Abstract
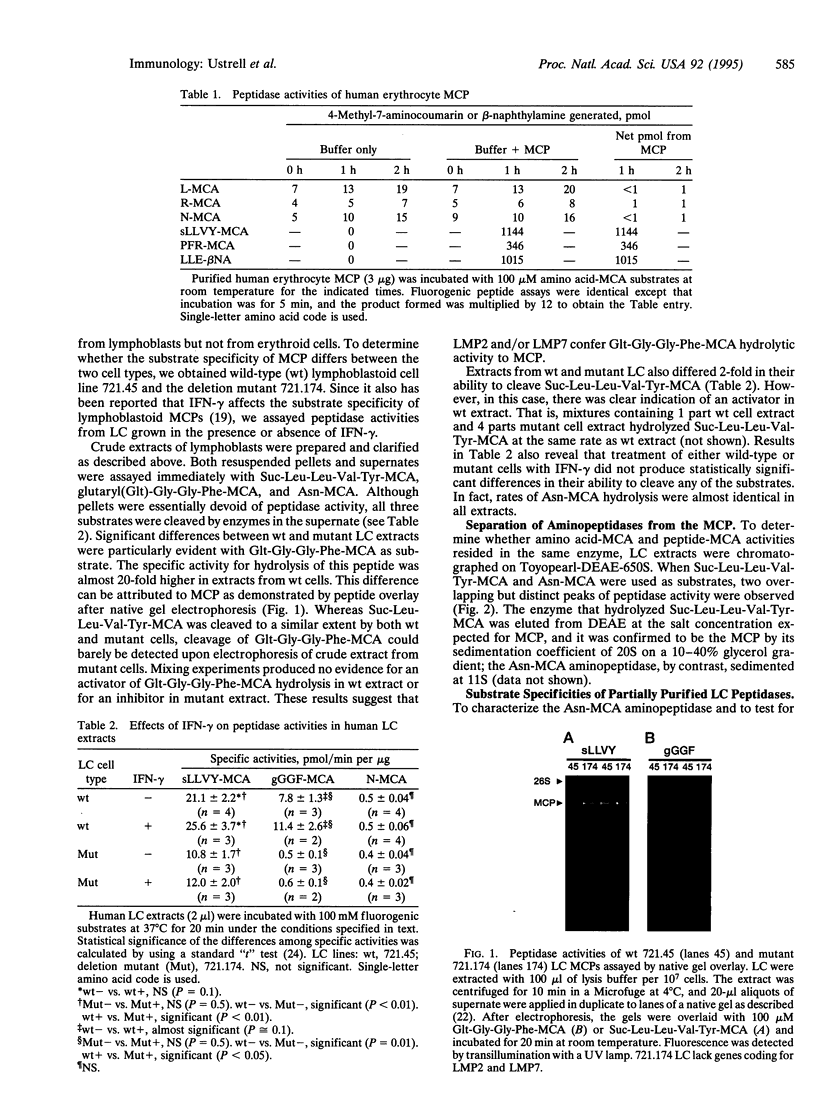
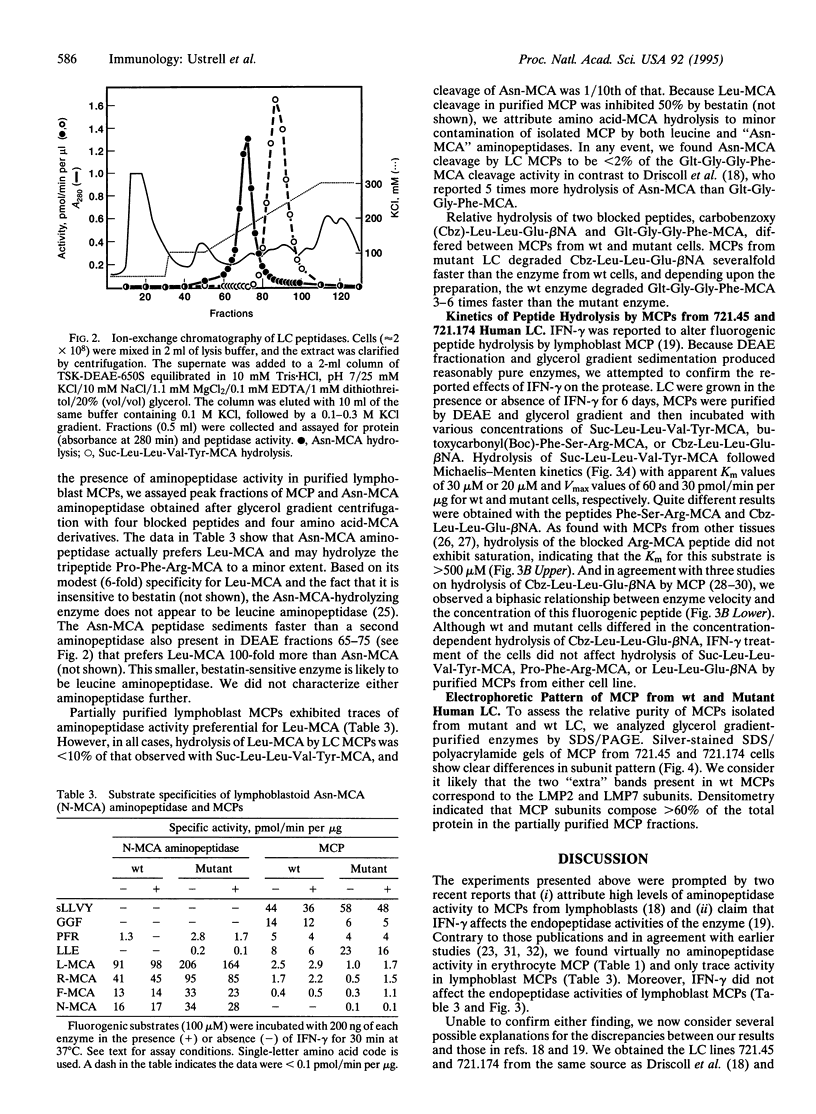
We have examined several peptidase activities of human multicatalytic protease (MCP) purified from the lymphoblastoid cell line 721.45 and a deletion mutant derivative, 721.174, lacking MCP subunits encoded in the major histocompatibility complex (MHC) class II region. Wild-type lymphoblast MCP hydrolyzed a specific peptide, glutaryl-Gly-Gly-Phe-4-methylcoumaryl-7-amide (-MCA), several times faster than the mutant enzyme did, suggesting that MHC-encoded subunits may provide this activity. Contrary to a recent report [Driscoll, J., Brown, M. G., Finley, D. & Monaco, J J. (1993) Nature (London) 365, 262-264], we did not detect significant aminopeptidase associated with lymphoblast MCPs. Our results also differ markedly from those of Gaczynska et al. [Gaczynska, M., Rock, K. L. & Goldberg, A L. (1993) Nature (London) 365, 264-267], who reported that gamma interferon (IFN-gamma) alters the peptidase activities of lymphoblast MCPs. We found that IFN-gamma did not produce significant differences in the peptidase activities of purified MCPs. Moreover, our measurements of Vmax and Km for succinyl-Leu-Leu-Val-Tyr-MCA hydrolysis differ 600-fold and 15-fold, respectively, from those reported by Gaczynska et al. On balance, the findings presented here do not support the idea that IFN-gamma induces major changes in the peptidase activity of purified MCPs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992 Nov 12;360(6400):171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- Arribas J., Castaño J. G. Kinetic studies of the differential effect of detergents on the peptidase activities of the multicatalytic proteinase from rat liver. J Biol Chem. 1990 Aug 15;265(23):13969–13973. [PubMed] [Google Scholar]
- Bennett K., Levine T., Ellis J. S., Peanasky R. J., Samloff I. M., Kay J., Chain B. M. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur J Immunol. 1992 Jun;22(6):1519–1524. doi: 10.1002/eji.1830220626. [DOI] [PubMed] [Google Scholar]
- Boes B., Hengel H., Ruppert T., Multhaup G., Koszinowski U. H., Kloetzel P. M. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J Exp Med. 1994 Mar 1;179(3):901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaballah H., Rivett A. J. Peptidylglutamyl-peptide hydrolase activity of the multicatalytic proteinase complex: evidence for a new high-affinity site, analysis of cooperative kinetics, and the effect of manganese ions. Biochemistry. 1992 Apr 28;31(16):4133–4141. doi: 10.1021/bi00131a033. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Brown M. G., Finley D., Monaco J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993 Sep 16;365(6443):262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Dubiel W., Ferrell K., Pratt G., Rechsteiner M. Subunit 4 of the 26 S protease is a member of a novel eukaryotic ATPase family. J Biol Chem. 1992 Nov 15;267(32):22699–22702. [PubMed] [Google Scholar]
- Dubiel W., Pratt G., Ferrell K., Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem. 1992 Nov 5;267(31):22369–22377. [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Goldberg A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993 Sep 16;365(6443):264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994 Jan 28;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hoffman L., Pratt G., Rechsteiner M. Multiple forms of the 20 S multicatalytic and the 26 S ubiquitin/ATP-dependent proteases from rabbit reticulocyte lysate. J Biol Chem. 1992 Nov 5;267(31):22362–22368. [PubMed] [Google Scholar]
- Honoré B., Leffers H., Madsen P., Celis J. E. Interferon-gamma up-regulates a unique set of proteins in human keratinocytes. Molecular cloning and expression of the cDNA encoding the RGD-sequence-containing protein IGUP I-5111. Eur J Biochem. 1993 Dec 1;218(2):421–430. doi: 10.1111/j.1432-1033.1993.tb18392.x. [DOI] [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Ma C. P., Slaughter C. A., DeMartino G. N. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain). J Biol Chem. 1992 May 25;267(15):10515–10523. [PubMed] [Google Scholar]
- Mason R. W. Characterization of the active site of human multicatalytic proteinase. Biochem J. 1990 Jan 15;265(2):479–484. doi: 10.1042/bj2650479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Cardozo C., Hidalgo M. C., Michaud C. Regulation of the peptidylglutamyl-peptide hydrolyzing activity of the pituitary multicatalytic proteinase complex. Biochemistry. 1991 Jun 18;30(24):5999–6005. doi: 10.1021/bi00238a025. [DOI] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Ortiz-Navarrete V., Seelig A., Gernold M., Frentzel S., Kloetzel P. M., Hämmerling G. J. Subunit of the '20S' proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991 Oct 17;353(6345):662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Realini C., Dubiel W., Pratt G., Ferrell K., Rechsteiner M. Molecular cloning and expression of a gamma-interferon-inducible activator of the multicatalytic protease. J Biol Chem. 1994 Aug 12;269(32):20727–20732. [PubMed] [Google Scholar]
- Rechsteiner M., Hoffman L., Dubiel W. The multicatalytic and 26 S proteases. J Biol Chem. 1993 Mar 25;268(9):6065–6068. [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 1989 Jul 25;264(21):12215–12219. [PubMed] [Google Scholar]
- Taylor A. Aminopeptidases: structure and function. FASEB J. 1993 Feb 1;7(2):290–298. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Wilk S., Pearce S., Orlowski M. Identification and partial purification of a cation-sensitive neutral endopeptidase from bovine pituitaries. Life Sci. 1979 Jan 29;24(5):457–464. doi: 10.1016/0024-3205(79)90218-2. [DOI] [PubMed] [Google Scholar]
- Yang Y., Waters J. B., Früh K., Peterson P. A. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]




