Abstract
Thrombospondins are a family of large multi-domain glycoproteins described as matricelluar proteins based on their ability to interact with a broad range of receptors, matrix molecules, growth factors or proteases and to modulate array of cellular functions including intracellular signaling, proliferation and migration. Two members of the thrombospondin family, thrombospondin 1 (TSP1) and thrombospondin 2 (TSP2) are studied extensively to determine their structure and function. While expressed at low levels in normal adult tissues their increased expression is seen predominantly in response to cellular perturbations. Despite structural similarities a notable functional difference between TSP1 and TSP2 includes the ability of former to activate of latent TGF-β and its competitive inhibition by the latter. Both these thrombospondins are reported to play important roles in TGF-β rich ocular environment with most reports related to TSP1. Expressed by many ocular cell types and detectable in the aqueous and vitreous humor TSP1 and TSP2 influence many cellular interactions in the eye such as angiogenesis, cell migration, wound healing, TGF-β activation and regulation of inflammatory immune responses. Together these processes are known to contribute to the immune privilege status of the eye. Emerging roles of TSP1 and TSP2 in ocular functions and pathology are reviewed here.
INTRODUCTION
a. Thrombospondins
Thrombospondins are a family of large extracellular glycoproteins that can bind specific receptors expressed on various cells and modulate their functions such as migration, proliferation or cell death. So far five members have been identified (TSP-1 to TSP-5)1. Due to their interactions with cell surface receptors, growth factors, cytokines or components of ECM thrombospondins can influence many complex tissue-specific processes. Evolutionarily thrombospondins are conserved proteins with greater than 95% homology between murine and human proteins.
b. Functional domains of Thrombospondins
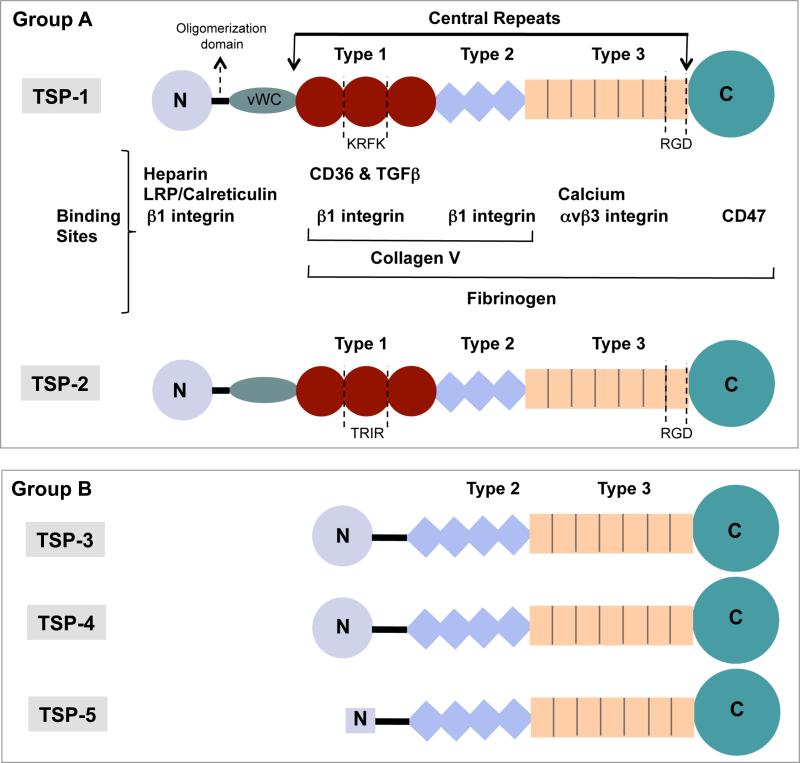
Structurally thrombospondins contain multiple domains as shown in figure 1. These include an N-terminal and lectin-like globular C-terminal domain at two ends with an oligomerization and a vWF type C region and three types (type I, II and II) of repeat sequences between the two terminal domains. Based on the oligomerization domains thrombospondins are divided in two groups – trimer-forming group A thrombospondins and pentamer-forming group B thrombospondins. Group A includes TSP-1 and TSP-2 that are distinct from group B members with a presence of a single vWF domain and three properdin-like type I repeats (TSRs) which include regions that bind receptor CD36 and latent TGF-β. Integrin b1 binding regions are distributed throughout the TSP structure including the terminal domains and all three repeat sequences. Type II repeats are comprised of EGF-like modules. The total number of type II modules differs in group A (3) and group B TSPs (4). No evidence was found for binding of these repeats to EGFR 2. Type III domain includes 13 calcium binding repeats and on average each repeat binds two calcium ions. Overall 31 calcium-binding sites are predicted to be distributed through type II, type III and C-terminal domains 3. The presence of an RGD sequence within type III repeats may allow binding of TSPs to avb3 integrins. Additionally, a region in C-terminal domain binds CD47 receptor, while b1 integrin binding sites are distributed through the type I and type II repeats and N-terminal domain. Thrombospondin-1 can also bind other extracellular matrix ligands including fibrinogen, fibronectin and some collagens, heparin, neutrophil elastase and some matrix metalloproteases (MMPs).
Figure 1. Functional domains of thrombospondins.
Schematic diagram of the multi-domain structures of the members of thrombospondin family. Group A thrombospondins include TSP-1 and TSP-2 that are found in trimeric conformation, while Group B includes TSP-3, TSP-4 and TSP-5/COMP that form pentamers. (vWC = von Willebrand type C domain).
c. Tissue and cellular expression of group A thrombospondins
In a normal healthy adult TSP-1 expression is limited with most abundant protein in alpha granules of platelets and thus very low levels in plasma (100-200 ng/ml) and higher levels (50 – 75 μg/ml) in serum 4, 5. In human tissue constitutive expression of TSP-1 was detected by immunostaining in peritubular connective tissue of kidneys, subendothelial region of aortic vessels, dermal-epidermal junction of the skin, base of the epithelial cells in the sweat glands in the dermis and skeletal muscle 6 and cells of the trabecular meshwork, cornea and conjunctiva of the eye 7, 8. Distribution of TSP-1 and TSP-2 in different ocular compartments is summarized in Table 1.
Table 1.
Expression of Group A Thrombospondins in the eye
| Ocular site | TSP-1 | TSP-2 |
|---|---|---|
| Aqueous Humor | Sheibani et al.19 | |
| Trabelcular meshwork | Flugel-Koch et al.7 | |
| Haddadin et al.20 | Haddadin et al.20 | |
| Cornea - | ||
| Epithelium | Uno et al.21, Hiscott et al.22 | |
| Keratinocytes | Uno et al.21 | |
| Endothelium | Uno et al.21, Hiscott et al.22 | |
| Conjunctiva – | ||
| Dendritic cells | Gandhi et al.23 | |
| Iris and Ciliary Body – | ||
| Epithelium | Futagami et al. 24 | Futagami et al.24 |
| Stroma | Cursiefen et al.25 | Cursiefen et al.25 |
| Retina – | ||
| Ganglion cells | Huang J. et al.26 | Huang J et al.26 |
| Inner nuclear layer (bipolar cells, horizontal cells) | ||
| Outer plexiform layer | Suzuma et al.27 | |
| Photoreceptors | ||
| Vascular cells | ||
| Epithelial cells | Carron JA et al.28 | Carron JA et al.28 |
| Miyajima-Uchida et al.29 | Futagami et al.24 | |
| Futagami et al.24 | ||
| Glial cells | Cinati et al.30 | Cinati et al.30, Huang J. et al.26 |
In most cell types TSP-1 is induced by wounding or during tissue remodeling. Exposure of cells to factors like TGF-β, retinoic acid, vitamin A or progesterone also increases TSP-1 expression 9-13. In addition to oncogenes (Ras and Myc) mediated negative regulation of TSP-1 expression in tumor cells 14, in some cells such as macrophages inflammatory stimulation has been reported to reduce TSP-1 expression 15, 16. Among immune cells immature dendritic cells 17 express TSP-1 and increase it in response to microbial stimuli, PGE2 or TGF-β. Similarly macrophages increase TSP-1 expression in response to TGF-β 9. While T lymphocytes store TSP-1 intracellularly their adhesion to fibronectin and collagen type IV enhances their cell surface expression of TSP-1. Endogenous TSP-1 in T cells regulates their migration in the absence of exogenous TSP-1 18
d. Functions of TSP-1
(1) Cell adhesion and migration
By binding various extracellular matrix proteins TSP-1 is involved in cell adhesion as well as cell migration. However, multiple receptors engaged by TSP-1 makes this function differ based on cell types such that an inhibitory effect on cellular migration is reported in the case of vascular endothelial cells 31 and dendritic cells 32, while promotion of migration is noted in the case of neural crest cells 33, fibroblasts 34, corneal endothelial cells 35 and T cells 36. In the case of tumor cells migration is correlated with metastasis; with inhibition reported in some tumor cells 37 and tumor progression in others 38, 39. These differences are partly due to the fact that tumor cell migration is downstream of other functions influenced by TSP-1 such as activation of latent TGF-β, inhibition of angiogenesis or up-regulation of matrix metalloproteinases 40. These differences also translate to different outcomes e.g. the absence of TSP-1 inhibits migration of fibroblasts delaying wound healing 35, 41, but also enhances DC migration to draining lymph nodes promoting antigen sensitization and subsequent immune response 32.
(2) Inhibition of angiogenesis
The antiangiogenic effect of TSP-1 is delivered directly via CD36 and CD47 receptors expressed on vascular endothelial cells regulating their migration, proliferation and apoptosis 42, 43 and subsequently inhibiting growth of new blood vessels. The lymphangiogenic effect of TSP-1 is mediated indirectly via ligation of CD36 on tissue macrophages and inhibiting their expression of the VEGF-c 44. These mechanisms contribute to the observed increase in both hem- and lymphangiogenesis in TSP-1 deficient mice 25, 44, 45.
(3) Activation of latent TGF-β
Thrombospondin-1 mediates activation of latent TGF-β through a specific sequence located in type I repeats of TSP-1 (KRFK) 46 which binds LSKL sequence located in the amino terminus of LAP of latent TGF-β to release biologically active TGF-β 47, independently of RGD:integrin interactions. This TSP-1 binding sequence is conserved in LAPs associated with all isoforms of TGF-β making their activation possible by TSP-1 47, 48. However, due to the absence of integrin binding RGD sequence in the LAP isoform (LAPβ2) associated with TGF-β2 it cannot be activated by integrins 49, 50. This may explain the importance of TSP-1 as a major TGF-β activator in the ocular tissues where TGF-β2 expression predominates 51-54 further supporting the spontaneous development of ocular pathology in TSP-1 deficient mice 55. In type 1 repeats of TSP-2 however a TRIR sequence corresponding to the KRFK sequence does not activate latent TGF-β 46. In addition to direct activation of latent TGF-β, TSP-1 is also known to induce expression of metalloproteases MMP-2 and MMP-9 capable of activating latent TGF-β 56, 57.
ANGIOGENESIS
Retina
Retinal vascular development in the mouse occurs postnatally mainly through angiogenesis. The newly forming vessels begin spreading from the optic nerve towards the retinal periphery following a scaffolding pattern laid out by the retinal astrocytes thus forming the superficial layer of retinal vasculature. This is normally complete by the first week of postnatal life. During the second and third postnatal week of life, these vessels sprout deep into the retina and form the deep and intermediate layers of retinal vasculature. Thus, the primary retinal vascular plexus is completed by the third postnatal week of life. The retinal vasculature continues undergoing pruning and remodeling and by six-weeks of postnatal life the formation of the retinal vasculature is complete58. These processes are regulated by a tightly balanced production of positive and negative factors, whose alterations under various pathological conditions may result in retinal neovascularization and growth of abnormal vessels as occurs in retinopathy of prematurity and diabetic retinopathy.
The normal developing retinal vasculature exhibits a highly restricted pattern of organization which does not extend beyond the inner limiting membrane into the vitreous or beyond the outer plexiform layer in the inner retina. It was hypothesized that the presence of some factor(s) at these sites may be responsible for the restricted patterns of normal vessel growth. Furthermore, alterations in the production of such factors may contribute to various pathologies with a neovascular component. A number of such factors have now been identified which include both proangiogenic factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) and negative regulators of angiogenesis such as thrombospondin-1 (TSP1) and pigmented epithelium derived factor (PEDF)59-61. Alterations in production of these factors are linked to ocular neovascularization in various eye diseases.
We showed TSP1 is present at very high levels in vitreous and aqueous humor prepared from various species including mice, rat, bovine, and humans19. Most interestingly, we showed a significant decline in production of TSP1 in aqueous humor and vitreous samples of diabetic rats, and humans with diabetic retinopathy. We and others have shown that PEDF is also present in vitreous samples prepared from humans with and without diabetes62. The physiological roles of these molecules in retinal vascular development and pathogenesis of various ocular diseases remains an area of significant importance for investigation.
a. Retinal vascular development
The presence of significant levels of TSP1 in the vitreous and its decreased production during diabetes suggested an important role for TSP1 in retinal vascular homeostasis. In addition, although an anti-angiogenic activity was previously demonstrated for TSP163, the physiological role TSP1 played during vascular development and angiogenesis was not known. Mice deficient in TSP1 have been generated and are viable 64, 65. These mice exhibited defects in the development of alveolar structures in the lung, which was mainly attributed to lack of sufficient TGF-β activity, since TSP1 is capable of activating TGF-β, at least in some tissues. These mice were also shown to exhibit increased vascular density and defects in wound healing.
Using these mice we investigated how lack of TSP1 impact postnatal retinal vascular development and neovascularization during oxygen induced ischemic retinopathy (OIR). Examining the postnatal developing retinal vasculature at one, two, and three weeks of postnatal life showed no significant differences in the density and pattern of developing retinal vasculature. However, a significant difference in density of retinal vasculature was observed at six weeks of age. Retinas from six-week-old TSP1-deficient mice showed increased vascular density compared with wild type mice. This was concomitant with increased density of retinal endothelial cells, but not retinal pericytes, in TSP1-deficient mice58. Together these results established, for the first time, an important role for TSP1 expression during vascular remodeling and pruning of the developing vasculature.
We also asked whether lack of TSP1 affects retinal neovascularization during OIR. Although, an increase in degree of neovascularization at postnatal day 17 (P17; when maximum neovascularization occurs in this model) was observed in TSP1 deficient mice compared with wild type mice, it was not significant. However, we observed a significant decrease in degree of vessel obliteration following exposure to hyperoxia. Furthermore, we examined the expression of TSP1 and VEGF during OIR. Increased expression of VEGF is a driving force during the hypoxic phase of OIR. We observed a significant increase in VEGF expression during OIR independent of TSP1 status. In addition, we showed that TSP1 expression is not responsive to hyperoxia/hypoxia during OIR suggesting a passive role for TSP1 function. Our data suggested that there is a threshold level for VEGF expression during angiogenesis and vascular remodeling and pruning such that when the VEGF level is above this threshold it results in endothelial cell activation and angiogenesis, and when VEGF drops below this threshold TSP1 will eliminate those vessels. Furthermore, we showed that postnatal retinal vascularization and neovascularization during OIR were attenuated in mice with increased levels of TSP1 expression in their eye66. These results are consistent with the proposed physiological role of TSP1 during vascular pruning and remodeling.
b. Pathogenesis of diabetic retinopathy
Our early studies in rat demonstrated a significant decrease in TSP1 levels in the vitreous and aqueous humor samples prepared from diabetic animals suggesting an important role for TSP1 in the development and progression of diabetic retinopathy19. We also showed that incubation of microvascular endothelial cells under high glucose results in decreased levels of TSP1. We have now confirmed the result of culture studies using retinal endothelial cells. We showed that exposure to high glucose results in decreased production of TSP1 and enhanced migration of retinal endothelial cells without affecting other functions67. In addition, retinal endothelial cells prepared from TSP1 null mice exhibit enhanced migration and proangiogenic activity 68, 69. These studies are all consistent with our previous work showing that expression of TSP1 in the endothelial cells promotes a differentiated, quiescent phenotype 45, 70.
To further demonstrate the important role of TSP1 in the development and progression of diabetes, we hypothesized that a decreased level of TSP1 will exacerbate the pathogenesis of diabetic retinopathy. To test this hypothesis we bred our TSP1 deficient mice with the Akita/+ mice, which develop diabetes at four weeks of age. We and others have shown that Akita/+ mice exhibit some of the early retinopathy changes including thickening of vascular basement membrane, loss of pericytes, and acellular capillaries with a relatively long duration of diabetes (at least 10 months) 71, 72. In contrast, we recently showed that Akita/+ mice deficient in TSP1 develop more severe retinopathies with 7 months of diabetes72. In addition, we showed that TSP1-deficient mice made diabetic by streptozotocin, or bitransgenic Akita/+ mice where down-regulation of TSP1 in the endothelium occurs at will, also developed more severe retinopathy with a relatively short duration of diabetes. Thus, down regulation of TSP1, most likely in the endothelium, during diabetes contributes to the pathogenesis of diabetic retinopathy and is consistent with detection of reduced levels of TSP1 in vitreous samples from diabetic patients62. These studies also provide support for potential use of TSP1 as a biomarker for severity of diabetic retinopathy and its potential use as target for therapy.
c. Choroidal neovascularization
Our studies as well as studies from other groups have also suggested an important role for TSP1 in the regulation of choroidal vasculature73-75. Lutty and colleagues demonstrated a significant decrease in TSP1 levels in Bruch's membranes prepared from patients with exudative Age related Macular Degeneration (AMD)74. Using TSP1-deficient mice we asked whether TSP1 expression impacts choroidal neovascularization in the mouse model of laser-induced choroidal neovascularization. We recently showed that lack of TSP1 exacerbates the laser-induced choroidal neovascularization in TSP1-deficient mice compared with wild type mice. We showed that this was associated with the significant enhancement of macrophages recruited to the wound area in TSP1−/− mice75. Furthermore, to test the therapeutic of use of TSP1 in inhibition of choroidal neovascularization, we used a TSP1 antiangiogenic mimetic peptide in wild type and TSP1−/− mice subjected to laser induced choroidal neovascularization. We observed a significant inhibition in animals receiving intravitreal injection of this peptide. Thus, TSP1 and its antiangiogenic mimetic peptides provide a suitable approach for inhibition of choroidal neovascularization and are the subject of further investigation in our laboratory.
d. Uveal melanoma
Uveal melanoma is the most common primary intraocular malignant tumor in humans, and it occurs predominantly in a nonhereditary, sporadic manner 76, 77. Although some patients get successful treatment, approximately half of all patients ultimately develop metastases and die within a year. This is generally associated with expansion of its metastasis in the liver with very poor prognosis. This behavior is suggestive of production of anti-angiogenic factors by the primary tumor, which keep the metastasis in check and upon removal of primary tumor, the metastasis began to expand. We hypothesized that changes in the expression of TSP1 may contribute to the development and progression of uveal melanoma.
Using a murine transgenic pigmented ocular tumor model 78 we asked whether changes in TSP1 level occurs with progression of the tumor. In these mice the tumors begin to develop by 3 weeks of age and by 12 weeks of age the tumor fills the whole eye cavity. We showed at 3 weeks there is significant expression of TSP1 in the primary tumors and its level significantly decreases as the tumor grows and becomes highly vascularized. We also showed increased ocular expression of TSP1 in these mice is sufficient to suppress tumor growth66. Similar results were observed when these mice were treated with TSP1-anti-angiogenic mimetic peptide75. Thus, alterations in production of TSP1 may also make a significant contribution to the pathogenesis of uveal melanoma and provides a suitable target for detection and treatment of uveal melanoma.
e. TSP2 and retinal vasculature
TSP2 is a closely related subfamily member of the TSP family of matricellular proteins with antiangiogenic activity79. Very little is known about the physiological role of TSP2 in vascular development and function. Mice deficient in TSP2 exhibit mainly connective tissue defects presented as collagen fibril formation defects80. These mice also exhibit additional abnormalities under various challenges including wound healing and oxidative stress 81, 82. Our preliminary studies using these mice indicate minimal effects on postnatal retinal vascular development and neovascularization during OIR. These mice also do not significantly differ from their wild type counterparts in response to laser induced choroidal neovascularization and streptozotocin induced diabetes. In culture, we have shown that both retinal endothelial cells and pericytes express TSP2, and TSP2 levels are significantly higher in retinal pericytes 83, 84. In addition, our studies with TSP2-reporter mice, which express GFP driven by TSP2 promoter, indicate retinal pericytes are a major source of TSP2. Our studies also suggest that the increased production of TSP2 under oxidative stress conditions may have inhibitory effects on angiogenesis 83, 84
Cornea
The normal cornea, the transparent windscreen of the eye, is devoid of both blood and lymphatic vessels (for review see ref.85). This avascularity is actively maintained and has been described as “corneal (lymph)angiogenic privilege.” Corneal avascularity is mandatory for corneal transparency and good visual acuity and is therefore evolutionary highly conserved 44, 86 In addition, there is a huge overlap in the mechanisms contributing to corneal angiogenic privilege and “corneal immune privilege.” In fact, both together allow for the remarkable success of unmatched penetrating keratoplasties.
Corneal (lymph)angiogenic privilege can be described by several characteristics:
- it is present already during early development in utero, characterized by fetal absence of corneal blood and lymphatic vessels, in contrast to adjacent vascularised conjunctiva,
- it is redundantly organized, i.e. failure of one defence mechanism does not spontaneously cause lack of avascularity, and
- it follows a threshold concept, i.e. low grade inflammatory and angiogenic stimuli (the eye is constantly exposed to) do not cause pathologic corneal lymphangiogenesis. When a certain threshold is supervened, massive neovascular ingrowths can occur.
Corneal angiogenic privilege is maintained by several mechanisms (for review see ref. 1):
Endogenous inhibitors, primarily localized at the epithelial/basement membrane and the Descemet's layer.
Receptor decoy mechanism, such as ectopically expressed VEGF receptor 3, and soluble forms of VEGF R1 and R2.(2)
Mechanisms interfering with hypoxia driven VEGF upregulation (IPAS).
Thrombospondins are a family of matrix factors, which are known to act as antiangiogenic agents extraocularly. What is the role of thrombospondins (TSP), and especially TSP 1 in corneal (lymph)angiogenic privilege?
Expression of TSP 1 and 2 has been reported in the eye, especially in the cornea and the iris both at the mRNA and protein level87. The absence of TSP 1 and TSP 2 or both in genetically altered mice does not cause spontaneous ingrowths of blood vessels into the cornea 87 This supports the concept of a redundantly organized angiogenic privilege. In contrast, lack of TSP 1 does cause an increased vascular density in other intraocular tissues 87 The iris vascularity in TSP 1 deficient mice is significantly higher compared to wild-type animals.
Under inflammatory conditions, TSP1 deficient mice show a significantly increased angiogenic and lymphangiogenic response. That indicates an immunomodulatory and thereby anti(lymph)angiogenic effect of primarily TSP 187
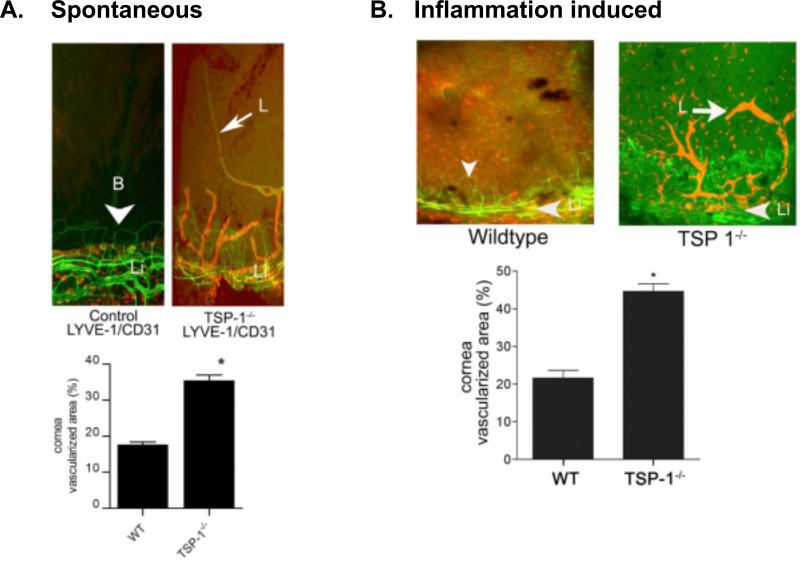
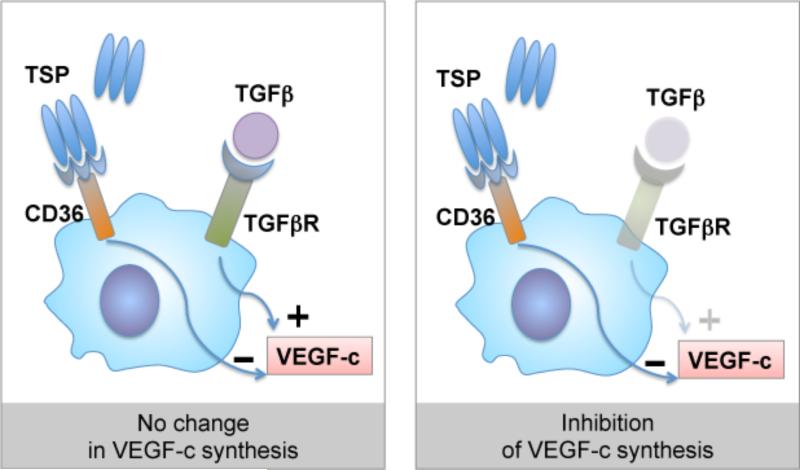
Even more interesting is the effect of TSP 1 deficiency on corneal lymphangiogenic privilege. Lack of TSP 1 in older mice causes spontaneous ingrowths of lymphatic vessels from the limbal vascular arcade into the normally avascular cornea44. This increased lymphangiogenesis is also observed at extraocular sites, e.g. at the ear skin and can be antagonized using topical TSP 1 application. The increased spontaneous corneal lymphangiogenesis in TSP1 deficient mice goes along with an increased induced lymphangiogenesis under inflammatory conditions (figure 2), e.g. in the mouse model of suture-induced corneal neovascularisation44. Increased lymphangiogenesis is in parallel to an increased influx of prolymphangiogenic macrophages into the cornea and increased tissue levels of prolymphangiogenic growth factor VEGF-C44. We hypothesized that the obvious antilymphangiogenic effect of TSP 1 may be regulated via lymphendothelial CD36, as is the antihemangiogenic effect of TSP 1. Indeed, CD36 deficient mice do show a similar corneal vascular phenotype as TSP 1 deficient mice44. But, CD36 is not expressed on lymphatic endothelial cells. Searching for potential other target cells, we identified a strong CD36 expression on corneal macrophages,(5) which are known to play a key role in inflammatory lymphangiogenesis87. We therefore tested the hypothesis that TSP 1, via CD36 on macrophages, inhibits lymphangiogenesis. Supporting that concept, bone marrow transplantation does abolish the prolymphangiogenic effect of CD36 deficiency. In addition, TSP 1 does downregulate the VEGF-C and D expression in macrophages, dependent on the presence of the CD36 receptor (figure 3)44. In summary, these findings indicate that TSP 1 via CD 36 signalling downregulates macrophage VEGF-C and D secretion and thereby contributes also to corneal lymphangiogenic privilege.
Figure 2. Increased lymphangiogenesis in TSP-1 deficient cornea.
Corneal flat mounts from WT and TSP-1 deficient mice stained with fluorescence conjugated anti-CD31 (green) and anti-LYVE-1 (red) under spontaneous (A) and suture-induced inflammatory conditions (B). Morphometric analysis of vascularized areas with LYVE-1+++ /CD31+ lymphatic vessels is presented below each set of staining. (* p < 0.05). From Cursiefen et al. J.Exp.Med. 2011, 208(5):1083
Figure 3. Regulation of lymphangiogenic factor VEGF-c expression in macrophages via CD36-TSP interaction.
Binding of CD36 by thrombospondin inhibits TGF-β-induced VEGF-c expression in macrophages. In a normal cornea such regulation is likely with TSP-2:CD36 interactions that may prevent VEGF-c secretion in response to any TGF-b activated during normal wound healing processes.
TSP 1 is an important endogenous modulator of both hem-and lymphangiogenesis and significantly contributes to corneal hem- and lymphangiogenic privilege. The cornea, due to its physiologic avascularity, is a useful model system to identify novel endogenous regulators of angiogenesis and lymphangiogenesis 88, 89.
WOUND HEALING
Transforming growth factor beta (TGF-β) being a major player in wound repair, many studies on TSP-1's role in wound repair have focused on its ability to bind the TGF-β latency-associated peptide (LAP) followed by a conformational change that allows TGF-β receptor to access active TGF-β.90 This review deals only with the role of TSP-1; readers are referred to Hiscott et al. (2006)91 for a discussion of other members of the TSP family in wound repair.
In the adult cornea, TSP-1 has been localized to the corneal endothelium and Descemet's Membrane (DM) in mouse,35 rat,92 bovine,22 and human22, 93. Little, if any, TSP-1 has been seen in the stromal keratocytes in any species22, 35, 92, 93. Localization in the corneal epithelium appears to vary between species with only minor amounts of TSP-1 observed in mouse21, 35 and rat;92 however, TSP-1 has been localized just below the corneal epithelium in human8. Also, in situ hybridization demonstrated that TSP-1 was expressed by the basal epithelial cells8. Interestingly, TSP-1 localization appeared to coincide with the Bowman's layer, with TSP-1 levels decreasing in the limbus and absent in the conjunctiva8. These authors postulated that TSP-1 bound the Bowman's layer and functioned as an anti-angiogenic molecule. Studies in TSP-1-deficient mice suggested that TSP-1 either was not required for development or that one of the other members of the TSP family had overlapping functions with TSP-1. Corneas of TSP-1-deficient mice appear morphologically normal except for a thinning of the cornea.20, 35
Although the role of TSP-1 in normal homeostasis of the cornea is unclear, several studies of corneal wound repair have suggested it has an important role8, 20-22, 92-96. These reports described three wound models. In the simplest wound model, corneal debridement, the epithelium was removed, leaving the basement membrane (BMZ) and underlying matrix undamaged. TSP-1 was rapidly induced after wounding, and within 30 minutes, TSP-1 was deposited in the wound area in mouse, rat, and human models.21, 92, 95 Little, if any, expression of TSP-1 was observed in the stromal cells. TSP-1 expression persisted until shortly after epithelial wound closure. At least 2 reports suggest the importance of TSP-1 expression in the debridement model. First, Uno et al. (2004)21 reported that in Vitamin A deficient mice, TSP-1 was not deposited after wounding, which correlated with slowed healing. Second, Uno et al. (2005)96 demonstrated that the addition of exogenous TSP-1 enhanced healing rates, while addition of anti-TSP-1 slowed healing rates.
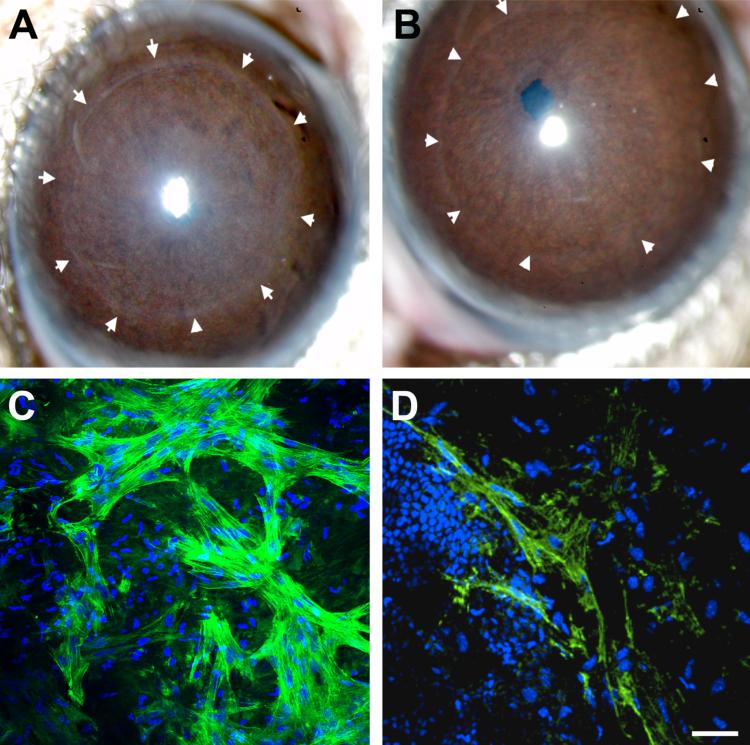
In the second wound model, superficial keratectomy, the epithelium, basement membrane, and a portion of the anterior stroma were removed. In contrast to the debridement wound, TSP-1 expression was observed at high levels in the stroma subjacent to the wound-healing epithelium. In the study by Matsuba et al.,92 TSP-1 was observed to be upregulated 24 hours after wounding and peaked at 7-8 days in rats. These studies did not indicate whether the TSP-1 was secreted from the epithelium or the wound-healing fibroblasts. Most interesting was the finding that TSP-1 co-localized with the myofibroblasts that were generated in response to the wound. These studies suggested that TSP-1 played a role in myofibroblast formation, perhaps through activation of TGF-β. However, this was not conclusively demonstrated. In support of this concept, superficial keratectomy wounds in TSP-1-deficient mice were examined. As seen in the Figure, haze formation, indicative of myofibroblast formation, was greatly reduced in TSP-1-deficient mice. In addition, examination of alpha-smooth muscle actin (SMA: a marker of myofibroblasts) in whole mounts demonstrated that myofibroblast formation also was greatly reduced in TSP-1-deficient mice (figure 4).
Figure 4. Abberations in wound healing of TSP-1 deficient cornea.
C57BL/6 mouse eyes 3 weeks (A, B) or 1-week (C, D) post-keratectomy in wild type (WT: A, C) and TSP-1-deficient mice (B, D). A) Slit lamp image of WT mouse cornea showing haze present within the circle of arrows, indicating original wound area. (B) Slit lamp image of TSP-1-deficient mouse cornea demonstrating lack of haze formation within original wound area (arrows). C) Whole mount immunolocalization of α-smooth muscle actin (SMA) within the stroma at the original wound site in WT mice. (D) TSP-1-deficient cornea demonstrating dramatic decrease in SMA. TOPRO3 was used as a counterstain to mark all cell nuclei. Bar = 50 μm.
In the third model, a penetrating wound was generated in which all levels of the cornea were damaged. Hiscott et al. (1999)93 demonstrated in a human organ culture model that after a penetrating wound, TSP-1 was deposited in the wound area. More recently, Blanco et al.35 compared healing in wild type (WT) and TSP-1-deficient mice. In WT mice, the corneas initially exhibited mild edema after wounding, closed the wound by 1 week, and by 2 weeks were clear with only a thin scar. In contrast, the TSP-1-deficient mice exhibited a gaping wound and persistent edema that lasted for the length of the experiment (8 weeks). In the WT mice, TSP-1 was first observed in the epithelium, and then spread into the wounded stroma. By one week after wounding, TSP-1 was expressed at high levels throughout the wound area, extending from the epithelium to the stroma. Obvious upregulation of TSP-1 was also seen in the endothelium. Expression of TSP-1 peaked between 1 and 2 weeks and gradually reduced to control levels by 4 weeks. When SMA was localized to demonstrate myofibroblast generation, there was an apparent co-localization of TSP-1 and SMA, indicating, as seen in the keratectomy wounds, that TSP-1 and SMA localize to the same area of the wound. When TSP-1-deficient mice were examined, there were several notable healing defects. Most interesting was the obvious defect in healing of the endothelium. In contrast to the WT mice where partial DM restoration was observed by 2 weeks after wounding, no DM restoration was observed in the TSP-1-deficient mice. In addition, the endothelial layer was restored with sparse, greatly elongated endothelial cells that had migrated to cover the wound area. This is in agreement with the finding of Scheef et al.94 who found that endothelial cells isolated from TSP-1-deficient mice migrated far slower in culture than WT cells. The TSP-1-deficient mice also had defects in healing of the posterior stroma exhibiting a gaping wound in contrast to the WT corneas, which sealed the wound. When SMA was localized in the TSP-1-deficient mice, there was an obvious decrease in the number of myofibroblasts. This lack of myofibroblasts might be responsible for the gaping wound, but it is also possible that the lack of stromal healing was the result of the lack of endothelial healing. The molecular mechanism of TSP-1 in these healing defects is yet to be determined.
In summary, the role of TSP-1 during corneal development and homeostasis is unclear. TSP-1-deficient corneas develop and function with no gross abnormalities. However, TSP-1 appears to be a major player in all wound models studied to date. Potential roles include enhancement of epithelial and endothelial migration, anti-angiogenesis, and myofibroblast generation. Many questions remain unanswered, including does TSP-1 function by activating TGF-β in these wound models and how does TSP-1 produced by epithelium, endothelium, and fibroblasts interact with TSP-1 produced by immune cells?
IMMUNE REGULATION
The immune privilege status of the eye depends on several immunoregulatory mechanisms that maintain the precise balance between effective immunity for pathogen clearance and protective immunity to maintain ocular tissue integrity. Cells in the eye with their ability to express TSP-1 contribute to such immuneregulation.
a. Ocular Antigen Presenting Cells
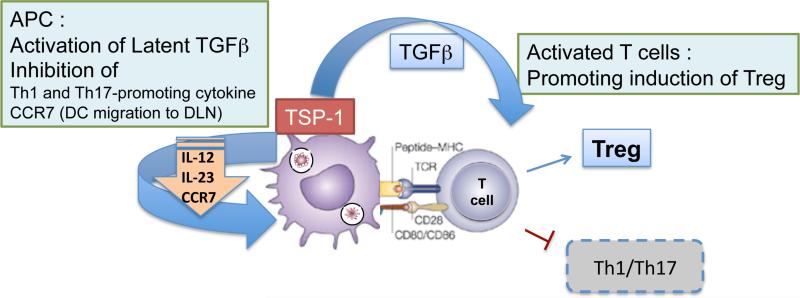
Normal eye derived antigen presenting cells (APCs) are capable of inducing a systemic regulatory immune response that can suppress inflammatory effector functions. Unlike conventional APCs in the periphery, ocular APCs are exposed to a TGF-β rich environment, which dictates their functional properties including migration to the spleen to induce regulatory immune response 97. Based on the preferential splenic localization of intravenously infused TGF-β-treated peripheral APCs, potential migration of ocular APCs via circulating blood was suggested rather than lymphatic channels as expected at other conventional sites 98, 99. This was previously considered possible in the absence of lymphatic drainage, but now appears paradoxical with the reported lymphatic drainage of ocular antigens 100, 101. In response to TGF-β, APCs increase their expression of TSP-1 9 which recently was reported to inhibit expression of CCR7, a receptor key to lymph-node migration of dendritic cells via afferent lymphatics32. This may explain the preferential migration of ocular APCs to the spleen.
Additionally, TSP-1 expressed by TGF-β-exposed APCs while remaining tethered to their surface via CD36 facilitates activation of their newly synthesized latent TGF-β 102, 103. Their dependence on TSP-1 to activate TGF-β over other available mechanisms such as integrins is likely associated with their predominant expression of TGF-β2 (unpublished observation) after being exposed to the same isoform abundantly available in the eye. Moreover, besides environmental TGF-β2, the exogenous TSP-1 also induces TGF-β2 expression by APCs 102. Thus ocular APCs successfully utilize local TSP-1 and TGF-β2 to initiate their own synthesis of these molecules. Membrane bound TSP-1 in APCs can also help activate TGF-β in the vicinity of responding T cells engaged during antigen presentation and by ligating CD47 on APCs, it inhibits expression of Th1-promoting inflammatory cytokine 9, 104. Thus TSP-1 expressing APCs promote development of regulatory T cells in the spleen that are critical to the downstream suppression of inflammatory immune response against ocular antigen (figure 5). Without TSP-1, TGF-b exposed APCs fail to suppress Th1-mediated inflammatory delayed type hypersensitivity response 102.
Figure 5.
Schematic representation of TSP-1 dependent regulation of immune responses
Immature dendritic cells are known to express TSP-1, which was reported to prevent their maturation and inhibit activation of Th1 effectors 17, 105. Similarly corneal APCs are dependent on their TSP-1 in maintaining immature phenotype under inflammatory conditions, which confers on the corneal tissue the advantage of prolonged survival in an allogeneic recipient without immunosuppression 106. In addition to regulating immunogenicity of corneal APCs, by impairing their expression of CCR7, TSP-1 prevents their lymph node migration and subsequent sensitization of the host against the alloantigen. In the retina, TSP-1 regulates the pro-inflammatory phenotype of microglia, the resident APCs, such that TSP-1 deficiency results in their activation with increased expression of inflammatory factors in the retina107.
Thus clearly TSP-1 is involved in the regulation of ocular APCs and contributes significantly to ocular immune regulation. The epithelial cells in the anterior as well as posterior segment of the eye have the potential to express TSP-1 8, 29 and therefore can help maintain a non-inflammatory phenotype of ocular APCs under normal conditions. Consistent with this possibility loss of immune privilege in TSP-1 deficient mice is evident from exacerbated uveitic inflammation of the retina that results in irreversible tissue damage 108. Together such immunoregulatory role of TSP-1 in the context of its anti- hem- and lymphangiogenic property and the ability to activate TGF-β2 makes it a critical molecule in preserving ocular immune privilege status.
b. TSP-1 deficiency and autoimmune Sjögren's syndrome
Deficiency of TSP-1 in mice is neither lethal nor does it result in gross developmental abnormalities but several studies report abnormal response to injury or immunologic perturbations in these mice 1. Although spontaneously increased expression of inflammatory markers TNF-α and iNOS was detected in TSP-1 deficient retina 107, evaluation of chronic inflammatory changes in the intraocular tissues in TSP-1 deficient mice remains to be examined. The absence of overt spontaneous inflammation in the anterior or posterior segment of TSP-1 deficient mice appears contrary to the significant role of this molecule in ocular immune privilege. However, expression of inflammatory markers (TNF-α, MCP-1 and MIP-2) that were previously associated with ocular surface inflammation are detectable in TSP-1 deficient cornea55. Disruption of corneal barrier integrity precedes this inflammation. Further characterization revealed spontaneous development of an ocular phenotype in TSP-1 deficient mice that involves inflammation of the ocular surface tissue associated with autoimmune Sjögren's syndrome.
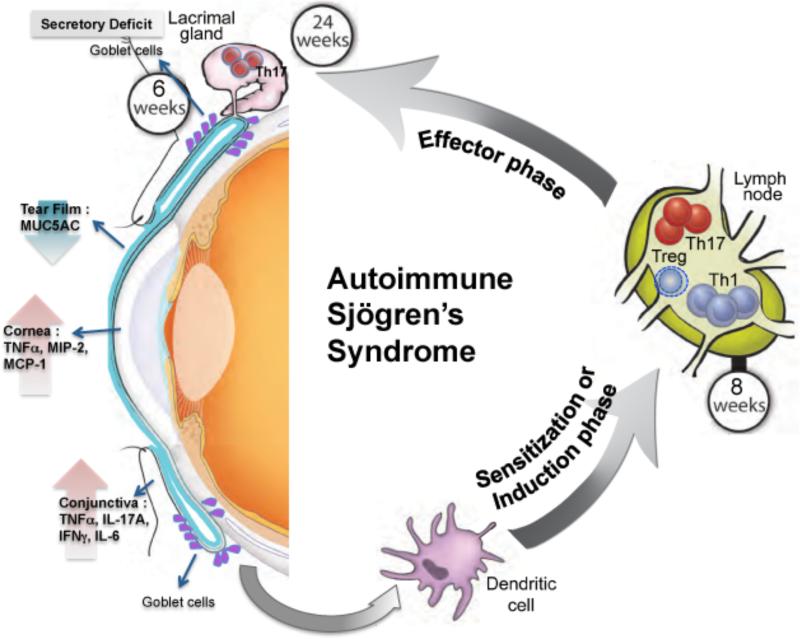
The ocular phenotype in TSP-1null mice develops progressively with age. The earliest detectable abnormality is the secretory deficit of conjunctival goblet cells at 6 weeks that results in reduced soluble mucin levels in tears32. At 8 weeks such secretory abnormality is also detected in lacrimal gland response to adrenergic stimulation55. However tear secretion in response to cholinergic stimulus remains normal. Since tear secretion is regulated by both adrenergic and cholinergic stimulation, loss of one signaling pathway results in a chronic but progressive compromise in tear quality. These changes in turn increase vulnerability of the ocular surface to commensal flora or pathogens that succeed in inducing inflammatory responses, presumably of the innate cells in the conjunctiva, leading to increased expression of inflammatory cytokines such as IL-17A, TNF-α, IFN-γ as well as IL-6 32. By 12 wks of age, inflammation in the conjunctiva is also accompanied by infiltration of the tissue by PMNs and increased lymphatic channels capable of facilitating migration of tissue dendritic cells to the draining cervical lymph nodes. The TSP-1 deficient DCs with their enhanced CCR7 expression successfully sensitize the host against ocular surface derived antigens thus initiating a systemic inflammatory immune response as represented by the elevated expression of transcription factors associated with inflammatory effectors such as Tbet (Th1) and RORγt (Th17 or NKT or γδ T cells109) in the draining lymph nodes. The appearance of such effectors in the lymph nodes begins by 8 weeks of age with a concurrent peripheral decline in Foxp3 expressing Tregs 32, 55. Such a peripheral imbalance in inflammatory and regulatory T cells has been identified in other autoimmune diseases. Between 12 and 24 weeks of age the inflammatory effectors gain access to the lacrimal gland as IL-17 secreting CD4+ cells are detected among the inflammatory infiltrates55. Interestingly, characteristic autoantibodies associated with Sjögren's syndrome are elevated during 12 -16 weeks of age further supporting the disease development55. The role of these antibodies in the disease development remains to be explored. With its persistent exposure to environmental factors and commensals, the ocular surface appears to be a vulnerable site that precipitates a chronic autoimmune disease process, which culminates in a severe ocular surface inflammation 55. Various stages of this process are depicted in figure 6.
Figure 6.
Overview of immunopathogenesis of autoimmune Sjögren's syndrome in TSP-1 deficient mice.
The development of autoimmunity in TSP-1 deficient mice suggests that the TSP-1 dependent immune regulation may not be limited to ocular immune privilege but may also extend to homeostatic clearance of apoptotic cells that involves activation of TGF-β to prevent aberrant sensitization of the host against tissue-derived autoantigens. Dysregulated TGF-β activation in clearance of apoptotic cells has been described in the immunopathogenesis of autoimmune diseases and is likely to be an underlying mechanism in the development of autoimmune Sjögren's syndrome in TSP-1 deficient mice. Whether TSP-1 deficiency results in other autoimmune diseases remains to be determined.
c. Association of THBS-1 polymorphism with ocular surface inflammation
In humans ocular surface inflammation is a very common condition after refractive surgery, a procedure with inherent damage to corneal nerves that induces local inflammation. Typically such inflammation is self-resolving, however in some individuals it develops into a chronic inflammatory condition. In a small targeted-gene association study, such individuals that developed post-refractive surgery chronic ocular surface inflammation were screened for selected 4 single nucleotide polymorphisms in their TSP-1 encoding gene THBS1 110. Significant correlation was detectable in the THBS1 polymorphism and the development of chronic ocular surface consistent with the observations in TSP-1 deficient mice. In particular, TSP-1 mutation in the promoter region correlated with a decreased expression of TSP-1 in the ocular surface epithelia and increased expression of inflammatory marker IL-1β. Interestingly, these results are consistent with a predominant expression of TGF-β2 in the ocular surface epithelial cells 54 as well as an increased expression of this isoform reported in the conjunctival epithelial cells derived from dry eye patients 111. A decline in the expression of TSP-1, a glycoprotein capable of activating latent form of TGF-β2, clearly supports the possibility of inflammation left unresolved due to unavailability of biologically active TGF-β which in turn results in a chronic inflammatory condition. Current treatment options for chronic inflammation include long-term use of immunosuppressive drugs like steroids with undesirable side effects. Extended immunosuppression itself is associated with several complications. Therefore THBS1 polymorphism may serve as a potential genetic biomarker for early identification of individuals that are likely to develop chronic ocular surface inflammation and allow determination of early treatment strategy to possibly avoid the chronic condition.
CONCLUSION
Over the past few years, data has accumulated to indicate that the matricellular protein TSP-1 plays an important role in ocular functions. These functions include some unique features of eye related tissues e.g. the need to maintain transparency of the corneal tissue or to protect delicate ocular anatomy from the damaging effects of inflammation and/or injury. The maintenance of corneal clarity to allow the passage of light involves specialized mechanisms to regulate development of blood and lymphatic vessels as well as wound healing. Similarly protecting specialized functions of the retinal structures involves avoiding growth of abnormal vessels, while throughout the eye effects of inflammatory immune responses are countered by immunoregulatory mechanisms. The ability of ocular cells to express TSP-1 supports several of these features of ocular responses to injury and inflammation that also make it an immunologically privileged site.
Studies using TSP-1 deficient mice have made it possible to understand mechanisms underlying retinal vascular homeostasis and vascular abnormalities detected in pathologic conditions that involve retinal neovascularization. In the corneal tissue TSP-1 appears to play a key role in maintaining corneal avascularity. This observation is supported by the increased hem- and lymphangiogenic responses invoked by corneal perturbations in TSP-1 deficient mice. Also an aberrant wound healing response to corneal injuries in these mice indicated an important role of TSP-1 in a highly regulated corneal wound repair process which is likely to be distinct from that noted at other non-ocular sites. The predominance of the TGF-β2 isoform in the ocular tissues possibly justifies the significance of TSP-1 at this site particularly with its ability to activate latent TGF-β in an integrin independent manner. Although the role of TSP-2 expression in the eye is not studied as extensively as that of TSP-1, its ability to competitively inhibit TSP-1 mediated TGF-β activation holds a strong potential in making it an important player that may finely regulate activation of a pro-fibrotic factor such as TGF-β and help prevent scar formation that can interfere with visual acuity. A potential close regulation between TSP-1 and TSP-2 in the eye remains to be explored.
The secretory deficit related to ocular surface inflammation in TSP-1 deficient mice has brought to light its potential involvement in regulating function of secretory epithelial cells in tissues like conjunctiva and lacrimal gland. Considering the predominant expression of TGF-β2 isoform in the former tissue, it is possible that other TGF-β2 expressing tissues are particularly affected by the absence of TSP-1.
While there are many TSP-dependent ocular mechanisms remain to be revealed in normal and disease states, emerging reports are promising translational opportunities ranging from a potential use of THBS-1 polymorphism as a genetic biomarker to the modulation of ocular angiogenesis using TSP-1 derived peptides in the treatment of various ocular pathologies.
References
- 1.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3(10):a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu A, Garg P, Yang S, Gong P, Pallero MA, Annis DS, et al. Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cgamma and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J Biol Chem. 2009;284(10):6389–402. doi: 10.1074/jbc.M809198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calzada MJ, Kuznetsova SA, Sipes JM, Rodrigues RG, Cashel JA, Annis DS, et al. Calcium indirectly regulates immunochemical reactivity and functional activities of the N-domain of thrombospondin-1. Matrix Biol. 2008;27(4):339–51. doi: 10.1016/j.matbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts DD. THBS1 (thrombospondin-1). Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2005;9(3):231. doi: 10.4267/2042/70774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ide M, Ishii H, Mukae H, Iwata A, Sakamoto N, Kadota J, et al. High serum levels of thrombospondin-1 in patients with idiopathic interstitial pneumonia. Respir Med. 2008;102(11):1625–30. doi: 10.1016/j.rmed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Wight TN, Raugi GJ, Mumby SM, Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- 7.Flugel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lussen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79(5):649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006;47(4):1352–8. doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- 9.Masli S, Turpie B, Hecker KH, Streilein JW. Expression of thrombospondin in TGF-βeta-treated APCs and its relevance to their immune deviation-promoting properties. J Immunol. 2002;168(5):2264–73. doi: 10.4049/jimmunol.168.5.2264. [DOI] [PubMed] [Google Scholar]
- 10.Uchida H, Kuroki M, Shitama T, Hayashi H. Activation of TGF-beta1 through up-regulation of TSP-1 by retinoic acid in retinal pigment epithelial cells. Curr Eye Res. 2008;33(2):199–203. doi: 10.1080/02713680701852090. [DOI] [PubMed] [Google Scholar]
- 11.Uchida H, Hayashi H, Kuroki M, Uno K, Yamada H, Yamashita Y, et al. Vitamin A up-regulates the expression of thrombospondin-1 and pigment epithelium-derived factor in retinal pigment epithelial cells. Exp Eye Res. 2005;80(1):23–30. doi: 10.1016/j.exer.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Iruela-Arispe ML, Porter P, Bornstein P, Sage EH. Thrombospondin-1, an inhibitor of angiogenesis, is regulated by progesterone in the human endometrium. J Clin Invest. 1996;97(2):403–12. doi: 10.1172/JCI118429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyder SM, Liang Y, Wu J, Welbern V. Regulation of thrombospondin-1 by natural and synthetic progestins in human breast cancer cells. Endocr Relat Cancer. 2009;16(3):809–17. doi: 10.1677/ERC-08-0311. [DOI] [PubMed] [Google Scholar]
- 14.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–5. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fordham JB, Hua J, Morwood SR, Schewitz-Bowers LP, Copland DA, Dick AD, et al. Environmental conditioning in the control of macrophage thrombospondin-1 production. Sci Rep. 2012;2:512. doi: 10.1038/srep00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65(1):79–84. [PubMed] [Google Scholar]
- 17.Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198(8):1277–83. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SS, Ivanoff A, Bergstrom SE, Sandstrom A, Christensson B, van Nerven J, et al. T lymphocyte expression of thrombospondin-1 and adhesion to extracellular matrix components. Eur J Immunol. 2002;32(4):1069–79. doi: 10.1002/1521-4141(200204)32:4<1069::AID-IMMU1069>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267(1):257–61. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 20.Haddadin RI, Oh DJ, Kang MH, Villarreal G, Jr., Kang JH, Jin R, et al. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2012;53(10):6708–17. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Oshima K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004;315(4):928–34. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- 22.Hiscott P, Seitz B, Schlotzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell and tissue research. 1997;289(2):307–10. doi: 10.1007/s004410050877. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FS, Rahman SA, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. J Leukoc Biol. 2013 doi: 10.1189/jlb.1012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Futagami Y, Sugita S, Vega J, Ishida K, Takase H, Maruyama K, et al. Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J Immunol. 2007;178(11):6994–7005. doi: 10.4049/jimmunol.178.11.6994. [DOI] [PubMed] [Google Scholar]
- 25.Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45(4):1117–24. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhou L, Wang H, Luo J, Zeng L, Xiong K, et al. Distribution of thrombospondins and their neuronal receptor alpha2delta1 in the rat retina. Exp Eye Res. 2013;111:36–49. doi: 10.1016/j.exer.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Suzuma K, Takagi H, Otani A, Oh H, Honda Y. Expression of thrombospondin-1 in ischemia-induced retinal neovascularization. Am J Pathol. 1999;154(2):343–54. doi: 10.1016/S0002-9440(10)65281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carron JA, Hiscott P, Hagan S, Sheridan CM, Magee R, Gallagher JA. Cultured human retinal pigment epithelial cells differentially express thrombospondin-1, -2, -3, and -4. Int J Biochem Cell Biol. 2000;32(11-12):1137–42. doi: 10.1016/s1357-2725(00)00065-0. [DOI] [PubMed] [Google Scholar]
- 29.Miyajima-Uchida H, Hayashi H, Beppu R, Kuroki M, Fukami M, Arakawa F, et al. Production and accumulation of thrombospondin-1 in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(2):561–7. [PubMed] [Google Scholar]
- 30.Cinatl J, Jr., Bittoova M, Margraf S, Vogel JU, Cinatl J, Preiser W, et al. Cytomegalovirus infection decreases expression of thrombospondin-1 and -2 in cultured human retinal glial cells: effects of antiviral agents. J Infect Dis. 2000;182(3):643–51. doi: 10.1086/315779. [DOI] [PubMed] [Google Scholar]
- 31.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138(3):707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival Inflammation in Thrombospondin-1 Deficient Mouse Model of Sjogren's Syndrome. PLoS One. 2013;8(9):e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker RP, Hagios C, Chiquet-Ehrismann R, Lawler J, Hall RJ, Erickson CA. Thrombospondin-1 and neural crest cell migration. Dev Dyn. 1999;214(4):312–22. doi: 10.1002/(SICI)1097-0177(199904)214:4<312::AID-AJA4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood JA, Murphy-Ullrich JE. Signaling of de-adhesion in cellular regulation and motility. Microsc Res Tech. 1998;43(5):420–32. doi: 10.1002/(SICI)1097-0029(19981201)43:5<420::AID-JEMT8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Blanco-Mezquita JT, Hutcheon AE, Zieske JD. Role of thrombospondin-1 in repair of penetrating corneal wounds. Invest Ophthalmol Vis Sci. 2013;54(9):6262–8. doi: 10.1167/iovs.13-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SS, Forslow A, Sundqvist KG. Autocrine regulation of T cell motility by calreticulin-thrombospondin-1 interaction. J Immunol. 2005;174(2):654–61. doi: 10.4049/jimmunol.174.2.654. [DOI] [PubMed] [Google Scholar]
- 37.Wei W, Kong B, Yang Q, Qu X. Hepatocyte growth factor enhances ovarian cancer cell invasion through downregulation of thrombospondin-1. Cancer Biol Ther. 2010;9(2):79–87. doi: 10.4161/cbt.9.2.10280. [DOI] [PubMed] [Google Scholar]
- 38.Firlej V, Mathieu JR, Gilbert C, Lemonnier L, Nakhle J, Gallou-Kabani C, et al. Thrombospondin-1 triggers cell migration and development of advanced prostate tumors. Cancer Res. 2011;71(24):7649–58. doi: 10.1158/0008-5472.CAN-11-0833. [DOI] [PubMed] [Google Scholar]
- 39.Nucera C, Porrello A, Antonello ZA, Mekel M, Nehs MA, Giordano TJ, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci U S A. 2010;107(23):10649–54. doi: 10.1073/pnas.1004934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albo D, Shinohara T, Tuszynski GP. Up-regulation of matrix metalloproteinase 9 by thrombospondin 1 in gastric cancer. J Surg Res. 2002;108(1):51–60. doi: 10.1006/jsre.2002.6452. [DOI] [PubMed] [Google Scholar]
- 41.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831–9. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagavandoss P, Wilks JW. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun. 1990;170(2):867–72. doi: 10.1016/0006-291x(90)92171-u. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6(1):41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 44.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208(5):1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8(7):1329–41. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, et al. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270(13):7304–10. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274(19):13586–93. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 48.Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, et al. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-beta1 complex. J Biol Chem. 2010;285(22):17029–37. doi: 10.1074/jbc.M110.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGF-βeta3. FEBS Lett. 2002;511(1-3):65–8. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 50.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 51.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32(8):2201–11. [PubMed] [Google Scholar]
- 52.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9(10):963–9. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 53.Knisely TL, Bleicher PA, Vibbard CA, Granstein RD. Production of latent transforming growth factor-beta and other inhibitory factors by cultured murine iris and ciliary body cells. Curr Eye Res. 1991;10(8):761–71. doi: 10.3109/02713689109013870. [DOI] [PubMed] [Google Scholar]
- 54.Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, Yamamoto S. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and -beta 3 latency-associated peptide in human cornea. Invest Ophthalmol Vis Sci. 1994;35(8):3289–94. [PubMed] [Google Scholar]
- 55.Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175(3):1136–47. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee T, Esemuede N, Sumpio BE, Gahtan V. Thrombospondin-1 induces matrix metalloproteinase-2 activation in vascular smooth muscle cells. J Vasc Surg. 2003;38(1):147–54. doi: 10.1016/s0741-5214(02)75468-2. [DOI] [PubMed] [Google Scholar]
- 57.Qian X, Wang TN, Rothman VL, Nicosia RF, Tuszynski GP. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp Cell Res. 1997;235(2):403–12. doi: 10.1006/excr.1997.3681. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228(4):630–42. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 59.Aiello LP. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 1997;29(5):354–62. doi: 10.1159/000268033. [DOI] [PubMed] [Google Scholar]
- 60.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–93. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 61.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 62.Wang S, Gottlieb JL, Sorenson CM, Sheibani N. Modulation of thrombospondin 1 and pigment epithelium-derived factor levels in vitreous fluid of patients with diabetes. Arch Ophthalmol. 2009;127(4):507–13. doi: 10.1001/archophthalmol.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101(5):982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 66.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn. 2006;235(7):1908–20. doi: 10.1002/dvdy.20837. [DOI] [PubMed] [Google Scholar]
- 67.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295(6):C1647–57. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–8. [PubMed] [Google Scholar]
- 69.Wang Y, Wang S, Sheibani N. Enhanced proangiogenic signaling in thrombospondin-1-deficient retinal endothelial cells. Microvasc Res. 2006;71(3):143–51. doi: 10.1016/j.mvr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Sheibani N, Frazier WA. Thrombospondin 1 expression in transformed endothelial cells restores a normal phenotype and suppresses their tumorigenesis. Proc Natl Acad Sci U S A. 1995;92(15):6788–92. doi: 10.1073/pnas.92.15.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46(6):2210–8. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 72.Sorenson CM, Wang S, Genderon R, Paradis H, Sheibani N. Thrombospondin-1 deficiency exacerbates the pathogenosis of diabetic retinopathy. Journal of Diabetes & Metabolism. 2013 doi: 10.4172/2155-6156.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He S, Incardona F, Jin M, Ryan SJ, Hinton DR. Thrombospondin-1 expression in RPE and choroidal neovascular membranes. Yan Ke Xue Bao. 2006;22(4):265–74. [PubMed] [Google Scholar]
- 74.Uno K, Bhutto IA, McLeod DS, Merges C, Lutty GA. Impaired expression of thrombospondin-1 in eyes with age related macular degeneration. Br J Ophthalmol. 2006;90(1):48–54. doi: 10.1136/bjo.2005.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Sorenson CM, Sheibani N. Lack of thrombospondin 1 and exacerbation of choroidal neovascularization. Arch Ophthalmol. 2012;130(5):615–20. doi: 10.1001/archopthalmol.2011.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18(1):75–84. viii. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Sheibani N, Albert DM. Angiogenesis and ocular tumorigenesis. In: Tombran-Tank JBC, editor. Ocular Angiogenesis: Diseases, Mechanisms and Therapies. Humana Press Inc.; Totowa, NJ: 2006. pp. 161–71. [Google Scholar]
- 78.Syed NA, Windle JJ, Darjatmoko SR, Lokken JM, Steeves RA, Chappell R, et al. Transgenic mice with pigmented intraocular tumors: tissue of origin and treatment. Invest Ophthalmol Vis Sci. 1998;39(13):2800–5. [PubMed] [Google Scholar]
- 79.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12(5):634–40. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 80.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140(2):419–30. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159(4):1255–62. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3(3-4):215–25. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palenski TL, Sorenson CM, Jefcoate CR, Sheibani N. Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells. Lab Invest. 2013;93(6):646–62. doi: 10.1038/labinvest.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, et al. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113(3):744–54. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103(30):11405–10. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, Chevet E, et al. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013;121(7):1229–37. doi: 10.1182/blood-2012-08-450502. [DOI] [PubMed] [Google Scholar]
- 89.Regenfuss B, Onderka J, Bock F, Hos D, Maruyama K, Cursiefen C. Genetic heterogeneity of lymphangiogenesis in different mouse strains. Am J Pathol. 2010;177(1):501–10. doi: 10.2353/ajpath.2010.090794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268(35):26784–9. [PubMed] [Google Scholar]
- 91.Hiscott P, Paraoan L, Choudhary A, Ordonez JL, Al-Khaier A, Armstrong DJ. Thrombospondin 1, thrombospondin 2 and the eye. Progress in retinal and eye research. 2006;25(1):1–18. doi: 10.1016/j.preteyeres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011;93(4):534–40. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hiscott P, Armstrong D, Batterbury M, Kaye S. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histology and histopathology. 1999;14(4):1309–20. doi: 10.14670/HH-14.1309. [DOI] [PubMed] [Google Scholar]
- 94.Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Molecular vision. 2007;13:1483–95. [PubMed] [Google Scholar]
- 95.Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci. 2002;43(9):2897–904. [PubMed] [Google Scholar]
- 96.Uno K, Kuroki M, Hayashi H, Uchida H, Kuroki M, Oshima K. Impairment of thrombospondin-1 expression during epithelial wound healing in corneas of vitamin A-deficient mice. Histology and histopathology. 2005;20(2):493–9. doi: 10.14670/HH-20.493. [DOI] [PubMed] [Google Scholar]
- 97.Hori J, Vega JL, Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocul Immunol Inflamm. 2010;18(5):325–33. doi: 10.3109/09273948.2010.512696. [DOI] [PubMed] [Google Scholar]
- 98.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4(3):130–7. [PubMed] [Google Scholar]
- 99.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146(9):3018–24. [PubMed] [Google Scholar]
- 100.Tam AL, Gupta N, Zhang Z, Yucel YH. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology. 2011;22(42):425101. doi: 10.1088/0957-4484/22/42/425101. [DOI] [PubMed] [Google Scholar]
- 101.Camelo S, Lajavardi L, Bochot A, Goldenberg B, Naud MC, Fattal E, et al. Drainage of fluorescent liposomes from the vitreous to cervical lymph nodes via conjunctival lymphatics. Ophthalmic Res. 2008;40(3-4):145–50. doi: 10.1159/000119866. [DOI] [PubMed] [Google Scholar]
- 102.Masli S, Turpie B, Streilein JW. Thrombospondin orchestrates the tolerance-promoting properties of TGF-βeta-treated antigen-presenting cells. Int Immunol. 2006;18(5):689–99. doi: 10.1093/intimm/dxl006. [DOI] [PubMed] [Google Scholar]
- 103.Masli S, Vega JL. Ocular immune privilege sites. Methods Mol Biol. 2011;677:449–58. doi: 10.1007/978-1-60761-869-0_28. [DOI] [PubMed] [Google Scholar]
- 104.Armant M, Avice MN, Hermann P, Rubio M, Kiniwa M, Delespesse G, et al. CD47 ligation selectively downregulates human interleukin 12 production. J Exp Med. 1999;190(8):1175–82. doi: 10.1084/jem.190.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. CD47 ligation selectively inhibits the development of human naive T cells into Th1 effectors. J Immunol. 2000;165(8):4624–31. doi: 10.4049/jimmunol.165.8.4624. [DOI] [PubMed] [Google Scholar]
- 106.Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol. 2010;185(8):4691–7. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ng TF, Turpie B, Masli S. Thrombospondin-1-mediated regulation of microglia activation after retinal injury. Invest Ophthalmol Vis Sci. 2009;50(11):5472–8. doi: 10.1167/iovs.08-2877. [DOI] [PubMed] [Google Scholar]
- 108.Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci. 2005;46(3):908–19. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 109.Price AE, Reinhardt RL, Liang HE, Locksley RM. Marking and quantifying IL-17A-producing cells in vivo. PloS one. 2012;7(6):e39750. doi: 10.1371/journal.pone.0039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Contreras-Ruiz L, Ryan DS, Sia RK, Bower KS, Dartt DA, Masli S. Polymorphism in THBS-1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Opthalmology. doi: 10.1016/j.ophtha.2014.01.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benito MJ, Calder V, Corrales RM, Garcia-Vazquez C, Narayanan S, Herreras JM, et al. Effect of TGF-beta on ocular surface epithelial cells. Exp Eye Res. 2013;107:88–100. doi: 10.1016/j.exer.2012.11.017. [DOI] [PubMed] [Google Scholar]