Abstract Abstract
Pulmonary arterial hypertension is a manifestation of a group of disorders leading to pulmonary vascular remodeling and increased pulmonary pressures. The right ventricular (RV) response to chronic pressure overload consists of myocardial remodeling, which is in many ways similar to that seen in left ventricular (LV) failure. Maladaptive myocardial remodeling often leads to intraventricular and interventricular dyssychrony, an observation that has led to cardiac resynchronization therapy (CRT) for LV failure. CRT has proven to be an effective treatment strategy in subsets of patients with LV failure resulting in improvement in LV function, heart failure symptoms, and survival. Current therapy for pulmonary arterial hypertension is based on decreasing pulmonary vascular resistance, and there is currently no effective therapy targeting the right ventricle or maladaptive ventricular remodeling in these patients. This review focuses on the RV response to chronic pressure overload, its effect on electromechanical coupling and synchrony, and how lessons learned from left ventricular cardiac resynchronization might be applied as therapy for RV dysfunction in the context of pulmonary arterial hypertension.
Keywords: pulmonary hypertension, pulmonary arterial hypertension, right ventricular failure, dyssynchrony, cardiac resynchronization therapy
Pulmonary arterial hypertension is a manifestation of a group of disorders that cause pulmonary vascular remodeling, alterations in pulmonary vascular resistance, and reduced compliance. Ultimately, increased pulmonary pressures may induce alterations in right ventricular structure and function. Current therapy is largely based on targeting biochemical pathways to decrease pulmonary vascular resistance. Despite this, the final common pathway for many patients remains right ventricular failure and death.1
Although left ventricular (LV) failure has been well characterized, leading to multiple therapies with proven mortality benefit, less is known about the failing right ventricle (RV). In fact, early evaluation of the role of RV function in disease states (i.e., RV free wall infarction/ablation, Fontan physiology, or lung transplantation) suggested that the RV was highly adaptable and perhaps superfluous to overall cardiac function in the absence of sustained pulmonary arterial hypertension. However, recent improvements in imaging and technology, including strain imaging, tissue Doppler imaging (TDI), and magnetic resonance imaging (MRI) have led to a better understanding of the adaptation of the RV to chronic pressure overload.
Many similarities between RV and LV failure have been observed, including changes in mechanical function, RV global and myocyte structure and function,2 myocardial blood flow,3 and electrophysiologic properties.4 In a limited number of mechanical interventions in the pulmonary circulation (lung transplantation and pulmonary artery endarterectomy), RV reverse remodeling has been noted, suggesting a reversible component to RV remodeling related to pulmonary arterial hypertension.5,6 Although these findings give hope for potential therapies to reverse RV remodeling and failure, there are currently no proven therapies targeting the failing RV. Furthermore, there is no consistent evidence that treatment of elevated pulmonary artery pressure either restores RV function or forestalls further deterioration of RV function. As recent reviews suggest, the similarities between RV and LV failure and past success in treatment of LV failure may offer valuable insight when it comes to exploring potential therapeutic options for RV failure.7,8
This review focuses on the RV response to chronic pressure overload, its effect on electromechanical coupling and synchrony, and how lessons learned from left ventricular cardiac resynchronization might be applied as therapy for right ventricular dysfunction in the context of pulmonary arterial hypertension.
RV remodeling in response to chronically elevated pulmonary arterial pressures
The RV is a thin-walled structure with a design well suited for adaptation to a varying preload in the setting of low pulmonary vascular resistance. However, pulmonary vasoconstriction and vascular remodeling lead to chronically elevated pulmonary vascular resistance/reduced pulmonary artery compliance, which is viewed by the RV as increased static and pulsatile afterload. In turn, these changes increase RV wall stress, leading to adaptive remodeling. The response of the RV to chronically elevated pulmonary pressure follows the law of Laplace, resulting in increased RV wall thickness, ventricular dilatation, increased ventricular wall stress, and increased preload. This response, similar to that of the LV, frequently results in reduced systolic function.9 As a result of more recent investigations, we now know changes to the molecular structure,2 metabolism,10 electrophysiologic,4 and mechanical properties2 of the RV accompany the more traditional hallmarks of remodeling.
Altered gene expression leading to change in molecular structure has been observed in the LV and RV of failing hearts. Specifically, the balance in isoforms of myosin heavy chain (MHC) is altered, favoring an almost exclusive dependence on β-MHC.2 α-MHC has been shown to have significantly faster contractile activity than β-MHC,11 presumably leading to more rapid contraction and relaxation. Normally, α-MHC messenger RNA (mRNA) makes up 25%–35% of total ventricular MHC mRNA, translating to approximately 10% of the total ventricular MHC.12 In patients with right heart failure related to pulmonary arterial hypertension, α-MHC mRNA is down-regulated by 64%–84%, leading to decreased α-MHC expression and an increased reliance on β-MHC.2 Decreased expression of α-MHC has been linked to decreased power output in isolated rat cardiac myocytes as well as in isolated working hearts.13 The shift in balance in isoforms of MHC likely has significant detrimental effect on overall cardiac function and output.
Another structural response to chronic RV pressure overload involves changes to the extracellular cardiac structure. Animal studies have shown increased collagen deposition related to pressure overload in LV and RV remodeling.14,15 More recently, the increase in collagen content of diseased RVs related to pressure overload was characterized and revealed increased myocardial fibrosis with greater nonsoluble collagen content over time.16 The increase in myocardial fibrosis was found to lag behind myocyte hypertrophy and is regulated by postsynthetic processing of procollagen.16 The increase in myocardial fibrosis related to chronic pressure overload likely contributes to pump dysfunction and inefficiency by reducing the flexibility of the myocyte support structure and delimiting the diffusion of nutrient substrates.
In addition to molecular and fibrotic changes, myocardial hypertrophy is a known response to chronic pressure overload. Hypertrophy, along with increased afterload, increases metabolic demand and increased myocardial blood flow may be required for optimal function. However, incremental increases in right coronary artery (RCA) blood flow may not develop. In fact, reduced systolic RCA blood flow relative to hypertrophy has been shown in a dog model of pulmonary arterial hypertension17 and was more recently confirmed in humans with pulmonary arterial hypertension.3 RCA blood flow, which is relatively monophasic in healthy hearts, was shown to become biphasic with systolic flow impediment proportional to RV pressure and mass in patients with pulmonary arterial hypertension.3 In addition, the microvascular circulation may also be altered in the setting of RV hypertrophy related to pressure overload, as evidenced by the finding of decreased RV capillary density in a rat model of pulmonary arterial hypertension.18 The relative decrease in blood flow to the diseased RV has been shown to result in ischemia by myocardial scintigraphy in 9 of 23 patients with pulmonary arterial hypertension.19 The patients demonstrating ischemia by myocardial scintigraphy were found to have significantly higher right atrial pressures and RV end-diastolic pressures, suggesting a link between myocardial perfusion and performance.
It has been suggested that the above mentioned supply-demand mismatch leading to myocardial ischemia may alter metabolism in the RV leading to further remodeling and impaired electromechanical coupling.10 Fatty acid oxidation is the energy substrate of choice for normal myocardium; however, glycolytic metabolism is a common response to ischemia.20 Increased glucose metabolism, as demonstrated by increased uptake of 18-fluorodoexyglucose by positron emission tomography, has been shown in the RV of patients with pulmonary hypertension.21 Animal models of pulmonary arterial hypertension and RV hypertrophy have demonstrated a similar shift toward glycolytic metabolism.22,23 This metabolic shift has been linked to decline in RV function and altered expression of ion channels leading to electrical remodeling in two rat models of pulmonary arterial hypertension.23 As these findings suggest, altered metabolism in the failing RV may be the result of relative ischemia induced by the above-mentioned adaptations of the RV to chronic pressure overload.
RV electrophysiology is not immune to chronic pressure overload. RV myocytes in patients with LV failure have shown electrophysiologic abnormalities similar to those of LV myocytes. Specifically, prolonged action potentials related to reduced transient outward potassium current (Ito1), inward rectifier potassium current (Ik1), and slow component of delayed rectifier potassium current (Iks) have been demonstrated.4 To further characterize the electrophysiologic properties of the diseased right ventricle, Chen et al.24 performed epicardial mapping in patients undergoing pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. They found that conduction delay in the right bundle was present, regardless of the presence of right bundle branch block on the surface electrocardiogram (ECG). The RV was also found to complete activation and repolarization later than the LV in these patients.24 Hardziyenka et al.25 performed epicardial mapping in a similar group of patients undergoing pulmonary endarterectomy and showed that epicardial activation was later in the RV free wall than LV lateral wall due to significantly slower longitudinal and transverse conduction velocities in the RV. TDI echocardiography in these patients revealed that mechanical diastolic interventricular delay correlated with the RV to LV activation delay.25 This evidence underscores the importance of electrophysiologic remodeling and its role in undermining electromechanical coupling and promoting disturbances due to anisotropic conduction, even in instances in which the surface ECG does not indicate gross conduction delay.
Intraventricular dyssynchrony
The RV is comprised of distinct inflow, apical, and outflow compartments that contribute individually to systolic function.26 In response to chronic pressure overload, normal RV mechanics are disturbed. As the RV enlarges, its sphericity increases, and septal deformation into the LV occurs; this results in altered oblique muscle alignment and loss of the classical twisting and shortening motion of the interventricular septum. Thus, the main contractile force of the RV is generated by the transversely oriented fiber of the basal lateral free wall.27
Loss of RV compartmental individuality and abnormal strain have been characterized by two-dimensional (2D), speckle tracking, and TDI echocardiography. Use of 2D and three-dimensional (3D) echocardiography in patients with World Health Organization (WHO) group 2 pulmonary hypertension related to ischemic cardiomyopathy revealed a complete loss of compartmental independence and alterations in ventricular strain in the pulmonary hypertension group.28 In addition, speckle-tracking echocardiography of patients with WHO group 1 and WHO group 2 pulmonary hypertension has revealed altered longitudinal strain curves in the RV free wall. Specifically, the RV free wall reached lower peak strain later in the cardiac cycle compared with controls.29 Systolic and early diastolic tissue velocities were also reduced in these patients.29 RV dyssynchrony appears to be present even in the absence of a right bundle branch block (RBBB) on surface ECG, as evidenced by speckle-tracking echocardiography showing delayed contraction of the basal and mid RV free wall in patients with pulmonary arterial hypertension without RBBB when compared with control subjects.30 Evidence of RV dyssynchrony by TDI echocardiography has been shown to be present in the absence of abnormalities in RV size and function by standard echocardiographic indices and has been linked to disease severity.30 These findings suggest that traditional evaluation of conduction delay and RV function is insufficient to evaluate RV dyssynchrony.
Altered RV mechanics result in inefficient contraction and likely have effects on cardiomyocyte structure and function. Studies of both isolated LV and RV dysfunction have suggested that intraventricular dyssynchrony is accompanied by energy-inefficient shifting of blood from high wall stress to low wall stress regions within the ventricle rather than ejecting blood from the ventricle. Marcus et al.31 have estimated that 18% of RV ejection time is wasted by nonejecting contraction within the RV, resulting in reduced stroke volume, stroke work, and coronary perfusion. Furthermore, studies of left ventricular dyssynchrony have suggested that molecular remodeling in high- and low-load areas may differentially alter contractile proteins, energy efficiency, and calcium handling.32
Interventricular dyssynchrony and ventricular interdependence
The RV and LV are coupled anatomically and mechanically.27 Therefore, changes to one ventricle will inevitably affect the performance of the other.33 Although the effects of chronic pressure overload are most obviously seen in the RV, mechanical dyssynchrony leads to biventricular dysfunction and LV remodeling. The presence of elevated RV pressures leads to dyssynchronous ventricular contraction and leftward ventricular septal bowing, which has been characterized by 2D and 3D echocardiography,28 speckle-tracking echocardiography,34 TDI,35 and MRI.36
Interventricular dyssynchrony may be the result of delayed activation in regions of the RV due to intraventricular conduction disturbances via mechanisms reviewed above. As noted above, epicardial mapping in patients with chronic thromboembolic pulmonary hypertension has demonstrated delayed activation of the RV free wall compared with the LV lateral wall.25 Studies in both MRI and TDI echocardiography have revealed RV delay to peak strain compared with the LV in patients with pulmonary hypertension.37 MRI strain imaging suggests that this delay in RV peak strain represents a functionally unproductive RV postsystolic isovolumetric contraction period that is followed by a normal relaxation phase.37 The delayed RV contraction persists into the early LV diastolic period (low LV pressure), producing an RV to LV pressure gradient and enhanced bowing of the septum into the LV. Maximal septal bowing coincides with peak RV shortening as assessed by MRI.31 LV septal bowing is most prominent during early LV diastole and has been shown to impair LV diastolic filling, thereby reducing LV preload and increasing pulmonary venous pressure.38,39 Further characterization of interventricular dyssyncrony includes MRI strain evidence of increased early diastolic LV free wall lengthening,40 suggesting the effects of LV septal bowing extend to the LV free wall.
In addition to impaired diastolic filling, evidence suggests alterations to LV structure and function in patients with impaired RV function. Acute and chronic RV pressure overload has been shown to affect adversely LV mechanical synchrony and myocardial performance by speckle-tracking echocardiography.41 In addition, epicardial electrical mapping of chronic thromboembolic pulmonary hypertension patients has shown alterations in conduction. Specifically, patients with RV failure (right heart failure defined as tricuspid annular plane systolic excursion [TAPSE] <16 mm) had significantly longer PQ and QTc duration, longer LV effective refractory period, and significantly reduced LV longitudinal conduction velocity when compared with patients without RV failure.42 MRI evidence suggests that patients with chronic thromboembolic pulmonary hypertension also have decreased LV free wall mass as a result of either impaired LV loading or secondary to left intraventricular load shifting, compared with control subjects, which improved after pulmonary endarterectomy.43 Thus, it may be LV underperformance induced by right ventricular dysfunction that sets the stage for terminal deterioration in patients with advanced pulmonary arterial hypertension.
Cardiac resynchronization for left ventricular failure
LV failure is characterized by LV structural, molecular, electrophysiological, and mechanical remodeling similar to changes observed in pulmonary arterial hypertension–induced RV failure. Cardiac resynchronization therapy (CRT) acts to restore mechanical synchrony of the LV contraction, improving biventricular dynamics, and has been demonstrated to lead to reverse cardiac remodeling associated with clinically relevant reductions in morbidity and mortality.44,45
There is evidence that CRT may induce positive extracellular matrix remodeling. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) have been shown to play key roles in extracellular matrix remodeling in congestive heart failure.46 Increased levels of MMP-2 and decreased levels of TIMP-2 have been demonstrated to correlate with maladaptive cardiac remodeling.46 CRT has been shown to decrease MMP-2 levels and increase TIMP-2 levels, indicating positive extracellular matrix remodeling.47 CRT has also been shown to have anti-inflammatory effects with decreased levels of interleukin 6 (IL-6) and IL-8.47 Positive extracellular matrix remodeling resulting from improvements in myocyte tethering and/or energy substrate diffusion may lead to a decrease in myocardial fibrosis and play a role in improved cardiac function.
LV CRT has been shown to positively affect many of the abnormalities observed in RV failure related to chronic pressure overload. CRT has been shown to partially restore dyssynchronous heart failure–induced ion channel remodeling, as evidenced by partial restoration of Ik1 and Ito1 currents, and to decrease action potential duration in an animal study.46 There is evidence to support partial restoration of altered metabolism from an animal model where CRT was shown to increase the expression of many mitochondrial enzymes that fuel pyruvate metabolism and, therefore, lead to increased adenosine triphosphate production.48 Endomyocardial biopsy specimens obtained from patients with heart failure before and after CRT revealed increased mRNA levels of α-MHC and an increased ratio of α-MHC to β-MHC mRNA in post-CRT samples, indicating restoration of the MHC balance seen in healthy hearts.49 Thus, electrical stimulation to induce mechanical synchrony results in molecular-level alterations that either restore LV function or offset molecular changes that exert adverse contractile responses.
Although these findings are encouraging and have led to great improvements in many patients with heart failure, there remain a significant proportion of patients who do not respond to CRT. Exactly why some patients do not respond to CRT is not completely understood; however, evidence suggests that baseline RV function and, potentially, the location of RV pacing play important roles in responsiveness to CRT.50-52 Poor baseline right ventricular function as assessed by TAPSE has been shown to correlate to reduction in LV end-systolic volume following initiation of CRT, indicating baseline RV function is a relevant predictor of CRT response.50 Several small studies have shown conflicting results regarding the effect of RV lead position (RV midseptal position versus RV apex) in CRT.51,52
Cardiac resynchronization for the failing right ventricle
There is preliminary evidence that resynchronization therapy may be beneficial for RV failure in the setting of increased afterload. Studies to date have been focused on animal models of pulmonary arterial hypertension and on patients with congenital heart disease and chronic thromboembolic pulmonary hypertension. These offer support for the validity of cardiac resynchronization therapy for RV failure. RV pacing has been shown to be beneficial in patients with RV dysfunction and RBBB in the setting of tetralogy of Fallot and aortic stenosis status after Ross procedure.53 In 2010, Berruezo et al.54 published a case report of a patient with WHO group III pulmonary hypertension, RV failure, and RBBB who developed complete atrioventricular block requiring pacemaker implantation. Because of the presence of RV dysfunction, dP/dt was measured while pacing various sites before placing leads in the RA appendage and His bundle region. RV mapping was then performed, and a third lead was placed at the site of latest activation, the free wall of the RV outflow tract. The patient demonstrated clinical and echocardiographic improvement with bifocal RV pacing. At 3 months of follow-up, the patient had markedly improved clinical status from New York Heart Association class IV to II, ability to walk more than 450 m on 6-minute walk test, and slight improvement in global RV function by echocardiography.54
Experience with left ventricular CRT has suggested that pacing in the latest activated site optimizes the chance of inducing ventricular function improvements.55,56 It is interesting to note that all of the studies that look at mechanical activation in the context of pulmonary hypertension find the RV free wall to consistently be the latest site of mechanical activation.35,37,57 Lumens et al.58 developed a computer simulation of severe pulmonary arterial hypertension and showed improvements in cardiac pump function with simulated RV free wall pacing. Pacing of the RV free wall also showed improvement in RV systolic function (as assessed by RV dP/dtmax), a decrease in the time difference between RV and LV peak pressure and shortened duration of RV and LV contraction in an animal model of pulmonary arterial hypertension.59 More recently, Hardziyenka et al.60 performed a study that included 14 patients with chronic thromboembolic pulmonary hypertension and diastolic interventricular delay as assessed by TDI echocardiography. Temporary sequential RA-RV pacing at the RV apex at the optimal atrioventricular interval resulted in an average 13% increase in LV outflow tract velocity time integral, a reduction in diastolic interventricular delay from 50 ± 19 milliseconds to 3 ± 22 milliseconds, reduced RV end systolic area, increased LV end diastolic area, and significant improvements in RV global and longitudinal contractility and LV longitudinal contractility.60 A similar study is currently underway to evaluate the acute effects of attempted cardiac resynchronization in patients with pulmonary arterial hypertension.61
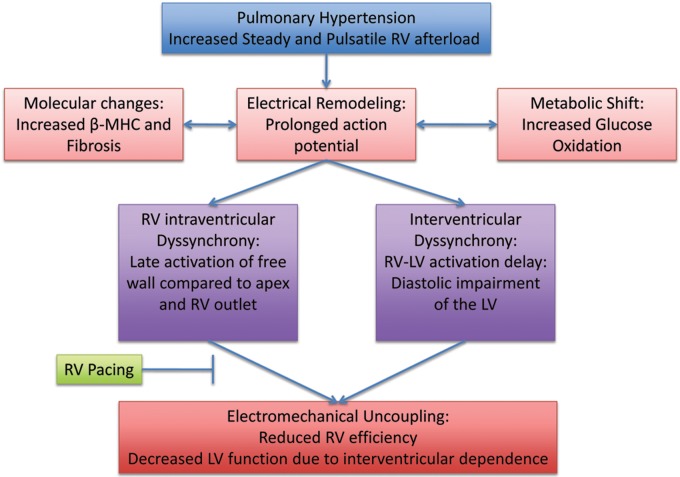
These studies have shown evidence for acutely improved hemodynamics as a result of cardiac resynchronization in patients with pulmonary arterial hypertension and associated dyssynchrony (Fig. 1). It is not yet known whether similar improvements would be seen in other forms of pulmonary hypertension, or whether acutely improved hemodynamics would be maintained and translate to improved clinical outcomes in the long term.
Figure 1.
The right ventricular (RV) response to chronic pressure overload leading to intraventricular and interventricular dyssynchrony. RV pacing could potentially inhibit or reverse maladaptive remodeling. LV: left ventricle/ventricular; MHC: myosin heavy chain.
Summary
Pulmonary arterial hypertension often leads to RV failure and death. The RV response to chronic pressure overload consists of myocardial remodeling, which is in many ways similar to that seen in LV failure. Changes in myocardial structure, metabolism, regional blood flow, and electrophysiologic ion currents have been observed in the failing RV. As a result, RV dyssynchrony is observed and has been shown to have detrimental effects on overall cardiac function.
Recent advances in the understanding of LV failure suggest alterations in electromechanical coupling and hemodynamics that have responded dramatically to cardiac resynchronization therapy. Given the similarities observed in RV and LV remodeling in response to dyssynchrony, it is reasonable to entertain the possibility of resynchronization therapy for the failing RV. Animal studies, a computer simulation analysis, and one study involving patients with chronic thromboembolic pulmonary hypertension have shown acute improvements in cardiac function and hemodynamics with RV pacing. It is not yet clear whether these promising results would apply to other types of pulmonary hypertension, and it remains to be seen whether resynchronization for RV failure could forestall further deterioration or improve ventricular vascular coupling in the long term. Further study focusing on these issues will be needed to determine whether resynchronization therapy for RV failure in patients with pulmonary arterial hypertension will become a viable treatment option.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst, RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–1619. [DOI] [PubMed]
- 2.Lowes BD, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, et al. Changes in gene expression in the intact human heart: downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 1997;100(9):2315–2324. [DOI] [PMC free article] [PubMed]
- 3.Van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 2008;29:120–127. [DOI] [PubMed]
- 4.Li GR, Lau CP, Leung TK, Nattel S. Ionic abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm 2004;1(4);460–468. [DOI] [PubMed]
- 5.Ritchie M, Waggoner AD, Davila-Roman VG, Barzilai B, Trulock EP, Eisenberg PR. Echocardiographic characterization of the improvement in right ventricular function in patients with severe pulmonary hypertension after single-lung transplantation. J Am Coll Cardiol 1993;22(4):1170–1174. [DOI] [PubMed]
- 6.Reesnik HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk-Noordegraaf A, Bresser P. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg 2007;133(1):58–64. [DOI] [PubMed]
- 7.Freidberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 2014;129(9):1033–1044. [DOI] [PubMed]
- 8.Handoko ML, de Man FS, Allaart CP, Paulus WJ, Westerhof N, Vonk-Noordegraaf A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev 2010;19(115):72–82. [DOI] [PMC free article] [PubMed]
- 9.Bogaard HJ, Abe K, Vonk-Noordegaaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right heart failure in pulmonary hypertension. Chest 2009;135(3):794–804. [DOI] [PubMed]
- 10.Archer SL, Fang Y, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 2013;3(1):144–152. [DOI] [PMC free article] [PubMed]
- 11.Stelzer JE, Brickson, SL, Locher MR, Moss RL. Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J Physiol (Lond) 2007;579(1):161–173. [DOI] [PMC free article] [PubMed]
- 12.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 2000;86:386–390. [DOI] [PubMed]
- 13.Korte SF, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol 2005;289:H801–H812. [DOI] [PubMed]
- 14.Brooks A, Schinde V, Bateman AC, Gallagher PJ. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart 2003;89:1255–1256. [DOI] [PMC free article] [PubMed]
- 15.Marino TA, Kent RL, Uboh CE, Fernandez E, Thompson EW, Cooper G. Structural analysis of pressure versus volume overload hypertrophy of cat right ventricle. Am J Physiol 1985;249:H371–H379. [DOI] [PubMed]
- 16.Baicu CF, Li J, Zhang Y, Kasiganesan H, Cooper G, Zile MR, Bradshaw AD. Time course of right ventricular pressure-overload induced myocardial fibrosis: relationship to changes in fibroblast postsynthetic procollagen processing. Am J Physiol Heart Circ Physiol 2012;303:H1128–H1134. [DOI] [PMC free article] [PubMed]
- 17.Murray PA, Baig H, Fishbein MC, Vatner ST. Effects of experimental right ventricular hypertrophy on myocardial blood flow in conscious dogs. J Clin Invest 1979;64:421–427. [DOI] [PMC free article] [PubMed]
- 18.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;129:1951–1960. [DOI] [PubMed]
- 19.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 2001;38:1137–1142. [DOI] [PubMed]
- 20.Taegtmeyer H, King LM, Jones BE. Energy substrate metabolism, myocardial ischemia, and targets for pharmacotherapy. Am J Cardiol 1998;82(5A):54K–60K. [DOI] [PubMed]
- 21.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, et al. Increased [18F] fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005;45:1849–1855. [DOI] [PubMed]
- 22.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 2004;286:H1185–H1192. [DOI] [PubMed]
- 23.Piao L, Fang YH, Cadete VJ, Wiethold C, Urboniene D, Toth PT, Marsboom G, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med 2010;88:47–60. [DOI] [PMC free article] [PubMed]
- 24.Chen PS, Moser KM, Dembitsky WP, Auger WR, Daily PO, Calisi CM, Jamieson SW, Feld GK. Epicardial activation and repolarization patterns in patients with right ventricular hypertrophy. Circulation 1991:83:104–118. [DOI] [PubMed]
- 25.Hardziyenka M, Campian ME, Bouma BJ, Linnenbank AC, Bruin-Bon R, Kloek JJ, van der Wal AC, et al. Right-to-left ventricular diastolic delay in chronic thromboembolic pulmonary hypertension is associated with activation delay and action potential prolongation in right ventricle. Circ Arrhyth Electrophysiol 2009;2:555–561. [DOI] [PubMed]
- 26.Geva T, Powell AJ, Crawford EC, Chung T, Colan SD. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation 1998;98:339–345. [DOI] [PubMed]
- 27.Saleh S, Liakopoulos O, Buckberg G. The septal motor of biventricular function. Eur J Cardiothorac Surg 2006;29(suppl 1):S126–S138. [DOI] [PubMed]
- 28.Calcutteea A, Chung R, Linqvist P, Hodson M, Henein M. Differential right ventricular regional function and the effect of pulmonary hypertension: three dimensional echo study. Heart 2011;97(12):1004–1011. [DOI] [PubMed]
- 29.Brili S, Stamatopoulos I, Misailidou M, Chrysohoou C, Tousoulis D, Tatsis I, Stefanadis C. Longitudinal strain curves in the RV free wall differ in morphology in patients with pulmonary hypertension compared to controls. Int J Cardiol 2013;167(6):2753–2756. [DOI] [PubMed]
- 30.Kalogeropoulos AP, Georgiopoulou VV, Howell S, Pernetz MA, Fisher MR, Lerakis S, Martin RP. Evaluation of right intraventricular dyssynchrony by two-dimensional strain echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr 2008;21(9):1028–1034. [DOI] [PubMed]
- 31.Marcus TJ, Gan CT, Zwanenburg JJ, Boonstra A, Allaart CP, Gotte MJW, Vonk-Noordegraaf A. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left to right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008;51(7):750–757. [DOI] [PubMed]
- 32.Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res 2013;113:765–776. [DOI] [PMC free article] [PubMed]
- 33.Santamore WP, Dell’Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 1998;40:289–308. [DOI] [PubMed]
- 34.Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, Sharp J, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010;121:259–266. [DOI] [PMC free article] [PubMed]
- 35.Lopez Candales A, Dohi K, Rajagopalan N, Suffoletto M, Murali S, Gorcsan J, Edelman K. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound 2005;3:23. [DOI] [PMC free article] [PubMed]
- 36.Marcus JT, Vonk-Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension. Chest 2001;119:1761–1765. [DOI] [PubMed]
- 37.Mauritz G, Marcus JT, Westerhof N, Postmus PE, Vonk-Noordegraaf A. Prolonged right ventricular post-systolic isovolumetric period in pulmonary arterial hypertension is not a reflection of diastolic dysfunction. Heart 2011;97(6):473–478. [DOI] [PubMed]
- 38.Louie EK, Rich S, Brundage B. Doppler echocardiographic assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J Am Coll Cardiol 1986;8:1298–1306. [DOI] [PubMed]
- 39.Lazar JM, Flores AR, Grandis DJ, Orie JE, Schulman DS. Effects of chronic right ventricular pressure overload on left ventricular diastolic function. Am J Cardiol 1993;72:1179–1182. [DOI] [PubMed]
- 40.Lumens J, Arts T, Marcus TJ, Vonk-Noordegraaf A, Delhaas T. Early diastolic left ventricular lengthening implies pulmonary hypertension-induced right ventricular decompensation. Cardiovasc Res 2012;96:286–295. [DOI] [PubMed]
- 41.Ichikawa K, Dohi K, Sugiura E, Sugimoto T, Takamura T, Ogihara Y, Nakajima H, et al. Ventricular function and dyssynchrony quantified by speckle-tracking echocardiography in patients with acute and chronic right ventricular pressure overload. J Am Soc Echocardiogr 2013;26(5):483–492. [DOI] [PubMed]
- 42.Hardziyenka M, Campain ME, Verkerk AO, Surie S, van Ginneken ACG, Hakim S, Linnenbank AC, et al. Electrophysiologic remodeling of the left ventricle in pressure overload-induced right heart failure. J Am Coll Cardiol 2012;59(24):2193–2202. [DOI] [PubMed]
- 43.Hardziyenka M, Campain ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass. J Am Coll Cardiol 2011;57(8):921–928. [DOI] [PubMed]
- 44.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, et al. Cardiac resynchronization therapy for mild to moderate heart failure. N Engl J Med 2010;363:2385–2395. [DOI] [PubMed]
- 45.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed]
- 46.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation 2009;119:1220–1230. [DOI] [PMC free article] [PubMed]
- 47.Stanciu AE, Vatasescu RG, Stanciu MM, Lorgulescu C, Vasile AI, Dorobantu M. Cardiac resynchronization therapy in patients with chronic heart failure is associated with anti-inflammatory and anti-remodeling effects. Clin Biochem 2013;46(3):230–234. [DOI] [PubMed]
- 48.Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet 2010;3:78–87. [DOI] [PMC free article] [PubMed]
- 49.Vanderheyden M, Mullins W, Delrue L, Goethals M, de Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F, Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy: responders versus nonresponders. J Am Coll Cardiol 2008;51(2):129–136. [DOI] [PubMed]
- 50.Cappelli F, Porciani MC, Ricceri I, Perrotta L, Ricciardi G, Michelucci A, Padeletti L. Tricuspid annular plane systolic excursion evaluation improves selection of cardiac resynchronization therapy patients. Clin Cardiol 2010;33(9):578–582. [DOI] [PMC free article] [PubMed]
- 51.Riedlbauchoca L, Cihak R, Bytesnik J, Vancura V, Fridl P, Hoskova L, Kautzner J. Optimization of right ventricular lead position in cardiac resynchronization therapy. Eur J Heart Fail 2006;25:609–614. [DOI] [PubMed]
- 52.Haghjoo M, Bonakdar H, Jorat M, Fazelifar A, Alizadeh A, Ojaghi-Haghjghi Z, Esmaielzadeh M, Sadr-Ameli MA. Effect of right ventricular lead location on response to cardiac resynchronization therapy in patients with end-stage heart failure. Europace 2009;11(3):356–363. [DOI] [PubMed]
- 53.Dubin AM, Feinstein JA, Reddy M, Hanley FL, Van Hare GF, Rosenthal DN. Electrical resynchronization a novel therapy for the failing right ventricle. Circulation. 2003;107:2287–2289. [DOI] [PubMed]
- 54.Berruezo A, DePotter T, Sitges M, Mansour F, Mont L, Brugada J. Bifocal right ventricular resynchronization for the failing right ventricle. Pacing Clin Electrophysiol 2011;34(8):e78–e81. [DOI] [PubMed]
- 55.Saba S, Marek J, Schwartzman D, Jain S, Adelstein E, White P, Oyenuga OA, Onishi T, Soman P, Gorcsan J. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy. Circ Heart Fail 2013;6:427–434. [DOI] [PubMed]
- 56.Kahn FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy. J Am Coll Cardiol 2012;59(17):1509–1518. [DOI] [PubMed]
- 57.Lopez-Candales A, Dohi K, Bazaz R, Edelman K. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue Doppler imaging. Am J Cardiol 2005;96:602–606. [DOI] [PubMed]
- 58.Lumens J, Arts T, Broers B, Boomars KA, van Paassen P, Prinzen FW, Delhaas T. Right ventricular free wall pacing improves cardiac pump function in severe pulmonary arterial hypertension: a computer simulation analysis. Am J Physiol Heart Circ Physiol 2009;297(6):H2196–H2205. [DOI] [PubMed]
- 59.Handoko ML, Lamberts RR, Redout EM, de Man FS, Boer C, Simonides WS, Paulus WJ, Westerhof N, Allaart CP, Vonk-Noordegraaf A. Right ventricular pacing improves right heart function in experimental pulmonary hypertension: a study in the isolated heart. Am J Physiol Heart Circ Physiol 2009;297:H1752–H1759. [DOI] [PubMed]
- 60.Hardziyenka M, Surie S, de Groot JR, de Bruin-Bon R, Knops RE, Remmelink M, Yong ZY, et al. Right ventricular pacing improves haemodynamics in right ventricular failure from pressure overload: an open observational proof-of-principle study in patients with chronic thromboembolic pulmonary hypertension. Europace 2011;13:1753–1759. [DOI] [PubMed]
- 61.Milliez P-U; University Hospital, Caen. Resynchronisation therapy of right ventricle in pulmonary arterial hypertension. Bethesda, MD: National Library of Medicine, 2000. http://clinicaltrials.gov/show/NCT01905189. Accessed April 16, 2014. NLM identifier: NCT01905189.