Abstract
The establishment of an optimal noninvasive method for diagnosing invasive aspergillosis (IA) is needed to improve the management of this life-threatening infection in patients with hematological disorders, and a number of noninvasive tests for IA that target different fungal components, including galactomannan, (1→3)-β-d-glucan (BDG), and Aspergillus DNA, have been developed. In this study, we prospectively evaluated the diagnostic potential of three noninvasive tests for IA that were used in a weekly screening strategy: the double-sandwich enzyme-linked immunosorbent assay (ELISA) for galactomannan (Platelia Aspergillus), a real-time PCR assay for Aspergillus DNA (GeniQ-Asper), and an assay for BDG (β-glucan Wako). We analyzed 149 consecutive treatment episodes in 96 patients with hematological disorders who were at high risk for IA and diagnosed 9 proven IA cases, 2 probable IA cases, and 13 possible invasive fugal infections. In a receiver-operating characteristic (ROC) analysis, the area under the ROC curve was greatest for ELISA, using two consecutive positive results (0.97; P = 0.036 for ELISA versus PCR, P = 0.055 for ELISA versus BDG). Based on the ROC curve, the cutoff for the ELISA could be reduced to an optical density index (O.D.I.) of 0.6. With the use of this cutoff for ELISA and cutoffs for PCR and BDG that give a comparable level of specificity, the sensitivity/specificity/positive predictive value/negative predictive value of the ELISA and the PCR and BDG tests were 1.00/0.93/0.55/1.00, 0.55/0.93/0.40/0.96, and 0.55/0.93/0.40/0.96, respectively. In conclusion, among these weekly screening tests for IA, the double-sandwich ELISA test was the most sensitive at predicting the diagnosis of IA in high-risk patients with hematological disorders, using a reduced cutoff of 0.6 O.D.I.
Invasive aspergillosis (IA) is one of the most serious complications in patients with hematological malignancies. It has an extremely high mortality rate (11) and affects not only terminally ill patients with refractory leukemia or lymphoma but also patients who could otherwise be expected to experience a potential cure of the underlying leukemia or lymphoma. Among several factors that contribute to the high mortality rate, difficulties in establishing a reliable diagnosis early enough for successful intervention have been repeatedly discussed (10). A definitive diagnosis usually requires invasive tissue sampling, which is often hampered by the critical condition of the patients, while a delay in initiating antifungal therapy, or, conversely, a hasty use of empiric or prophylactic amphotericin B before making a definitive diagnosis may result in treatment failure for full-blown infection or excess toxicity, respectively.
To overcome this problem and to improve the treatment outcome, advances have been made over the past decade in the fields of both diagnostics and therapeutics, including improvements in diagnostic imaging (7, 8, 18) and histopathology (1), and the development of broad-spectrum antifungal agents with low toxicities (4, 24, 29, 33). In the field of diagnostics, much attention has recently been given to the development of several types of noninvasive laboratory tests for IA. These tests are designed to sensitively detect circulating Aspergillus components and include a double-sandwich enzyme-linked immunosorbent assay (ELISA) for galactomannan (GM) antigen (Platellia Aspergillus) (30), tests for (1→3)-β-d-glucan (BDG) (β-glucan Wako or FungiTec G test) (23, 25), and a number of PCR-based assay systems for Aspergillus DNA (5, 6, 12, 34).
The ELISA for GM uses a rat monoclonal antibody directed against the 1→5-β-galactofuranoside side chains of the GM molecule as both the capture and detection antibodies for ELISA and can detect as little as 1.0 ng of circulating GM per ml (30). The excellent sensitivity and specificity of this assay have been repeatedly demonstrated and validated in tests of patients with hematological disorders (22, 27, 32). BDG is a ubiquitous component of diverse fungal species and a possible target for the diagnostic detection of IA. Two assay systems are currently available for the sensitive detection of circulating BDG, and both are based on the Limulus reaction, in which a trace amount of BDG can trigger a horseshoe crab coagulation cascade through factor G (23, 25). The BDG test is a useful method for screening for invasive fungal infection (IFI) and is widely used in Japan. The other test that has long been under intensive investigation for the sensitive detection of IA is PCR amplification of Aspergillus DNA, mainly of the 18S ribosomal gene (5, 6, 12, 34). Moreover, recently introduced real-time PCR designs have made it possible to quantitatively evaluate a fungal load with high sensitivity (9, 17, 21).
With regard to an antifungal strategy, it would be interesting to determine which of these tests is the best for diagnosing IA in patients with hematological disorders. Although high sensitivity and specificity are reported for PCR-based assays, the question whether PCR-based assays are superior to GM ELISA is still controversial (3, 5, 19, 34). Previously, we developed a sensitive real-time PCR system for detecting Aspergillus 18S ribosomal DNA, with which as few as 40 copies of aspergillus DNA per ml of plasma could be stably detected. We reported that the sensitivity of our real-time PCR for IA in 33 IA patients was higher than those of the double-sandwich ELISA for GM and the BDG test, with only a slightly lower specificity than that of GM ELISA (17). However, this previous study may have been biased by its partially retrospective design, limited sampling points in each case or infectious episode, and use of an inappropriately high cutoff value for ELISA. In the present purely prospective analysis, we consecutively enrolled 96 patients with hematological disorders who were at high risk for IA, monitored the levels of Aspergillus DNA, GM, and BDG in plasma, as well as the development of IA, at weekly intervals, and evaluated their diagnostic potentials by using receiver-operating characteristic (ROC) analyses.
MATERIALS AND METHODS
Study population and design.
From March 2001 through April 2002, a consecutive series of adult patients with hematological disorders who had been admitted to our hospital and were thought to be at high risk for IA were enrolled in the study, and their levels of Aspergillus DNA in plasma and GM in serum, and BDG in plasma were monitored weekly. Patients were considered to be at high risk for IA if (i) they underwent chemotherapy and were expected to be neutropenic (less than 500 neutrophils per μl) for at least 10 days, (ii) they had refractory disease or were neutropenic and presented for more than 96 h with persistent fever that was refractory to appropriate broad-spectrum antibacterial treatments, (iii) they had presented with acute graft-versus-host disease (GVHD) of grade 2 or greater or had extensive chronic GVHD, or (iv) they had received corticosteroids for more than 3 weeks within the previous 60 days. Plasma Aspergillus DNA levels, serum GM levels, and plasma BDG levels were to be measured once weekly whenever the patients were thought to be at high risk. Each period during which measurement was performed was defined as one treatment episode. Omission of sampling was permitted unless two consecutive samples were lacking. Treatment episodes with only one or two samples for each test were excluded from the analysis.
The level of Aspergillus DNA in plasma was measured using real-time PCR, as described previously (17). The ELISA for GM (Platelia Aspergillus; Sanofi Diagnostics Pasteur, Marnes-La-Cosuette, France) and the β-glucan Wako test (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) were performed as specified by the manufacturers. Each sample was tested twice for GM and BDG, and the average of the two measurements was taken.
Antifungal prophylaxis consisted of daily administration of 200 mg of fluconazole or itraconazole capsules with or without 15 mg of aerosolized amphotericin B or 10 mg of intravenous amphotericin B for patients with a suspected history of IA. Neutropenic fever was treated with broad-spectrum antibiotics in accordance with the published guidelines (16). Blood samples were used for bacterial, mycobacterial, and fungal cultures prior to the initiation of antibiotics. When IFI was suspected, treatment with 1 mg intravenous amphotericin B per kg was initiated. During the febrile period, patients were intensively surveyed for possible sites of infection and causative microorganisms. Diagnostic procedures included routine cultures of urine and stools, repeated cultures of blood and sputum, weekly chest X rays, high-resolution computed tomography (CT) scan of the chest, and, when possible, bronchoscopic examinations and open biopsies.
Case definitions.
For each treatment episode, a diagnosis was made following the published case definition criteria for invasive fungal infections from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC-IFICG and NIAID-MSG) (2), with the necessary modification that the plasma GM level was not included in the microbiological criteria.
Statistical analysis.
As described by Maertens et al. (22), we made a set of different estimates (A/B, C, and D) for the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each test, where different definitions of disease status for an episode were used to calculate these statistical indexes, since there is an intrinsic uncertainty regarding the true disease status of IA so that the calculation of these values could be significantly affected by the definition of the disease status. Estimate A/B defines “proven IA” and “probable IA” as truly positive and only “no IA” as truly negative, whereas estimates C and D incorporate “possible IFI” into the truly positive and truly negative groups, respectively. In all of the estimates, “no-IA” episodes were considered truly negative. Since our objective was to validate and compare the potentials of different diagnostic tests in a setting where these tests are performed weekly to monitor the development of IA, the positivity or negativity of a test was defined for each episode, where an episode was considered positive if at least one sample (method I), or any two consecutive samples (method II) became positive. There is also a practical reason for this approach. The onset and resolution of an IA episode are not always clear and, indeed, are rather poorly defined in many cases. Even in proven cases, there might be several febrile episodes and the onset might be insidious. In this setting, the sample-based calculation of sensitivity and specificity might be severely biased. In addition, we determined a proper cutoff value for each test through a ROC analysis, in which sensitivity and specificity were calculated as a function of the cutoff value, (1 − specificity) was plotted against the sensitivity, and the areas under the ROC curves (AUCs) were calculated. The significance of the difference in the AUCs of any two diagnostic measures was statistically tested as described above, and P values were calculated by the paired method under the null hypothesis that the two ROC curves represent random samples from similar underlying data for sensitivities and specificities (13). Therefore, the P values can be used only to compare two ROC curves at a time. The calculated P values reflect the one-tailed significance of difference between two ROC curves.
RESULTS
Study episodes.
There were 149 treatment episodes in 96 consecutive patients, including 9 proven IA, 2 probable IA, 13 possible IFI, and 125 no-IA episodes. Of these, 56 episodes (38%) were associated with stem cell transplantation. The patient characteristics and sample distributions are summarized in Table 1. Nineteen treatment episodes had no host factors. Overall, 1,251 samples were analyzed by the real-time PCR assay, 1,233 were analyzed by double-sandwich ELISA for GM, and 1,243 were analyzed by the BDG test. On average, approximately eight samples were examined for each treatment episode. The characteristics of the 24 episodes of proven IA, probable IA, and possible IFI are shown in Table 2. There were 24 fatal episodes, of which 8 were proven IA, 1 was probable IA, 4 were possible IFI, and 11 were no IA. Autopsies were performed in 14 episodes (58%), including 6 proven IA and 8 no-IA cases. In the remaining 10 fatal episodes, autopsy was not permitted by the patients' families. The 3 proven IA episodes were diagnosed based on histopathology of a pharyngeal biopsy specimen, a surgical specimen of the brain, and a skin biopsy specimen, respectively. Although postmortem examinations disclosed superinfections of disseminated Trichosporon infection and atypical mycobacteriosis in episode 1 and episode 9, respectively, no invasive candidiasis was documented during the study period.
TABLE 1.
Patient characteristics
| Characteristic | Patients with:
|
Totalb | |||
|---|---|---|---|---|---|
| Proven IA | Probable IA | Possible IFI | No IA | ||
| No. of episodes | 9 | 2 | 13 | 125 | 149 (96) |
| No. of deaths | 8 | 1 | 4 | 11 | 24 |
| No. of autopsies | 6 | 0 | 0 | 8 | 14 |
| Age (yr) | |||||
| Mean | 46 | 47 | 43 | 45 | 45 |
| Median | 42 | 47 | 40 | 47 | 46 |
| Range | 19-69 | 40-53 | 18-68 | 17-74 | 17-74 |
| Sex (no. male/no. female) | 6/3 | 2/0 | 12/1 | 82/43 | 102/47 (67/29) |
| No. with diseasea | |||||
| AML | 3 | 1 | 5 | 48 | 57 (29) |
| ALL | 1 | 0 | 4 | 26 | 31 (19) |
| CML | 0 | 1 | 2 | 8 | 11 (9) |
| MDS | 3 | 0 | 2 | 11 | 16 (14) |
| NHL | 2 | 0 | 0 | 28 | 30 (21) |
| AA | 0 | 0 | 0 | 2 | 2 (2) |
| Other | 0 | 0 | 0 | 2 | 2 (2) |
| No. with allografts | 4 | 2 | 6 | 44 | 56 |
| Duration of episode (days) | |||||
| Mean | 126 | 92 | 78 | 50 | 57 |
| Median | 135 | 92 | 57 | 37 | 43 |
| Range | 36-234 | 50-134 | 35-172 | 11-181 | 11-234 |
| No. with host factor: | |||||
| Neutropenia | 7 | 1 | 8 | 86 | 102 |
| Fever | 6 | 1 | 7 | 37 | 51 |
| GVHD | 2 | 2 | 5 | 17 | 26 |
| Steroid | 2 | 1 | 4 | 28 | 35 |
| None | 1 | 0 | 0 | 18 | 19 |
| Duration of neutropenia (days) | |||||
| Mean | 63 | 10 | 42 | 16 | 21 |
| Median | 37 | 10 | 18 | 14 | 15 |
| Range | 0-205 | 0-20 | 0-162 | 0-120 | 0-205 |
| No. of samples tested | |||||
| PCR | 154 | 25 | 146 | 926 | 1,251 |
| Mean (per episode) | 17.1 | 12.5 | 11.2 | 7.4 | 8.4 |
| Median (per episode) | 17 | 13 | 9 | 6 | 6 |
| Range (per episode) | 7-32 | 6-19 | 4-24 | 3-26 | 3-32 |
| GM | 155 | 24 | 140 | 914 | 1,233 |
| Mean (per episode) | 17.2 | 12.0 | 10.8 | 7.3 | 8.3 |
| Median (per episode) | 18 | 12 | 9 | 5 | 6 |
| Range (per episode) | 7-30 | 5-19 | 5-24 | 2-26 | 2-30 |
| BDG | 158 | 24 | 147 | 914 | 1,243 |
| Mean (per episode) | 17.6 | 12.0 | 11.3 | 7.3 | 8.3 |
| Median (per episode) | 19 | 12 | 9 | 6 | 6 |
| Range (per episode) | 7-31 | 5-19 | 6-24 | 3-23 | 3-31 |
AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; CLL, chronic nyelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; AA, aplastic anemia.
Values in parentheses are numbers of patients. Other values refer to numbers of episodes.
TABLE 2.
Diagnosis of IA and its documentation
| Episode no. | Patient characteristicsa:
|
Host factors | Clinical evidence | Culture and its source | Histological evidence | Maximum value (method I/method II)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Sex | IA | Primary disease | Status of primary disease | Out- come | PCR (copies/ml) | GM (O.D.L) | BDG (ng/ml) | |||||
| 1 | 41 | F | P | AML M1 | Post-allo, RD | Dead | NF | Erosion of sinus walls | A. flavus and A. fumigatus from pharyngeal mucosa | Biopsy | 2,000/200 | 3.8/3.6 | 19.7/4.7 |
| 2 | 32 | M | P | MDS (RAEB-t) | Post-allo, CR | Dead | GS | Dyspnea, pleural effusion | Autopsy | 32/0 | 1.3/1.0 | 60.5/36.5 | |
| 3 | 58 | M | P | AML M1 | RD | Dead | NF | Halo sign | Autopsy | 90/42.5 | 7.7/6.4 | 25/1.5 | |
| 4 | 38 | F | P | AML M2 | Post-allo, CR | Alive | NS | Cavity within area of consolidation | A. fumigatus from broncheal lavage fluid | Biopsy | 33.5/0 | 1.9/1.7 | 2.8/0 |
| 5 | 51 | M | P | Macroglobulinemia | Stable disease | Dead | None | Extensive skull base destruction | A. fumigatus from epidural abscess | Biopsy | 0/0 | 1.2/0.8 | 37.4/7.1 |
| 6 | 19 | M | P | MDS RA | RD | Dead | NF | Multiple nodular lesions in the lung field, pleural effusion | Autopsy | 3,500/1,000 | 2.5/1.5 | 155.5/59.2 | |
| 7 | 42 | M | P | MDS/AML | Post-allo, RD | Dead | NFG | Dyspnea, pleural effusion | Autopsy | 24/9 | 2.4/0.6 | 0/0 | |
| 8 | 63 | F | P | ATL acute type | RD | Dead | NF | Dyspnea, pleural effusion | Autopsy | 50/12.5 | 1.9/0.7 | 2.4/0 | |
| 9 | 69 | M | P | ALL PreB | RD | Dead | NF | No specific clinical evidence | Autopsy | 100,000/5,000 | 4.2/1.1 | 171.7/12.6 | |
| 10 | 53 | M | PP | AML M2 | Post-allo, CR | Dead | FG | Dyspnea, pleural effusion | A. spergillus spp. from broncho- alveolar lavage fluid | NAb | 5/0 | 5.3/0.7 | 4.5/2.2 |
| 11 | 40 | M | PP | CML CP1 | Post-allo, CR | Alive | NGS | Halo sign | A. fumigatus from sputum | NA | 11.5/7.5 | 2.3/2.0 | 0/0 |
| 12 | 68 | M | PPP | MDS/AML | RD | Dead | NF | Multiple nodular lesions in the lung field, intraparenchymal brain mass lesion, seizure, hemiparesis | NA | 155/100 | 2.2/1.5 | 18.3/16.6 | |
| 13 | 24 | M | PPP | AML M4E | CR, HDAraC | Alive | NF | Nodular skin lesion without any other explanation, multiple nodular lesions in the lung field | NA | 20.5/0 | 4.5/0.3 | 0/0 | |
| 14 | 61 | M | PPP | AML M4E | CR, HDAraC | Alive | N | Halo sign | NA | 1,000/9 | 0.2/0.1 | 3.5/2.9 | |
| 15 | 30 | M | PPP | ALL precursor B | Post-allo, CR | Alive | NFGS | Nonspecific abnormal shadow in lung field, pleural effusion | NA | 60/60 | 0.6/0.4 | 0/0 | |
| 16 | 61 | M | PPP | AML M2 | RD | Dead | NF | Multiple nodular lesions in the lung field, halo sign, cavity within area of consolidation | NA | 84.5/0 | 1.1/0.7 | 2/0 | |
| 17 | 68 | M | PPP | CML BC | RD | Dead | NS | Dyspnea, pleural effusion | NA | 165/0 | 0.3/0.2 | 0/0 | |
| 18 | 25 | M | PPP | ALL precursor B | RD | Alive | NG | Cavity within area of consolidation | NA | 400/0 | 0.7/0.6 | 3.2/0 | |
| 19 | 32 | M | PPP | ALL PreB | Post-allo, CR | Dead | FGS | Dyspnea, pleural effusion | NA | 27/1 | 0.7/0.5 | 3.7/2.4 | |
| 20 | 18 | F | PPP | AML M2 | CR, HDAraC | Alive | N | Halo sign | NA | 0/0 | 0.6/0.1 | 0/0 | |
| 21 | 55 | M | PPP | MDS RA | Stable disease | Alive | F | Cough, dyspnea, pleural effusion | NA | 19/4 | 0.8/0.3 | 0/0 | |
| 22 | 28 | M | PPP | CML CP1 | Post-allo, CR | Alive | G | Cough, dyspnea, pleural effusion | NA | 0/0 | 0.4/0.3 | 0/0 | |
| 23 | 40 | M | PPP | CML CP1 | Post-allo, CR | Alive | GS | Cough, dyspnea, new infiltrate not fulfilling the major radio- logical criteria without an alternative diagnosis | NA | 6/0 | 0.5/0.4 | 0/0 | |
| 24 | 54 | M | PPP | ALL precursor B | Post-allo, CR | Alive | F | Dyspnea, new infiltrate not ful- filling the major radiological criteria without an alternative diagnosis | NA | 10.5/0 | 0.5/0.3 | 0/0 | |
F, female; M, male; P, proven; PP, probable; PPP, possible; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; RA, refractory anemia; RAEB-t, RA with excess of blasts in transformation; ALL, acute lymphoblastic leukemia/lymphoma; CML, chronic myelogenous leukemia; CP, chronic phase; BC, blastic crisis; allo, allogencic hematopoietic stem cell transplantation; CR, complete remission; RD, refractory disease; HDAraC, high-dose cytrabine; N, neutropenia; F, persistent fever; G, GVHD, S, prolonged use of corticosteroid.
NA, not available.
Among the 125 no-IA episodes, 11 deaths occurred, and the diagnosis of no IA was confirmed by autopsy in 8. The other three fatal episodes were not confirmed by autopsy and included two respiratory failures following chemotherapy and one case of severe stomatitis following a second bone marrow transplantation. One respiratory failure was due to bacterial pneumonia, in which Pseudomonas aeruginosa was cultured from the sputum and the blood. In the other episode, respiratory failure developed in association with rapid tumor growth. Although no pathogen was identified despite repeated cultures, we could not completely exclude a possible infectious origin of this episode. The episode of severe stomatitis became suddenly fatal after the patient aspirated the clot and was asphyxiated.
ROC analysis.
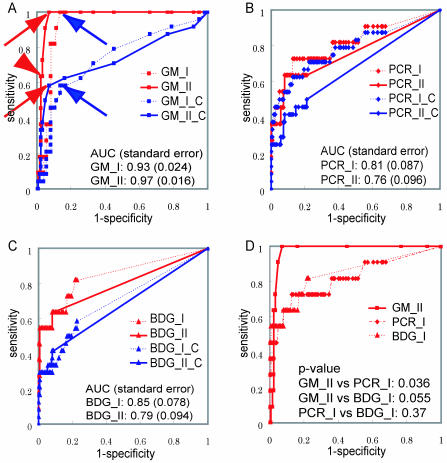
Figure 1 shows ROC curves for each test, using different definitions of the disease status. First, we examined the behaviors of the ROC curves for different diagnostic tests by using an “ideal” estimate (estimate A/B), in which episodes were expected to be most accurately defined. ELISA has a larger AUC in both method I (ELISA, 0.93; PCR, 0.81; BDG, 0.85) and method II (ELISA, 0.97; PCR, 0.76; BDG, 0.79). To increase the sensitivity for GM, we could more easily decrease its cutoff value with a small decrease in specificity. In contrast, a higher sensitivity could be obtained for the PCR and BDG tests by decreasing their cutoff values, but this would be at a significant cost in terms of specificity. When we shifted the diagnostic algorithm from method I (one positive sample) to method II (two consecutive positive samples), the AUC for the GM test was further increased while those for the PCR and BDG tests decreased, indicating that the GM test has higher reproducibility than the other two tests. The comparison of ROC curves of ELISA (method II), PCR (method I), and BDG (method I) is presented in Fig. 1D. When estimate C was applied for ROC analyses, these characteristics of the ROC curve for GM were partially obscured. In estimate C, a large decrease in sensitivity shifted the ROC curve downward and caused a significant reduction in AUC for the ELISA and BDG test, as expected. On the other hand, the ROC curve for the PCR test did not significantly change, since an expected decrease in sensitivity due to false-positive episodes in the possible IFI group is thought to be counterbalanced by a gain due to false-positive PCR results in these episodes. The ROC curves for the GM test in estimates A/B, C, and D, which is not presented but is similar to that for A/B, represent extreme cases, and the unknown “real” ROC curve might be mapped between these extremes.
FIG. 1.
(A to C) ROC curves of the GM (A), PCR (B), and BDG (C) tests for screening for IA. Both methods I and II were used. The ROC curves obtained by estimate A/B are shown in red, and those obtained by estimate C are shown in blue. The ROC curves obtained by method II are indicated by solid lines, and those obtained by method I are indicated by dotted lines. (D) Combination of ROC curves of the GM test (method II) and those of the PCR and BDG tests (method I).
Optimal cutoff value.
Determination of an optimal cutoff value may be somewhat arbitrary depending on the purpose of the diagnostic test. A loss of specificity may be allowed to obtain a higher sensitivity. Based on the conventional or manufacturer-recommended cutoff values, an optical density index (O.D.I.) of 1.0, in two serial samples for GM (2, 22), i.e., 40 copies/ml for PCR and 11 pg/ml for the BDG test, all tests showed excellent specificity (0.98) in estimate A/B whereas their sensitivity was generally low (0.64, for GM, 0.45 for the PCR test, and 0.55 for the BDG test) even in estimate A/B, with further decreases as low as 0.33 for the GM test and 0.29 for the BDG test in estimate C. The current standard for ELISA (red arrowhead in Fig. 1A) seems to be inadequate. It could be reduced to 0.6 O.D.I. in method II (red arrows in Fig. 1A), or the criteria for positivity could be relaxed to those in method I while retaining the same cutoff (1.0 O.D.I.) (blue arrows), without great loss of specificity. With regard to specificity, the former may be recommended (P = 0.0334 by Fisher's direct test), which reflects a more leftward displacement of the ROC curve for method II. Both cutoff values represent the inflexion point of each ROC curve, around which the diagnostic efficacy is maximum for both cutoffs. The sensitivity/specificity and PPV/NPV of the GM test are 1.0/0.93 and 0.55/1.0 for a cutoff value of 0.6 O.D.I. in method II and 1.0/0.86 and 0.38/1.0 for a cutoff value of 1.0 O.D.I. in method I. Various diagnostic statistical parameters in different calculations are presented in Table 3. We may improve the diagnostic efficiency by using two or three tests in combination. In our analyses, however, we could not obtain better sensitivity by combination use of multiple tests employing much reduced cutoff values while maintaining high specificity (data not shown). This is also accompanied by significant delay of diagnosis.
TABLE 3.
Statistics for some selected thresholds
| Method and threshold | Sensitivity A/B (C) | Specificity A/B (D) | PPV A/B (D) | NDV A/B (C) | Efficacy A/B (C) |
|---|---|---|---|---|---|
| Method I | |||||
| GM (O.D.I.) | |||||
| 0.5 | 1.00 (0.88) | 0.34 (0.33) | 0.12 (0.11) | 1.00 (0.93) | 0.40 (0.43) |
| 0.6 | 1.00 (0.79) | 0.55 (0.54) | 0.16 (0.15) | 1.00 (0.93) | 0.59 (0.59) |
| 1.0 | 1.00 (0.58) | 0.86 (0.85) | 0.38 (0.34) | 1.00 (0.91) | 0.87 (0.81) |
| 1.5 | 0.82 (0.46) | 0.90 (0.89) | 0.41 (0.38) | 0.98 (0.90) | 0.89 (0.83) |
| PCR (copies/ml) | |||||
| 5 | 0.91 (0.88) | 0.43 (0.41) | 0.12 (0.11) | 0.98 (0.95) | 0.47 (0.30) |
| 10 | 0.82 (0.79) | 0.60 (0.55) | 0.15 (0.13) | 0.97 (0.94) | 0.62 (0.63) |
| 20 | 0.73 (0.67) | 0.78 (0.75) | 0.23 (0.19) | 0.97 (0.92) | 0.78 (0.77) |
| 40 | 0.45 (0.46) | 0.98 (0.93) | 0.63 (0.36) | 0.95 (0.90) | 0.93 (0.89) |
| BDG (ng/ml) | |||||
| 2 | 0.82 (0.58) | 0.77 (0.76) | 0.24 (0.21) | 0.98 (0.91) | 0.78 (0.74) |
| 3 | 0.64 (0.46) | 0.84 (0.82) | 0.26 (0.23) | 0.96 (0.89) | 0.82 (0.78) |
| 5 | 0.55 (0.29) | 0.92 (0.92) | 0.38 (0.35) | 0.96 (0.87) | 0.89 (0.82) |
| 11 | 0.55 (0.29) | 0.98 (0.97) | 0.67 (0.60) | 0.96 (0.88) | 0.94 (0.87) |
| Method II | |||||
| GM (O.D.I.) | |||||
| 0.5 | 1.00 (0.63) | 0.84 (0.83) | 0.35 (0.31) | 1.00 (0.92) | 0.85 (0.81) |
| 0.6 | 1.00 (0.58) | 0.93 (0.91) | 0.55 (0.48) | 1.00 (0.92) | 0.93 (0.87) |
| 1.0 | 0.64 (0.33) | 0.98 (0.97) | 0.70 (0.64) | 0.97 (0.88) | 0.95 (0.87) |
| 1.5 | 0.45 (0.25) | 0.98 (0.97) | 0.63 (0.56) | 0.95 (0.87) | 0.93 (0.86) |
| PCR (copies/ml) | |||||
| 5 | 0.64 (0.43) | 0.87 (0.86) | 0.30 (0.27) | 0.96 (0.89) | 0.85 (0.80) |
| 10 | 0.45 (0.30) | 0.94 (0.93) | 0.38 (0.33) | 0.95 (0.88) | 0.90 (0.84) |
| 20 | 0.36 (0.26) | 0.98 (0.97) | 0.67 (0.50) | 0.95 (0.88) | 0.93 (0.87) |
| 40 | 0.36 (0.26) | 1.00 (0.99) | 1.00 (0.67) | 0.95 (0.88) | 0.95 (0.89) |
| BDG (ng/ml) | |||||
| 2 | 0.64 (0.42) | 0.91 (0.90) | 0.39 (0.33) | 0.97 (0.89) | 0.89 (0.83) |
| 3 | 0.55 (0.29) | 0.95 (0.95) | 0.50 (0.66) | 0.96 (0.88) | 0.92 (0.85) |
| 5 | 0.55 (0.29) | 0.98 (0.97) | 0.67 (0.60) | 0.96 (0.88) | 0.94 (0.87) |
| 11 | 0.45 (0.25) | 0.99 (0.99) | 0.83 (0.71) | 0.95 (0.87) | 0.95 (0.87) |
Time interval between the first positive result and the antemortem diagnosis.
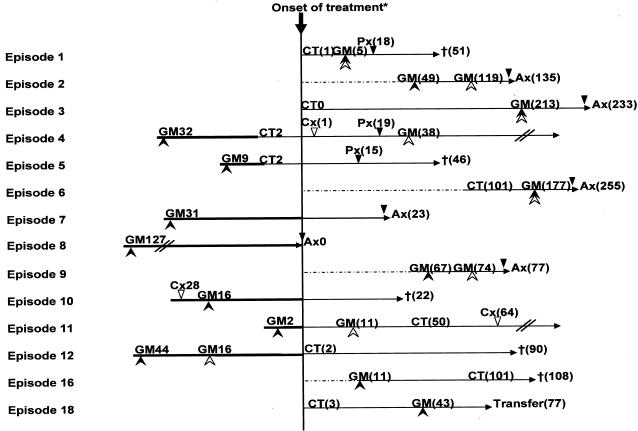
Chronological relationships between the first positive results of different screening tests, histopathology, and diagnostic imaging are summarized in Fig. 2 and 3. For the PCR and BDG tests, the conventional cutoff was used, while the second of the first two consecutive results equal to or greater than 0.6 or 1.0 O.D.I. was plotted for ELISA. When the new reduced cutoff was used, the first positive date for GM was brought forward by a median of 10 (0 to 70, n = 9, mean = 24) days compared to the conventional cutoff value. Using the conventional cutoff, only one episode was identified to have a positive ELISA result before definitive treatment was started. In contrast, with the new reduced cutoff, the first positive ELISA result preceded the initiation of broad-spectrum antifungal treatment in seven IA-positive episodes (median, 31 days; range, 2 to 127 days; mean, 28 days). It became positive 51 days before a positive histopathology result (10 to 127 days; mean, 31 days).
FIG. 2.
Number of days from when GM assays become positive to the onset of treatment, using a threshold of 0.6 O.D.I. by method II (solid arrowheads) or 1.0 O.D.I. by method II (open arrowheads), or positive findings on CT. Open triangles indicate the date of positive culture, and solid triangles indicate when the histopathological diagnosis was made (Px, biopsy; Ax, autopsy). The values in parentheses indicate the number of days after the onset of treatment. For example, for episode 11, CT showed specific findings 50 days after the onset of treatment and the GM assay become positive 2 days before treatment. Episode numbers correspond to those in Table 2. Episodes whose GM assays did not reach the threshold are not shown. For episodes 2 and 9, a CT scan was not performed, and for episodes 7, 8, 10, 17, 19, 21, and 22, the CT findings were nonspecific and could not be used for decision-making. Each treatment was started at the discretion of the physician, taking into account various prices of clinical information, including CT findings and the results of GM assays. For Episode 8, IA was not suspected and no antifungal agent was administered. Therefore, the date of death was used instead of the date of treatment onset.
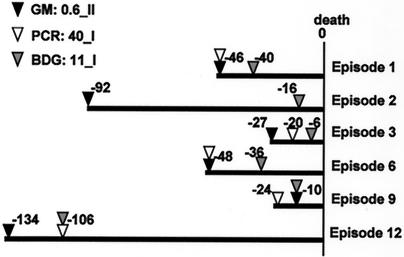
FIG. 3.
Number of days before death that each test gave positive results. Solid triangles indicate the date when GM became positive, using a threshold of 0.6 O.D.I. by method II; open triangles indicate the date when PCR exceeded a cutoff value of 40 copies/ml; and shaded triangles indicate the date when the BDG test exceeded a cutoff value of 11 ng/ml, by method I. In episode 2, PCR never exceeded the cutoff value. Episode numbers correspond to those in Table 2.
Unfortunately, chronological comparisons between the three different assays were possible for only six episodes, in which patients had refractory leukemia and their IA tended to have a rapidly progressive course as a terminal infection (Fig. 3). In these episodes, ELISA gave positive findings earlier than (five episodes) or at the same time as (one episode) the BDG test (median, 16.5 days; range, 0 to 76 days). The PCR test was positive in 11 of 24 IA patients in estimate C. A comparison was possible in 5 of the 11 episodes, which were also positive for ELISA, but there was no significant difference in the date of the first positive result between ELISA and the PCR tests.
DISCUSSION
In this study, we compared the diagnostic potential of three different laboratory tests used to screen for IA in a prospective setting, where GM, DNA, and BDG levels in a cohort of patients at high risk for IA were measured weekly. The statistical parameters of a diagnostic test can be dramatically affected by the predetermined cutoff value, and when there is some uncertainty regarding the disease status, as in this case, they can also be influenced by the definition of the disease status. Therefore, to meaningfully compare the diagnostic potentials of these different tests, we performed an ROC analysis for each test by using the same cohort of patients with different positive result criteria (methods I and II) and various definitions of the disease status (estimates A/B, C, and D). As a result, the ROC curve for the GM test seemed to be better than those for the other two tests.
We previously reported that this real-time PCR for Aspergillus DNA was highly sensitive in vitro and with clinical samples (17): it could stably detect as few as 40 copies/ml in vitro and showed a higher sensitivity (79%) than those of the GM (58%) and BDG (67%) tests. In the present prospective analysis with consecutive patients, however, these results were not reproduced. This may be partly explained by the fact that our previous study included many retrospective samples. Furthermore, we intentionally selected IA patients and used a higher cutoff value for the GM test. Although several authors have also reported excellent sensitivity in PCR assays for IA (5, 6, 14, 34), we cannot directly compare those results with ours since there were differences in the target genes, methods of DNA extraction, starting materials, and designs of the PCR amplifications. Some form of standardization is required to make an international comparison possible. We used our real-time PCR system (GeniQ-Asper) (17) because it is most widely used in Japan. Several authors, including Loeffler et al. and Costa et al., also published excellent real-time PCR detection systems for Aspergillus DNA (9, 21, 26, 28), and their systems might produce superior results in the diagnosis of IA, which should be addressed in future studies.
As a diagnostic test, PCR requires more time and more complicated processing and thus costs more than the BDG and GM tests. It costs six times (15,700 yen/test) as much as the BDG and GM assays (2,700 yen/test) in Japan. A specialized laboratory as well as an expensive assay system and reagents are also required. These problems should be addressed before PCR is widely accepted as a standard screening test for IA, although it still seems to have value in making a diagnosis when a variety of clinical samples are used (20, 26, 28, 31).
The BDG test has also been widely used in Japan as a noninvasive diagnostic test for IFI. While it covers wide ranges of fungal species and may be potentially more useful as a screening test for IFI, it can cause frequent nonspecific reactions to various medical materials. Three kinds of assay systems for BDG have been developed in Japan: a chromogenic assay (FungiTec G test), β-glucan test Maruha) and a kinetic assay (β-glucan test Wako), but there is still some debate regarding their diagnostic potential. According to a sample-based analysis by Yoshida et al. (35), the chromogenic assay seems to be more sensitive (87.9 and 72.7%, respectively) than the kinetic assay but much less specific (43.3 and 75.2%, respectively) when the cutoff values recommended by the manufacturers are used. In the present study, where we used a kinetic assay, we could not obtain sufficient sensitivity even with the cutoff being maximally reduced. Furthermore, even if positive results were obtained, the positive results with the BDG test tended to occur later in the clinical course. The present result (55% sensitivity and 98% specificity) is consistent with our previous results (67% sensitivity and 84% specificity) using the chromogenic assay and also with other reports. This seems to be an inherent limitation of BDG assays for the diagnosis of IA, although they show a very high sensitivity and specificity for candidiasis (25).
The diagnostic potential of double-sandwich ELISA for GM has been repeatedly validated in recent large-scale studies (15, 22). However, a direct comparison of the results of different studies, including ours, is not always easy and in fact can be quite difficult or impractical. Many factors can influence the apparent sensitivity and specificity and of course the PPV and NPV. Therefore, the important point is the way in which these results should be interpreted, and this depends on the objective and design of each study. From this perspective, our results are comparable to those of Maertens et al. (22) but in contrast to those of Herbrecht et al. (15). The latter addressed principally the diagnostic potential of the GM test in the presence of an unknown neutropenic fever or some respiratory signs and symptoms in cancer patients. On the other hand, in our study as well as in that of Maertens et al., the principal concern was the potential of the test in serial screenings with multiple measurements throughout the entire period of hematology care. For example, the mean numbers of measurements per episode in our study and that of Maertens et al. (8.3 and 11.2 per episode, respectively, with GM measured weekly) are significantly different from that in the study of Herbrecht et al. (5.5 per episode, with GM measured daily or weekly), consistent with the study designs. The difference becomes more prominent for proven IA episodes (17.3 and 19 versus 6.8). The differences in the mean number and timing of measurements clearly affect the apparent sensitivity and specificity of the studies. Hence, the apparent statistical values obtained by Herbrecht et al. are expected to be lower than ours and those of Maertens et al., but they should provide a better approximation of the corresponding sample-based statistics, even though the patient population was more heterogeneous.
According to the ROC analysis of double-sandwich ELISA, the conventionally used cutoff seems to be too high: our recommendation is 0.6 O.D.I., and two consecutive positive results should be taken into consideration. With these new criteria, the GM test showed an excellent chronological profile. It gave the first positive diagnostic result in 9 of 14 GM-positive IA episodes and in 5 of 9 IA or possible IFI episodes where both CT and GM were positive. It preceded the initiation of empiric or definitive antifungal therapy in seven episodes. Using the novel criteria, positivity was ascertained a median of 10 days before conventional positivity was noted, and in six cases the GM test gave positive results only with the novel criteria. These chronological advantages were not observed with a threshold of 1.0 O.D.I. by method II: for episodes 5, 7, 8, and 10, the GM assay did not become positive; for episode 4, the GM assay exceeded the criteria 38 days after the onset of treatment; for episode 12, the GM assay gave positive results 16 days before the onset of treatment. According to the high PPV with the novel cutoff criteria (0.55 for proven or probable IA and 0.48 for proven, probable, or possible IFI) and the early timing of its positivity, we could have initiated antifungal therapy in a preemptive manner for episodes 4, 5, 7, 8, 10, 11, and 12.
Our result does not justify a discontinuation or moratorium of empiric antifungal treatment based only on a single negative result in the face of an impending threat of IA. It should be stressed that the extremely high NPVs provided here are episode-based calculations. Sample-based NPVs should be much lower, especially when patients are at high risk. We could not exclude a possibility of other IFI. Similarly, PPV does not always represent the probability of currently having IA but, rather, predicts the probability that the subject has or will have IA. In addition, while there was a sufficient number of no-IA episodes in this study to permit reliable estimations of specificity and NPV, there is much uncertainty regarding the estimations of the absolute values of sensitivity and PPV because of the small number of IA patients.
REFERENCES
- 1.Andreas, S., S. Heindl, C. Wattky, K. Moller, and R. Ruchel. 2000. Diagnosis of pulmonary aspergillosis using optical brighteners. Eur. Respir. J. 15:407-411. [DOI] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Becker, M. J., S. de Marie, D. Willemse, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2000. Quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis in an experimental rat model. J. Clin. Microbiol. 38:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden, R., P. Chandrasekar, M. H. White, X. Li, L. Pietrelli, M. Gurwith, J. A. van Burik, M. Laverdiere, S. Safrin, and J. R. Wingard. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359-366. [DOI] [PubMed] [Google Scholar]
- 5.Bretagne, S., J. M. Costa, E. Bart-Delabesse, N. Dhedin, C. Rieux, and C. Cordonnier. 1998. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clin. Infect. Dis. 26:1407-1412. [DOI] [PubMed] [Google Scholar]
- 6.Buchheidt, D., C. Baust, H. Skladny, J. Ritter, T. Suedhoff, M. Baldus, W. Seifarth, C. Leib-Moesch, and R. Hehlmann. 2001. Detection of Aspergillus species in blood and bronchoalveolar lavage samples from immunocompromised patients by means of 2-step polymerase chain reaction: clinical results. Clin. Infect. Dis. 33:428-435. [DOI] [PubMed] [Google Scholar]
- 7.Caillot, D., O. Casasnovas, A. Bernard, J. F. Couaillier, C. Durand, B. Cuisenier, E. Solary, F. Piard, T. Petrella, A. Bonnin, G. Couillault, M. Dumas, and H. Guy. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J. Clin. Oncol. 15:139-147. [DOI] [PubMed] [Google Scholar]
- 8.Caillot, D., J. F. Couaillier, A. Bernard, O. Casasnovas, D. W. Denning, L. Mannone, J. Lopez, G. Couillault, F. Piard, O. Vagner, and H. Guy. 2001. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 19:253-259. [DOI] [PubMed] [Google Scholar]
- 9.Costa, C., J. M. Costa, C. Desterke, F. Botterel, C. Cordonnier, and S. Bretagne. 2002. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 40:2224-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 11.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 12.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley, J. A., and B. J. McNeil. 1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839-843. [DOI] [PubMed] [Google Scholar]
- 14.Hebart, H., J. Loffler, C. Meisner, F. Serey, D. Schmidt, A. Bohme, H. Martin, A. Engel, D. Bunje, W. V. Kern, U. Schumacher, L. Kanz, and H. Einsele. 2000. Early detection of aspergillus infection after allogeneic stem cell transplantation by polymerase chain reaction screening. J. Infect. Dis. 181:1713-1719. [DOI] [PubMed] [Google Scholar]
- 15.Herbrecht, R., V. Letscher-Bru, C. Oprea, B. Lioure, J. Waller, F. Campos, O. Villard, K. L. Liu, S. Natarajan-Ame, P. Lutz, P. Dufour, J. P. Bergerat, and E. Candolfi. 2002. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 20:1898-1906. [DOI] [PubMed] [Google Scholar]
- 16.Hughes, W. T., D. Armstrong, G. P. Bodey, A. E. Brown, J. E. Edwards, R. Feld, P. Pizzo, K. V. Rolston, J. L. Shenep, and L. S. Young. 1997. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin. Infect. Dis. 25:551-573. [DOI] [PubMed] [Google Scholar]
- 17.Kami, M., T. Fukui, S. Ogawa, Y. Kazuyama, U. Machida, Y. Tanaka, Y. Kanda, T. Kashima, Y. Yamazaki, T. Hamaki, S. Mori, H. Akiyama, Y. Mutou, H. Sakamaki, K. Osumi, S. Kimura, and H. Hirai. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504-1512. [DOI] [PubMed] [Google Scholar]
- 18.Kami, M., Y. Tanaka, Y. Kanda, S. Ogawa, T. Masumoto, K. Ohtomo, T. Matsumura, T. Saito, U. Machida, T. Kashima, and H. Hirai. 2000. Computed tomographic scan of the chest, latex agglutination test and plasma (1AE3)-beta-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745-752. [PubMed] [Google Scholar]
- 19.Kawamura, S., S. Maesaki, T. Noda, Y. Hirakata, K. Tomono, T. Tashiro, and S. Kohno. 1999. Comparison between PCR and detection of antigen in sera for diagnosis of pulmonary aspergillosis. J. Clin. Microbiol. 37:218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawazu, M., Y. Kanda, S. Goyama, M. Takeshita, Y. Nannya, M. Niino, Y. Komeno, T. Nakamoto, M. Kurokawa, S. Tsujino, S. Ogawa, K. Aoki, S. Chiba, T. Motokura, N. Ohishi, and H. Hirai. 2003. Rapid diagnosis of invasive pulmonary aspergillosis by quantitative polymerase chain reaction using bronchial lavage fluid. Am. J. Hematol. 72:27-30. [DOI] [PubMed] [Google Scholar]
- 21.Loeffler, J., N. Henke, H. Hebart, D. Schmidt, L. Hagmeyer, U. Schumacher, and H. Einsele. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the light cycler system. J. Clin. Microbiol. 38:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 23.Mori, T., H. Ikemoto, M. Matsumura, M. Yoshida, K. Inada, S. Endo, A. Ito, S. Watanabe, H. Yamaguchi, M. Mitsuya, M. Kodama, T. Tani, T. Yokota, T. Kobayashi, J. Kambayashi, T. Nakamura, T. Masaoka, H. Teshima, T. Yoshinaga, S. Kohno, K. Hara, and S. Miyazaki. 1997. Evaluation of plasma (1→3)-beta-d-glucan measurement by the kinetic turbidimetric Limulus test,for the clinical diagnosis of mycotic infections. Eur. J. Clin. Chem. Clin. Biochem. 35:553-560. [PubMed] [Google Scholar]
- 24.Nakai, T., J. Uno, K. Otomo, F. Ikeda, S. Tawara, T. Goto, K. Nishimura, and M. Miyaji. 2002. In vitro activity of FK463, a novel lipopeptide antifungal agent, against a variety of clinically important molds. Chemotherapy 48:78-81. [DOI] [PubMed] [Google Scholar]
- 25.Obayashi, T., M. Yoshida, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshima, S. Kohno, A. Horiuchi, A. Ito, et al. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 26.Rantakokko-Jalava, K., S. Laaksonen, J. Issakainen, J. Vauras, J. Nikoskelainen, M. K. Viljanen, and J. Salonen. 2003. Semiquantitative detection by real-time PCR of Aspergillus fumigatus in bronchoalveolar lavage fluids and tissue biopsy specimens from patients with invasive aspergillosis. J. Clin. Microbiol. 41:4304-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonen, J., O. P. Lehtonen, M. R. Terasjarvi, and J. Nikoskelainen. 2000. Aspergillus antigen in serum, urine and bronchoalveolar lavage specimens of neutropenic patients in relation to clinical outcome. Scand. J. Infect. Dis. 32:485-490. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti, M., B. Posteraro, L. Pagano, G. Pagliari, L. Fianchi, L. Mele, M. La Sorda, A. Franco, and G. Fadda. 2003. Comparison of real-time PCR, conventional PCR, and galactomannan antigen detection by enzyme-linked immunosorbent assay using bronchoalveolar lavage fluid samples from hematology patients for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:3922-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone, E. A., H. B. Fung, and H. L. Kirschenbaum. 2002. Caspofungin: an echinocandin antifungal agent. Clin. Ther. 24:351-377; discussion, 329. [DOI] [PubMed] [Google Scholar]
- 30.Stynen, D., A. Goris, J. Sarfati, and J. P. Latge. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang, C. M., D. W. Holden, A. Aufauvre-Brown, and J. Cohen. 1993. The detection of Aspergillus spp. by the polymerase chain reaction and its evaluation in bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 148:1313-1317. [DOI] [PubMed] [Google Scholar]
- 32.Verweij, P. E., D. Stynen, A. J. Rijs, B. E. de Pauw, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1995. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J. Clin. Microbiol. 33:1912-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petersen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, E. Anaissie, and J. Lee. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]
- 34.Yamakami, Y., A. Hashimoto, I. Tokimatsu, and M. Nasu. 1996. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J. Clin. Microbiol. 34:2464-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida, K., Y. Niki, H. Mitekura, M. Nakajima, H. Kawane, and T. Matsushima. 2001. A discrepancy in the values of serum (1-3)-beta-d-glucan measured by two kits using different methods. Nippon Ishinkin Gakkai Zasshi 42:237-242. (In Japanese.) [DOI] [PubMed] [Google Scholar]