Abstract Abstract
Two defining characteristics of stem cells are their multilineage differentiation potential (multipotency or pluripotency) and their capacity for self-renewal. Growth factors are well-established regulators of stem cell differentiation and self renewal, but less is known about the influence of the metabolic state on stem cell function. Recent studies investigating cellular metabolism during the differentiation of adult stem cells, human embryonic stem cells (ESCs), and induced pluripotent stem cells have demonstrated that activation of specific metabolic pathways depends on the type of stem cells as well as the lineage cells are differentiating into and that these metabolic pathways can influence the differentiation process. However, some common patterns have emerged, suggesting that undifferentiated stem cells primarily rely on glycolysis to meet energy demands. Our own data indicate that undifferentiated ESCs not only exhibit a low mitochondrial membrane potential but also express high levels of the mitochondrial uncoupling protein 2 and of glutamine metabolism regulators when compared with differentiated cells. More importantly, interventions that target stem cell metabolism are able to either prevent or enhance differentiation. These findings suggest that the metabolic state of stem cells is not just a marker of their differentiation status but also plays an active role in regulating stem cell function. Regulatory metabolic pathways in stem cells may thus serve as important checkpoints that can be modulated to direct the regenerative capacity of stem cells.
Keywords: stem cells, metabolism, mitochondria, glutamine, UCP2, uncoupling protein 2, mesenchymal stem cells, Warburg, embryonic stem cells, induced pluripotent stem cells, differentiation
Stem cells are characterized by their multipotency/pluripotency (capacity to differentiate into multiple lineages) and their self renewal (capacity to proliferate without differentiating and without undergoing senescence). Broadly, stem cells can be divided into two categories: adult stem cells and pluripotent stem cells (PSCs). Adult stem cells are found in a variety of tissues, where they perform important regenerative and reparative functions after birth. Tissues with high rates of postnatal cellular turnover, such as the bone marrow, skin, or intestinal organs, typically require highly active adult stem cell populations to continuously replenish mature cells. Examples of adult stem cells are hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) in the bone marrow, epidermal stem cells in the skin, and intestinal stem cells. Most adult stem cells are multipotent but not pluripotent; in other words, they can give rise to multiple lineages but not to cell types from all three germ layers (endoderm, ectoderm, and mesoderm).
PSCs have a much broader differentiation capacity than adult stem cells. Until recently, research on PSCs focused primarily on embryonic stem cells (ESCs). However, in 2006, a pioneering paper demonstrated that adult somatic cells, such as fibroblasts, can be converted into a stem cell state (induced PSCs [iPSCs]) that approximates that of ESCs in terms of self renewal and pluripotency by overexpressing selected transcription factors.1 This discovery has revolutionized the field of stem cell research, because it now allows for the generation of PSCs from any individual by “reprogramming” adult fibroblasts obtained from simple skin biopsy into iPSCs, which can then be further differentiated into a very broad range of mature cell types. Differentiated cells derived from iPSCs can be used to repair or regenerate diseased organs and even engineer functional tissues.
Much of the research on the mechanisms governing the differentiation and self renewal of adult stem cells or ESCs/iPSCs has focused on how specific growth factors and extracellular matrix signals can elicit responses in stem cells. Knowledge of these mechanisms is key to the development of regenerative therapies, because derivation of a functional and mature cell type requires a distinct, lineage-specific set of differentiation cues. Unsuccessful or incomplete differentiation of stem cells represents a liability, because uncommitted stem cells that are implanted into patients could potentially differentiate into unwanted cell types (e.g., spontaneous bone or fat formation of stem cells implanted in the heart) or give rise to tumors, such as teratomas.
In recent years, there is an emerging realization that, in addition to growth factors and extracellular matrix cues, metabolic pathways may also provide important signals that direct stem cell self renewal or differentiation. A few recent review articles provide a comprehensive overview of the burgeoning field of metabolism research in stem cells.2-5 Even though this field is fairly young, the broad area of “cellular metabolism” refers to a vast and complex interplay of various anabolic and catabolic pathways. Since the recognition that not only do undifferentiated stem cells and mature stem cells have distinct metabolic phenotypes but the metabolic pathways are mechanistically involved in the differentiation processes, research groups have attempted to target a plethora of metabolic pathways in stem cells. Instead of cataloging all these studies, the current article will focus on a few selected findings and highlight concepts that point toward important future directions of metabolism research in stem cells.
Glycolysis versus mitochondrial glucose oxidation during pluripotent stem cell differentiation
There are two major pathways by which glucose can contribute to the energy production in a cell: glycolysis and mitochondrial glucose oxidation. Glycolysis occurs in the cytosol and involves the conversion of glucose to pyruvate, which generates a net gain of 2 moles of adenosine triphosphate (ATP) per mole of glucose. Pyruvate can then be converted to lactate in the cytosol without providing any additional energy yield. Alternatively, pyruvate generated in the cytosol can enter the mitochondrial tricarboxylic acid (TCA) cycle for further decarboxylation and oxidation, a process during which nicotinamide adenine dinucleotide (NADH) is generated and subsequently oxidized in the mitochondrial electron transport chain (ETC) to produce additional ATP. This process of glycolysis coupled with mitochondrial oxidation provides a significantly higher energy yield than glycolysis alone: a total of 36 moles of ATP per mole of glucose. However, the higher ATP yield of mitochondrial glucose oxidation depends on the availability of adequate oxygen for the ETC, and mitochondrial glucose oxidation releases reactive oxygen species (ROS), which could be potentially harmful depending on the amount of ROS generated.
The discovery by the pioneering researcher and Nobel laureate Otto Warburg that cancer cells rely on glycolysis even in the presence of oxygen (aerobic glycolysis or the so-called Warburg phenomenon) roughly a century ago6 still holds true for many tumors and malignant cells, but understanding the precise advantages of forfeiting the higher ATP yield continues to elude researchers. This question has regained relevance today, because recent studies on PSCs, including both iPSCs and ESCs, suggest that undifferentiated PSCs are similar to cancer cells in that they also appear to primarily rely on glycolysis instead of mitochondrial glucose oxidation for ATP generation.7,8 Specifically, undifferentiated ESCs and iPSCs have higher expression levels of glycolytic enzymes, higher glycolytic rates and lactate production, and lower rates of glucose oxidation than their differentiated counterparts.7,8
The shift toward increased glycolysis during induction of pluripotency
Although multiple previous studies were able to show that, as PSCs become more differentiated, they shift from glycolysis to mitochondrial glucose oxidation,2 more recent studies have investigated the corollary: Do mature, differentiated cells become more glycolytic during their reprogramming to pluripotency?9,10
Folmes and colleagues9 were able to show that the metabolic shift from a pro-oxidative state to glycolysis is a crucial step in the conversion of somatic cells into iPSCs, characterized by upregulating glycolytic genes, such as hexokinase and lactate dehydrogenase, as well as downregulating mitochondrial ETC proteins. More importantly, successful reprogramming of mature cells into iPSCs is enhanced by promoting glycolysis and impaired when inhibiting glycolysis or stimulating mitochondrial glucose oxidation.9 These findings mirror previously observed findings that indicated that hypoxia increases iPSC generation at least three- to fourfold.11 Together, the data underscore that metabolic shifts are not just passive indicators of stem cell differentiation states, but that the ratio of glycolysis/mitochondrial glucose oxidation affects the differentiation status of cells.
The global metabolic profiles of undifferentiated iPSCs and ESCs are very similar, especially when iPSCs are maintained for multiple passages in an undifferentiated state.10 However, iPSCs do differ in the levels of some metabolites when compared with ESCs. For example, certain ESCs have higher levels of ω-6 and ω-3 fatty acids, whereas iPSCs tend to have higher levels of metabolites involved in the S-adenosyl methionine (SAM) cycle, such as SAM, 5’methylthioadenosine, hypoxanthine, and inosine,10 possibly reflecting higher levels of DNA methylation in human iPSCs. It has to be emphasized that the extent of genomic and phenotypic differences between iPSCs and ESCs remains controversial, and such comparisons are often based on selected clonal cell lines. Some of the observed differences may therefore be due to the genetic variability between the individual somatic cells from which the iPSC clones were derived. The high glycolytic rates and low levels of mitochondrial glucose oxidation seen in undifferentiated iPSCs and ESCs, on the other hand, have been consistently observed by multiple groups. It is thus likely that this metabolic phenotype is mechanistically linked to the pluripotency state.
The Warburg effect in PSCs: uncoupled states and glutamine metabolism
Initial studies suggested that undifferentiated PSCs have reduced mitochondrial mass as well as limited mitochondrial oxidative capacity and that they thus do not rely on mitochondrial glucose oxidation.7,8 However, more recent work indicates that undifferentiated PSCs can have similar mitochondrial mass and mitochondrial DNA copy number as differentiated cells and that mitochondria in PSCs are consuming oxygen.12,13 One explanation for this discrepancy is that there may be no universal rule regarding metabolic differences between undifferentiated PSCs and differentiated cells. Depending on whether one uses a generic differentiation protocol (i.e., generating embryoid bodies) or a directed differentiation protocol (i.e., generating highly purified neurons or cardiomyocytes), each differentiated cell type is likely to have a distinct metabolic profile. Therefore, comparisons between undifferentiated and differentiated cells need to be interpreted in the context of lineage-specific differentiation.
Furthermore, another set of findings may reconcile the observation that undifferentiated ESCs and iPSCs exhibit high levels of glycolysis and lactate generation with the presence of active mitochondrial respiration. Zhang and colleagues13 were able to demonstrate that undifferentiated human ESCs express increased levels of uncoupling protein 2 (UCP-2). Uncoupling proteins such as UCP-2 have multiple functions, including the suppression of ROS production and the uncoupling of mitochondrial respiration from ATP generation. If mitochondria in undifferentiated PSCs are indeed uncoupled, then the mitochondria oxidize NADH equivalents without being able to maintain a mitochondrial proton gradient and membrane potential necessary for ATP production.
Our own data on the mitochondrial membrane potential of undifferentiated human ESCs support this notion. As shown in Figure 1A, use of the potentiometric dye JC-1 reveals that mitochondria in undifferentiated human ESCs are predominantly green (i.e., depolarized or uncoupled) when compared with a mature cell type, in which mitochondria exhibit red fluorescence. Furthermore, when mitochondrial oxygen consumption is measured (Fig. 1B), human ESCs (hESCs) and human aortic smooth muscle cells (SMCs) have similar rates of baseline oxygen consumption. However, when SMCs are challenged with the chemical uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, they markedly augment their oxygen consumption, whereas hESCs are affected to a lesser extent. This supports the notion that mitochondria of undifferentiated ESCs may be uncoupled and depolarized even before the application of a chemical uncoupler. Lastly, expression levels of UCP-2 are substantially higher in undifferentiated ESCs than in SMCs (Fig. 1C), similar to what was reported by Zhang et al.13
Figure 1.
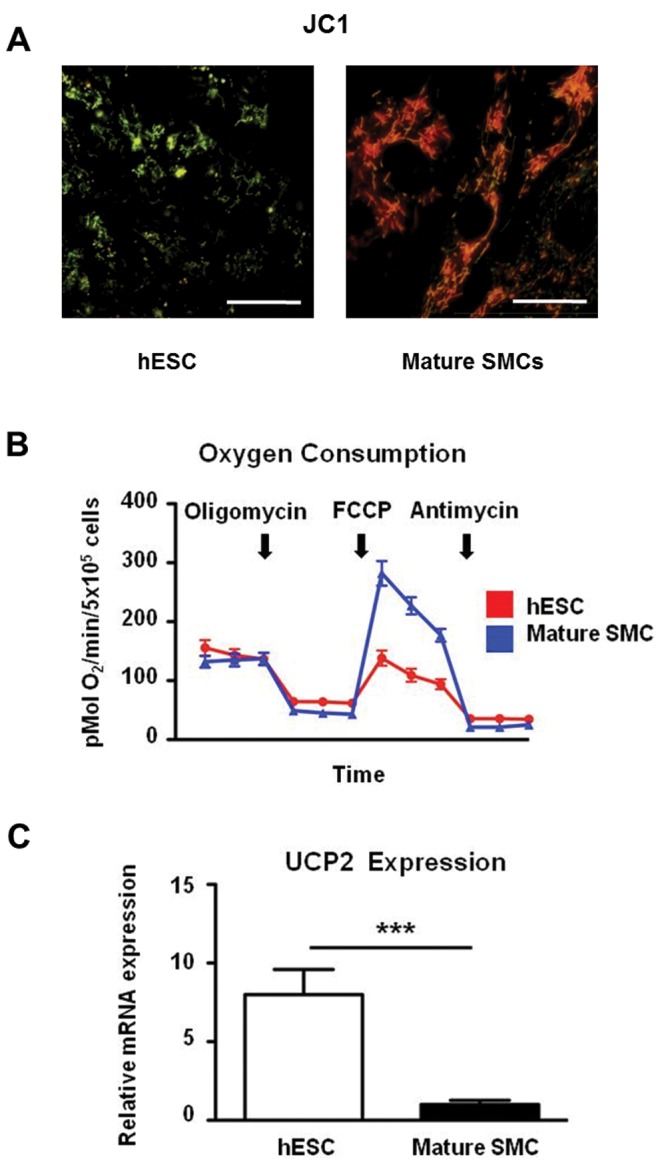
Uncoupled state in undifferentiated human embryonic stem cells (hESCs). Staining of an undifferentiated hESC (H1 cell line) colony (A, left) and adult human aortic smooth muscle cells (SMCs; A, right) with the potentiometric mitochondrial dye JC-1. Scale bar: 25 μm. Green indicates low mitochondrial membrane potential, and red indicates higher mitochondrial membrane potential. Oxygen consumption rate (OCR) is measured by the Seahorse XF-24 analyzer in adherent hESCs and aortic SMCs over time, when exposed to the adenosine triphosphate (ATP) synthase inhibitor oligomycin, the uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and the respiration inhibitor antimycin (B). Relative expression of the uncoupling protein 2 (UCP-2) in undifferentiated hESCs and SMCs as assessed by quantitative polymerase chain reaction (C). mRNA: messenger RNA.
If mitochondria in undifferentiated PSCs actively consume oxygen but are depolarized or uncoupled and thus unable to generate ATP, it would explain why the cells have to rely on a high glycolytic flux for ATP generation. From a teleological point of view, it is still difficult to understand the advantages of such a high glycolytic flux if it does not lead to increased ATP generation. In an attempt to explain the similar Warburg phenomenon in cancer cells, newer models of cancer cell metabolism emphasize the importance of glycolysis and the mitochondrial TCA cycle for cell proliferation.14 In addition to generating NADH that can be used for ATP production, mitochondrial metabolism of glucose also generates important metabolites that can be used for anabolic process, such as providing carbons to generate proteins, lipids, and carbohydrates required for cell proliferation.14 This view of glucose as a carbon source and not just a source of energy is a highly provocative but very plausible explanation of the low rates of mitochondrial ATP generation in cancer cells. It is quite possible that this explanation also applies to undifferentiated ESCs and iPSCs, which also exhibit extremely high rates of proliferation and biosynthesis.
The realization that proliferative cells not only require ATP but also need increased metabolic flux through the mitochondrial TCA cycle independent of ATP generation to ensure biosynthesis prompted a search for alternate carbon sources to fuel the TCA cycle. One such additional carbon source is the amino acid glutamine. Glutamine is the most abundant free amino acid in human plasma and can serve as a carbon source after it undergoes conversion to glutamate and then alpha-ketoglutarate, a key component of the mitochondrial TCA cycle. The recent discovery that cancer cells express high levels of glutaminase, the major enzyme that metabolizes glutamine, and that glutamine metabolism is a therapeutic target in cancer15 further substantiates the idea that highly proliferative cancer cells require continuous supply of nonglucose carbon sources. For highly proliferative cells, glutamine also offers the added advantage that it can serve as a nitrogen source as well as a carbon source.14 Warburg also described the high levels of ammonia production in cancer cells when he published his seminal work.6 Because ammonia is released as a by-product of glutamine metabolism, it is likely that Warburg’s observation foreshadowed the discovery of how central glutamine metabolism is for cancer cells.
We recently investigated whether undifferentiated human ESCs similarly rely on glutamine to fuel their proliferative state. As shown in Figure 2A, undifferentiated human ESCs show a marked drop in their proliferative rate upon removal of glutamine from the culture medium. More importantly, upon differentiation into embryoid bodies (generic differentiation resulting in the differentiation of cells into all three germ layers), human ESCs show a substantial downregulation of glutaminase 2 (also known as mitochondrial glutaminase) and the cellular glutamine transporters SLC1A5 and SLC7A5 (Fig. 2B). The specific mechanisms that link glutamine metabolism to the pluripotency of ESCs or iPSCs still need to be determined.
Figure 2.
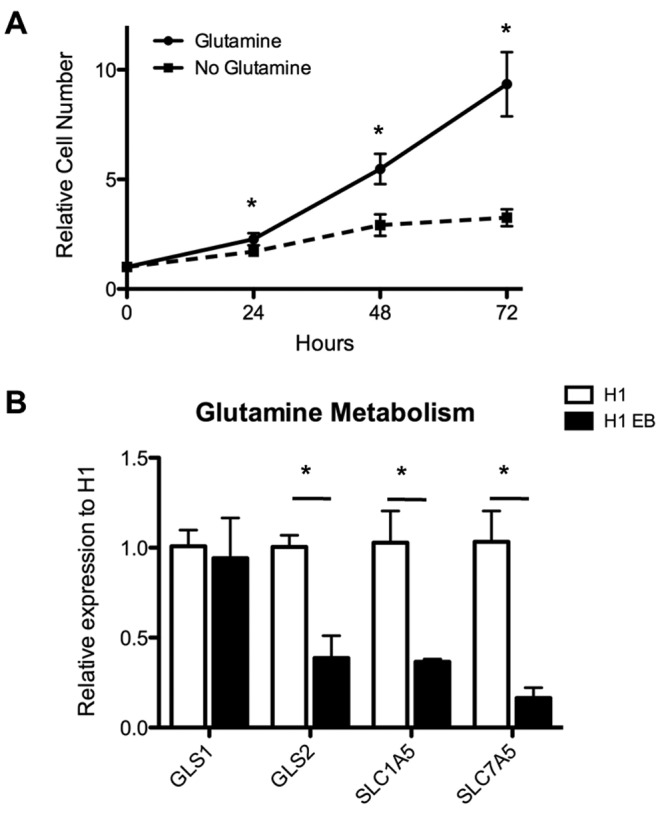
Glutamine metabolism in human embryonic stem cells (hESCs). hESC (H1 cell line) growth rate decreases rapidly upon removal of glutamine from the culture medium (A). Upon differentiation of hESCs into embryoid bodies, the expression levels of mitochondrial glutaminase (GLS2) and the glutamine transporters SLC1A5 and SLC7A5 as assessed by quantitative polymerase chain reaction are markedly reduced (B). EB: embryoid body; GLS1: glutaminase 1.
Investigating metabolic pathways to differentiate stem cells
Understanding the interplay of metabolic pathways and shifts between bioenergetic states is of great interest to stem cell biologists studying the mechanisms that govern differentiation of PSCs into mature cells or the reprogramming of mature cells to PSCs. However, uncovering metabolic signals may also yield important practical benefits for the purpose of tissue engineering and regenerative medicine. One recent study by Tohyama and colleagues16 exemplifies this. In this study, cardiomyocytes were derived from PSCs, and a metabolic profile analysis revealed that cardiomyocytes were able to use lactate as a fuel, whereas undifferentiated PSCs and noncardiomyocytes were not. Replacement of glucose that is commonly present in the differentiation medium with lactate resulted in the selective purification of differentiated, functional cardiomyocytes, most likely because other cell types were unable to survive in the glucose-free, high-lactate medium. Not only was this cardiomyocyte-directed differentiation protocol able to generate a 99% pure population of cardiomyocytes, it also avoided the formation of teratomas when the differentiated cells were transplanted.16 Undifferentiated iPSCs and ESCs have the capacity to form teratomas, and this concern is one major impediment to using PSC-derived cells in clinical trials, because even highly efficient differentiation protocols cannot guarantee the removal of all hidden, residual pluripotent cells within the differentiated cells that may still retain their teratoma-forming capacity. Whether similar metabolic interventions can also increase the differentiation efficacy into other lineages, such as neurons or hepatocytes, is not yet known. For PSC-derived cardiomyocytes, this metabolic protocol represents a major step forward in terms of providing large quantities of purified regenerative cells that could be used for clinical applications.
Mitochondrial function in adult stem cells
Undifferentiated adult stem cells appear to be similar to PSCs in their reliance on glycolysis instead of mitochondrial oxidation. The adult quiescent stem cells, such as long-term repopulating HSCs, express higher levels of glycolytic enzymes and lower levels of oxidative phosphorylation (OXPHOS) proteins.17,18 Similar to what has been surmised for PSCs, the reduced level of ROS production during nonoxidative glycolysis is thought to be the underlying reason for the reliance on glycolysis, thus allowing HSCs to minimize oxidative damage and maintain long-term self renewal and regenerative capacity.18
Mesenchymal stem cells (MSCs) are adult stem cells found in the bone marrow that are able to differentiate into osteogenic, adipogenic, and chondrogenic lineages. Assessment of mitochondrial biogenesis and mitochondrial respiration indicates that mitochondrial mass, mitochondrial oxygen consumption, and antioxidant defenses that can protect against mitochondrial ROS generated by mitochondrial ETC activity all increase in parallel during osteogenic differentiation of human MSCs.19 A recent study similarly evaluated mitochondrial respiration during adipogenic differentiation of human MSCs and also found a shift toward higher mitochondrial respiration in the postdifferentiated state when compared with undifferentiated MSCs.20 The signaling mechanisms responsible for initiating or enhancing adipogenic differentiation in MSCs appear to involve the generation of mitochondrial ROS released by the ETC.21
Suppression of mitochondrial biogenesis by knockdown of mitochondrial transcription factor A (TFAM), which binds mitochondrial DNA and is essential for the transcription of ETC components encoded by the mitochondrial DNA, as well as chemical inhibition of mitochondrial respiration or hypoxic suppression of mitochondrial respiration were all able to mitigate the differentiation of MSCs into adipocytes.20 Exposure of adult human MSCs to hypoxia or to a prolyl hydroxylase inhibitor, which mimics hypoxia by activating hypoxia-inducible factor signaling, activates the embryonic transcription factors Oct-4 and Nanog, which are normally not expressed in adult stem cells (Fig. 3). It is not yet clear whether this activation of embryonic genes in adult stem cells is sufficient to increase the pluripotency of MSCs, but it may explain why reprogramming of adult somatic cells to iPSCs is enhanced when the cells are concomitantly exposed to hypoxia.11
Figure 3.
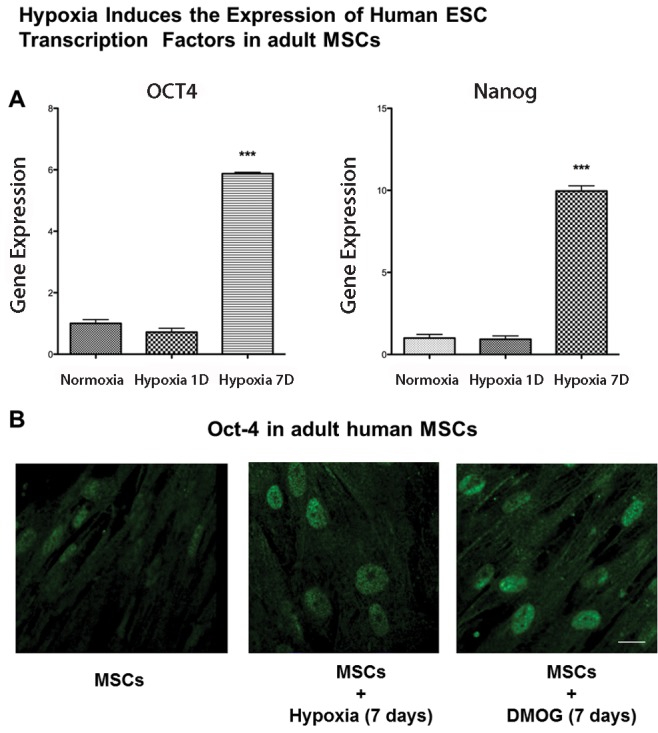
Hypoxia induces embryonic transcription factors in adult mesenchymal stem cells (MSCs). Exposure of adult human MSCs to hypoxia (1% for 7 days) increases the expression of the embryonic pluripotency genes Oct-4 and Nanog as assessed by quantitative polymerase chain reaction. Y-axis indicates relative gene expression normalized to normoxic control gene expression levels (A). Immunofluorescence staining for Oct-4 confirms increased nuclear levels of Oct-4 in hypoxia (1% for 7 days) as well as when exposed to the prolyl hydroxylase inhibitor dimethyloxallyl glycine (DMOG; B). ESC: embryonic stem cell. Scale bar: 20 μm.
Even though suppression of mitochondrial function appears to prevent MSC differentiation, it may also serve an important therapeutic function. A number of regenerative effects of MSCs are independent of their ability to differentiate into mesenchymal lineages. For examples, MSCs can promote the formation of vascular networks and angiogenesis by secreting guidance molecules or other growth factors and acting as pericytes.22 If MSCs are therapeutically used for the purpose of vascular repair and regeneration in ischemic tissue, suppression of mitochondrial respiration could prevent or limit the spontaneous differentiation of MSCs into fat cells or osteoblasts.
The mitochondria of human MSCs have been associated with another therapeutic effect that involves the transfer of mitochondria to cardiomyocytes by partial cell fusion23 or into injured alveoli during acute inflammatory lung injury.24 Similar to the accessory function of MSCs for angiogenesis,22 this newly uncovered mitochondrial rescue function also appears to be independent of their ability to differentiate into mesenchymal lineages. The exact mechanisms and functional impacts of such mitochondrial transfers from MSCs to mature cells need to be further elucidated.
Conclusions and future perspectives
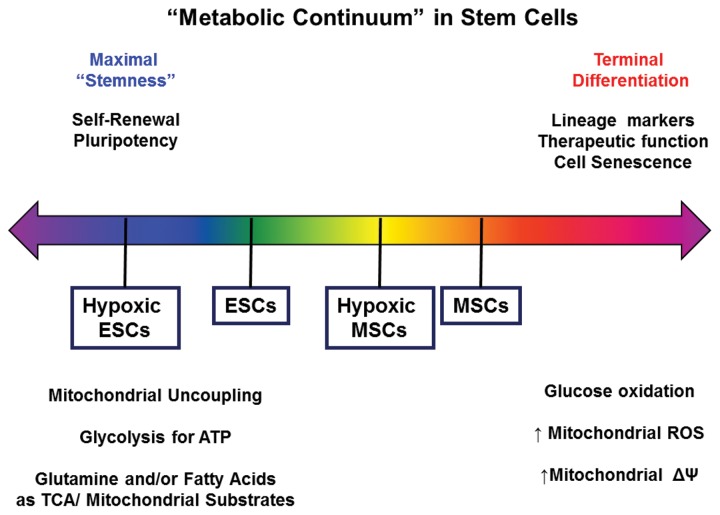
PSCs and adult stem cells vary significantly in terms of their degree of pluripotency/multipotency. It appears that this spectrum of pluripotency goes hand in hand with a similar spectrum or continuum of metabolism and bioenergetic states (Fig. 4). Much of the data published on metabolism in PSCs and adult stem cells has been primarily associative, but there are enough indications that suggest that metabolic pathways can in part regulate stem cell differentiation potential, self renewal capacity, and other aspects of stem cell function. This concise overview highlighted some of the recent developments in the metabolic research of stem cells; numerous important questions remain unanswered and will form the basis of future research, such as the following: (1) Does the modulation of stem cell metabolism lead to improved regenerative function of stem cells? In cardiovascular disease, cell therapy with stem and progenitor cells has shown only rather modest benefits,25,26 and it is possible that one reason for this is the lack of metabolic adaptation or maturation of the transplanted regenerative cells. (2) How do shifts in metabolism specifically affect transcription factors, epigenetic regulators, or microRNAs that regulate pluripotency, differentiation, and self-renewal? Current findings suggest that stem cell states regulate cellular metabolism and that, conversely, metabolism can also modulate stem cell states, but very little is known about the specific signaling pathways. Using metabolic cues to direct cells into specific mature cell lineages requires a precise understanding of how distinct metabolites, sources of carbon and nitrogen, or signaling molecules such as ROS affect the molecular switches that regulate stem cell function and differentiation. (3) Does the mitochondrial network structure impact stem cell differentiation? Recent developments in cancer research indicate that it is not only mitochondrial function but even mitochondrial network structure that can impact the proliferation of cancer cells. Inhibition of mitochondrial fission, for example, prevents cancer cell proliferation.27 Little is known about whether similar mechanisms also exist in adult stem cells or PSCs. (4) Can modulation of metabolism in endogenous cells affect their regenerative potential? A recent study has shown that sprouting blood vessels are characterized by endothelial cells, which are highly glycolytic.28 Adult stem cells, progenitor cells, or other proliferative regenerative cells exist in many adult tissues. Many such regenerative cell types are quiescent or unable to provide adequate regeneration in diseased states, and it is possible that modulation of cellular metabolism may enhance their regenerative capacity.
Figure 4.
Metabolic continuum in stem cells. The pluripotency continuum correlates with a metabolic continuum in stem cells. ATP: adenosine triphosphate; ESC: embryonic stem cell; MSC: mesenchymal stem cell; ROS: reactive oxygen species; TCA: tricarboxylic acid.
These are just a few questions emerging in the exciting new field of stem cell metabolism. It is very likely that, as more pathways and mechanisms are uncovered, new questions will arise.
Source of Support: This work is supported in part by the National Institutes of Health (R01-GM094220 to JR).
Conflict of Interest: None declared.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed]
- 2.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl) 2010;88:981–986. [DOI] [PMC free article] [PubMed]
- 3.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development 2013;140:2535–2547. [DOI] [PMC free article] [PubMed]
- 4.Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab 2013:18:325–332. [DOI] [PubMed]
- 5.Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 2012;11:589–595. [DOI] [PMC free article] [PubMed]
- 6.Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Z 1924;152:309–344.
- 7.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 2010;28:721–733. [DOI] [PubMed]
- 8.Varum S, Rodrigues AS, Moura MB, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 2011;6:e20914. [DOI] [PMC free article] [PubMed]
- 9.Folmes CD, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 2011;14:264–271. [DOI] [PMC free article] [PubMed]
- 10.Panopoulos AD, Yanes O, Ruiz S, et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res 2012;22:168–177. [DOI] [PMC free article] [PubMed]
- 11.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009;5:237–241. [DOI] [PubMed]
- 12.Birket MJ, Orr AL, Gerencser AA, et al. A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci 2011;124:348–358. [DOI] [PMC free article] [PubMed]
- 13.Zhang J, Khvorostov I, Hong JS, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 2011;30:4860–4873. [DOI] [PMC free article] [PubMed]
- 14.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441–464. [DOI] [PubMed]
- 15.Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009;458:762–765. [DOI] [PMC free article] [PubMed]
- 16.Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013;12:127–137. [DOI] [PubMed]
- 17.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010;7:380–390. [DOI] [PMC free article] [PubMed]
- 18.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011;9:298–310. [DOI] [PubMed]
- 19.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008;26:960–968. [DOI] [PubMed]
- 20.Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation ofhuman mesenchymal stem cells. PLoS One 2013;8:e77077. [DOI] [PMC free article] [PubMed]
- 21.Tormos KV, Anso E, Hamanaka RB, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 2011;14:537–544. [DOI] [PMC free article] [PubMed]
- 22.Paul JD, Coulombe KL, Toth PT, et al. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol 2013;64:124–131. [DOI] [PMC free article] [PubMed]
- 23.Acquistapace A, Bru T, Lesault PF, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 2011;29:812–824. [DOI] [PMC free article] [PubMed]
- 24.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:759–765. [DOI] [PMC free article] [PubMed]
- 25.Rehman J. Feeling the elephant of cardiovascular cell therapy. Circulation 2010;121:197–199. [DOI] [PMC free article] [PubMed]
- 26.Rehman J. Bone marrow tinctures for cardiovascular disease: lost in translation. Circulation 2013;127:1935–1937. [DOI] [PMC free article] [PubMed]
- 27.Rehman J, Zhang HJ, Toth PT, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J 2012;26:2175–2186. [DOI] [PMC free article] [PubMed]
- 28.De Bock K, Georgiadou M, Schoors S, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013;154:6651–6663. [DOI] [PubMed]