Abstract
Background
Mast cells are immune cells derived from hematopoietic precursors that mature in the tissue microenvironment. Mast cells are critical for allergic, immune and inflammatory processes, many of which involve TNF. Mast cells uniquely store TNF in their secretory granules. Upon stimulation, mast cells rapidly (30 min) secrete beta-hexosaminidase (beta-hex) and granule-stored TNF through degranulation, but also increase TNF mRNA and release de novo synthesized TNF 24 hr later. Regulation of these two distinct pathways is poorly understood.
Methods
human LAD2 leukemic mast cells are stimulated by substance P (SP). TNF secretion and gene expression were measured by ELISA and Real-Time PCR. Live cell mitochondrial dynamics was observed under Confocal Microscopy. Cell energy consumption were measured in term of oxygen consumption rate.
Results
Here we show that granule-stored TNF is preformed and its secretion from LAD2 mast cells stimulated by SP exhibits (a) higher energy consumption and is inhibited by the mitochondrial ATP pump blocker oligomycin, (b) rapid increase of intracellular calcium levels, and (c) reversible mitochondrial translocation, from a perinuclear distribution to the cell surface, as compared to de novo synthesized TNF release induced by lipopolysaccharide (LPS). This mitochondrial translocation is confirmed using primary human umbilical cord blood-derived mast cells (hCBMCs) stimulated by an allergic trigger (IgE/streptavidin).
Conclusion
These findings indicate that unique mitochondrial functions distinguish granule-stored from newly synthesized TNF release from human mast cells, thus permitting the versatile involvement of mast cells in different biological processes.
Keywords: Lipopolysaccharide, Mast cells, Mitochondria, Substance P, TNF
Introduction
Mast cells are bone marrow-derived immune cells that can secrete pre-stored mediators such as histamine and tryptase through rapid (5 to 30 min) degranulation, as well as delayed (12 to 24 hr) newly synthesized cytokines including interleukin-4 (IL-4) IL-6, IL-8, IL-13 and TNF in response to allergic or neuropeptide triggers 1, 2. In fact, mast cells uniquely store TNF in secretory granules 3, 4. Stimulation of LAD2 5 cells by SP induces degranulation and secretion of pre-stored TNF 6, while stimulation with LPS induces selective de novo synthesis and release of TNF without degranulation 7, 8, 9.
Other secretory cell types, like eosinophils, use distinct mechanisms for secretion, such as exocytosis of large storage granules, and release from small secretory vesicles 10. Mast cells can also release mediators selectively without degranulation 11, first reported for release of serotonin without histamine 12, and later for IL-6 without histamine 13. In both cases, this selective release involved release from small vesicles (diameter=80 nm) rather than from the typical secretory granules (diameter=1000 nm) 14, 13. This ability, mast cell stimulation by allergic and non-immune triggers, 15 as well as the synergistic stimulation by cytokines and neuropeptides 16 may allow mast cells to participate in a variety of distinct pathophysiological settings, in addition to allergy17. These include innate and acquired immunity 18, inflammation 11, autoimmunity 19, wound healing20 and cancer growth 21, as well as atherosclerosis and obesity 22. However, little is known about what distinguishes rapid degranulation from delayed selective cytokine release.
Degranulation from rat peritoneal mast cells requires metabolic energy and calcium 23. Mitochondria are the primary sources of energy production in eukaryotic cells and also have the ability to buffer calcium locally 24. Moreover, mitochondria are dynamic organelles that that participate in many complicated cell functions through morphological and localization changes 25. Increasing evidence indicates the importance of mitochondrial dynamics in immune cell regulation. For instance, local ATP production by mitochondria is required for T cell chemotaxis 26. Moreover, mitochondrial translocation is required for T cell “immune synapse” formation and sustainable calcium influx 27. On the other hand, local intracellular calcium changes can also regulate mitochondrial dynamics and subcellular localization 28.
In this study, we show that SP-induced granule-stored TNF secretion, unlike newly synthesized selective TNF release, requires high mitochondrial energy consumption, intracellular calcium increase, and mitochondrial translocation to the cell surface.
Materials and methods
Cell lines and reagents
LAD2 culture cells 5 (from Dr. A.S. Kirshenbaum, NIH, Bethesda, MD, USA) were cultured in StemPro-34 Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 100 ng/ml recombinant human stem cell factor (rhSCF, from Biovitrum AB, Stockholm, Sweden) and 100 U/ml penicillin/streptomycin. Cells were grown in an incubator in 5% CO2 and air at 37 °C. All cells were used during their logarithmic growth period. They were stimulated by either SP (10 μM) or LPS (10 ng/ml) dissolved in distiled water.
Human cord blood-derived mast cells (hCBMCs) were grown from human cord blood obtained during normal deliveries in accordance with established institutional guidelines 29. Briefly, mononuclear cells were isolated by layering heparin-treated cord blood onto Lymphocyte Separation Medium (INC Biomedical, Aurora, OH, USA). CD34+ progenitor cells were isolated from mononuclear cells by positive selection of AC133 (CD133+/CD34+) cells by magnetic cell sorting (Miltenyi Biotech, Auburn, CA, USA). hCBMCs were derived by the culture of CD34+ progenitor cells with minor modifications. For the first six weeks, CD34+ cells were cultured in AIM medium (Gibco, Grand Island, NY, USA) supplemented with 100 ng/ml rhSCF and after six weeks 50 ng/ml IL-6 (Chemicon, Billerica, MA, USA) was added and cultured at 37 °C in 5% CO2 balanced air. Mast cell viability was determined by trypan blue (0.3%) exclusion. They were stimulated first passively sensitized with human monoclonal IgE (EMD Bioscience, Darmstadt, Germany, 1 μg/ml) for 24 hr and then with streptavidin (Sigma, 125 ng/ml) for 30 min as indicated 30.
TNF secretion assay
LAD2 cells were treated with SP (Sigma, 10 μM), in order to achieve the strongest possible degranulation, or LPS (Sigma, at 10 ng/ml) for 30 min, 6 hr and 24 hr. TNF release was measured by ELISA (R&D system, Minneapolis, MN, USA) in the supernatant fluid. In certain experiments, LAD2 cells wer pretreated with the transcriptional inhibitor, Actinomycin D (Sigma, 15 μM, 1 hr) before stimulation with SP.
Mast cell degranulation assay
β-hexosaminidase (Beta-hex) secretion, as an index of mast cell degranulation, was assayed using a fluorometric assay as previously reported. Briefly, β-hexosaminidase activity in the supernatant fluid and cell lysates (LAD2 cells, 0.5×105/tube, were lysed with 1% Triton X-100 to measure residual cell-associated Beta-hex) were incubated with substrate solution (p-nitrophenyl-N-acetyl-β-D-glucosaminide from Sigma, St Louis, MO, USA) in 0.1 M NaOH/0.2 M glycine. Absorbance was measured at 405 nm in an enzymelinked immunosorbent assay reader, and the results are expressed as the percentage of Beta-hex released over the total.
Quantitative RT-PCR
Total RNA from cultured mast cells was isolated using Trizol reagent (Invitrogen) and RNeasy Mini Kit (Qiagen, Valencia, CA, USA) respectively, according to the manufacturer's instructions. Reverse transcription was performed with 300 ng of total RNA using the iScript cDNA synthesis kit (BIO-RAD, Hercules, CA, USA). In order to measure TNF gene expression, quantitative real time PCR was performed using Taqman gene expression assays. The following probes (Applied Biosystems, Carlsbad, CA, USA) were used: TNF: Hs00542477_m1. Samples were run at 30 cycles using Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems). Relative mRNA abundance was determined from standard curves run with each experiment and TNF expressions were normalized to GAPDH (Hu, VIC TAMRA) as endogenous control.
Intracellular calcium measurement
LAD2 cells were loaded with 5 μM Fura-2 AM (Invitrogen) for 20 min, washed and then incubated for another 20 min at 37 C. Cells were then treated with either SP (10 μM) or LPS (10 ng/ml). Fluorescence signals were acquired on Flexstation II (Bucher Biotech, Basel, Switzerland). Cytosolic calcium was calculated after subtraction of the background fluorescence by measuring the ratio of the two emission intensities (excitation at 340 nm and 380 nm,). Each experiment was repeated three times independently.
Cell energy consumption measurement
LAD2 cell oxygen consumption rates were measured by Searhorse XF-24 Flux analyzer (Seahorse Bioscience Inc, North Billerica, MA, USA). LAD2 cells were treated with SP (10 μM) or LPS (10 ng/ml). Energy consumption was inhibited by the mitochondrial ATP pump blocker oligomycin. Cells were incubated with oligomycin (Sigma, 2 μM for 20 min) and then treated with SP (Sigma, 10 μM) or LPS (Sigma, 10 ng/ml). Experiments were conducted three times and results were similar.
Confocal microscopy
Human mast cells were incubated with 20 nM MitoTracker deep red probe (Invitrogen) for 20 min and 50 nM LysoTracker DND (Invitrogen) for 30 min. Cells were washed, moved to glass bottom culture dishes (MatTek, Ahsland, MA, USA) and observed using a Leica TCS SP2 Confocal microscopy (Leica, Buffalo Grove, IL, USA). Percentages of cells with mitochondrial translocation were counted from 100 randomly selected mast cells in each experiment by three independent operators. Confocal digital images were processed using the National Institute of Health ImageJ 1.32 and Adobe Photoshop 7.0 Programs.
Data Analysis
Statistical significance differences between experimental samples and controls were determined by the Student'-t test using the SigmaPlot 9.0 (SPSS, Chicago, IL, USA). Differences were considered significant if p<0.05.
Results
Time course of SP and LPS-induced TNF secretion and gene expression
SP stimulation (10 μM) results in secretion of granule-stored TNF at 30 min (Fig. 1A), as well as de novo synthesized TNF 24 hr later (Fig. 1A). Stimulation with LPS (10 ng/ml) for 30 min has no effect on degranulation and granule-stored TNF (Fig. 1A), but incubation for 24 hr induces de novo synthesis and release of TNF without degranulation (Fig. 1A). The gene expression level of TNF is significantly increased at 6 hr and 24 hr both after SP and LPS stimulation (Fig. 1B), indicating that SP and LPS induce de novo TNF synthesis. Under light microscopy, LAD2 cells stimulated by SP at 30 min show clear signs of degranulation (Fig. 1C), but no signs of degranulation at 24 hr (Fig. 1D). Beta-hex is also measured to confirm degranulation and secretion occurred in parallel with preformed TNF (Fig. 1E). In order to confirm that TNF secreted at 30 min is preformed, LAD2 cells are treated with RNA synthesis blocker actinomycin D (15 μg/ml) 1 hr before SP stimulation. There is no significant difference in TNF release amounts at 30 min with or without actinomycin D (15 μg/ml) have (Fig. 1F).
Figure 1. Time course of SP and LPS-induced TNF secretion, mRNA expression and light microscopy of LAD2 cells.
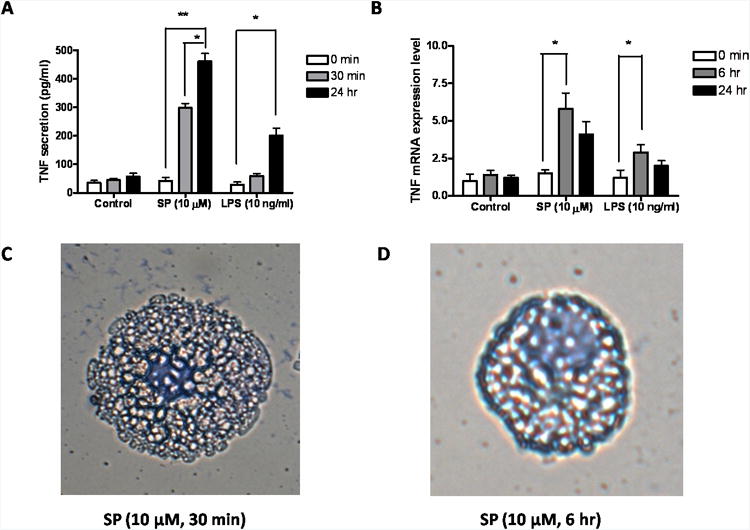
LAD2 cells were treated with SP (10 μM) or LPS (10 ng/ml) for the time indicated. (A) TNF release was measured by ELISA (R&D System) in the supernatant fluid (n=3; * p<0.05, **p<0.01). (B) TNF mRNA expression was measured by Real Time PCR (n=3;p, *P<0.05). (C, D) LAD2 cells were stained with Tuidine blue and were observed under light microscopy. LAD2 cells were treated with SP (10 μM) for (C) 30 min and (D) 24 hr. (Scale bars = 5 μm). (E) Beta-hex release was measured (n=3; * P<0.05, **P<0.01). (F) TNF release was measured from cells treated with SP with or without Actinomycin D pre-incubation (15 μg/ml for 60 min) (n=3; * p<0.05, **p<0.01).
Intracellular calcium levels of SP and LPS-induced TNF secretion
SP (10 μM) triggers a rapid significant cytosolic calcium increase within 1 min (Fig. 2A). SP resulted cytosolic calcium level returns to the same level of control after 50 min. LPS (10 ng/ml) has no effect on intracellular level (Fig. 2B).
Figure 2. Time course of SP and LPS stimulation on LAD2 mast cell cytosolic calcium changes.
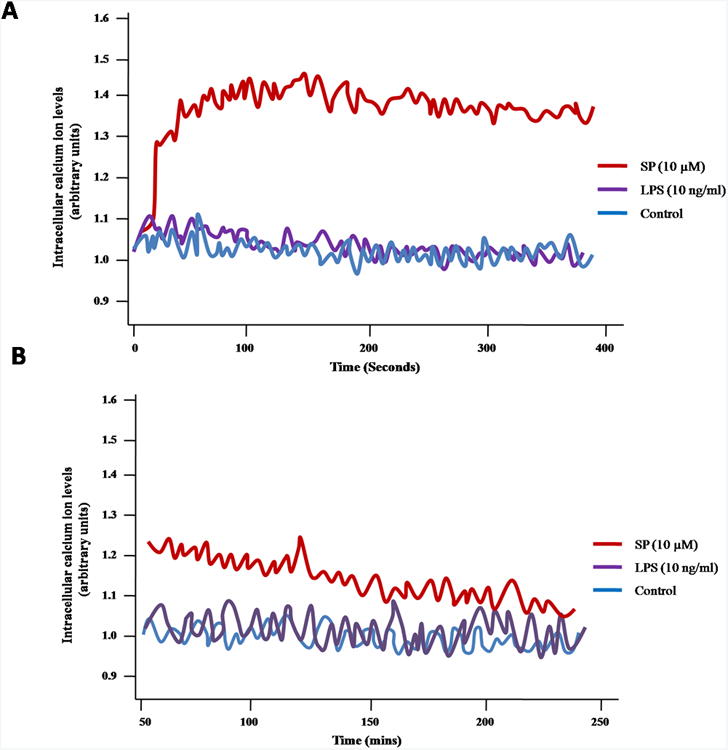
LAD2 cells were loaded with 5 μM fura-2 AM (Invitrogen) for 20 min at 37°C, washed and then incubated for another 20 min. Cells were then treated with either SP (10 μM) or LPS (10 ng/ml). Fluorescence signals were acquired on Flexstation II. The figure is representative of three repeats with similar results.
Energy consumption during SP-stimulated preformed and LPS-induced de novo synthesized TNF release
To test if degranulation and de novo TNF secretion have different energy requirements, mitochondrial oxygen consumption was investigated during these two processes in LAD2 cells. SP induced a significant oxygen consumption spike, while there was almost no difference between LPS stimulated and control cells (Fig. 3A). In order to investigate if the mitochondrial energy production is required for degranulation, mitochondrial energy production was blocked by pre-treating LAD2 cells with the ATP synthase inhibitor oligomycin (2 μM) for 30 min. Oliygomycin treatment dropped the metabolic baseline of both SP and LPS- treated cells to 30% of normal. In addition, the energy consumption spike (Fig. 3B) was inhibited. Oliygomycin treatment also inhibits SP-simulated granule-stored TNF secretion at 30 min (Fig. 3C). In contrast, there is no significant difference in SP-induced TNF release at 24 hr with or without treatment with oliygomycin (Fig. 3D).
Figure 3. Energy consumption in LAD2 cells stimulated by SP and LPS.
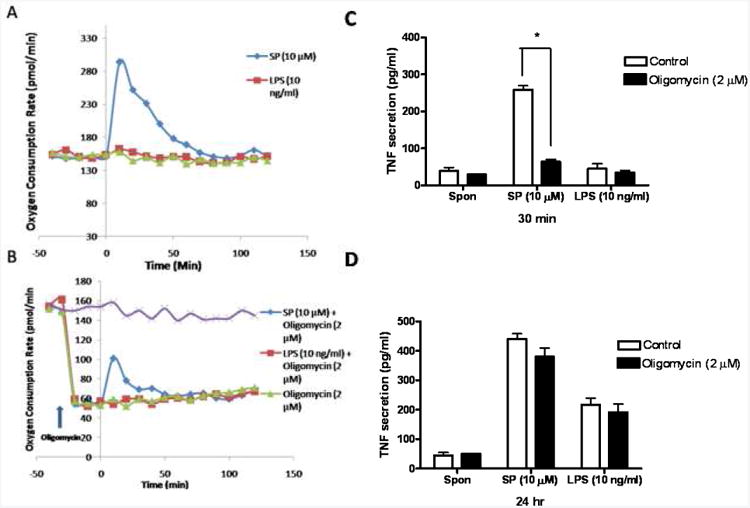
(A,B) Cell oxygen consumption rates were measured by Searhorse XF-24 Flux analyzer (Seahorse Bioscience Inc,) in LAD2 cells treated with either SP (10 μM) or LPS (10 ng/ml). LAD2 cells were pretreated (B) by the mitochondrial ATP pump blocker oligomycin (2 μM) for 20 min and then were treated with SP (10 μM) or LPS (10 ng/ml) for 30 min or 24 hr. Experiments were conducted three times and one representative experiment is shown. TNF secretion was measured by ELISA as indicated before (n=3; *p<0.05).
Degranulation, but not de novo cytokine secretion, results in mitochondrial translocation
Examination of resting live LAD2 cells by Confocal microscopy shows that mitochondria stained with MitoTracker red are located around the nucleus as a “mitochondria pool” (indicated by the white dashed circle); very few mitochondria could be found close to the cell surface (Fig. 4A). Since the average pH of mast cell granules is 5.5 31, the lysosome dye LysoTracker 32 was used to stain secretory granules. After SP (10 μM) stimulation for 30 min at 37 C, mast cells undergo rapid degranulation as indicated by the content of numerous granules stained with LysoTracker outside the cell (Fig. 4B, left panel). Following degranulation, many mitochondria appear to be smaller (Fig. 4B middle panel) and have translocated close to the cell surface region (Fig. 4B, right panel). This phenomenon is not observed in LPS-stimulated mast cells (Fig. 4C).
Figure 4. Degranulation, but not de novo TNF release, is associated with mitochondrial translocation.
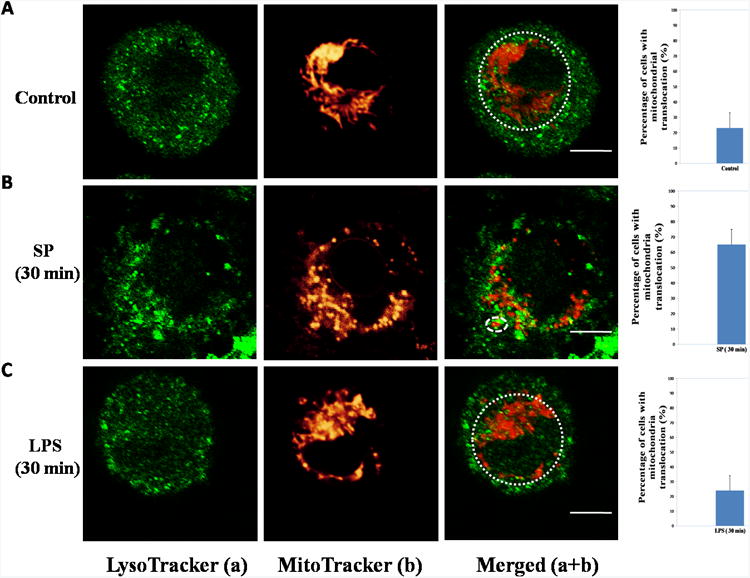
LAD2 cells were stained with MitoTracker deep red probe (20 nM) for 20 min and LysoTracker DND green probe (50 nM) for 30 min. The cells were harvested in glass bottom culture dishes and observed under the Leica TCS SP2 Confocal microscope. The left panels depict granules in green and the middle panels represent mitochondrial fluorescence shown in red. The right panels represent images merged from the two previous panels. The white dashed circles on the right panels outline the “mitochondrial pool”. The graphs shown at the far right represent percentages of mast cells with mitochondrial translocation obtained from 100 randomly selected cells. (A) Control groups. (B) SP (10 μM, 30 min). (C) LPS (10 ng/ml, 30 min). Each experiment was repeated three times and was evaluated by three independent operators. Scale bars = 5 μm.
Degranulation-associated mitochondrial translocation is reversible
Confocal images of SP-activated mast cells at 24 hr show that there is no evidence of degranulation (Fig. 5B). At 24 hr, it is obvious that most mitochondria were found again in the perinuclear region (Fig. 5B). Only about 20% of cells contain translocated mitochondria, which is no different from that of controls. This finding indicates degranulation-induced mitochondrial translocation is reversible. Just there is no mitochondrial translocation 30 min after LPS stimulation (Fig. 4C), there is no translocation at 24 hr either; LPS-stimulated mast cells still contained intact mitochondria located in the perinuclear region (Fig. 5C).
Figure 5. Degranulation-associated mitochondrial translocation is reversible.
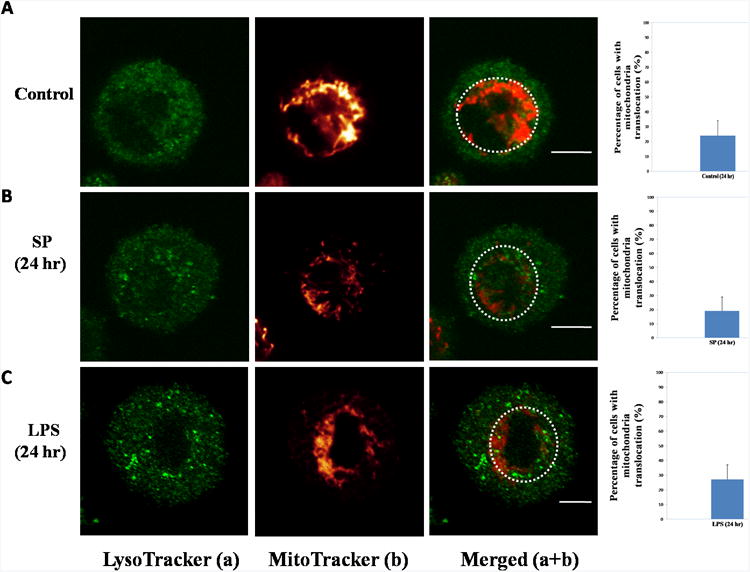
LAD2 cells were stained with MitoTracker deep red probe (20 nM) for 20 min and LysoTracker DND green probe (50 nM) for 30 min. The left panels depict granules in green and the middle panels represent mitochondrial fluorescence. The right panels represent images merged from the two previous panels. The graphs at the far right represent percentages of mast cells with mitochondrial translocation from 100 randomly selected cells. (A) Control groups. (B) SP (10 μM, 24 hr). (C) LPS (10 ng/ml, 24 hr). Each experiment was repeated three times and was calculated by three independent operators. Scale bars = 5 μm.
IgE/streptavidin stimulated hCBMCs secrete preformed TNF is associated with mitochondrial translocation
Under Confocal microscopy, unstimulated hCBMCs (6A, upper panels), as well as those treated only with IgE (1 μg/ml) for 30 minutes (Fig 6A, middle panels), had most of their mitochondria interconnected in a “net” located in the perinuclear region (within the dashed circles). In contrast, hCBMCs stimulated by IgE (1 μg/ml) and streptavidin (125 ng/ml) show rapid (30 minutes) degranulation (Fig 6C) at up to 35% Beta-hex release (Fig. 6D), and mitochondrial translocation (Fig 6C, lower panels).
Figure 6. IgE/streptavidin-triggered hCBMCs degranualtion and TNF secretion is associated with mitochondrial translocation.
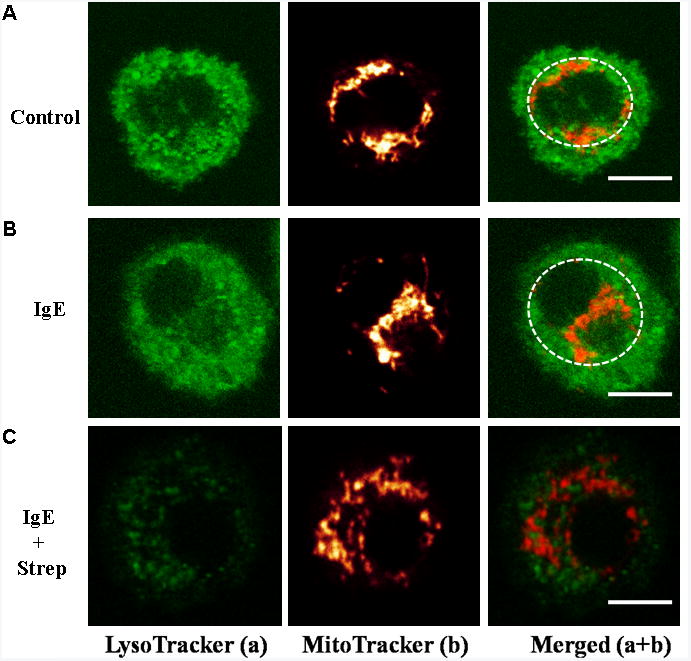
Mitochondrial translocation and degranulation in hCBMCs observed by Confocal microscopy. (A) Mitochondrial distribution in resting (panels 1-3), (B) IgE-incubated (1 μg/ml) (panels 4-6), and (C) IgE (1 μg/ml) + streptavidin (125 ng/mL)–stimulated (panels 7-9) cells for 30 min. Cells were stained with LysoTracker (a, green) and MitoTracker (b, red). (D) Beta-hex release was measured (n=3; * P<0.05)
Discussion
The present findings show that TNF secretion from human mast cells can occur through distinct pathways with different energy and calcium requirements, as well as mitochondrial dynamics. Degranulation was known to require calcium 33 and energy 34. Here we show that the process requires much more energy and calcium than de novo synthesized TNF release. The preformed nature of rapid released TNF was confirmed by the treatment of transcriptional inhibitor Actinomycin D which has little effects on blocking rapid TNF release. We used high SP concentration (10 μM) in order to induce maximum degranulation. Such high concentration had also previously been used to induce maximal LAD2 cell activation 6. Moreover, only SP concentrations higher than 1 μM were reported to be able to enhance the rate of oxygen consumption of isolated cardiac cell mitochondria 35. Other authors had reported secretion of preformed TNF from mast cells in different species by different triggers 4, 36, 37. However, there was no attempt to investigate how this secretion differs from de novo synthesis and release. Delayed (24 hr) TNF release could not be from continuous degranulation because morphological observations of SP-stimulated mast cells showed no degranulation at 24 hr. It would have been desirable, instead of SP, to use peptidoglycan, which has been reported to induce degranulation from rodent mast cells 38. However, LAD2 cells are unresponsive to peptidoglycan 39.
We specially show that mast cells preformed TNF secretion is associated with mitochondrial translocation to the cell surface. Mitochondrial translocation was confirmed using hCBMCs stimulated by IgE/streptavidin. These results indicate such mitochondrial translocation is not due to any specific trigger or leukemic nature of LAD2 cell. We recently showed that mast cell degranulation is tightly associated with mitochondrial morphological changes 40 and functions 41. One possible explanation for our findings is that mitochondria translocate close to the secretory granules in order to provide energy locally, possibily for the granules to fuse with the plasma membrane and undergo exocytosis as shown for lymphocyte chemotaxis 26. Mitochondrial translocation may also be needed to maintain optimal local calcium levels necessary for exocytosis 23, most likely for the calcium dependent proteins involved in degranulation, such as the Soluble NSF Attachment Protein (SNARE) and the Vesicle-Associated Membrane Protein 8 (VAMP-8) 42. It was previously shown that mitochondrial translocation was necessary to keep calcium channels open at the “immunological synapse” in activated T cells 27.
Mitochondrial health is maintained through autophagy 43,44. The normal appearance and distribution of mitochondria in mast cells 24 hr after SP stimulation may be either because fragmented mitochondria underwent fusion and translocated back to the perinuclear region or there was biogenesis of new mitochondria and digestion of old mitochondria through autophagy. The latter possibility is supported by a recent paper reporting that inhibition of autophagy blocks mast cell secretion 45. Unlike degranulation, mast cell selective release of de novo synthesized mediators 11 could involve vesicular traffic that requires negligible and hard to measure calcium and energy levels. For instance, differential release of serotonin without degranulation involved small vesicles shuttling serotonin from secretory granules to the cell surface 46. Similarly, IL-1 induced selective release of de novo synthesized IL-6 contained within small vesicles 13. Vesicular secretion may also be involved in the ability of corticotropin-releasing hormone (CRH) to induce selective release of vascular endothelial growth factor (VEGF) without degranulation 47.
The regulatory processes investigated here may be applicable to release of the mediators and could help explain how human mast cells participate in diverse biological processes. Mitochondrial dynamics may be necessary for a rapid mast cell response to an environmental trigger, as opposed to a delayed mediator release in other conditions, such as inflammation 48, innate and acquired immunity 1, or metabolic diseases 22. An implied importance of our findings could be the possibility of inhibition of mast cell mitochondrial translocation could be targeted as anti-allergic therapy, while permitting de novo synthesis of molecules, such as VEGF which is useful in would healing. Better understanding of the mitochondrial dynamics involved in mast cell activation may permit individualized therapy for allergic and inflammatory disorders.
Acknowledgments
This work was supported in part by NIH grant R01 AR47652 to TCT. We thank Biovitrum AB (Stockholm, Sweden) for their kind gift of rhSCF and Dr. A.S. Kirshenbaum (National Institutes of Health, Bethesda, Md.) for the supply of LAD2 mast cells. Bodi Zhang was supported by a graduate fellowship from Galenica, SA (Athens, Greece). Konstantinos-Dionysios Alysandratos and Asimenia Angelidou are recipients of postgraduate scholarships from the Hellenic State Scholarships Foundation (Athens, Greece). Konstantinos-Dionysios Alysandratos' present address: Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Tx., USA; Asimenia Angelidou's present address: Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, Tx., USA.
Abbreviations
- LPS
lipopolysaccharide
- rhSCF
recombinant human stem cell factor
- SP
Substance P
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest: The authors declare no conflicts of interests.
References
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol. 2007;178(9):5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- 5.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 6.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2007;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 8.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 9.Kubo Y, Fukuishi N, Yoshioka M, Iriguchi S, Imajo S, Yasui Y, Matsui N, Akagi M. Bacterial components regulate the exxpression of Toll-like receptor 4 on human mast cells. Inflamm Res. 2007;56(2):70–75. doi: 10.1007/s00011-006-6064-4. [DOI] [PubMed] [Google Scholar]
- 10.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166(8):4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 11.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 12.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 13.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171(9):4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 14.Tamir H, Theoharides TC, Gershon MD, Askenase PW. Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H] serotonin. J Cell Biol. 1982;93:638–647. doi: 10.1083/jcb.93.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107(9):4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinstein E, Mekori YA, Mor A. Autoimmunity and mast cell-related diseases. Expert Rev Clin Immunol. 2008;4(2):267–274. doi: 10.1586/1744666X.4.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20(13):2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Conti P. Mast cells: the JEKYLL and HYDE of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Theoharides TC, Sismanopoulos N, Delivanis DA, Zhang B, Hatziagelaki EE, Kalogeromitros D. Mast cells squeeze the heart and stretch the gird: Their role in atherosclerosis and obesity. Trends Pharmacol Sci. 2011;32(9):534–542. doi: 10.1016/j.tips.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Douglas WW. CIBA Foundation Symposium. Vol. 54. Elsevier/N; Holland: 1978. Stimulus-secretion coupling: variations on the theme of calcium- activated exocytosis involving cellular and extracellular sources of calcium; pp. 61–90. [DOI] [PubMed] [Google Scholar]
- 24.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11(9):678–684. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203(13):2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104(36):14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. Int J Biochem Cell Biol. 2009;41(10):1972–1976. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 30.Sundstrom JB, Hair GA, Ansari AA, Secor WE, Gilfillan AM, Metcalfe DD, Kirshenbaum AS. IgE-FcepsilonRI interactions determine HIV coreceptor usage and susceptibility to infection during ontogeny of mast cells. J Immunol. 2009;182(10):6401–6409. doi: 10.4049/jimmunol.0801481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RG, Carty SE, Fingerhood BJ, Scarpa A. The internal pH of mast cell granules. FEBS Lett. 1980;120(1):75–79. doi: 10.1016/0014-5793(80)81050-7. [DOI] [PubMed] [Google Scholar]
- 32.Wu M, Baumgart T, Hammond S, Holowka D, Baird B. Differential targeting of secretory lysosomes and recycling endosomes in mast cells revealed by patterned antigen arrays. J Cell Sci. 2007;120(Pt 17):3147–3154. doi: 10.1242/jcs.007260. [DOI] [PubMed] [Google Scholar]
- 33.Douglas WW. Stimulus-secretion coupling: variations on the theme of calcium- activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;54:61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- 34.Johanson T. Energy metabolism in rat mast cells in relation to histamine secretion. Pharmacol Toxicol. 1987;61(2):1–20. doi: 10.1111/j.1600-0773.1987.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 35.Prabhakar NR, Runold M, Kumar GK, Cherniack NS, Scarpa A. Substance P and mitochondrial oxygen consumption: evidence for a direct intracellular role for the peptide. Peptides. 1989;10(5):1003–1006. doi: 10.1016/0196-9781(89)90182-4. [DOI] [PubMed] [Google Scholar]
- 36.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111(8):4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mor A, Ben-Moshe O, Mekori YA, Kloog Y. Inhibitory effect of farnesylthiosalicylic acid on mediators release by mast cells: preferential inhibition of prostaglandin D(2) and tumor necrosis factor-alpha Release. Inflammation. 34(5):2010. 314–8. doi: 10.1007/s10753-010-9236-x. [DOI] [PubMed] [Google Scholar]
- 38.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109(10):1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshioka M, Fukuishi N, Iriguchi S, Ohsaki K, Yamanobe H, Inukai A, Kurihara D, Imajo N, Yasui Y, Matsui N, Tsujita T, Ishii A, Seya T, Takahama M, Akagi M. Lipoteichoic acid downregulates FcepsilonRI expression on human mast cells through Toll-like receptor 2. J Allergy Clin Immunol. 2007;120(2):452–461. doi: 10.1016/j.jaci.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, Weng Z, Miniati A, Vasiadi M, Katsarou-Katsari A, Miao B, Leeman SE, Kalogeromitros D, Theoharides TC. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J Allergy Clin Immunol. 127(6):2011. 1522–31. doi: 10.1016/j.jaci.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Alysandratos KD, Angelidou A, Kempuraj D, Tagen M, Vasiadi M, Asadi S, Theoharides TC. TNF secretion from human mast cells is regulated by mitochondrial dynamics and mitochondrial uncoupling protein 2 (UCP2) J Immunol. 2010;184(135):11. [Google Scholar]
- 42.Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A. 2008;105(7):2580–2585. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyde BB, Twig G, Shirihai OS. Organellar vs cellular control of mitochondrial dynamics. Semin Cell Dev Biol. 21(6):2010. 575–81. doi: 10.1016/j.semcdb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ushio H, Ueno T, Kojima Y, Komatsu M, Tanaka S, Yamamoto A, Ichimura Y, Ezaki J, Nishida K, Komazawa-Sakon S, Niyonsaba F, Ishii T, Yanagawa T, Kominami E, Ogawa H, Okumura K, Nakano H. Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol. 2011;127(5):1267–76.e6. doi: 10.1016/j.jaci.2010.12.1078. [DOI] [PubMed] [Google Scholar]
- 46.Kops SK, Theoharides TC, Cronin CT, Kashgarian MG, Askenase PW. Ultrastructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tissue Res. 1990;262:415–424. doi: 10.1007/BF00305238. [DOI] [PubMed] [Google Scholar]
- 47.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 48.Theoharides TC. Mast cells and pancreatic cancer. N Engl J Med. 2008;358(17):1860–1861. doi: 10.1056/NEJMcibr0801519. [DOI] [PubMed] [Google Scholar]


