs are under way in order to confirm these data.
Acknowledgments
This study was supported by a grant from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Sao Paulo, Brazil).
We thank Elletra Grene for English correction.
REFERENCES
- 1.Alves, J. R. 1996. Comparação entre três métodos sorológicos no seguimento de pacientes com paracoccidioidomicose. M.S. thesis. Universidade Federal de São Paulo, São Paulo, Brazil.
- 2.Blotta, M. H. S. L., and Z. P. Camargo. 1993. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationships with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 31:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Camargo, Z. P., J. L. Guesdon., E. Drouhet, and L. Improvisi. 1984. Enzyme-linked immunosorbent assay (ELISA) in paracoccidioidomycosis. Mycopathologia 88:31-37. [DOI] [PubMed] [Google Scholar]
- 5.Camargo, Z. P., C. Unterkircher, S. P. Campoy, and L. R. Travassos. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion test. J. Clin. Microbiol. 26:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo, Z. P., C. S. Unterkircher, and L. R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis in serological tests. J. Med. Vet. Mycol. 27:407-412. [PubMed] [Google Scholar]
- 7.Cano, L. E., and A. Restrepo. 1987. Predictive value of serologic tests in the diagnosis and follow-up of patients with paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 29:276-283. [DOI] [PubMed] [Google Scholar]
- 8.Conti-Diaz, I. A., J. E. Mackinnon, L. Calegari, and S. Casserone. 1978. Estudio comparativo de la inmunoelectroforesis-inmunodifusión (IEOF-ID) y de la inmunoelectroforesis (IEF) en el diagnóstico de la paracoccidioidomicosis. Mycopathologia 63:161-165. [DOI] [PubMed] [Google Scholar]
- 9.Dupont, B., M. Huber, S. J. Kim, and J. E. Bennett. 1987. Galactomannan antigenemia and antigenuria in aspergillosis studies in patients and experimentally infected rabbits. J. Infect. Dis. 155:1-11. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira, R. P., B. Yu, Y. Niki, and D. Armstrong. 1990. Detection of Candida antigenuria in disseminated candidiasis by immunoblotting. J. Clin. Microbiol. 28:1075-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco, M. 1986. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 25:5-18. [DOI] [PubMed] [Google Scholar]
- 12.Freitas-da-Silva, G., and M. C. Roque-Barreira. 1992. Antigenemia in paracoccidioidomycosis. J. Clin. Microbiol. 30:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, N. M., G. B. Del Negro, H. P. Martins, and C. S. Lacaz. 1987. Detection of Paracoccidioides brasiliensis circulating antigens by immunoelectrophoresis-immunodiffusion technique: preliminary report. Rev. Med. Trop. Sao Paulo 29:327-328. [DOI] [PubMed] [Google Scholar]
- 14.Gómez, B. L., J. I. Figueroa, A. J. Hamilton, B. Ortiz, M. A. Robledo, R. J. Hay, and A. Restrepo. 1997. Use of monoclonal antibody in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J. Clin. Microbiol. 35:3278-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, K. A., J. P. Latge, and T. R. Rogers. 1990. Detection of Aspergillus antigens associated with invasive infection. J. Clin. Microbiol. 28:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magaldi, S. W., and D. W. R. Mackenzie. 1986. Detección de antigenemía y anticuerpos de paracoccidioides mediante procedimentos electroforéticos invertidos, p. 80. In Proceedings Coloquio Internacional sobre la Paracoccidioidomicosis. Corporación para Investigaciones Biologicas, Medeilín, Colombia.
- 17.Magaldi, S. W., D. W. R. Mackenzie, and M. B. Albornoz. 1989. Detection of Paracoccidioides brasiliensis circulating antigen by the passive hemagglutination inhibition in patients sera, p. 18. In Proceedings Encuentro International sobre Paracoccidioidomicosis. Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela.
- 18.Marques da Silva, S. H. M., A. L. Colombo., M. H. S. L. Blotta., J. D. Lopes., F. Queiroz-Telles, and Z. P. Camargo. 2003. Detection of circulating gp43 antigen in serum, cerebrospinal fluid and bronchoalveolar lavage of patients with paracoccidioidomycosis. J. Clin. Microbiol. 41:3675-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathews, R. C. 1996. Comparative assessment of the detection of candidal antigens as a diagnostic tool. J. Med. Vet. Mycol. 34:1-10. [DOI] [PubMed] [Google Scholar]
- 20.McEwen, J. G., A. M. Garcia, B. L. Ortiz, S. Botero, and A. Restrepo. 1995. In search of the natural habitat of Paracoccidioides brasiliensis. Arch. Med. Res. 26:305-306. [PubMed] [Google Scholar]
- 21.Mendes-Giannini, M. J., M. E. Camargo, C. A. Lacaz, and A. W. Ferreira. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 20:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes-Giannini, M. J. S., J. P. Bueno, M. A. Skikanai-Yasuda, A. W. Ferreira, and A. Masuda. 1989. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 27:2842-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes-Giannini, M. J., J. P. Bueno, M. A. Shikanai-Yassuda, A. M. S. Stolf, A. Masuda, V. Amato-Neto, and A. W. Ferreira. 1990. Antibody responses to 43-kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am. J. Trop. Med. Hyg. 43:200-206. [DOI] [PubMed] [Google Scholar]
- 24.Mendes-Giannini, M. J., G. B. Del Negro, and A. M. Siqueira. 1994. Serodiagnosis, p. 345-363. In M. Franco, C. S. Lacaz, A. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 25.Neves, A. R., R. L. Mamoni, Z. P. Camargo, and M. H. S. L. Blotta. 2003. Negative immunodiffusion tests in sera of paracoccidioidomycosis patients may be related to low avidity IgG2 antibodies directed against carbohydrate epitopes. Clin. Diagn. Lab. Immunol. 10:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puccia, R., S. Schenkman, P. A. J. Gorin, and L. R. Travassos. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect. Immun. 53:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puccia, R., and L. R. Travassos. 1991. The 43-kDa glycoprotein from the human pathogen Paracoccidioides brasiliensis and its deglycosylated form: excretion and susceptibility to proteolysis. Arch. Biochem. Biophys. 289:298-302. [DOI] [PubMed] [Google Scholar]
- 28.Puccia, R., and L. R. Travassos. 1991. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restrepo, A. 1966. La prueba de inmunodifusíon en el diagnóstico de la paracoccidioidomicosis. Sabouraudia 4:223-230. [PubMed] [Google Scholar]
- 30.Rodrigues, M. C., C. M. Cassaguerra, and C. S. Lacaz. 1984. Antigenemia in paracoccidioidomycosis: probable demonstration of circulating antigen by counterimmunoelectrophoresis test. Rev. Inst. Med. Trop. São Paulo 26:285-287. [DOI] [PubMed] [Google Scholar]
- 31.Salina, M. A., M. A. Shikanai-Yasuda, R. P. Mendes, B. Barraviera, and M. J. Mendes-Giannini. 1998. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidioidomycosis patients before and during treatment. J. Clin. Microbiol. 36:1723-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stambuk, B. U., R. Puccia, M. L. C. Almeida, L. R. Travassos, and S. Schenkman. 1988. Secretion of the 43-kDa glycoprotein antigen by Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 26:367-373. [DOI] [PubMed] [Google Scholar]
- 33.Taborda, C. P., and Z. P. Camargo. 1993. Diagnosis of paracoccidioidomycosis by passive haemagglutination assay of antibody using a purified and specific antigen: gp43. J. Med. Vet. Mycol. 31:155-160. [DOI] [PubMed] [Google Scholar]
- 34.Taborda, C. P., and Z. P. Camargo. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32:554-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanke, B., and A. T. Londero. 1994. Epidemiology and paracoccidioidomycosis infection, p. 109-120. In M. Franco, C. S. Lacaz, A. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 36.Wheat, L. J., R. B. Kohler., and R. P. Tewari. 1986. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N. Engl. J. Med. 314:83-88. [DOI] [PubMed] [Google Scholar]