Abstract
The “gold standard” for epidemiological typing of Streptococcus pneumoniae (pneumococcus) is the capsular reaction test (Neufeld test) with antisera against the 90 pneumococcal polysaccharide capsules, i.e., serotyping. The method is labor intensive and requires a certain level of experience to be performed satisfactory, and thus it has been restricted for use in specialized reference or research laboratories. Surveillance of the serotype distribution of pneumococci that cause infections is important to secure an optimal composition of pneumococcal vaccines and to monitor antibiotic resistance in pneumococci. At Statens Serum Institut, a simple latex agglutination test for serotyping of pneumococci has been developed. The Pneumotest-Latex kit consists of 14 different pooled pneumococcus antisera (pools A to I and pools P to T) applied to latex particles. In a blind test of 352 isolates (with all 90 serotypes represented), 336 (95.5%) were typed or grouped correctly by the Pneumotest-Latex; in addition, 2 (7%) of 30 strains regarded as nontypeable or rough strains were serotyped, and the serotypes of these two isolates were confirmed by the capsular reaction test with type-specific antisera. The Pneumotest-Latex seems to be a sensitive method for serotyping or grouping of the majority of pneumococcal strains. By use of this ready-to-perform latex agglutination kit (Pneumotest-Latex), serotyping of pneumococci can gain more ground as a tool in prevention of pneumococcal diseases.
The bacterium Streptococcus pneumoniae (pneumococcus) is a major cause of invasive diseases and upper respiratory tract infections (1). Prevention strategies against pneumococcal infections have been based on the immunogenicity of the polysaccharide capsule. At present, 90 different capsular types have been described, and type-specific polysaccharide antibodies for typing have been raised in rabbits (5). The “gold standard” method to perform epidemiological serotyping of pneumococci is the capsular reaction test, also known as the Quellung reaction or the Neufeld test, as it was first described by Neufeld in 1902. This method, however, is labor intensive and requires a certain level of experience to be performed correctly, and therefore it has hardly been used outside specialized reference or research laboratories (1, 7). In recent years, the introduction of new pneumococcal conjugate vaccines has raised the need for a simpler and easier method for serotyping of pneumococcal strains (1). Likewise, the emergence of antibiotic resistance and the spread of resistant strains have brought focus on epidemiological typing of pneumococci.
Several alternative methods to the capsular reaction test, such as coagglutination, counterimmunoelectrophoresis, enzyme-linked immunosorbent assay, Ouchterlony test, and DNA techniques, have been described for serotyping of pneumococci. However, the sensitivity, specificity, and the labor needed have shown that these techniques are not an alternative to the Neufeld method (1, 2, 3, 7, 18).
Latex agglutination tests are widely used methods for identification and typing of many clinical important microorganisms (6, 13). Earlier latex methods have been used for serotyping of pneumococci, using different combinations of pneumococcal antisera for research purposes (1, 7, 13, 16). Based on the established chessboard system (Pneumotest) for serotyping of pneumococci, we have developed a new latex agglutination test for identification of pneumococcal groups or types that is suitable for routine diagnostic use. The aim of the present study is to present and evaluate the new prototype latex kit (Pneumotest-Latex) against the standard procedure, the Neufeld capsular reaction test.
MATERIALS AND METHODS
Preparation of latex reagents.
Each of the 14 described pneumococcal polyclonal pool antisera (pools A to I and P to T) was purified by use of the caprylic acid purification method (10). Briefly, untreated pooled sera and acetate buffers were mixed. Caprylic acid was added, and the solution was mixed for 30 min, followed by centrifugation for another 30 min. Phosphate-buffered saline-EDTA was added to the supernatant, and the pH was adjusted to 7.4, followed by cooling to 2 to 8°C. Ammonium sulfate was added to the solution under constant stirring for 30 min at 2 to 8°C, and thereafter the solution was centrifuged for 30 min at 5,000 rpm (Heraeus Sepatech Contifuge 17RS instrument). The supernatant was removed, and the pellet was diluted in a small amount of PBS and dialyzed overnight at 2 to 8°C in 5 liters of PBS. The next day the solution was filtered through a 0.2-μm-pore-size filter (Minisart; Sartorius), and then the optical density was measured.
For each of the 14 pooled sera, a latex suspension in glycine buffer was prepared by coating with one pool of purified and absorbed pneumococcal polyclonal antiserum raised in rabbits as described by Slotved et al. (14). The concentration of the pooled antiserum in each latex reagent was determined in latex tests by use of different concentrations of pooled serum in the latex suspension (10 to 50 μl per ml) and by testing against their respective positive controls of pneumococcal reference strains (Statens Serum Institut [SSI]). Each of the 14 latex reagents was tested in a latex test against strains representing all 90 serotypes to check for cross-reactions. The cross-reactions that appeared were absorbed from the antiserum with various amounts of vaccines. The absorption was performed by adding vaccine of the cross-reactive serotype to the pooled antiserum, followed by incubation overnight at 2 to 8°C, centrifugation for 10 min at 4,000 rpm, and filtration through a 0.2-μm-pore-size filter (Minisart; Sartorius) (9). After absorption, all latex reagents were examined to verify that the cross-reactions were removed without affecting the test results obtained by examination with the remaining serotypes.
Latex agglutination test.
The latex agglutination was performed by mixing an aliquot of 10 μl of a pneumococcal broth culture (Todd-Hewitt broth [TH broth]; SSI Diagnostica, Hillerøod, Denmark) with 10 μl of latex reagent on a microscope slide, and thereafter the slide was manually rocked for 5 to 8 s. A positive reaction (the mixture became fluffy, i.e., an agglutination) could be read with the naked eye within 5 to 10 s if the latex reagent contained antiserum homologous to the capsule of the pneumococcal culture. Agglutinations observed after more than 30 s were nonspecific. Therefore, late agglutinations must be regarded as false-positive reactions. Interpretation of the serotype or serogroup was done from reactions with the 14 latex reagents by use of the chessboard system (Table 1).
TABLE 1.
Distribution in the chessboard system of the 352 pneumococcal strains according to their serotypes and number tested with the Pneumotest-Latex
| Pool | Serotype(s) or group(s)a (n) with pool:
|
Total no. of isolates | |||||
|---|---|---|---|---|---|---|---|
| P | Q | R | S | T | Nonvaccine groups or types | ||
| A | 1 (8) | 18F (1), 18A (2), 18B (1), 18C (7) | 4 (8) | 5 (7) | 2 (5) | 39 | |
| B | 19F (8), 19A (8), 19B (4), 19C (1) | 6A (5), 6B (7) | 3 (7) | 8 (7) | 47 | ||
| C | 7F (8), 7A (2), 7B (2), 7C (5) | 20 (5) | 24F (5), 24A (3), 24B (1), 31 (5), 40 (2) | 38 | |||
| D | 9A (1), 9L (5), 9N (7), 9V (8) | 11F (1), 11A (8), 11B (1), 11C (1), 11D (1) | 16F (5), 16A (1), 36 (2), 37 (5) | 46 | |||
| E | 12F (8), 12A (4), 12B (1) | 10F (4), 10A (8), 10B (2), 10C (1) | 33F (8), 33A (1), 33B (5), 33C (3), 33D (1) | 21 (5), 39 (5) | 56 | ||
| F | 17F (6), 17A (2) | 22F (7), 22A (6) | 27 (2), 32F (1), 32A (1), 41F (3), 41A (2) | 30 | |||
| G | 29 (4) | 34 (5) | 35F (5), 35A (1), 35B (5), 35C (2) | 42 (2) | 47F (1), 47A (1) | 26 | |
| H | 14 (7) | 23F (7), 23A (5), 23B (5) | 15F (1), 15A (5), 15B (7), 15C (5) | 13 (6), 28F (4), 28A (3) | 55 | ||
| I | 25F (1), 25A (1) | 38 (5) | 43 (1) | 44 (1) | 45 (1) | 46 (2), 48 (3) | 15 |
| Total no. of isolates | 59 | 50 | 63 | 58 | 56 | 66 | 352 |
Boldface indicates that the group or type is included in the 23-valent pneumococcal vaccine.
Capsular reaction test.
Capsular reaction tests were performed as described by the manufacturer by use of type-specific pneumococcal rabbit antisera (SSI) (15).
Strains.
All strains were obtained from the SSI Streptococcus strain collection, under the World Health Organization Collaborating Centre for Reference and Research on Pneumococci, the Streptococcus Unit, SSI, Copenhagen, Denmark. Pneumococcal reference strains representing all 90 serotypes (5) were used during the development of the Pneumotest-Latex.
For evaluation of the performance of the kit, 352 pneumococcal isolates were used (Table 1). They consisted of the 47 reference strains previously used, 238 clinical isolates kept in storage, and 67 strains from an external quality exchange study of serotyping performed by a number of reference laboratories in the European Union (H. B. Konradsen et al., personal communication). During the evaluation process, all testing was performed under blinded conditions and by technicians who were not extensively trained in pneumococcal typing. The isolates were chosen to cover all 90 serotypes, with an average of five isolates of each type. However, for the very rare serotypes, it was possible to test only one or two isolates (Table 1), and therefore we included some reference strains.
Moreover, 30 noncapsulated (rough) pneumococci were selected from the collection for examination. Noncapsulated pneumococci were identified by opthochin sensitivity and bile solubility tests (4). Likewise, five isolates of Streptococcus mitis and five isolates of Streptococcus oralis were tested against the 14 latex reagents for cross-reaction.
All strains were stored either lyophilized or in nutrient beef broth with 10% glycerin (SSI Diagnostica) at −80°C, and 10% horse blood agar plates (SSI Diagnostica) were used for subculture. All cultures were incubated overnight at 33 to 37°C. The plate cultures were kept at 5°C for a week before subculturing.
The latex agglutination test was performed from a pneumococcal culture in TH broth after overnight incubation. The next day the TH broth culture was inspected for diffuse growth before testing with the Pneumotest-Latex and thereafter was discarded. Streptococci were also cultured in TH broth.
Serum broth (SSI Diagnostica) and Mueller-Hinton broth (MH broth) (SSI Diagnostica) were tested for their capacity to present antigen when used to cultivate pneumococci. Both media were compared with the TH broth by use of our 90 pneumococcal reference strains.
Tests for stability of latex reagents.
The stability of the latex reagents was tested after storage at 2 to 8°C for 1, 3, 6, and 9 months by performance of the latex test against positive controls. Likewise, stability during normal use was tested twice a week for 4 weeks, keeping the latex reagents at 2 to 8°C during the night and at room temperature for 8 h a day.
RESULTS
Table 1 shows the distribution in the chessboard system of the 352 pneumococcal strains examined according to their serotypes and number tested with the Pneumotest-Latex.
Initially during evaluation, cross-reactions and slow reactions were observed when typing clinical strains with particular latex reagents (pools E, S, and T) (Table 2). This was done while the kit was being developed, and the latex reagents had been tested with the 90 reference strains only. During improvement of the kit, we were able to remove the cross-reactions by absorption of the pooled antiserum with specific vaccines (clinical strains) and also by adjusting the sensitivity of the latex reagent pools by increasing the antiserum concentrations.
TABLE 2.
Unexpected reactions observed in 17 strains during examination of the 352 isolates with the 14 latex reagents
| Serotype confirmed by Neufeld test (no. of strains) | Expected reaction (pool 1/pool2) | Unexpected reaction |
|---|---|---|
| 20 (1) | C/T | Slow reaction with pool T |
| 12A (2) | E/R | Cross-reaction with pool S |
| 23B (1) | H/Q | Cross-reaction with pool E |
| 31 (3) | C | Cross-reaction with pool T |
| 34 (4) | G/Q | Cross-reaction with pool E |
| 35F (1) | G/R | Cross-reaction with pool E |
| 41F (1) | F | Cross-reaction with pool S |
| 46 (1) | I | Cross-reaction with pool S |
| 47F (1) | G/T | Cross-reaction with pool E |
| 48 (2) | I | Cross-reaction with pool S |
All of the latex reagent pools reacted with a strain carrying the homologous capsule, showing an agglutination reaction within 10 s, except for one occasion where a strain showed a slower agglutination reaction (within 20 s) with latex reagent pool T (Table 2). However, by changing the concentration of pool T antiserum, it was possible to improve the reaction time.
Tested under blinded conditions with the Pneumotest-Latex kit, 336 (95.5%) of the 352 strains were given the correct serotype or serogroup identification compared to the results obtained with the capsular reaction test. Included in the 352 tested isolates were 67 isolates used in a European Union project, which were also typed correctly. For 16 strains (4.5%) (covering nine serotypes), a reaction with an incorrect latex reagent (pools E, S, and T) was observed, giving an inconclusive result (Table 2). All of these 16 strains had a nonvaccine serotype, and therefore nine of the strains gave a positive reaction with latex reagent pool G or I. Therefore, any typing situation giving a positive reaction with latex reagent pool G or I should be interpreted with caution.
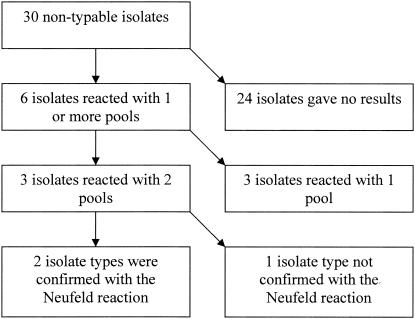
Six isolates (20%) of our 30 strains that were regarded as nontypeable or noncapsulated reacted with one or more latex reagent (Fig. 1). Three isolates reacted with two latex reagent pools each, indicating a serotype, and we were thereafter able to confirm the serotypes of two strains (serotypes 14 and 15C) by the capsular reaction test. The four other nontypeable strains which reacted with the latex pools will be further characterized to clarify the cause of the positive reactions.
FIG. 1.
Thirty isolates regarded as nontypeable or noncapsulated pneumococci (based on the Neufeld reaction) and tested with the Pneumotest-Latex kit.
We found three cross-reactions with three different S. oralis isolates and one cross-reaction with an S. mitis strain. Three of the cross-reactions were observed with latex reagent pool E, two against S. oralis and one against S. mitis. One cross-reaction was observed with latex reagent pool D against S. mitis.
Serum broth, used in our laboratory as the standard broth medium for cultivation of pneumococci, was shown to be the most efficient broth, followed by TH broth. In TH broth there was generally a good growth; however, a few serotypes, such as 12A and 12B, did sometimes show negative growth. MH broth was the least efficient medium for cultivation of pneumococci; most of the tubes showed weak growth (data not shown). When performing the latex agglutination test, there were no differences in agglutination patterns between the three media.
The stability of the latex reagents when kept at 2 to 8°C was excellent, showing no changes in the agglutination test against the positive controls after 1, 3, 6, and 9 months (data not shown). There was no sign of decreased reactivity with the positive control during agglutination when the latex reagents were kept at 2 to 8°C at night and at room temperature for 8 h a day for 4 weeks (data not shown).
DISCUSSION
To be able to detect changes in the serotype distribution of disease-causing pneumococci during introduction and maintenance of pneumococcal disease prevention programs, it is necessary to have an easy and rapid method to serotype pneumococci. The standard Neufeld capsular reaction test is not suitable for large-scale typing because it is labor-intensive and time-consuming, and requires a certain level of experience to be performed satisfactorily (1, 7). Compared with other latex agglutination methods (1, 3, 13, 18), the latex method presented here is simpler and faster. In previously described latex methods for serotyping of pneumococci, the time to get a positive agglutination was up to 1 min or even longer (1, 3, 18), or the typing procedure needed to be carried out under certain laboratory conditions such as in a moist chamber (13). Under these conditions, the latex method was not suitable for high-throughput serotyping of pneumococci.
Because the Pneumotest-Latex is based on the established Pneumotest, technicians found the new method to be a simple alternative to the usual capsular reaction test. Even technicians who are not familiar with serotyping of pneumococci quickly were adapted to the method and performed at a high level. The speed of the observed agglutination reaction (positive reaction within 10 s) was experienced as a great advantage. The same is true for the ease of interpreting a reaction as positive compared to that in the normal capsular reaction test, in which one looks for capsular swelling or agglutination in the microscope. This is the main explanation for the reduction of time spent during serotyping, which makes the Pneumotest-Latex suitable for large-scale studies (1, 7).
The Pneumotest-Latex kit was tested against all 90 serotypes and was found to have sensitivity and specificity comparable to those of the Neufeld capsular reaction test, which was also observed by Arai et al. (1).
The selection of TH broth as a medium for culturing the strains for typing by the Pneumotest-Latex was based on the observation made by Lafong and Crothers (7). They found that TH broth gave a high concentration of free antigen. It is, however, not the best medium to use for culturing pneumococcal strains, and we observed that strains with serotypes 12A and 12B were particularly difficult to grow in TH broth. As an alternative to TH broth, we tested both serum broth and MH broth. We found that serum broth was a more suitable broth than the two other broths because it showed very good growth of all 90 reference strains. However, serum broth is a broth used mainly at SSI, and it is not well known outside Denmark. Among the three broth media, MH broth was the least able to support the growth of pneumococci. However, if there was sufficient growth, then there were no significant differences in the typing results obtained with the three media (data not shown). Because TH broth is a broth medium used around the world, this broth was chosen as a standard broth for the Pneumotest-Latex (7, 11, 12).
The use of latex agglutination for serotyping of pneumococcal colonies directly from blood agar plates was tested; however, this was not possible due to many nonspecific agglutination reaction (data not shown).
The results show that the reagents are stable during storage for many months and during normal daily work in the laboratory. The test for stability during storage will continue for 3 years.
Like previous studies that used latex-based serotyping of pneumococci, the Pneumotest-Latex aims at typing pneumococci included in the 23-valent polysaccharide vaccine and the 7- to 11-valent pneumococcal conjugate vaccines (1, 7). Compared to the Pneumotest, the Pneumotest-Latex is extended to include latex reagent pools G and I, which makes initial grouping or typing of some nonvaccine types possible. However, our preliminary results showed that when a serotype or group was identified by latex reagent pool G or I, there was a possibility of unexpected cross-reactions (Table 2), which underlines the need for extra caution when interpreting the serotypes of isolates reacting in latex reagent pool G or I. The cross-reactions and slow reactions were observed primarily with pools E, S, and T (Table 2). We were able to remove the cross-reactions by further absorptions and by adjusting the sensitivity by increasing the serum concentration. The explanation for these cross-reactions was the fact that the adjustment of the sensitivity and specificity was done only with the 90 reference strains without including multiple clinical strains.
The great majority of pneumococci causing serious diseases can be serotyped or grouped by this new Pneumotest-Latex method. Further characterization of isolates within groups must be done by the conventional capsular reaction test for the time being. We recommend control of the type of isolate identified by the Pneumotest-Latex by the traditional capsular reaction test. The reason for this is that with the Pneumotest-Latex, we were able to serotype two isolates which we were not able to type before with the conventional capsular reaction test and which had been regarded as rough pneumococci or nontypeable (Fig. 1). The serotypes of these two isolates were verified by the capsular reaction test as true serotypes. We therefore suspect that serotyping by use of the pooled sera is more sensitive with the latex agglutination test than with the capsular reaction (Neufeld) test. However, the situation became more complex as we found four other strains regarded as being rough pneumococci reacting in different latex reagent pools without being able to detect a capsular reaction in the Neufeld test. Furthermore, S. mitis and S. oralis reacted in some of the latex reagent pools. Because of the close relationship between viridans streptococci of the mitis group and pneumococci, this was not unexpected, and cross-reactions between particular pneumococcal types and other species, such as Escherichia coli, Klebsiella spp., etc., are well known (9, 17). During introduction of new typing methods, such phenomena have to be carefully evaluated. It must therefore here be emphasized that, for the moment, the Pneumotest-Latex is recommended for serotyping of isolates identified as pneumococci that phenotypically present polysaccharide capsules by growing diffusely in broth medium. It is therefore recommended only as a method for typing of strains that have been confirmed as capsulated pneumococci by growing diffusely in the broth and as optochin sensitive and bile soluble (4, 8). At present, we also recommend accepting as true serotyping results only those which can be verified by the capsular reaction test with type-specific antisera.
Even with these temporary restrictions on the use of the Pneumotest-Latex, the kit can be a great help in a laboratory conducting serotyping of pneumococci because of its speed and ease of performance. The Pneumotest-Latex will be further developed, and it is our hope that in the future serotyping of pneumococci will be attractive for use also outside reference laboratories, as a tool in both diagnosis and prevention of pneumococcal diseases.
The major advantage of our Pneumotest-Latex is the simple procedure and fast reaction compared to those seen in other studies on the development of a pneumococcal latex tests (1, 7, 13, 16). The latex method in this study has been tested against all 90 known pneumococcal strains, which showed that the method can be used for a general serotyping or grouping of pneumococcal isolates and not only for the serotypes included in the pneumococcal vaccines (1, 7).
Finally, people with little knowledge of typing and microscopy can perform this test. Due to these results, we will make use of the latex assay in our laboratory and use the Neufeld method only to confirm the serotype.
REFERENCES
- 1.Arai, S., T. Konda, A. Wada, Y. Matsunaga, N. Okabe, H. Watanabe, and S. Inouye. 2001. Use of antiserum-coated latex particles for serotyping Streptococcus pneumoniae. Microbiol. Immunol. 45:159-162. [DOI] [PubMed] [Google Scholar]
- 2.Brito, D. A., M. Ramirez, and H. de Lencastre. 2003. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 41:2378-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandler, L. J., B. S. Reisner, G. L. Woods, and A. K. Jafri. 2000. Comparison of four methods for identifying Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 37:285-287. [DOI] [PubMed] [Google Scholar]
- 4.Facklam, R. 2002. What happened to the streptococci: overview of taxonomy and nomenclature. Clin. Microbiol. Rev. 15:613-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, Y. H., H. C. Chang, and T. C. Chang. 2001. Development of a latex agglutination test for rapid identification of Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 20:97-103. [DOI] [PubMed] [Google Scholar]
- 7.Lafong, A. C., and E. Crothers. 1988. Simple latex agglutination method for typing pneumococci. J. Clin. Pathol. 41:230-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund, E. 1959. Diagnosis of pneumococci by the optochin and bile tests. Acta Pathol. Microbiol. Scand. 47:308-315. [DOI] [PubMed] [Google Scholar]
- 9.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods in microbiology, vol. 12. Academic Press, London, United Kingdom.
- 10.McKinney, M. M., and A. Parkinson. 1987. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J. Immunol. Methods 96:271-278. [DOI] [PubMed] [Google Scholar]
- 11.Park, M. K., D. E. Briles, and M. H. Nahm. 2000. A latex bead-based flow cytometric immunoassay capable of simultaneous typing of multiple pneumococcal serotypes (multibead assay). Clin. Diagn. Lab. Immunol. 7:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell, H., R. R. Facklam, J. F. Padula, and R. Cooksey. 1978. Capillary precipitin typing of Streptococcus pneumoniae. J. Clin. Microbiol. 8:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal, A., M. K. Lalitha, T. J. John, K. Thomas, P. Raghupathy, S. Jacob, and M. C. Steinhoff. 1996. Modified latex agglutination test for rapid detection of Streptococcus pneumoniae and Haemophilus influenzae in cerebrospinal fluid and direct serotyping of Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 15:472-477. [DOI] [PubMed] [Google Scholar]
- 14.Slotved, H. C., J. Elliott, T. Thompson, and H. B. Konradsen. 2003. Latex assay for serotyping of Group B streptococcus isolates. J. Clin. Microbiol. 41:4445-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen, U. B. 1993. Typing of pneumococci by using 12 pooled antisera. J. Clin. Microbiol. 31:2097-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuertz, K., I. Merx, H. Eiffert, E. Schmutzhard, M. Mäder, and R. Nau. 1998. Enzyme immunoassay detecting teichoic and lipoteichoic acids versus cerebrospinal fluid culture and latex agglutination for diagnosis of Streptococcus pneumoniae meningitis. J. Clin. Microbiol. 36:2346-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasallo, F. J., I. López-Miragaya, A. Rodríguez, and J. Torres. 2001. Rapid identification of Streptococcus pneumoniae in blood cultures using a latex agglutination assay. Eur. J. Clin. Microbiol. Infect. Dis. 20:594-595. [DOI] [PubMed] [Google Scholar]
- 18.Wellstood, S. 1992. Evaluation of a latex test for rapid detection of pneumococcal antigens in sputum. J. Clin. Microbiol. Infect. Dis. 11:449-451. [DOI] [PubMed] [Google Scholar]