Abstract
Despite the widespread evidence that lowering glycemic levels reduces the risks of complications in diabetic patients, there has been little improvement in glycemic control among patients in the United States and Europe in recent years. Although widely used, there has been considerable controversy surrounding the role of self-monitoring of blood glucose (SMBG) as a means for achieving glycemic control. The high cost of test strips has made considerations regarding appropriate recommendations a priority, especially given our current climate of health care cost-containment. Existing clinical recommendations lack specific guidance to patients and clinicians regarding SMBG practice intensity and frequency, particularly for those not treated with insulin. Previous studies of the association between SMBG and glycemic control found often weak and sometimes conflicting evidence. Several areas in need of development were identified. A re-examination of the role of SMBG is needed with special attention to the unique needs of patients using different diabetes treatments, within special clinical sub-populations, and during initiation of SMBG versus its ongoing use. Further understanding of the intensity and frequency of SMBG needed to capture variability in glycemic patterns would facilitate more specific guideline development. Patient education programs geared toward teaching patients appropriate SMBG practice, glycemic targets and actions to be taken in response to readings would be beneficial. Continuing medical education (CME) programs that provide guidance regarding ways to utilize patients’ SMBG records to tailor medication regimens should be developed. To facilitate communicating SMBG reports to providers, a standardized format that extracts key data elements and facilitates quick review for healthcare providers would be useful. Finally, the practice of SMBG is very expensive, and thus the health economic aspects of SMBG need to be carefully assessed.
Keywords: Self-monitoring of blood glucose, glycemic control, diabetes mellitus
Introduction
Several landmark studies have demonstrated that improved glycemic control reduces the risk of diabetic complications in type 1 (The Diabetes Control and Complications Trial (DCCT)1,2 and type 2 diabetes (United Kingdom Prospective Diabetes Trial (UKPDS)3, Kumanoto Study4. Despite this strong evidence supporting the importance of tight glycemic control, there has been little improvement in glycemic control in the United States5,6 and Europe7–9 in recent years. Clearly, novel interventions or better implementation of existing approaches are needed to help patients achieve tighter glycemic control and reduce the serious complications associated with poor control. Most of the quality improvement efforts to address suboptimal glycemic control have been focused on aspects of health care (e.g., A1C screening rates, more intensive use of polypharmacy), while patient self-management (e.g., self-monitoring of blood glucose (SMBG), medication taking adherence, exercise and diet) have received less attention. This paper will focus on the role of SMBG as a tool to achieving better glycemic control, discuss briefly emerging evidence for its effectiveness, some barriers which stand in the way of its use, and suggest several areas that need to be developed.
Conceptual Framework
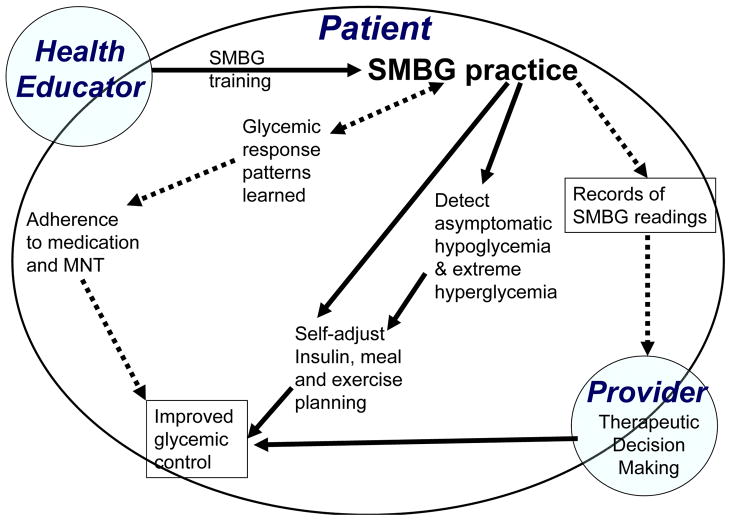
SMBG may lead to improved glycemic control through a multitude of pathways SMBG provides immediate feedback to patients regarding their levels of glycemia. This feedback may help patients achieve better control if it is used to adjust the time, type or dose of insulin therapy. Careful monitoring may reduce the risk of undetected, asymptomatic hypoglycemia and thereby enabling a patient to safely intensify insulin therapy to achieve near-normoglycemia. It is important to note that in all the landmark trials (DCCT1, UKPDS3, Stockholm Trial2 and Kumamoto Study4) patients utilized SMBG to help patients achieve glycemic goals in the intensive therapy arms. SMBG practice may facilitate improved control through several other mechanisms as well. SMBG practice can be used routinely to detect and prevent acute metabolic events due to extreme hyperglycemia10,11. The timing of SMBG is important. Monitoring may useful for identifying asymptomatic hypoglycemic at bedtime, glycemic excursions and postprandial hyperglycemic spikes, a known cardiovascular risk factor12 after meals. A recent study showed the variability (and the extremes) of SMBG readings were the best predictor of hypoglycemia.13 SMBG practice helps patients understand their usual diurnal cycles (“glucose profile”) and glycemic dynamics in response to changes in diet, exercise, transition onto new antihyperglycemic therapy and abnormal clinic states (e.g., intercurrent illness, pregnancy, stress, travel, systemic glucocorticoid treatment). SMBG may provide an important safety factor for individuals employed in high-risk occupations or activities in which a metabolic event could have serious consequences. The process of using SMBG to learn about ones physiologic state and making behavioral and therapeutic adjustments may be empowering, providing patients with a sense of control over their own disease process. Some studies suggest that SMBG practice may lead to better medication adherence, which should in turn benefit glycemic control.14, 15 Benefits for patients treated with oral agents may also be mediated through physician modifications in type and dosing of medication in response to recorded home glucose readings.
While we identify several pathways through which SMBG practice could theoretically lead to improved glycemic control, it is important to keep in mind that all of these pathways are rarely fully taken advantage of by patients and providers. Patient motivation, knowledge regarding actions to be taken in response to readings, and appropriateness of recommendations, among others, play a role. Thus what is theoretically possible (“efficacy”) is rarely duplicated in the real world (“effectiveness”). The discrepancy between efficacy and effectiveness poses a challenge and raises questions. It is unknown whether interventions could strengthen the pathways leading to better control, thereby increasing effectiveness, or whether only patients who utilize certain pathways should be encouraged to practice SMBG.
SMBG Utilization Patterns
In a 1994–1997 survey-based study16 of SMBG utilization patterns in 44,181 patients from the Kaiser Permanente Northern California Diabetes Registry, we assessed adherence to The American Diabetes Association (ADA) guidelines for SMBG practice.17 Although most patients reported some level of SMBG monitoring, 60% of those with type 1 diabetes and 67% of those with type 2 diabetes reported practicing SMBG less than recommended (3–4 times daily for type 1, daily for type 2 treated pharmacologically) (Figure 2). Since the DCCT findings were published, diabetes health education efforts have placed a greater emphasis on the importance of SMBG, resulting in a steady increase in SMBG practice in the United States. Despite this trend, widespread under-utilization persists, and remains much more prevalent than over-utilization.
Figure 2.
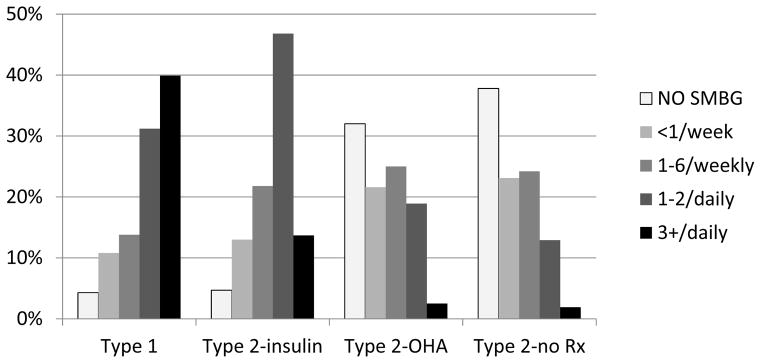
Self-reported SMBG practice among 50,943 patients with diabetes who were>19 years of age by type of diabetes and treatment: the Kaiser Permanente Northern California Diabetes Registry 1995–1997.
Barriers to SMBG
Because test strips are expensive, patients who lack insurance coverage for strips often bear the full financial burden of purchasing the strips themselves. With appropriate utilization for a patient with type 1 diabetes, the annual, out-of-pockets costs for strips alone can approach $1000. This cost represents a financial barrier, particularly for the poorer patients lacking health insurance benefits that cover testing supplies. We have previously reported that utilization of SMBG was inversely associated with out-of-pocket costs, and this “price elasticity” was significantly greater in the poorest patients.16 In a nationwide study (Translating Research Into Action for Diabetes—TRIAD), we again found that patients reduced SMBG utilization when they had to pay full price for strips, although the size of the reduction in utilization did not differ across income.18 Nyomba and colleagues have also confirmed a reduction in strip use with increased expenditure using a trial that randomized patients to either receive free test strips or pay full price for test strips.19 Bowker et al. showed that in a Canadian sample with uniform coverage for medical care and medications, those without public insurance for testing supplies had poorer glycemic control.20
Language and functional health literacy may create another barrier to the practice of SMBG. We found that patients who could not speak English were much less likely to utilize SMBG as recommended.16 Also, patients who have inadequate functional health literacy in general21 may be unable to grasp the complex instructions needed for appropriate SMBG practice, and may not be adequately communicate questions to their healthcare provider. Other significant, independent predictors of non-adherent practice of SMBG include longer time since diagnosis of diabetes, less intensive diabetes therapy, few medical visits, being male, elderly or an ethnic minority, having lower educational attainment, living in a low income neighborhood, current smoking, and excessive alcohol consumption.16,22
Emerging evidence of SMBG effectiveness
Although self-monitoring of blood glucose is widely recommended as a component of diabetes management, there exists substantial controversy about this costly practice, especially for patients not treated with insulin. It has been argued that existing evidence, particularly that pertaining to SMBG’s ability to improve glycemic control, is weak and does not support specific recommendations nor reimbursement for test strips (e.g., by private and governmental health plans)23,24. Although most of the supporting evidence comes from patients with type 1 diabetes25, its usefulness is still sometimes questioned for these patients.26–28 It has even been argued that self-monitoring may cause psychological harm.29 However a study of SMBG in elderly diabetic individuals found no negative influence on their perception of quality of life.14
Historically, there has been even less supportive evidence for SMBG’s use in patients with type 2 diabetes23,28,30–36. Many observational studies have failed to detect a relationship between SMBG and glycemic control.31,33,37 Although there have been several positive anecdotal reports25,26,31,38–41,25,26,31,38–42, the results of most trials have been negative32,43,44.32,35,43,44
A meta-analysis of randomized studies among patients with type 2 diabetes failed to find a positive effect of SMBG on glycemic control.45 However, the authors acknowledged several important limitations in many of the including studies, naming lack of statistical power (the largest study included only 208 patients), inconsistencies in recommended monitoring frequency, lack of standardization of training (and even whether they were trained at all), no or insufficient advice given on how to use SMBG to modify insulin therapy, insufficient study duration, and substantial loss to follow-up. Another meta-analysis stated that most of the previous observational studies also had methodological shortcomings35 including lack of controls, short follow-up, low power (sample sizes ranged from 12 to 250 patients), and failure to stratify patients by type of treatment. Therefore, conclusions from many of the previous studies, experimental or observational, must be evaluated carefully in light of potential study design flaws, whether the results (all or in part) support or deny the value of SMBG.
A newer generation of SMBG studies is emerging. These have used more sophisticated methodologic approaches and avoided many of the design flaws that limited past studies. Soumerai and collegues15 conducted a longitudinal study 2 years before and after implementation of a managed care coverage policy requiring full insurance coverage of blood glucose monitors. These authors used a quasi-experimental (interrupted time-series) analysis to assess whether this policy change increased SMBG practice and improved glycemic control. They showed that this policy change resulted in increased SMBG practice. Moreover there was a 0.63 point improvement in mean A1C among those with poor baseline glycemic control (A1C < 10%) who initiated monitoring, although no significant difference was seen for patients with good or adequate baseline glycemic control. Compared with non-initiators, initiators of blood glucose testing also showed significant improvements in adherent diabetes medication use. Other authors have also reported SMBG was associated with better medication compliance.14 This study provides strong evidence given that the exposure, i.e., policy change, was an external force that, while influencing SMBG practice, is otherwise outside of the sphere of influence of the patient. While we hypothesize that our exposure of interest (e.g., SMBG practice) influences a clinical outcome (e.g., A1C), it is not uncommon that the direction of influence goes the other way around as well; patients may increase or be told to increase SMBG frequency in response to poor A1C values. Such “reverse causality” could introduce a powerful bias unless analyzed carefully. This quasi-experimental design largely circumvents the major methodologic problem caused by reverse causality.
In a randomized, controlled trial, Nyomba et al investigated the effect of randomized provision of free test strips vs. having to purchase strips on SMBG practice and, subsequently, on glycemic control.19 The study was conducted in insulin treated patients matched for age, sex, education, income, type and duration of diabetes, years of insulin treatment, number of daily insulin injections, and A1C. Self-monitoring of blood glucose frequency was significantly higher (p<0.03) in the group who received test strips free-of-charge vs. in the control group who paid for strips (2.0 ± 0.2 tests/day vs 1.4 ± 0.1 tests/day). Mean A1C remained stable over the 12 months in the group receiving free testing strips, whereas glycemic control worsened in the control group and differed significantly (P < 0.002) between groups after 6 months. This evidence suggests that financial barriers to SMBG practice exist and simply providing access to free testing supplies may lead to increased frequency of SMBG practice, and improved glycemic control in pharmacologically treated patients. This is a particularly powerful design not only because it is randomized, but also because the exposure (test strip cost) is outside of the sphere of influence of a patient, and therefore minimizes the impact of reverse causality.
We have previously shown strong associations between baseline SMBG frequency and subsequent (one year later) glycemic control in diabetic patients, even those not treated pharmacologically.46 However a shortcoming with this lagged, cross-sectional design, and most observational studies, is that it does not distinguish between newly initiated SMBG use and ongoing SMBG use. Pharmacoepidemiologists now suggest “new user cohort designs” because 1) new initiators often have a very different baseline clinical profile and different response to an intervention than ongoing users and 2) ongoing users have better outcomes simply because those failing treatment (i.e., SMBG doesn’t help) or adverse events (e.g., excessive discomfort) discontinue use of SMBG, leaving the treatment successes and biasing the results.47
In a longitudinal study of SMBG, we assessed how 4-year changes in SMBG practice influenced changes in glycemic control separately in patients who were previous non-users and newly initiated SMBG and ongoing users. Preliminary finding suggest a surprisingly different effect in these two groups. Among patients who did not previously practice SMBG, initiating SMBG once daily resulted in a significant lowering of A1C in all diabetes sub-groups (i.e., patients using no medication, oral agents only, or insulin treated). Among ongoing SMBG users, we observed a significant effect only in patients treated pharmacologically and the effect size was much smaller than among new users. The observed relationships between strip frequency and A1C, showed a clear dose response with an expected diminishing effect at higher SMBG frequencies. However, among ongoing users of SMBG who were not treated pharmacologically, changes in SMBG frequency had no discernable effect on A1C. These new findings suggest that separate clinical recommendations for new and ongoing users are indicated. The larger sizes in new users who are not treated with insulin may be attributable partially to the large increase in self-awareness facilitated by new SMBG. This important educational aspect may be enhanced by asking patients to perform intensive monitoring for periodically (e.g., 7-point profile on one or two days) to refresh their understanding of diurnal patterns and glycemic response to diet, exercise and therapy.
Healthcare cost considerations and policy changes
Cost concerns are the primary reason SMBG is so hotly debated. The annual direct cost for SMBG test strips alone in the U.S. is estimated to exceed $3 billion. Test strips are the 4th largest pharmacy expenditure and represent 2% of total pharmacy budget at Kaiser Permanente and represent a substantial portion of the total pharmacy budget at the Veterans Administration Hospitals as well (John Piette, Personal Communications). In 2002, United Kingdom’s Nation Health Service (NHS) in a report published by the National Prescribing Centre, described spending about 40% more on test strips than for oral hypoglycemic agents.48 In recent years, California and 37 other states have passed legislation that mandated health plans to provide diabetes supplies, including equipment and strips for SMBG. Health plans across the nation have been concerned about the cost implications of such legislation. On the other hand, patients are also concerned given the trend toward increasing cost-sharing (patient pays bigger share of healthcare costs). Issues of cost will require careful consideration since under-utilization is common even when strips are provided free of charge and utilization will likely decline as the out-of-pocket share increases.16
Decisions about SMBG recommendations have important economic implications for health plans, healthcare providers and patients. While challenging, a careful estimation of cost-effectiveness must be undertaken. Unlike pharmaceuticals, SMBG is a tool for feedback and monitoring, and has no direct effect on health in isolation. For example, the effects of SMBG on glycemic control are theoretically mediated through a variety of mechanisms such as self-regulation of insulin, meal planning, ability to communicate glycemic patterns to the provider and subsequent adjustments of medication regimen and possibly medication-taking adherence. Cost-effectiveness analysis of medications and their clinical effect is relatively routine. However, an objective balancing of the cost of SMBG and its potential indirect impact on glycemic control, as well as risk of hypoglycemia and patient empowerment, will pose a greater challenge.
Patient Health Education
The weak past evidence for SMBG effectiveness is likely attributable in part to the lack of consistent actions, if any, taken by patients or providers in response to SMBG readings. Thus a re-examination of the current training available for patients and providers is needed. A study showed that among patients treated with oral agents or insulin, only 30% and 58% respectively were able to identify their low glycemic targets for home monitoring.49 Low patient comfort with sliding scale insulin adjustments in response to SMBG is not well studied, but may play a factor. This is important because a significantly greater proportion of the patients treated with medications who knew their low target took appropriate action when their blood glucose values were low.
Lack of understanding of behavioral responses to SMBG readings limits the value of SMBG, suggesting the importance of patient education programs. The effectiveness of SMBG may increase as we refine our ability to teach self-management skills, instill greater awareness of their importance, motivate patients to make behavioral changes in response to readings, and enhance self-confidence50,51.42,50–52 For example, well-trained patients more readily modify insulin dose and timing in response to home glucose readings, and improved insulin administration is one of the best way to improve glycemic control.53 Because lifestyle changes such as improved diet and exercise have limited sustainability, patients may benefit from the feedback about the impact of their efforts to make lifestyle changes provided by regular monitoring. Research is needed to confirm whether special training, enhanced patient motivation and confidence (self-efficacy) would improve the effectiveness of self-monitoring.
The design of patient SMBG training programs needs to involve behavioral medicine and health education specialists in addition to endocrinologists and/or diabetologists. The program must be designed to enable even low literacy patients to understand the complexity of SMBG in their self-management.54 The training program also needs to be culturally appropriate and sensitive to patients’ motivation level (e.g., using self-referral to weed out unmotivated patients who will unlikely benefit), discuss cost concerns with the patient, and use up-to-date health educational approaches (e.g., “shared decision-making”).
Continuing Medical Education for the Healthcare Provider
Given the multitude of pharmaceutical options available, tailoring medication regimens as a function of glycemic patterns derived from SMBG data is a complex task. Decision trees could be based on algorithms that incorporate key SMBG summary statistics (e.g., fasting, bedtime, 2 hour post-meal SMBG readings) to make medication regimen decisions. Continuing medical education (CME) that provide advanced training on how to best utilize SMBG data for fine-tuning of medication regimens may be beneficial. Additionally, healthcare providers and health educators should be trained to assess patient motivation and readiness to change; SMBG training is likely to provide the most benefit in the motivated and ready to change patients. Providers need to be trained to confirm that patients in fact understand the complex information communicated regarding SMBG.54 The “teach-back method”, where patients are asked to feedback to their provider or educator what they just learned as a way of confirming that the information was correctly understood has been shown to be a particularly effective way of training complex self-care tasks.21
New Technologies and Healthcare System Factors
Noninvasive (continuous) glucose monitoring may someday replace SMBG as we know it, providing a painless way to monitor glucose automatically and frequently.55 This technological advance could provide detailed information on glucose patterns and trends, facilitating an even better optimization of glycemic control and an early warning system for hypoglycemic events. Patients are more likely to utilize a technology that causes no pain. However, such new technologies are expensive, and future work is needed to evaluate whether they will be cost effective.
Continuous monitoring will generate voluminous amounts of data that will need to be summarized if it is to be useful for healthcare providers. However, even the amount of data accumulated through traditional SMBG can be overwhelming to the healthcare provider, especially when a patient hands in a journal with a year’s recordings of multiple daily SMBG readings at the beginning of a 15 minute clinic appointment. A standard reporting format (preferably electronic) that summarizes the most informative elements from patient SMBG records would be very useful. The current technology facilitates downloading of data from most glucose meters and such reporting could be automated and include graphic representation. However, at this point, the wide range of meter technologies and associated software, and frequent use of paper and pencil records likely creates a confusing and inefficient transfer of data from the patient to the provider.
Conclusions
Given the historically lackluster evidence of SMBG effectiveness and expense of test strips, managed care and governmental decision makers are struggling with decisions around whether and to what extent to support SMBG. There is emerging evidence that SMBG should play an important role in glycemic control efforts for both the patient and healthcare provider. There are clearly avenues that would further strengthen the effectiveness and cost-effectiveness of this expensive intervention. Diabetes care is a complex intervention, of which SMBG is only one facet. We cannot expect interventions aimed this single facet of a complex intervention to be highly efficacious.56. Roach (2004) summed it up nicely: “The effectiveness of any glucose monitoring program is highly dependent on the ability of patients and providers to integrate the practice into an overall program of self-care and therapeutic decision making”.54 A renewed effort toward tightly weaving SMBG into all aspects of care and self-care will increase its value.
Recommendations
Clinical guidelines: Develop separate clinical recommendations for new SMBG users and ongoing SMBG users, and further stratified by diabetes therapy. Ideally, newly initiated SMBG practice would be integrated as a teaching tool into a health education program soon after the diagnosis of diabetes. The guidance for the development of specific SMBG recommendations provided by additional scientific studies is likely limited. Science can only go so far. Guidelines will need to merge expert opinion with a careful review of the scientific evidence though a consensus process. Just such an effort was recently convened (Global Consensus Conference on SMBG, October, 2004) by the International Diabetes Center in Minneapolis. A publication with clinical recommendations is forthcoming.
SMBG health education: Develop health education training programs that teach patients how to perform SMBG, identifies glycemic targets, and trains which specific actions should be taken based on readings (including adjusting insulin dose/timing, preventive action for hypoglycemia, meal planning). Programs need to be geared so that this complex information is fully understood by patients with low functional health literacy and non-English speaking patients.
Continued Medical Education: Train healthcare providers to identify glycemic profiles from patient’s SMBG records and utilize to tailor pharmacotherapy. Healthcare providers also need to be trained on approaches to teaching SMBG to their patients. These should include how to discuss actions to be taken in response to readings and discussions regarding the cost implications of SMBG. Training should also cover how to assess patient motivation.
SMBG reports: Develop a standard reporting format (preferably electronic) that can be used to summarize the key elements from patient SMBG records for a quick assessment by healthcare providers. Minimally, the reports should identify patterns (frequency and timing) of hypoglycemic and hyperglycemic excursions in graphically in tabular form.
Access: Ensure that high risk patients who would benefit from SMBG don’t fall through the cracks due to barriers to care (e.g., financial or language barriers).
Health economics evaluation: Assess cost-effectiveness of SMBG.
Figure 1.
Conceptual model of the potential pathways that link self-monitoring of blood glucose to glycemic control.
Acknowledgments
Financial support: Funded by National Institute of Health (NIDDK): R01 DK61678-02
Reference List
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med. 1991;230:101–108. doi: 10.1111/j.1365-2796.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 5.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Ubink-Veltmaat LJ, Bilo HJ, Groenier KH, Houweling ST, Rischen RO, Meyboom-de Jong B. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: a prospective population-based study in The Netherlands (ZODIAC-1) Eur J Epidemiol. 2003;18:793–800. doi: 10.1023/a:1025369623365. [DOI] [PubMed] [Google Scholar]
- 8.Gatling W, Budd S, Walters D, Mullee MA, Goddard JR, Hill RD. Evidence of an increasing prevalence of diagnosed diabetes mellitus in the Poole area from 1983 to 1996. Diabet Med. 1998;15:1015–1021. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1015::AID-DIA719>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Berger B, Stenstrom G, Sundkvist G. Incidence, prevalence, and mortality of diabetes in a large population. A report from the Skaraborg Diabetes Registry. Diabetes Care. 1999;22:773–778. doi: 10.2337/diacare.22.5.773. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. . Clinical Practice Recommendations 1998. Diabetes Care. 1998;21 (Supplement 1):S1–S98. [PubMed] [Google Scholar]
- 11.Self-monitoring of blood glucose. American Diabetes Association. Diabetes Care. 1994;17:81–86. doi: 10.2337/diacare.17.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Cox DJ, Kovatchev BP, Julian DM, et al. Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79:1659–1662. doi: 10.1210/jcem.79.6.7989471. [DOI] [PubMed] [Google Scholar]
- 14.Gilden JL, Casia C, Hendryx M, Singh SP. Effects of self-monitoring of blood glucose on quality of life in elderly diabetic patients. J Am Geriatr Soc. 1990;38:511–515. doi: 10.1111/j.1532-5415.1990.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 15.Soumerai SB, Mah C, Zhang F, et al. Effects of health maintenance organization coverage of self-monitoring devices on diabetes self-care and glycemic control. Arch Intern Med. 2004;164:645–652. doi: 10.1001/archinte.164.6.645. [DOI] [PubMed] [Google Scholar]
- 16.Karter AJ, Ferrara A, Darbinian J, Ackerson LM, Selby JV. Self-monitoring of Blood Glucose: Language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23(4):477–483. doi: 10.2337/diacare.23.4.477. [DOI] [PubMed] [Google Scholar]
- 17.Supplement 1. American Diabetes Association: clinical practice recommendations 2000. Diabetes Care. 2000;23 (Suppl 1):S1–116. [PubMed] [Google Scholar]
- 18.Karter AJ, Stevens MR, Herman WH, et al. Out-of-pocket costs and diabetes preventive services: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2003;26:2294–2299. doi: 10.2337/diacare.26.8.2294. [DOI] [PubMed] [Google Scholar]
- 19.Nyomba BL, Berard L, Murphy LJ. The cost of self-monitoring of blood glucose is an important factor limiting glycemic control in diabetic patients. Diabetes Care. 2002;25:1244–1245. doi: 10.2337/diacare.25.7.1244-a. [DOI] [PubMed] [Google Scholar]
- 20.Bowker SL, Mitchell CG, Majumdar SR, Toth EL, Johnson JA. Lack of insurance coverage for testing supplies is associated with poorer glycemic control in patients with type 2 diabetes. CMAJ. 2004;171:39–43. doi: 10.1503/cmaj.1031830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163:83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Adams AS, Mah C, Soumerai SB, Zhang F, Barton MB, Ross-Degnan D. Barriers to self-monitoring of blood glucose among adults with diabetes in an HMO: a cross sectional study. BMC Health Serv Res. 2003;3:6. doi: 10.1186/1472-6963-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrick AW, Gill GV, MacFarlane IA, Cullen A, Power E, Wallymahmed M. Home glucose monitoring in type 2 diabetes: is it a waste of time? Diabet Med. 1994;11:62–65. doi: 10.1111/j.1464-5491.1994.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds RM, Strachan MW. Home blood glucose monitoring in type 2 diabetes. BMJ. 2004;329:754–755. doi: 10.1136/bmj.329.7469.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam KS, Ma JT, Chan EY, Yeung RT. Sustained improvement in diabetic control on long-term self-monitoring of blood glucose. Diabetes Res Clin Pract. 1986;2:165–171. doi: 10.1016/s0168-8227(86)80018-3. [DOI] [PubMed] [Google Scholar]
- 26.Mann NP, Noronha JL, Johnston DI. A prospective study to evaluate the benefits of long-term self-monitoring of blood glucose in diabetic children. Diabetes Care. 1984;7:322–326. doi: 10.2337/diacare.7.4.322. [DOI] [PubMed] [Google Scholar]
- 27.Willey KA, Twigg SM, Constantino MI, Yue DK, Turtle JR. Home blood glucose monitoring: how often? Practical Diabetes. 1993;10:22–25. [Google Scholar]
- 28.Belmonte MM, Schiffrin A, Dufresne J, Suissa S, Goldman H, Polychronakos C. Impact of SMBG on control of diabetes as measured by HbA1. 3-yr survey of a juvenile IDDM clinic. Diabetes Care. 1988;11:484–488. doi: 10.2337/diacare.11.6.484. [DOI] [PubMed] [Google Scholar]
- 29.Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314:964–967. doi: 10.1136/bmj.314.7085.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worth R, Home PD, Johnston DG, et al. Intensive attention improves glycaemic control in insulin-dependent diabetes without further advantage from home blood glucose monitoring: results of a controlled trial. Br Med J (Clin Res Ed) 1982;285:1233–1240. doi: 10.1136/bmj.285.6350.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen BT, DeLong ER, Feussner JR. Impact of glucose self-monitoring on non-insulin-treated patients with type II diabetes mellitus. Randomized controlled trial comparing blood and urine testing. Diabetes Care. 1990;13:1044–1050. doi: 10.2337/diacare.13.10.1044. [DOI] [PubMed] [Google Scholar]
- 32.Fontbonne A, Billault B, Acosta M, et al. Is glucose self-monitoring beneficial in non-insulin-treated diabetic patients? Results of a randomized comparative trial. Diabete Metab. 1989;15:255–260. [PubMed] [Google Scholar]
- 33.Wieland LD, Vigil JM, Hoffman RM, Janis LW. Relationship between home glucose testing and hemoglobin Alc in type II diabetes patients. Am J Health Syst Pharm. 1997;54:1062–1065. doi: 10.1093/ajhp/54.9.1062. [DOI] [PubMed] [Google Scholar]
- 34.Tattersall R. Self monitoring of blood glucose concentrations by non-insulin dependent diabetic patients. BMJ. 1992;305:1171–1172. doi: 10.1136/bmj.305.6863.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects. A criteria-based literature review. Diabetes Care. 1997;20:1482–1486. doi: 10.2337/diacare.20.9.1482. [DOI] [PubMed] [Google Scholar]
- 36.Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319:83–86. doi: 10.1136/bmj.319.7202.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24:979–982. doi: 10.2337/diacare.24.6.979. [DOI] [PubMed] [Google Scholar]
- 38.Terent A, Hagfall O, Cederholm U. The effect of education and self-monitoring of blood glucose on glycosylated hemoglobin in type I diabetes. A controlled 18-month trial in a representative population. Acta Med Scand. 1985;217:47–53. doi: 10.1111/j.0954-6820.1985.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 39.Sonksen PH, Judd SL, Lowy C. Home monitoring of blood-glucose. Method for improving diabetic control. Lancet. 1978;1:729–732. doi: 10.1016/s0140-6736(78)90854-1. [DOI] [PubMed] [Google Scholar]
- 40.Walford S, Gale EA, Allison SP, Tattersall RB. Self-monitoring of blood-glucose. Improvement of diabetic control. Lancet. 1978;1:732–735. doi: 10.1016/s0140-6736(78)90855-3. [DOI] [PubMed] [Google Scholar]
- 41.Cohen M, Zimmet P. Self-monitoring of blood glucose levels in non-insulin-dependent diabetes mellitus. Med J Aust. 1983;2:377–380. doi: 10.5694/j.1326-5377.1983.tb122531.x. [DOI] [PubMed] [Google Scholar]
- 42.Muchmore DB, Springer J, Miller M. Self-monitoring of blood glucose in overweight type 2 diabetic patients. Acta Diabetol. 1994;31:215–219. doi: 10.1007/BF00571954. [DOI] [PubMed] [Google Scholar]
- 43.Gallichan MJ. Self-monitoring by patients receiving oral hypoglycemic agents: a survey and a comparative trial. Practical Diabetes. 1994;11:28–30. [Google Scholar]
- 44.Wing RR, Epstein LH, Nowalk MP, Scott N, Koeske R, Hagg S. Does self-monitoring of blood glucose levels improve dietary compliance for obese patients with type II diabetes? Am J Med. 1986;81:830–836. doi: 10.1016/0002-9343(86)90354-2. [DOI] [PubMed] [Google Scholar]
- 45.Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R. Self-monitoring in Type 2 diabetes mellitus: a meta-analysis. Diabet Med. 2000;17:755–761. doi: 10.1046/j.1464-5491.2000.00390.x. [DOI] [PubMed] [Google Scholar]
- 46.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 47.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 48.When and how should patients with diabetes mellitus test blood glucose? MeReC Bulletin (NHS National Prescribing Centre) 2002;13:1–4. [Google Scholar]
- 49.Bjorsness DK, Krezowski PA, Harwell TS, et al. Self-Blood Glucose Monitoring Practices: Do patients know and act on their target? Diabetes Care. 2003;26:3353–3354. doi: 10.2337/diacare.26.12.3353. [DOI] [PubMed] [Google Scholar]
- 50.Peyrot M, Rubin RR. Modeling the effect of diabetes education on glycemic control. Diabetes Educ. 1994;20:143–148. doi: 10.1177/014572179402000210. [DOI] [PubMed] [Google Scholar]
- 51.Rubin RR, Peyrot M, Saudek CD. Effect of diabetes education on self-care, metabolic control, and emotional well-being. Diabetes Care. 1989;12:673–679. doi: 10.2337/diacare.12.10.673. [DOI] [PubMed] [Google Scholar]
- 52.Peyrot M, Rubin RR. Modeling the effect of diabetes education on glycemic control. Diabetes Educ. 1994;20:143–148. doi: 10.1177/014572179402000210. [DOI] [PubMed] [Google Scholar]
- 53.Rubin RR, Peyrot M, Saudek CD. Differential effect of diabetes education on self-regulation and life-style behaviors. Diabetes Care. 1991;14:335–338. doi: 10.2337/diacare.14.4.335. [DOI] [PubMed] [Google Scholar]
- 54.Roach P. Better systems, not guidelines, for glucose monitoring. BMJ. 2004;329:E332. doi: 10.1136/bmj.329.7479.E332. [DOI] [PubMed] [Google Scholar]
- 55.Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282:1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 56.Mulrow CD, Pugh J. Making sense of complex interventions. J Gen Intern Med. 1995;10:111–112. doi: 10.1007/BF02600239. [DOI] [PubMed] [Google Scholar]