Abstract Abstract
Pulmonary arterial hypertension (PAH) is a fatal disease, and the ultimate cause of death is right ventricular (RV) failure. In this study, we investigated the acute hemodynamic effects of levosimendan in two rat models of RV hypertrophy and failure. Wistar rats were randomized to receive sham surgery (n = 8), pulmonary trunk banding (PTB; n = 8), or monocrotaline injection (MCT; n = 7). RV function was evaluated at baseline and after injection of placebo and two concentrations of levosimendan (12 and 60 μg/kg) using magnetic resonance imaging, echocardiography, and invasive pressure recordings. PTB and MCT injection caused hypertrophy, dilatation, and failure of the RV compared with sham surgery. Levosimendan increased RV end systolic pressure (sham surgery: 16.0% ± 3.8% [P = 0.0038]; MCT: 9.9% ± 3.1% [P = 0.018]; PTB: 24.5% ± 3.3% [P = 0.0001]; mean ± SEM) compared with placebo. Levosimendan markedly increased RV stroke volume (SV) in the MCT group (29.1% ± 8.3%; P = 0.012), did not change RV SV in the PTB group (0.4% ± 4.5%; P = 0.93), and decreased RV SV in the sham surgery group (−10.9% ± 3.7%; P = 0.020). Nitroprusside, which was used to mimic the systemic arterial vasodilator action of levosimendan, did not influence RV function. These data demonstrate that levosimendan acutely improves the failing right heart in a MCT model of PAH and that the mechanism involves a direct acute positive inotropic effect on the hypertrophic and failing RV of the rat.
Keywords: right ventricular function, heart failure, pulmonary hypertension, levosimendan
Introduction
Pulmonary arterial hypertension (PAH) is characterized by constriction or partial obstruction of the pulmonary vasculature, causing increased afterload and eventually right ventricular (RV) failure and death. Despite modern medical management, 3-year survival is only slightly better than 50%.1
RV function is the most decisive factor of longevity in patients with PAH.2 Under normal conditions, the RV works in a low-pressure system and adapts easily to minor changes in afterload. But with increased afterload in PAH, the RV undergoes hypertrophy and dilatation. This commences a vicious cycle with lowered cardiac output (CO), insufficiency of the tricuspid valve, and myocardial hypoperfusion as the disease progresses.3-5
Levosimendan is a calcium-sensitizing agent that increases contractility in cardiomyocytes without increasing intracellular calcium concentrations and oxygen demand.6,7 This makes levosimendan an optimal treatment strategy in a failing and hypoperfused ventricle. Levosimendan also dilates systemic and pulmonary blood vessels, possibly by opening of adenosine triphosphate–dependent potassium channels.8,9 Levosimendan is used in patients with acute left ventricular failure. But the hemodynamic profile of the drug might also be beneficial in the acute settings of the failing RV. In a few previous animal studies, levosimendan acutely improved the function of a failing RV directly or through afterload reduction.10-14 Data on levosimendan in patients with PAH are very sparse and conflicting.15-18
The aim of this study was to investigate the effects of levosimendan in a rat pulmonary trunk banding (PTB) model of RV hypertrophy and failure and in a monocrotaline (MCT) rat model of PAH. Use of the rat PTB model allows estimation of the direct RV myocardial effects of levosimendan independently of the pulmonary vasodilator action of this drug. In addition, the effects of nitroprusside were investigated in both models of RV failure to mimic the systemic arterial vasodilator effect of levosimendan.
Methods
Study design
Male Wistar rats were randomized to receive PTB (n = 8), sham surgery (n = 8), or MCT injection (n = 7). Evaluation was performed 28 days after PTB/sham surgery and 21 days after MCT injection when RV failure was present. A repeated-measurement design was performed for each rat regardless of randomization group: baseline measurement was followed by measurements after placebo (isotonic saline) injection and after two doses of levosimendan: Levo-12 (loading dose of 12 μg/kg followed by infusion of 0.1 μg/kg/min in 10 minutes)19 and Levo-60 (loading dose of 60 μg/kg followed by infusion of 0.5 μg/kg/min in 10 minutes; Fig. 1). This was repeated on two consecutive days in all animals, where magnetic resonance imaging (MRI) was performed on day 1 and echocardiography and invasive pressures were performed on day 2 (Fig. 1).
Figure 1.
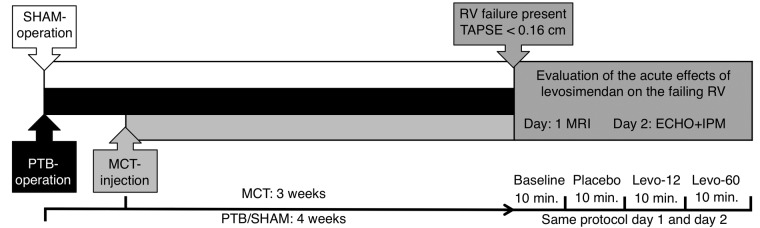
Study design. Rats were evaluated when right ventricular (RV) failure was present 28 days after pulmonary trunk banding (PTB)/sham surgery and 21 days after monocrotaline (MCT) injection. The evaluation protocol for each rat was magnetic resonance imaging (MRI) on day 1 and echocardiography (ECHO) and invasive pressure measurement (IPM) on day 2. TAPSE: tricuspid annular plane systolic excursion.
Drug administration
Placebo and levosimendan (Simdax; Orion, Espoo, Finland) were given at the smallest possible and equal volumes through a Venflon intravenous catheter dipped in heparin to avoid thrombosis. Levosimendan was diluted in saline prior to the experiments. The effects of levosimendan peak after about 10 minutes,6 and we aimed to evaluate the immediate acute effects. Doses were chosen as a clinically relevant dose (Levo-12) and a dose that was five times higher (Levo-60).6,19,20
Animals and ethics
Male Wistar rats (151 ± 3 g), obtained from M&B Taconic (Eiby, Denmark), were allowed access to water and food (Altromin 1324; Altromin, Lage, Germany) ad libitum. They were kept at a room temperature of 23°C, with a 12-h light-dark cycle. The study followed Danish law for animal research (approved under authorization nos. 2006-561-1180 and 2010-561-1829, Danish Ministry of Justice) and was conducted in accordance with guidelines from the National Institutes of Health.
PTB and sham surgery
PTB was performed as described previously.21 In brief, rats were placed on a heated pad and anesthetized with sevoflurane (Abbot Scandinavia, Solona, Sweden) in a 7% induction dose in a mixture with 67% O2 and 33% N2O. They were intubated and ventilated with 3.5% sevoflurane at 75 breaths per minute and a tidal volume of 10 mL/kg. Through a left thoracotomy, the pulmonary trunk and the aorta were carefully separated. With a modified Horizon applier (Horizon Applier, small; catalogue no. HZ137081; Weck Closure Systems, Research Triangle Park, NC), a titanium clip (Horizon Ligating Clips, ref. 001200; Weck Closure Systems) preset to an inner diameter of 0.5 mm was placed around the pulmonary trunk. The rats received 2 mL of isotonic saline and 0.10 mg/kg buprenorphine (Anorfin; GEA, Frederiksberg, Denmark) subcutaneously to prevent fluid loss and pain, respectively. Buprenorphine was added to the drinking water (7.4 μg/mL) for 3 days after the procedure to relieve postoperative pain. Rats undergoing sham surgery were treated the exact same way except for the placement of the titanium clip.
MCT
MCT (Crotaline; Sigma-Aldrich, St. Louis, MO) was dissolved in 6 M HCl, adjusted to pH 7.4 with 10 M NaOH, diluted in saline, and given intraperitoneally in a dose of 60 mg/kg while the rats were anesthetized. MCT treatment was initiated 1 week after PTB to ensure matching age groups and degree of disease on the day of evaluation.
Inclusion criteria for both PTB and MCT was that the rats survived the evaluation procedure and had a tricuspid annular plane systolic excursion (TAPSE) value of <0.16 mm.22
Nitroprusside
To investigate whether the RV effects of levosimendan could be secondary to systemic arterial vasodilation, another group of rats were randomized to receive PTB (n = 6), MCT injection (n = 6), or sham surgery (n = 6). After 28 days (PTB/sham surgery) or 21 days (MCT), all rats were evaluated with invasive pressures. After baseline and placebo (saline) measurement, sodium nitroprusside (Nitropress; Hospira, Lake Forest, IL) at 50 μg/mL (diluted in 5% glucose)23 was infused. Beginning with an infusion speed of 0.1 mL/h, the dose was increased by 0.1 mL/h every second minute until a reduction in mean arterial pressure (MAP) comparable to that achieved by levosimendan injection was reached.
MRI scanning
A 1.5-T whole-body MRI scanner (Magnetom Avanto; Siemens, Erlangen, Germany) was used. The rats were anesthetized with 3% sevoflurane, placed in a four-element wrist coil, and kept with spontaneous respiration during the scan. The electrocardiogram signal and respiration was recorded to trigger scanning between expiration and inspiration (SAII; Stony Brook, NY).
A stack of six contiguous slices encompassing the heart from base to apex in the cardiac short-axis orientation was acquired. We used a prospectively triggered spoiled gradient echo cine sequence.
The imaging parameters were as follows: repetition time (TR) = 9.26 ms, echo time (TE) = 3.96 ms, flip angle = 15°, acquisition matrix = 128 × 128, field of view = 60 mm × 60 mm, spatial in-plane resolution = 0.47 mm × 0.47 mm, slice thickness = 2 mm, and number of heart phases = 21. Data were evaluated using Segment (http://segment.heiberg.se) cardiac analysis software.
Stroke volume (SV) was measured in the pulmonary artery using a phase contrast sequence. The measurement position was centred after the pulmonary valves and in the PTB group before the clip. The sequence parameters were as follows: TR = 12.7 ms, TE = 3.56 ms, flip angle = 15°, acquisition matrix = 128 × 128, field of view = 80 mm × 80 mm, spatial in-plane resolution = 0.63 mm × 0.63 mm, slice thickness = 3 mm, and number of heart phases = 15. The velocity encoding parameter was optimized according to flow velocity in each animal, between 60 and 200 cm s−1. To increase temporal resolution, all flow measurements were repeated twice, with the second delayed by 6 ms relative to the first. This is reasonable because the data readout was finished after 6 ms, while the remaining TR time was “dead time.” The two data sets were combined and flow values obtained using specific analysis software (Siswin). All data evaluation was performed with the observer blinded to the sample source.
Echocardiography and catheter measurements
Rats were anesthetized and ventilated as described above. A 1.4-F Millar pressure tip catheter (Millar Instruments, Houston, TX) was calibrated and inserted through the right jugular vein into the RV.24 RV systolic and diastolic pressure, RV dP/dtmax, and RV dP/dtmin was recorded using NOTOCORD (NOTOCORD Systems SAS, Midland, NC). A similar catheter was inserted into the left carotid artery to measure systemic blood pressure (BP). Pulmonary vascular resistance—that is, RV afterload—was estimated as RV systolic pressure divided by RV CO.25
Echocardiography (Vivid 7; GE Healthcare, Wauwatosa, WI) was performed with an 11-MHz phased array pediatric transducer with a frame rate of about 150 Hz.22 We recorded tricuspid regurgitation and TAPSE.
Analysis of the images was performed offline using EchoPAC software (GE Healthcare), with the observer blinded to the source of the samples. Three consecutive heart cycles were analyzed for each individual rat, and the mean was used as a representative value.
Anatomical measurements
Rats were euthanized by drain of blood. We weighed the RV, liver, spleen, and lungs (Sartorius, Göttingen, Germany) and measured the length of the tibia. RVs were preserved in 4% formaldehyde (pH 7) histology. Blood was centrifuged, and the plasma was kept at −20°C.
Histology and enzyme-linked immunosorbent assay (ELISA)
RV tissue was handled as previously described.26 We calculated a mean fibrosis value from three randomly chosen images of each RV. The concentration of atrial natriuretic peptide (ANP) was measured using a commercial ELISA kit (E90225Ra, USCN Life Science, Wuhan, Hubei, China) in accordance with the manufacturer’s protocol. Absorbance was read at 450 nm (PHERAstar FS; BMG Labtech, Ortenberg, Germany).
Statistical analysis
All values are mean ± SEM. A one-way analysis of variance (ANOVA) for repeated measurements was performed. If significant, paired t tests between relevant groups were calculated. No post hoc multicomparison test was performed for easier interpretation of the results by the reader, as suggested by Saville.27 We used GraphPad Prism (ver. 5.0; GraphPad Software, San Diego, CA) as statistical software. Differences between groups were tested with unpaired t tests. If data were not normally distributed, equivalent nonparametric tests were performed. P < 0.05 was considered significant.
Results
Effects of PTB and MCT on RV hypertrophy and failure
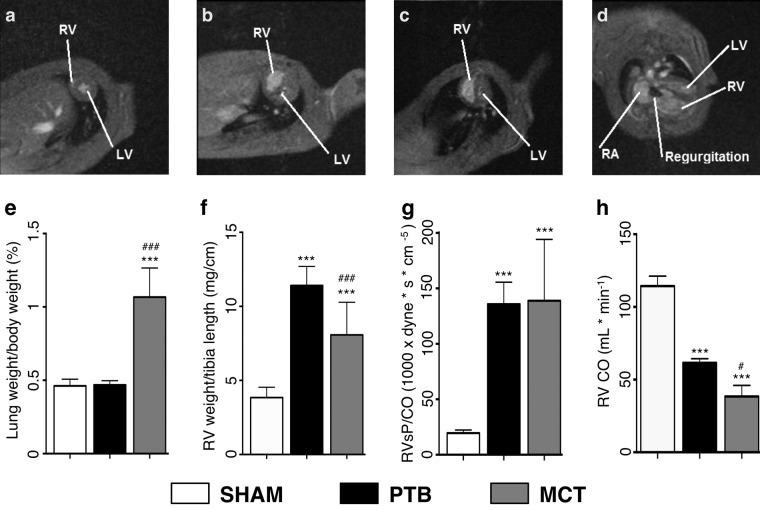
As seen in Table 1 and Figure 2, there was an increase in RV weight/tibia length in the PTB and MCT groups compared with the sham surgery group. Furthermore, the lungs of the MCT rats were heavier, whereas the PTB rat RVs had more fibrosis.
Table 1.
Animal characteristics at euthanasia
| Variable | Sham surgery (n = 8) | PTB (n = 8) | MCT (n = 7) |
|---|---|---|---|
| Liver weight/animal weight, % | 3.3 ± 0.03 | 3.0 ± 0.05** | 3.4 ± 0.06## |
| Spleen weight/animal weight, % | 0.23 ± 0.14 | 0.24 ± 0.14 | 0.20 ± 0.10# |
| Lung weight/animal weight, % | 0.46 ± 0.02 | 0.47 ± 0.01 | 1.07 ± 0.07**,# |
| RV weight/tibia length, g cm−1 | 0.004 ± 0.0003 | 0.011 ± 0.0005** | 0.008 ± 0.0008**,## |
| Fibrosis fraction, % | 0.29 ± 0.031 | 0.79 ± 0.089** | 0.31 ± 0.039### |
| ANP, pg mL−1 | 561 ± 94 | 708.± 101 | 1,515 ± 218*,## |
| Animal weight, g | 311 ± 4 | 275 ± 6** | 213 ± 8**,### |
Data are mean ± SEM. PTB: pulmonary trunk banding; MCT: monocrotaline; RV: right ventricle; ANP: atrial natriuretic peptide.
P < 0.05 versus sham.
P < 0.001 versus sham.
P < 0.05 versus PTB.
P < 0.01 versus PTB.
P < 0.001 versus PTB.
Figure 2.
Representative magnetic resonance imaging cardiac short-axis pictures of end systolic volumes from rats undergoing sham surgery (a), pulmonary trunk banding (PTB; b), or monocrotaline (MCT) injection (c). d, Cardiac long-axis picture of a PTB rat heart with enlarged right ventricle (RV) and right atrium (RA) and tricuspid insufficiency. LV: left ventricle. e, Lung weights normalized to body weight. f, RV free-wall weight normalized to tibia length. g, Baseline pulmonary vascular resistance, estimated as RV systolic pressure (RVsP) divided by cardiac output (CO). h, Baseline RV CO. One asterisk indicates P < 0.05 versus sham, three asterisks indicate P < 0.001 versus sham, one pound sign indicates P < 0.05 versus PTB, and three pound signs indicate P < 0.001 versus PTB. Data are mean ± SEM.
At baseline, the PTB and MCT rats had increased end systolic volume, lower CO, and lower TAPSE compared with the sham surgery rats (Table 2). Heart rate (HR), SV, and ejection fraction were lower compared with the sham surgery rats. The RVs in the PTB and MCT groups showed increased RV end systolic pressure (RVsP) and estimated pulmonary vascular resistance compared with RVs in the sham surgery group. Tricuspid insufficiency was present in all PTB and MCT rats, unlike in the sham surgery rats. Additionally, the PTB group was further hypertrophied and had even higher RVsP and dP/dtmax than the MCT group, whereas the MCT rats had lower RV SV, CO, and systemic BP than the PTB rats.
Table 2.
Baseline hemodynamics
| Variable | Sham surgery (n = 8) | PTB (n = 8) | MCT (n = 7) |
|---|---|---|---|
| Mean arterial pressure, mmHg | 92 ± 4 | 85 ± 4 | 68 ± 7**,# |
| RV end systolic pressure, mmHg | 27 ± 2 | 104 ± 6*** | 56 ± 4***,### |
| RV dP/dtmax, mmHg s−1 | 2,038 ± 194 | 5,261 ± 474*** | 2,784 ± 276*,### |
| RV dP/dtmin, mmHg s−1 | −1,932 ± 191 | −4,266 ± 281*** | −2,321 ± 179### |
| TAPSE, cm | 0.19 ± 0.006 | 0.12 ± 0.005*** | 0.11 ± 0.003*** |
| Tricuspid regurgitation, % | 25 | 100** | 100** |
| PVR, dyn × s × cm−5 | 19,400 ± 978 | 135,800 ± 6,982*** | 138,900 ± 20,900*** |
| Heart rate, bpm | 406 ± 14 | 339 ± 20* | 313 ± 21** |
| RV stroke volume, mL | 0.28 ± 0.01 | 0.18 ± 0.008** | 0.12 ± 0.02**,## |
| RV cardiac output, mL min−1 | 114 ± 7 | 62 ± 3*** | 38 ± 8***,# |
| RV end systolic volume, μL | 76 ± 10 | 228 ± 20*** | 212 ± 15*** |
| RV end diastolic volume, μL | 337 ± 18 | 425 ± 22** | 342 ± 9## |
| RV ejection fraction, % | 78 ± 2 | 47 ± 3*** | 39 ± 3*** |
Data are mean ± SEM. PTB: pulmonary trunk banding; MCT: monocrotaline; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; PVR: pulmonary vascular resistance (estimated as RV end systolic pressure divided by RV cardiac output); bpm: beats per minute.
P < 0.05 versus sham.
P < 0.01 versus sham.
P < 0.001 versus sham.
P < 0.05 versus PTB.
P < 0.01 versus PTB.
P < 0.001 versus PTB.
Acute hemodynamic effects of levosimendan
As seen in Figure 3, both RVsP and RV contractility measured by dP/dtmax increased compared with placebo in all groups after levosimendan infusion. Similarly, dP/dtmin improved in all groups with levosimendan injection compared with placebo injection. HR was increased only in the PTB group after Levo-60 infusion compared with placebo (7.4% ± 3.1%; P = 0.047).
Figure 3.
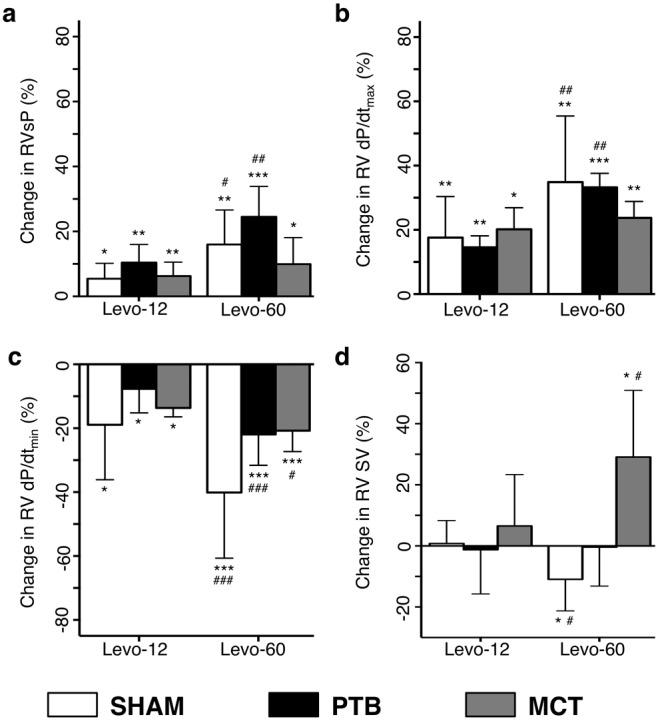
Hemodynamic changes after administration of Levo-12 and Levo-60 compared with placebo. a, Right ventricular (RV) systolic pressure (RVsP). b, RV dP/dtmax. c, RV dP/dtmin. d, RV stroke volume (RV SV). One asterisk indicates P < 0.05 versus placebo, two asterisks indicate P < 0.01 versus placebo, three asterisks indicate P < 0.001 versus placebo, one pound sign indicates P < 0.05 versus Levo-12, two pound signs indicate P < 0.01 versus Levo-12, and three pound signs indicate P < 0.001 versus Levo-12. Data are mean ± SEM. PTB: pulmonary trunk banding; MCT: monocrotaline.
Using MRI, we found that RV SV increased in the MCT group. No RV SV changes were recorded in the PTB group, and a decrease was found in the sham surgery rats.
No changes were recorded between baseline and placebo except for a decline in RV dP/dtmax and dP/dtmin in the PTB group and in mean systemic BP in the PTB and sham surgery groups. Infusion of Levo-60 reduced mean systemic BP in all groups (Fig. 4).
Figure 4.
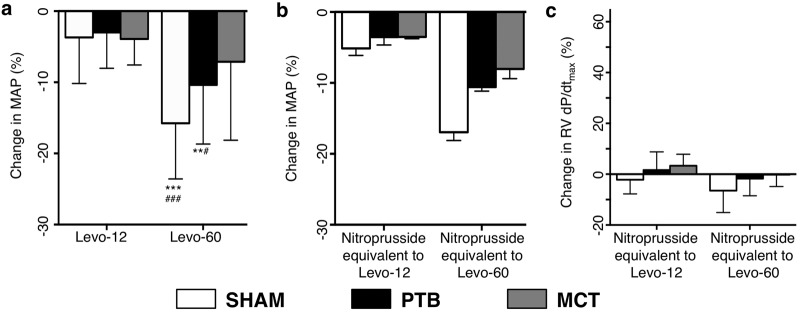
a, Changes in mean arterial blood pressure (MAP) after Levo-12 and Levo-60 administration compared with placebo. b, Hemodynamic effects of nitroprusside. Shown are changes in MAP compared with placebo. Compare this to the decrease in MAP seen in a. c, Changes in right ventricular (RV) dP/dtmax caused by the nitroprusside-induced decrease in MAP. All of these are nonsignificant. Compare this to Figure 3b. Two asterisks indicate P < 0.01 versus placebo, three asterisks indicate P < 0.001 versus placebo, one pound sign indicates P < 0.05 versus Levo-12, and three pound signs indicate P < 0.001 versus Levo-12. Data are mean ± SEM. PTB: pulmonary trunk banding; MCT: monocrotaline.
Nitroprusside
With nitroprusside, we obtained a decrease in MAP comparable to the reduction in MAP seen when infusing Levo-12 and Levo-60. At these levels, there were no changes in RV dP/dtmax in any of the three groups (Fig. 4).
Discussion
In this study, we demonstrate that levosimendan acutely improves the failing RV in the MCT rat model of PAH. Levosimendan also improves the failing RV in a PTB model, demonstrating a direct RV inotropic action of this drug. Nitroprusside did not influence RV function in the two animal models of RV failure, indicating that the observed effects of levosimendan on RV function was not related to the arterial vasodilator component of this drug.
Effects of PTB and MCT on RV hypertrophy and failure
Our models of PTB and MCT efficiently caused RV hypertrophy and failure compared with sham surgery. The MCT administration caused an increase in RV weight, lung weight, and RV dP/dtmax, in accordance with previous studies,28,29 and an RVsP comparable to that in rats injected with an even higher MCT dose.29
PTB caused hypertrophy and pathologic remodeling, evident by the increase in RV weight normalized for tibia length and increased fibrosis fractional area.26 Failure of the RV was evident by a decrease in CO, an increase in EDV, and tricuspid regurgitation compared with sham surgery.
In both models of RV failure, the body weight of the rats was lower than that of sham surgery rats at euthanasia, which indicates a general level of dysfunction, as seen by others30,31 (Table 1). Adjusted for tibia length, RV weights in both RV failure models increased to more than double to overcome the increased RV afterload compared with sham surgery. It was, however, only in the PTB group that fibrosis increased significantly. With the same inclusion criteria based on an equally lower TAPSE, the MCT model showed more signs of RV failure, including increased ANP levels in the blood.
These observations made us confident that the models were suitable to evaluate the effects of levosimendan in the failing hypertrophic right heart.
Effects of levosimendan on right heart hypertrophy and failure
Administration of levosimendan caused an increase in RV end systolic pressure and dP/dtmax with increasing levosimendan concentrations in sham surgery, MCT, and PTB animals. This finding is in accordance with previous studies in nondilated, hypertrophic RVs32,33 and reveals a contractile reserve even in a dilated, hypertrophied, and failing RV.
Compared with placebo, mean arterial BP decreased with increasing concentrations of levosimendan, which confirms a similar systemic vasodilatory effect of levosimendan in all groups (although nonsignificant in the MCT group), as described previously in healthy rats.20 We found no changes in RV dP/dtmax or other functional parameters at equivalent levels of BP reduction caused by nitroprusside (Fig. 4). This supports that the improved RV contractility is not caused or modified by systemic arterial vasodilation.
It was only in the MCT group that the RV managed to transform the increased contractility and pressure into a raised SV. Levosimendan has a well-described pulmonary vasodilatory effect,34 which likely explains the additional improvement compared with PTB animals with a fixed afterload. In the PTB rats, levosimendan was unable to decrease RV afterload due to the fixed constriction, and hence the RV SV was unchanged. In the sham surgery group there was a decrease in RV SV. This might be due to failure of levosimendan to further dilate the healthy pulmonary vascular tree, as described by others,14 combined with a systemic arterial vasodilatory action, peripheral pooling, and a reduction in preload. In the MCT and PTB groups, we would expect central venous pressure to be sufficiently high to maintain RV filling pressure despite peripheral pooling.
Increasing levosimendan doses also improved RV lusitropy measured by RV dP/dtmin in all three groups. This has been described in isolated septic guinea pig hearts but not in septic isolated rat hearts.35,36 The calcium-sensitizing effect of levosimendan is dependent on calcium concentration,6,37 explaining a decreased effect in diastole and hence an improved lusitropic state. Our results reveal that this positive lusitropic action also applies in a hypertrophied failing RV.
We barely observed changes in HR. This indicates that our observed changes in SV are of greater importance than changes in HR in order to increase CO, which also has been suggested in humans.9 Levosimendan increases HR in humans,9 but we and others find only small or no changes in HR in rats, implying a species-dependent chronotropic effect of levosimendan.14,35
In conclusion, we have demonstrated that levosimendan acutely improves the failing RV in a MCT model of PAH and that this beneficial profile involves a direct acute positive inotropic and lusitropic effect on the myocardium of the RV of the rat.
Limitations
All experiments were performed on Wistar Galas outbred rats. This should be taken into consideration, and further in vivo studies in other animal models are warranted before translating our findings to the clinical setting, as suggested by Lawrie.38
Source of Support: This study was supported by the Danish Ministry of Science (11-108410). The Danish Heart Foundation (grant R84-A3295) supported the research fellowship of MDV. Aase and Ejnar Danielsen’s Foundation; Trader Vald. Foersom and Wife Thyra Foersom, Born Otto’s Foundation; Andreassen and Hougaard’s General Foundation; and Commissioner of Police J. P. N. Colind and Wife Asmine Colind’s Foundation also made contributions to the study.
Conflict of Interest: None declared.
References
- 1.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163. [DOI] [PubMed]
- 2.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114:1883–1891. [DOI] [PubMed]
- 3.Mebazaa A, Karpati P, Renaud E, Algotsson L. Acute right ventricular failure—from pathophysiology to new treatments. Intensive Care Med 2004;30:185–196. [DOI] [PubMed]
- 4.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation 1981;63:87–95. [DOI] [PubMed]
- 5.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135:794–804. [DOI] [PubMed]
- 6.Figgitt DP, Gillies PS, Goa KL. Levosimendan. Drugs 2001;61:613–627. [DOI] [PubMed]
- 7.Pathak A, Lebrin M, Vaccaro A, Senard JM, Despas F. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther 2013;38:341–349. [DOI] [PubMed]
- 8.Fotbolcu H, Duman D. A promising new inotrope: levosimendan. Anadolu Kardiyol Derg 2010;10:176–182. [DOI] [PubMed]
- 9.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation 2000;102:2222–2227. [DOI] [PubMed]
- 10.Wiklund A, Kylhammar D, Radegran G. Levosimendan attenuates hypoxia-induced pulmonary hypertension in a porcine model. J Cardiovasc Pharmacol 2012;59:441–449. [DOI] [PubMed]
- 11.Missant C, Rex S, Segers P, Wouters PF. Levosimendan improves right ventriculovascular coupling in a porcine model of right ventricular dysfunction. Crit Care Med 2007;35:707–715. [DOI] [PubMed]
- 12.Kerbaul F, Gariboldi V, Giorgi R, et al. Effects of levosimendan on acute pulmonary embolism-induced right ventricular failure. Crit Care Med 2007;35:1948–1954. [DOI] [PubMed]
- 13.Kerbaul F, Rondelet B, Demester JP, et al. Effects of levosimendan versus dobutamine on pressure load–induced right ventricular failure. Crit Care Med 2006;34:2814–2819. [DOI] [PubMed]
- 14.Leather HA, Ver Eycken K, Segers P, Herijgers P, Vandermeersch E, Wouters PF. Effects of levosimendan on right ventricular function and ventriculovascular coupling in open chest pigs. Crit Care Med 2003;31:2339–2343. [DOI] [PubMed]
- 15.Ammirati E, Musca F, Oliva F, et al. Levosimendan reverted severe pulmonary hypertension in one patient on waiting list for heart transplantation. Int J Cardiol 2013;168:4518–4519. [DOI] [PubMed]
- 16.Cavusoglu Y, Beyaztas A, Birdane A, Ata N. Levosimendan is not effective in reducing pulmonary pressures in patients with idiopathic pulmonary arterial hypertension: report of two cases. J Cardiovasc Med 2009;10:503–507. [DOI] [PubMed]
- 17.Nieminen MS, Fruhwald S, Heunks LM, et al. Levosimendan: current data, clinical use and future development. Heart Lung Vessel 2013;5:227–245. [PMC free article] [PubMed]
- 18.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010;14:R169. [DOI] [PMC free article] [PubMed]
- 19.Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: improvement with levosimendan. Crit Care Med 2009;37:3017–3023. [DOI] [PubMed]
- 20.Segreti JA, Marsh KC, Polakowski JS, Fryer RM. Evoked changes in cardiovascular function in rats by infusion of levosimendan, OR-1896 [(R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)acetamide], OR-1855 [(R)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one], dobutamine, and milrinone: comparative effects on peripheral resistance, cardiac output, dP/dt, pulse rate, and blood pressure. J Pharmacol Exp Ther 2008;325:331–340. [DOI] [PubMed]
- 21.Andersen A, Nielsen JM, Holmboe S, Vildbrad MD, Nielsen-Kudsk JE. The effects of cyclic guanylate cyclase stimulation on right ventricular hypertrophy and failure alone and in combination with phosphodiesterase-5 inhibition. J Cardiovasc Pharmacol 2013;62:167–173. [DOI] [PubMed]
- 22.Hardziyenka M, Campian ME, de Bruin-Bon HA, Michel MC, Tan HL. Sequence of echocardiographic changes during development of right ventricular failure in rat. J Am Soc Echocardiogr 2006;19:1272–1279. [DOI] [PubMed]
- 23.Zahner MR, Kulikowicz E, Schramm LP. Recovery of baroreflex control of renal sympathetic nerve activity after spinal lesions in the rat. Am J Physiol Regul Integr Comp Physiol 2011;301:R1584–R1590. [DOI] [PMC free article] [PubMed]
- 24.Zimmer HG, Millar HD. Technology and application of ultraminiature catheter pressure transducers. Can J Cardiol 1998;14:1259–1266. [PubMed]
- 25.Watts JA, Gellar MA, Fulkerson MB, Das SK, Kline JA. Arginase depletes plasma l-arginine and decreases pulmonary vascular reserve during experimental pulmonary embolism. Pulm Pharmacol Ther 2012;25:48–54. [DOI] [PubMed]
- 26.Andersen A, Nielsen JM, Peters CD, Schou UK, Sloth E, Nielsen-Kudsk JE. Effects of phosphodiesterase-5 inhibition by sildenafil in the pressure overloaded right heart. Eur J Heart Fail 2008;10:1158–1165. [DOI] [PubMed]
- 27.Saville DJ. Multiple comparison procedures: the practical solution. Am Stat 1990;44:174–180.
- 28.Werchan PM, Summer WR, Gerdes AM, McDonough KH. Right ventricular performance after monocrotaline-induced pulmonary hypertension. Am J Physiol 1989;256:H1328–H1336. [DOI] [PubMed]
- 29.Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol 2006;291:H2424–H2430. [DOI] [PubMed]
- 30.Borgdorff MA, Bartelds B, Dickinson MG, Steendijk P, Berger RM. A cornerstone of heart failure treatment is not effective in experimental right ventricular failure. Int J Cardiol 2013;169:183–189. [DOI] [PubMed]
- 31.Schermuly RT, Kreisselmeier KP, Ghofrani HA, et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 2004;169:39–45. [DOI] [PubMed]
- 32.Brown L, Miller J, Dagger A, Sernia C. Cardiac and vascular responses after monocrotaline-induced hypertrophy in rats. J Cardiovasc Pharmacol 1998;31:108–115. [DOI] [PubMed]
- 33.Faber MJ, Dalinghaus M, Lankhuizen IM, et al. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am J Physiol Heart Circ Physiol 2006;291:H1580–H1586. [DOI] [PubMed]
- 34.De Witt BJ, Ibrahim IN, Bayer E, et al. An analysis of responses to levosimendan in the pulmonary vascular bed of the cat. Anesth Analg 2002;94:1427–1433. [DOI] [PubMed]
- 35.Behrends M, Peters J. The calcium sensitizer levosimendan attenuates endotoxin-evoked myocardial dysfunction in isolated guinea pig hearts. Intensive Care Med 2003;29:1802–1807. [DOI] [PubMed]
- 36.Zausig YA, Geilfus D, Missler G, Sinner B, Graf BM, Zink W. Direct cardiac effects of dobutamine, dopamine, epinephrine, and levosimendan in isolated septic rat hearts. Shock 2010;34:269–274. [DOI] [PubMed]
- 37.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002;360:196–202. [DOI] [PubMed]
- 38.Lawrie A. A report on the use of animal models and phenotyping methods in pulmonary hypertension research. Pulm Circ 2014;4:2–9. [DOI] [PMC free article] [PubMed]