Abstract Abstract
Endothelial nitric oxide synthase (eNOS) plays a major role in maintaining pulmonary vascular homeostasis. Tetrahydrobiopterin (BH4), an essential cofactor that stabilizes the dimerization of eNOS and balances nitric oxide (NO) and superoxide production, may have therapeutic potential in pulmonary hypertension. In the isolated perfused lung, we demonstrated a direct effect of exogenous administration of BH4 on pulmonary NO production, leading to acute vasorelaxation during the plateau phase of hypoxia-induced pulmonary vasoconstriction. In the chronic hypoxia-induced pulmonary hypertension rat model, chronic BH4 oral administration attenuated the pressor response to hypoxia (mean pulmonary artery pressure ± standard error of the mean, 31.8 ± 0.5 mmHg at 100 mg/kg/day; placebo group, 36.3 ± 0.6 mmHg; P < 0.05). During telemetric monitoring, right ventricular systolic pressure was reduced by approximately 50% after 1 week of BH4 treatment at 100 mg/kg/day. BH4 at 100 mg/kg/day reduced right ventricular hypertrophy (from 0.55 ± 0.01 to 0.50 ± 0.01; P < 0.05) and pulmonary vascular muscularization (from 79.2% ± 2% to 65.2% ± 3%; P < 0.01). BH4 treatment enhanced lung eNOS activity and reduced superoxide production, with a net increase in cyclic guanosine monophosphate levels. BH4 is effective in attenuating pulmonary hypertension in the hypoxic rat model when given as a rescue therapy.
Keywords: endothelium, nitric oxide, pulmonary hypertension, superoxide, tetrahydrobiopterin
Introduction
Pulmonary hypertension is a devastating disease characterized by pulmonary arterial vasoconstriction and vascular remodeling.1-3 The endothelium and, in particular, nitric oxide synthase (eNOS) play a major role in maintaining normal pulmonary vascular tone and structure. Dysfunctional eNOS can imbalance nitric oxide (NO) and superoxide production in the pulmonary vasculature, contributing to the pathogenesis of pulmonary hypertension.4
Tetrahydrobiopterin (BH4) is an essential cofactor that regulates eNOS activity. BH4 stabilizes the dimerization of eNOS, facilitates electron transfer, and balances NO production with superoxide generation.5,6 However, in pathological states in which BH4 bioavailability is reduced (e.g., oxidized by increased levels of free radicals, such as superoxide and peroxynitrite), eNOS becomes dysfunctional and its activity “uncoupled” to favor superoxide production.7,8 This imbalance in NO/superoxide production results in oxidative stress, a major contributing factor in a variety of vascular diseases,7,9,10 which also leads to detrimental effects on cardiac function.11
Previous studies in our group and others have shown that BH4 plays a pivotal role in regulating NO/superoxide balance in the pulmonary vasculature.12-14 It has been shown that hph-1 mice, deficient in GTP-cyclohydrolase 1 (GTPCH-1), the rate limiting enzyme for BH4 production, show pulmonary vascular remodeling and right ventricular hypertrophy under normoxic conditions, accompanied by significantly increased superoxide production in the lung. These mice were also more sensitive to hypoxia exposure and developed a more severe pulmonary hypertension phenotype compared with wild-type mice.
We demonstrated previously that BH4 administration attenuates the acute pulmonary vasoconstrictor response to hypoxia in a concentration-dependent manner via NO and hydrogen peroxide production and showed that BH4 has antioxidant properties.15 This led to the hypothesis that pharmacological treatment with BH4 may improve pulmonary endothelial function and has therapeutic potential in pulmonary hypertension. Employing a hypoxia-induced pulmonary hypertension rat model, we investigated the chronic pharmacological effects of BH4 on hemodynamics, right ventricular hypertrophy, and pulmonary vascular remodeling.
Methods
Animals
Male Sprague-Dawley (SD) rats (250–350 g) from Charles River (Margate, UK) were used for all of the experiments. All studies were conducted in accordance with UK Home Office Animals (Scientific Procedures) Act 1986.
Acute pharmacologic effects of BH4: the isolated perfused rat lung
The pulmonary vascular response to 6R-BH4 (sapropterin dihydochloride or BH4) was studied using the isolated rat lung preparation as previously described15 (Fig. S1). BH4 was supplied by BioMarin Pharmaceuticals (Novato, CA). Pulmonary artery pressure (PAP) was measured using a pressure transducer connected to PowerLab Data Acquisition system (ADInstruments). Hypoxic pulmonary vasoconstriction (HPV) was induced by ventilating the lung with 2% O2, 5% CO2, and 93% N2. BH4 (final concentration, 0.3 μg/mL and 1 μg/mL, freshly prepared as previously described15) or vehicle was added to the perfusate when consistent HPV had been achieved (usually after 3 hypoxic challenges). Perfusate samples were collected after passage through the lungs 1 minute before and 3 minutes after BH4 administration for determination of nitrate/nitrite (NOx) concentration. The effect of BH4 was calculated from the percentage of change in HPV response and NOx levels 4 minutes after BH4 administration compared with the HPV response and NOx levels before BH4 administration in the same animal.
Perfusate samples were centrifuged, and the supernatant was stored at −20°C. NOx concentration was measured using an ozone-based chemiluminescence method by an NO Analyzer (Sievers Model 280).16 Results are presented as the percentage of the NOx levels after BH4 administration in the perfusate compared with the levels before administration.
Chronic pharmacologic effects of BH4 treatment
SD rats were kept in normobaric hypoxia chamber (10% O2 and 90% N2).15 BH4 or placebo pellets were given after 2 weeks of hypoxia exposure and continued for an additional 2 weeks under hypoxia. Rats were divided into 5 groups: (1) normoxia control; (2) 2 weeks hypoxia; (3) 4 weeks hypoxia placebo control; (4) 4 weeks hypoxia with 2 weeks BH4 low dose (10 mg/kg/day) treatment; and (5) 4 weeks hypoxia with 2 weeks BH4 high-dose (100 mg/kg/day) treatment. BH4 pellets were prepared and supplied by BioMarin Pharmaceutical (Novato, CA) in 2 different dosages, 5 and 25 mg/pellet. Placebo pellets were also prepared and included all preservatives and core materials but without BH4. Rats were fasted 12 hours before the first dose and 8 hours every 24 hours for the next 2 doses to encourage compliance with treatment. BH4 pellets were kept in dark and dry conditions and tested in BioMarin every 6 months to assess the BH4/dihydrobiopterin (BH2) content.
Hemodynamic measurements, tissue collection, and histological examination
Rats were anesthetized (1∶1∶2 hypnorm∶midazolam∶water, 2.7 mL/kg of body weight, administered intraperitoneally). Right ventricular systolic pressure (RVSP) and PAP were measured via a precurved catheter inserted through the right jugular vein, and systemic blood pressure was recorded in the carotid artery cannulation using a PowerLab Data Acquisition system (ADInstruments).
The animals were then killed and tissues were collected, snap frozen, and stored at −80°C for biochemical measurements. Hearts were dissected and weighed, and the ratio of right ventricle (RV) to left ventricle plus the septum mass was used as an index of right ventricular hypertrophy (RVH). The left lung and RV were fixed with 10% formalin in phosphate-buffered saline and processed for elastic Van Gieson and hematoxylin and eosin (H&E) staining. Vessels less than 100 μm in peripheral lung were counted blindly under microscope (40×), and pulmonary vascular remodeling was expressed as the proportion of vessels with double elastic lamina (>50%) to total vessels counted (percentage total muscularized vessels).17 AxioVision software (Carl Zeiss) was used to quantifying RV cardiomyocyte cross section area and myocyte diameter from transversely cut cardiomyocytes (n ≥ 30) using H&E sections.
Radiotelemetry
A separate group of rats (n = 4) was implanted with radiotelemetry monitors (Dataquest A.R.T. 3.1; Data Sciences). RV pressures were measured by a fluid-filled sensing catheter inserted into the RV through the jugular vein and connected to the transmitter (model TA11PA-40), which sends the signal to a remote receiver (model RPC-1) and data exchange matrix connected to a computer. The pressure waveform was monitored online at 30-minute intervals. The animals were kept in separate cages, and subcutaneous injection of antibiotics (Baytril 5%, 10–20 mg/kg) and analgesics (buprenorphine, 0.1 mL/kg) were administered after surgery. A week after recovery from surgery, the animals were put in the hypoxia chamber and monitored daily.
Biochemical measurements
BH4 levels in lung tissue
Measurements of lung tissue BH4, BH2, and total biopterin were performed by high-performance liquid chromatography followed by serial electrochemical and fluorescent detection.18 Total biopterins were quantified by summing BH4, dihydrobiopterin, and biopterin. Biopterin levels were expressed as picomoles per gram of tissue.
Western blot analysis
Lungs were homogenized. Protein lysate (20 μg) was resolved in SDS-PAGE and transferred to nitrocellulose membranes. Purified mouse monoclonal antibody against human eNOS amino acid 1025–1203 (BD Biosciences, Oxford, UK; 1∶1,000) and horseradish peroxidase–conjugated secondary antibodies (1∶5,000) were used. The proteins were visualized by chemiluminescence (GE Healthcare). Optical densities of individual bands were measured. eNOS expression was normalized to β‐actin.
eNOS activity
NOS activity was determined by H3-L-arginine to H3-L-citrulline conversion using a commercial kit (Cayman Chemical) with minor modifications.19 The integrated H3-L-citrulline elution peak was expressed as a percentage of total H3 counts. The final results were normalized to total protein concentration per sample and expressed as cycles per minute per microgram of protein.
Superoxide levels
Total lung superoxide production was measured by lucigenin-enhanced chemiluminescence.20 Frozen lungs were homogenized in Krebs-HEPES buffer. Samples of homogenate corrected for total protein content were added to 2 mL Krebs-HEPES buffer containing lucigenin (5 mol/L) in a scintillation vial at 37°C, and chemiluminescence was quantified for 5 minutes after measurement of background. L-NG-nitroarginine methyl ester (1 mM) was added to the mixture, and the levels of superoxide were measured for 5 minutes. Superoxide production was expressed as relative light units per second per milligram dry weight.
NOx levels
Lung tissue homogenate NOx levels were measured by a commercial colorimetric assay kit (Cayman Chemical) according to the manufacturer’s instructions.
Cyclic guanosine monophosphate (cGMP) levels
Lung tissue samples were homogenized in cold 6% (weight/volume) trichloroacetic acid. After centrifugation, supernatants were washed 4 times with water-saturated diethyl ether. The aqueous extracts were then lyophilized and redissolved in the assay buffer. cGMP levels were measured by cGMP enzyme immunoassay Biotrak System (GE Healthcare) according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as mean values ± the standard error of the mean; n equals the number of animals per experiment. Statistical analysis was performed by using the Student t test when appropriate, nonparametric test with Mann-Whitney modification, or 1-way analysis of variance when applied to multiple group comparisons. A value of P < 0.05 was considered to be statistically significant.
Results
BH4 induces pulmonary vasodilation at HPV plateau
To examine its acute vasoactive properties, BH4 (0.3 μg/mL and 1 μg/mL) was administered during the plateau phase of HPV. BH4 at 1 μg/mL (final perfusate concentration) induced a significant (35% ± 5%) decrease in PAP in comparison with vehicle with a simultaneous increase in NOx (125% ± 27%) in the perfusate (Fig. 1).
Figure 1.
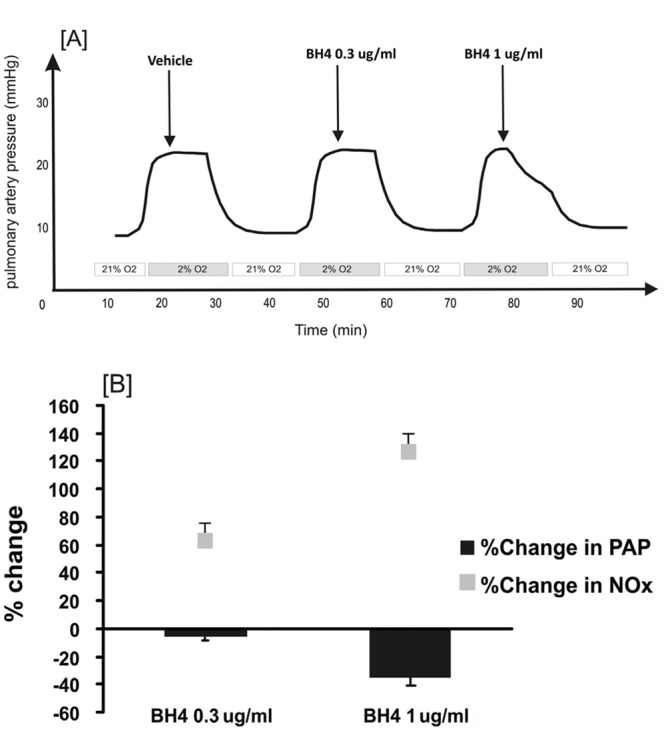
A, Representative pulmonary artery pressure (PAP) trace illustrating the protocol for tetrahydrobiopterin (BH4) administration during the stable phase of hypoxic pulmonary vasoconstriction (HPV) response in the rat isolated perfused lung. B, The effect of BH4 on PAP during HPV response. The effect of BH4 was calculated as the PAP reduction (ΔPAP) 3 minutes after drug administration expressed as a percentage of the maximum HPV response (HPVmax) in the same hypoxia challenge (black bars). Nitric oxide production was calculated as the percentage of the nitrate/nitrite (NOx) levels after BH4 administration in the perfusate compared with the levels before administration (n = 5).
Pharmacological effects of BH4 on chronic hypoxia–induced pulmonary hypertension
Radiotelemetry
Continuous radiotelemetric measurement of RVSP showed that pulmonary hypertension was established after 2 weeks hypoxia exposure (Fig. 2). BH4 (100 mg/kg/day) treatment for 1 week reduced RVSP from 63 ± 4 mmHg to 44.5 ± 4 mmHg in the awake free-living animal (Fig. 2).
Figure 2.
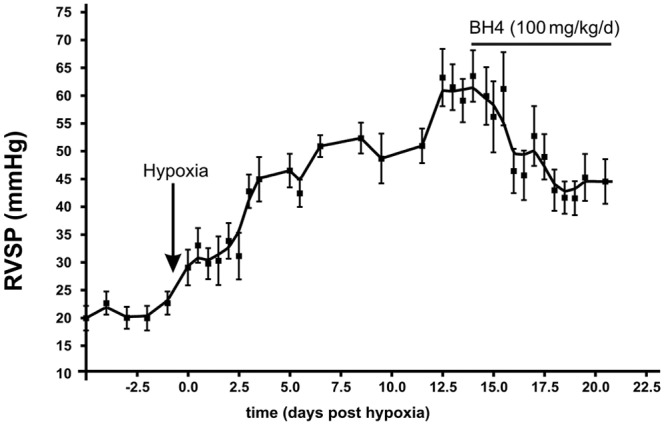
Tetrahydrobiopterin (BH4) treatment reverses pulmonary hypertension in rat hypoxia model. Effect of BH4 on right ventricular systolic pressure (RVSP) in hypoxia-induced pulmonary hypertension rat model measured by telemetry. Hypoxia was started on day 0; BH4 treatment was started on day 14 and continued for 1 week.
BH4 attenuated PAP and RVSP
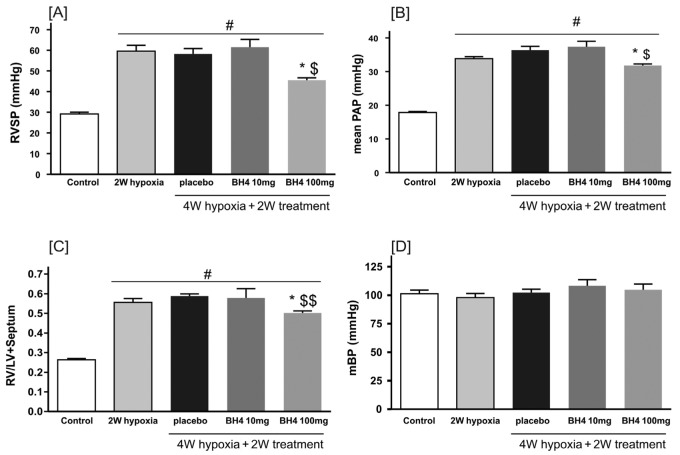
Hypoxia exposure for 2 weeks resulted in increases in PAP from 17.8 ± 0.3 mmHg to 33.9 ± 0.6 mmHg and RVSP from 29 ± 1 mmHg to 59.5 ± 3 mmHg; these levels were maintained at 4 weeks of hypoxia exposure (PAP, 36.3 ± 1 mmHg; RVSP, 58.2 ± 3 mmHg). Treatment with BH4 at 100 mg/kg/day from 2 weeks to 4 weeks significantly reduced RVSP (from 59.5 ± 3 mmHg to 45.5 ± 1 mmHg; P < 0.01; Fig. 3A) and PAP (from 33.9 ± 0.6 mmHg to 31.8 ± 0.5 mmHg; P < 0.05; Fig. 3B). Systemic blood pressure was not affected by hypoxia or BH4 treatment (Fig. 3D).
Figure 3.
Treatment with tetrahydrobiopterin (BH4) or placebo was started 2 weeks (W) after chronic hypoxia exposure and continued for another 2 W. Right ventricular systolic pressure (RVSP; A), mean pulmonary artery pressure (mPAP; B), and right ventricular over left ventricular and septum weight ratio (RV/LV+septum; C) in different groups are shown. In addition, the effect of hypoxia and drug treatment on mean systemic blood pressure (mBP) is presented in D. Asterisk: P < 0.05 compared with hypoxia placebo; dollar sign: P < 0.05 compared with 2 W hypoxia; double dollar sign: P < 0.01 compared with 2 W hypoxia; pound sign: P < 0.05 compared with control (n = 6–10 per group).
BH4 reduces RVH
Hypoxia exposure induced RVH (Fig. 3C). Treatment with BH4 at a dosage of 10 mg/kg/day did not influence RVH. However, BH4 treatment at 100 mg/kg/day significantly attenuated RVH (from 0.58 ± 0.0 mmHg to 10.50 ± 0.01 mmHg; P < 0.05; Fig. 3C), and this was associated with significant reduction in cardiomyocyte diameter in comparison with 4 weeks hypoxia-placebo treatment (24.9 ± 0.5 μm to 21.1 ± 0.5 μm; P < 0.05) and a significant reversal compared with 2 weeks hypoxia (23.5 ± 0.7 μm to 21.1 ± 0.5 μm; P < 0.05; Fig. 4).
Figure 4.
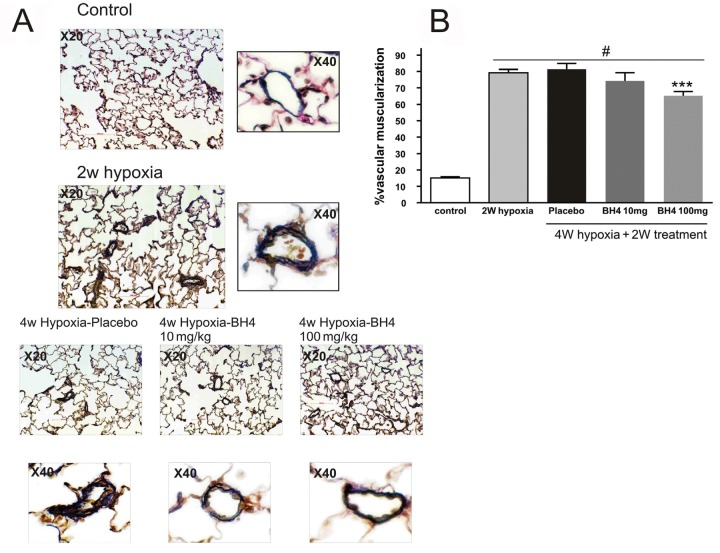
The effect of tetrahydrobiopterin (BH4) on distal lung vascular muscularization. A, Histological pictures taken to show the double elastic lamina seen with Van Gieson staining as indication for muscularized vessels. Notice the double elastic lamina with placebo-treated group, which is thinner in BH4 10 mg/kg and almost reduced to 1 elastic lamina in 100 mg/kg. Treatment with BH4 at a dosage of 100 mg/kg reversed lung vascular muscularization compared with placebo as well as 2 weeks (W) of hypoxia (the starting point of treatment; B). Three asterisks: P < 0.001 compared with 4 W of placebo treatment and 2 W of hypoxia; pound sign: P < 0.05 compared with control (n = 6 per group).
BH4 reverses pulmonary vascular muscularization
Pulmonary muscularization was significantly increased in rats exposed to 2 weeks of hypoxia compared with normoxia controls (79.2% ± 2% vs. 15.6% ± 1%; P < 0.01). Treatment with placebo or BH4 at a dosage of 10 mg/kg/day did not affect muscularization (81.4% ± 3% and 74.3% ± 5%, respectively), but BH4 at a dosage of 100 mg/kg/day induced significant reduction in vascular muscularization (65.2% ± 3%) compared with 2 weeks hypoxia alone or 4 weeks hypoxia alone (P < 0.01; Fig. 5).
Figure 5.
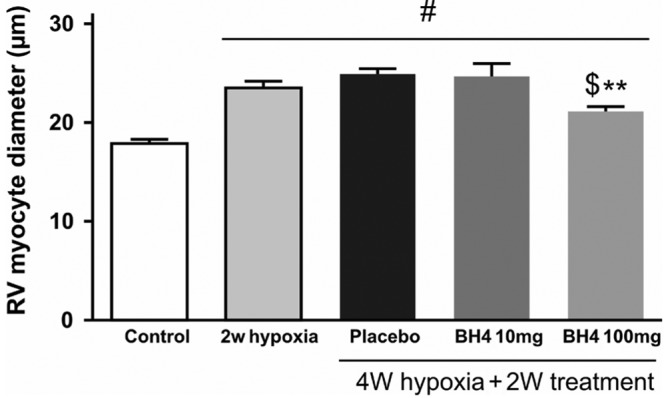
Myocyte diameter is measured in right ventricle (RV) sections in different groups as an index of RV hypertrophy. Two asterisks: P < 0.01 compared with placebo; dollar sign: P < 0.05 compared with 2 weeks (W) of hypoxia; pound sign: P < 0.05 compared with control (n = 5–8 per group); BH4: tetrahydrobiopterin.
Lung tissue BH4 levels
BH4 treatment did not influence lung homogenate BH4 levels in comparison with placebo or control (Table S1).
Effects of BH4 on eNOS protein levels and enzyme activity
eNOS protein levels were increased after 2 weeks of hypoxia and returned to normal levels after 4 weeks of hypoxia. Neither placebo nor BH4 treatment had any significant influence on eNOS protein expression in lung homogenates (Fig. 6A). eNOS activity in lung homogenates from the BH4 100 mg/kg/day treatment group was increased significantly in comparison with control, 2 weeks hypoxia, 4 weeks hypoxia-placebo, or 4 weeks hypoxia-BH4 (10 mg/kg/day; Fig. 6B).
Figure 6.
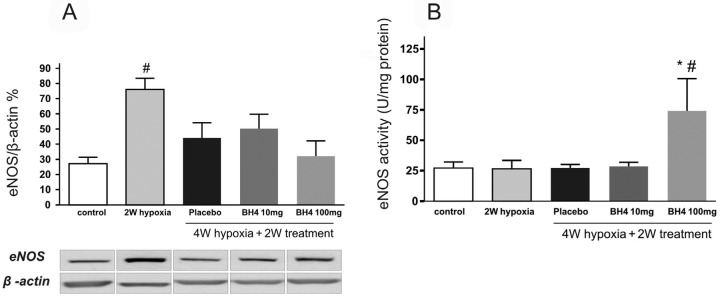
Illustration of the effects of tetrahydrobiopterin (BH4) treatment on levels of endothelial nitric oxide synthase (eNOS) protein expression (A; n = 6–8 per group) and eNOS activity (B; n = 4–8 per group) in lung homogenates in hypoxia recovery protocol. Pound sign: P < 0.05 compared with control; asterisk: P < 0.05 compared with 4 weeks (W) hypoxia placebo.
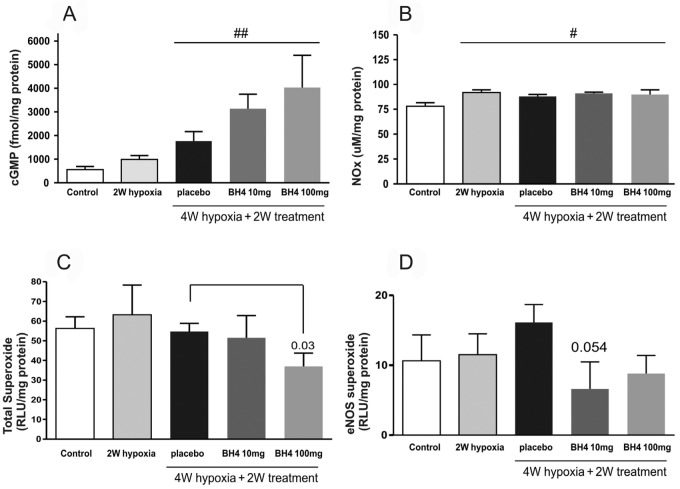
Effects of BH4 on cGMP, NOx, and superoxide levels
There was an increased cGMP level in lung homogenates with BH4 treatment (100 mg/kg/day) compared with placebo-treated group (Fig. 7; P < 0.01). NOx levels were increased in all hypoxic groups in comparison with control levels without significant differences in the BH4-treated group compared with placebo.
Figure 7.
Effects of tetrahydrobiopterin (BH4) recovery treatment on cyclic guanosine monophosphate (cGMP; n = 6–8 per group; A), nitrate/nitrite (NOx; n = 5–8 per group; B), superoxide (n = 6–8 per group; C), and endothelial nitric oxide synthase (eNOS)–related superoxide levels (n = 6–8; D) in lung homogenates in hypoxia recovery study. BH4 treatment did increase the levels of cGMP compared with the placebo-treated group but not to a statistically significant value. Nitric oxide levels were increased in all hypoxic groups in a similar manner. RLU: relative light units; pound sign: P < 0.05; two pound signs: P < 0.01 compared with control.
BH4 (100 mg/kg/day) significantly reduced the total superoxide levels in the lung homogenates. Furthermore, the fraction of eNOS-generated superoxide production was attenuated in the BH4-treated group compared with placebo (P = 0.054; Fig. 7).
Discussion
This study examined the effects of oral BH4 supplementation on hemodynamics, RVH, and pulmonary vascular remodeling in the chronic hypoxia-induced pulmonary hypertension rat model. We demonstrated that BH4 treatment (a) attenuated the increase in PAP and improved cardiac hemodynamics in this model and (b) reduced established pulmonary vascular muscularization and myocardial hypertrophy. Coincident with this, lung superoxide production was reduced, and cGMP levels were increased.
Pulmonary hypertension is a disease with a complex etiology. Dysfunctional endothelium and, in particular, eNOS activity that results in impaired NO and increased superoxide production is well documented and contributes to pulmonary vasoconstriction, excessive pulmonary vascular cell proliferation, inflammation, right ventricular dysfunction, and failure.2,4,21 Our previous observation that hph-1 mice exhibit a pulmonary hypertension phenotype under normoxia without overt systemic hypertension emphasized the essential role of BH4 in the regulation of the pulmonary circulation.12-14 BH4 therapy as a tool for manipulating NOS “uncoupling” has been explored extensively in a variety of cardiovascular diseases. BH4 supplementation can reverse endothelial pathophysiology by enhancing NO bioavailability and ameliorating oxidative stress.
Pulmonary hypertension has been a particular beneficiary of treatments developed for enhancing NO/cGMP signaling. These include currently approved clinical treatments, such as NO inhalation22,23 and PDE5 inhibitors,24,25 and more recently soluble gyuanylate cyclase stimulators. Our data from the isolated perfused lungs demonstrate a direct effect of exogenous BH4 administration on pulmonary NO production during hypoxic ventilation, leading to acute vasorelaxation during the plateau phase of hypoxia-induced pulmonary vasoconstriction. Exposing rats to chronic hypoxia induces an increase in PAP that peaks at around 2 weeks.26 Using radiotelemetry, we were able to examine the temporal relationship between BH4 administration and pulmonary pressure. A significant reduction in RVSP was observed (approximately 52%) after 1 week of treatment. Western blotting of lung homogenates revealed increased eNOS expression after 2 weeks of hypoxia exposure and a maintained baseline eNOS expression (similar to normoxia controls) after 4 weeks of hypoxia exposure. Preserved or increased lung eNOS expression after hypoxia exposure has also been reported by the other groups,27-29 which contrasts with the impairment of NO bioavailability in pulmonary hypertension. One possibility is a decrease in eNOS activity, as observed in isolated pulmonary arteries from rats exposed to hypoxia.30 Interestingly, although dosing the rats with BH4 after 2 weeks of hypoxia did not affect eNOS expression, it enhanced lung eNOS activity.
There is increasing evidence of oxidative stress in pulmonary hypertension,31,32 exemplified by nitrotyrosine formation (a marker of peroxynitrite production) in the lungs of patients with the disease.33 Vascular superoxide production has a number of important actions on vascular tissue, such as NO scavenging, peroxynitrite formation, and modulation of redox-sensitive signalling pathways,7,9,10 which may adversely affect vascular tone and structure. Furthermore, superoxide and peroxynitrite may oxidize BH4, and enhanced oxidative degradation is thought to be a major cause of reduced BH4 bioavailability and eNOS uncoupling in endothelial dysfunction states.34-36 Our data show that BH4 treatment significantly reduced total superoxide levels and, in particular, the fraction of eNOS-associated superoxide production in the lung. This fits well with our understanding of BH4-eNOS coupling. In addition, it is possible that exogenous BH4 acted as an antioxidant and scavenged reactive oxygen species such as superoxide.15
The effect of BH4 was dose dependent. BH4, administered at 100 mg/kg/day, effectively reduced pulmonary pressure, RVH, and pulmonary muscularization, whereas BH4 at 10 mg/kg/day did not have any significant effect. One possibility is that there was oxidation of active BH4 to inactive BH2 during hypoxia, thereby reducing exposure to the active molecule.37-39 This idea is consistent with previous findings that endothelial BH4 levels determine the susceptibility of mice to hypoxia-induced pulmonary hypertension; although BH4-deficient hph-1 mice are more susceptible, mice with GTPCH-1 overexpression for BH4 production are protected.12-14 In this study, we measured lung biopterin (BH4, BH2) levels, but the measurements were challenging, and there were no significant changes with BH4 treatment. Our understanding of how oral BH4 supplements reach the targeted tissue is still limited. Indeed, a recent clinical trial with oral BH4 treatment in coronary artery disease exhibited a concomitant increase in plasma BH2 levels, but BH4-eNOS coupling was tempered by BH4 oxidation, BH2 accumulation, and failure to increase BH4/BH2 ratio.40 This will complicate the future clinical application of BH4 as a treatment unless more stable BH4 analogs can be used.41
Nonetheless, the data from this study indicate that BH4 is effective in reversing pulmonary hypertension in a hypoxic rat model when given as a rescue therapy. Encouraging results also come from our recent clinical safety study of BH4 in patients with pulmonary arterial hypertension, where BH4 treatment at a dosage of 5 mg/kg/day was associated with a positive signal, an increase in 6-minute walk distance, and reduced plasma levels of monocyte chemoattractant protein 1.42 Collectively, these data suggest that BH4 supplementation has therapeutic potential for pulmonary hypertension.
Appendix. Supplemental material
Figure S1.
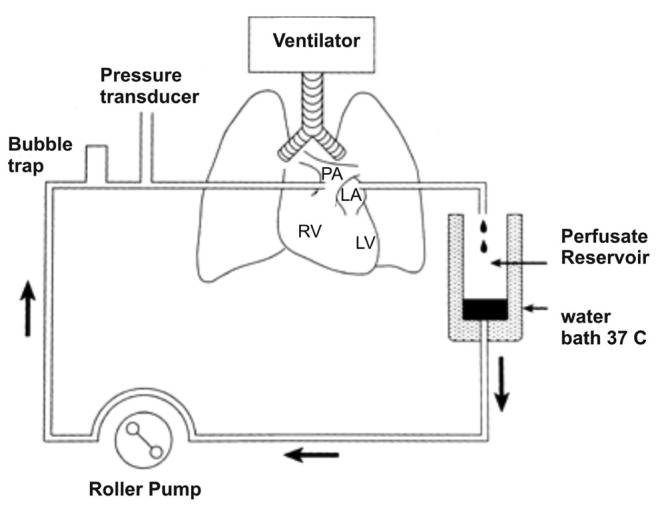
Schematic illustration of the basic components of perfused lung system used in acute pharmacological studies in rats. LA: left atrium; PA: pulmonary artery; RV: right ventricle; LV: left ventricle.
Figure S2.
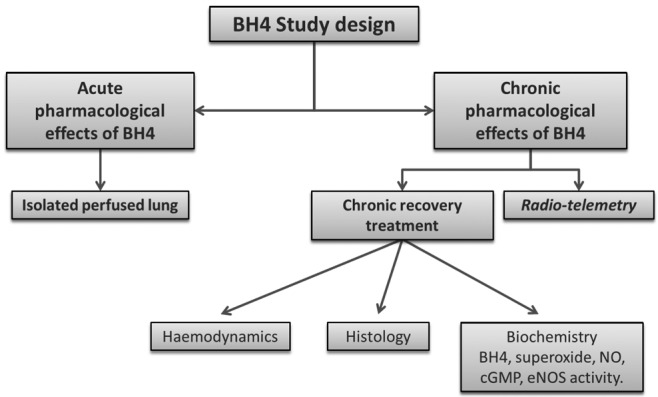
A general diagram showing the overall study design. BH4: tetrahydrobiopterin; cGMP: cyclic guanosine monophosphate; eNOS: endothelial nitric oxide synthase; NO: nitric oxide.
Table S1.
Measurements of lung tissue tetrahydrobiopterin (BH4), dihydrobiopterin (BH2), and total biopterin performed by high-performance liquid chromatography followed by serial electrochemical and fluorescent detection
| Variable | BH4 (pmol/mg protein) | BH4 : BH2 ratio | Total biopterin (pmol/mg protein) |
|---|---|---|---|
| Control (n = 10) | 18.3 ± 7.6 | 5.1 ± 2.4 | 22.2 ± 7.8 |
| Two weeks hypoxia (n = 5) | 28.5 ± 12.2 | 7.3 ± 2.9 | 33.2 ± 12.8 |
| Four weeks hypoxia placebo (n = 10) | 33.3 ± 17.3 | 4.8 ± 1.7 | 41.1 ± 21.8 |
| Four weeks hypoxia BH4 10 mg/kg (n = 5) | 35.7 ± 19.8 | 3.4 ± 1.6 | 45.9 ± 20.9 |
| Four weeks hypoxia BH4 100 mg/kg (n = 6) | 20.9 ± 17.5 | 3.1 ± 2.0 | 27.4 ± 16.8 |
Total biopterins were quantified by summing BH4, BH2, and biopterin. Biopterin levels were expressed as picomoles per gram tissue.
Source of Support: This research is supported by project grants from British Heart Foundation and BioMarin Pharmaceuticals.
Conflict of Interest: None declared.
Supplements
Appendix: Supplemental MaterialPulmCirc-004-462.s001.pdf (597.9KB, pdf)
References
- 1.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54(1 suppl):S10–S19. [DOI] [PubMed]
- 2.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8(8):443–455. [DOI] [PMC free article] [PubMed]
- 3.Tuder RM, Abman SH, Braun T, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 2009;54(1 suppl):S3–S9. [DOI] [PubMed]
- 4.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109(2):159–165. [DOI] [PubMed]
- 5.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 2004;24(3):413–420. [DOI] [PubMed]
- 6.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113(2):47–63. [DOI] [PubMed]
- 7.Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 2008;5(6):338–349. [DOI] [PubMed]
- 8.Tsikas D, Bohmer A, Flentje M. Tetrahydrobiopterin and endothelial nitric oxide synthase uncoupling. Hypertension 2012;59(2):e12. [DOI] [PubMed]
- 9.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25(1):29–38. [DOI] [PubMed]
- 10.Jeremy JY, Yim AP, Wan S, Angelini GD. Oxidative stress, nitric oxide, and vascular disease. J Card Surg 2002;17(4):324–327. [DOI] [PubMed]
- 11.Takimoto E, Champion HC, Li M, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 2005;115(5):1221–1231. [DOI] [PMC free article] [PubMed]
- 12.Khoo JP, Zhao L, Alp NJ, et al. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation 2005;111(16):2126–2133. [DOI] [PubMed]
- 13.Nandi M, Miller A, Stidwill R, et al. Pulmonary hypertension in a GTP-cyclohydrolase 1-deficient mouse. Circulation 2005;111(16):2086–2090. [DOI] [PubMed]
- 14.Nandi M, Leiper J, Arrigoni F, Hislop A, Vallance P, Haworth S. Developmental regulation of GTP-CH1 in the porcine lung and its relationship to pulmonary vascular relaxation. Pediatr Res 2006;59(6):767–772. [DOI] [PubMed]
- 15.Francis BN, Wilkins MR, Zhao L. Tetrahydrobiopterin (BH4) and the regulation of hypoxic pulmonary vasoconstriction. Eur Respir J 2010;36:323–330. [DOI] [PubMed]
- 16.Dias-Junior CA, Gladwin MT, Tanus-Santos JE. Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Radic Biol Med 2006;41(12):1764–1770. [DOI] [PubMed]
- 17.Zhao L, Sebkhi A, Ali O, et al. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J 2009;34(4):948–957. [DOI] [PubMed]
- 18.Cosentino F, Hurlimann D, Delli GC, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 2008;94(4):487–492. [DOI] [PubMed]
- 19.Giraldez RR, Zweier JL. An improved assay for measurement of nitric oxide synthase activity in biological tissues. Anal Biochem 1998;261(1):29–35. [DOI] [PubMed]
- 20.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007;49(4):717–727. [DOI] [PMC free article] [PubMed]
- 21.Wilkins MR. Pulmonary hypertension: the science behind the disease spectrum. Eur Respir Rev 2012;21(123):19–26. [DOI] [PMC free article] [PubMed]
- 22.Turanlahti M, Pesonen E, Pohjavuori M, Lassus P, Fyhrquist F, Andersson S. Plasma cyclic guanosine monophosphate reflecting the severity of persistent pulmonary hypertension of the newborn. Biol Neonate 2001;80(2):107–112. [DOI] [PubMed]
- 23.Beghetti M, Habre W, Friedli B, Berner M. Continuous low dose inhaled nitric oxide for treatment of severe pulmonary hypertension after cardiac surgery in paediatric patients. Br Heart J 1995;73(1):65–68. [DOI] [PMC free article] [PubMed]
- 24.Tessler R, Wu S, Fiori R, Macgowan CK, Belik J. Sildenafil acutely reverses the hypoxic pulmonary vasoconstriction response of the newborn pig. Pediatr Res 2008;64(3):251–255. [DOI] [PubMed]
- 25.Zhao L, Mason NA, Morrell NW, et al. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 2001;104(4):424–428. [DOI] [PubMed]
- 26.Zhao L. Chronic hypoxia-induced pulmonary hypertension in rat: the best animal model for studying pulmonary vasoconstriction and vascular medial hypertrophy. Drug Discov Today Dis Models 2010;7:83–88.
- 27.Le Cras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol Lung Cell Mol Physiol 1996;270(1 Pt 1):L164–L170. [DOI] [PubMed]
- 28.Resta TC, Gonzales RJ, Dail WG, Sanders TC, Walker BR. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am J Physiol 1997;272(2 Pt 2):H806–H813. [DOI] [PubMed]
- 29.Tyler RC, Muramatsu M, Abman SH, et al. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 1999;276(2 Pt 1):L297–L303. [DOI] [PubMed]
- 30.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 2002;277(46):44085–44092. [DOI] [PubMed]
- 31.Cracowski JL, Cracowski C, Bessard G, et al. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med 2001;164(6):1038–1042. [DOI] [PubMed]
- 32.Robbins IM, Morrow JD, Christman BW. Oxidant stress but not thromboxane decreases with epoprostenol therapy. Free Radic Biol Med 2005;38(5):568–574. [DOI] [PubMed]
- 33.Bowers R, Cool C, Murphy RC, et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 2004;169(6):764–769. [DOI] [PubMed]
- 34.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278(25):22546–22554. [DOI] [PubMed]
- 35.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111(8):1201–1209. [DOI] [PMC free article] [PubMed]
- 36.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circ 2001;103(9):1282–1288. [DOI] [PubMed]
- 37.Hoshikawa Y, Ono S, Suzuki S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol 2001;90(4):1299–1306. [DOI] [PubMed]
- 38.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 2006;290(1):L2–L10. [DOI] [PubMed]
- 39.Wu W, Platoshyn O, Firth AL, Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007;293(4):L952–L959. [DOI] [PubMed]
- 40.Cunnington C, Van AT, Shirodaria C, et al. Systemic and vascular oxidation limits efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 2012;125:1356–1366. [DOI] [PMC free article] [PubMed]
- 41.Kunuthur SP, Milliken PH, Gibson CL, Suckling CJ, Wadsworth RM. Tetrahydrobiopterin analogues with NO-dependent pulmonary vasodilator properties. Eur J Pharmacol 2011;650(1):371–377. [DOI] [PubMed]
- 42.Robbins IM, Hemnes AR, Simon GJ, et al. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res 2011;37(1):26–34. [DOI] [PubMed]