Abstract
Background and purpose
Single Dose Radiation Therapy (SDRT) provides remarkably high rates of control even for tumors resistant to fractionated radiotherapy. SDRT tumor control depends on acute acid sphingomyelinase-mediated endothelial cell injury and monoclonal antibodies targeting Vascular Endothelial Cell Growth Factor (VEGF) signaling radiosensitized tumor endothelium when delivered immediately prior to irradiation. Here we evaluate the ability of the oral VEGF receptor inhibitor, axitinib, to sensitize tumor endothelium and increase tumor control with SDRT.
Methods and materials
Axitinib was added to primary cultured endothelial cells, or administered orally to Sv129/BL6 mice bearing radiosensitive MCA/129 sarcoma or radioresistant B16F1 melanoma flank tumors, followed by SDRT. Endothelial apoptosis was assessed by TUNEL assay or bis-benzamide staining. Mice with irradiated tumors were followed for 90 days to evaluate the impact of axitinib on SDRT tumor control.
Results
Pre-treatment with axitinib increased acute endothelial cell apoptosis following SDRT in vitro, and in vivo for both MCA/129 and B16F1 tumors. Axitinib correspondingly increased SDRT tumor growth delay and complete response rate (by 40%) for both tumors. Administration precisely 1 h before SDRT was critical for radiosensitization.
Conclusions
Axitinib radiosensitizes tumor endothelial cells and enhances tumor cure with SDRT, which may permit dose de-escalation and significantly expand the range of clinical indications for SDRT.
Keywords: Radiosurgery, Stereotactic body radiation therapy, Vascular endothelial growth factor, Acid sphingomyelinase, Ceramide, Anti-angiogenic
Single Dose Radiotherapy or SDRT (also known as Radiosurgery or Stereotactic Body Radiotherapy) represents an emerging form of cancer treatment using ultra-high single doses of radiation to ablate primary or metastatic human tumors. While previous studies in animal tumor models have suggested that tumor microvascular damage yields no critical contribution to tumor cure by radiation [1–3], genetic and pre-clinical studies in our laboratory found disruption of tumor vasculature obligate for cure with SDRT at exposures exceeding 10 Gy [4–6]. This endothelial cell dysfunction results from activation of acid sphingomyelinase (ASMase), converting sphingomyelin to the second messenger ceramide in the endothelial plasma membrane.
The vast majority of cancer patients treated with radiation therapy will receive fractionated radiotherapy. Treatment exposures are typically repeated daily until maximal tolerable normal tissue doses are reached. Overall tumor cure with fractionated radiotherapy is ~65% for all tumors treated with curative intent. SDRT, facilitated by image guidance (IGRT) and intensity modulation (IMRT) technologies that improve precision in tumor targeting to reduce the risk of normal tissue toxicity, has revolutionized cancer treatment with local control rates above 90%, even in tumors considered resistant to conventional fractionated radiotherapy [7, 8]. Despite improved efficacy, SDRT is still limited by close proximity of tumor to critical normal tissues in many clinical settings. Further optimization of the therapeutic ratio is thus required to provide a safe approach to local tumor cure, and to expand clinical indications to currently untreatable settings.
Consistent with an obligatory role of ASMase-mediated microvascular damage in the SDRT response [4], our research indicates that angiogenic factors that protect against endothelial damage diminish the SDRT response, while select anti-angiogenic drugs improve SDRT local tumor control [9]. In this regard, we showed that ionizing radiation (IR)-induced damage to endothelial cells is initiated by ASMase trafficking to plasma membranes within seconds to minutes, generating the second messenger ceramide therein, an event obligate for propagation of SDRT endothelial injury [10]. Further, the angiogenic factors bFGF, VEGF-121 or VEGF-165 repress IR-induced ASMase translocation, post-translational activation, consequent ceramide generation, and endothelial apoptosis. The ability to reverse the VEGF effect by re-addition of exogenous ceramide to irradiated endothelium identifies ASMase, and not some other factor regulated by VEGF, as critical to the anti-apoptogenic VEGF effect. Conversely, anti-angiogenic agents, such as anti-VEGFR2 Ab DC101 (Imclone/Eli Lilly), de-repress ASMase activity, synergistically increasing IR-induced ceramide elevation, enhancing IR-induced apoptosis [9]. That ceramide is critical for anti-angiogenic radiosensitization is evidenced by anti-ceramide Ab inhibition of DC101-enhanced endothelial apoptosis [9].
These results translate in vivo, as i.v. anti-VEGFR2 DC101 (ImClone) or anti-VEGF G6–31 (Genentech/Roche) synergistically increase radiation-induced endothelial apoptosis in MCA/129 sarcomas, and enhance tumor response to SDRT [9]. Critically, anti-angiogenic antibody delivery must be administered immediately prior (0.5–2 h) to irradiation, but not earlier or after IR, in order to de-repress ASMase acutely. At longer intervals between drug delivery and irradiation the system appears to counter-regulate, re-setting the ceramide-generating capability of ASMase at or near the original setting. In contrast, tumors in asmase−/− mice, which provide apoptosis-resistant vasculature, are unaffected by either anti-angiogenic agent. These studies thus define an ASMase-dependent endothelial response that appears to dictate the outcome of tumor cure by SDRT and is modulated by VEGF.
The current study was designed to test whether the VEGFR-selective small molecule inhibitor axitinib (AG-013736, Pfizer) might recapitulate the biologic effectiveness of anti-VEGF and anti-VEGFR antibodies. Axitinib is an oral, potent and selective receptor tyrosine kinase inhibitor of VEGFR1, 2, 3 (with 10-fold lower activity for PDGFR-B and c-Kit) currently approved for 2nd line treatment of advanced renal cell cancer. As only a rapid, transient VEGFR inhibition is required for synergism with SDRT, we posited that axitinib has multiple properties that make it potentially superior to other available anti-angiogenic agents for this indication. Axitinib is a rapidly absorbed PO and possesses a short biologic half-life of 2–6 h [11]. These attributes support the clinical potential of axitinib for radiosensitization, as chronic VEGF inhibition using antibodies with half-lives of weeks violates the precise time-window for radiosensitization, and may unfavorably reset the ceramide rheostat for subsequent treatment. Furthermore, prolonged VEGF inhibition unnecessarily increases risk of significant high-grade toxicities [12, 13]. Here, we demonstrate that axitinib effectively enhances tumor endothelial cell injury and tumor cure when delivered prior to SDRT in pre-clinical studies.
Materials and methods
Drug formulation and administration
Axitinib (AG-13736, form IV, Pfizer, Inc.) was provided as a powder and suspended in 0.5% sodium carboxy-methyl cellulose solution for administration by oral gavage.
In vivo experiments
Wild type, Sv129/BL6 mice, males, 6–8 weeks old, were purchased from The Jackson Laboratory. Mice were housed at the Research Animal Resource Center (RARC) of Memorial Sloan-Kettering Cancer Center. This facility is approved by the American Association for Accreditation of Laboratory Animal Care and is maintained in accordance with the regulation and standards of the U.S. Department of Agriculture and the Department of Health and Human Services, NIH.
MCA/129 fibrosarcoma and B16F1 melanoma cells were maintained in DMEM high glucose supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37 °C in a humidified 5% CO2 chamber. Cells, 1 × 106/100 µl, were gently resuspended into PBS and injected subcutaneously into the right flank of mice [4]. Once tumors reached a size of 100–150 mm3 mice were either treated with IR and/or axitinib. Radiation was delivered using a Pantak Siefert Systems X-ray 320 at 117 cGy/min (50 cm source to skin distance). Mice were lightly sedated with ketamine (0.1 mg/g) and xylazine (0.02 mg/g) and only tumor, surrounding skin and subcutaneous tissues were exposed using a specialized lead jig. Tumor volumes, based on caliper measurements, were calculated daily according to the formula of Kim et al. [14]. Complete response was defined as no evidence of measureable tumor. For Kaplan–Meier analysis of progression-free survival, tumor progression was defined as a 25% increase in tumor size over baseline.
Quantification of apoptosis
Apoptosis was quantified in vivo in the endothelium of tumor specimens following double staining with TUNEL, to detect apoptotic cells, and the endothelial cell surface marker MECA-32, to identify tumor endothelium [4]. Briefly, tumor specimens were obtained at the indicated time points after treatment, fixed in 4% paraformaldehyde, embedded in paraffin, and 5-µm sections were stained. Apoptotic endothelial cells display a red-brown TUNEL-positive nuclear signal surrounded by a dark blue plasma membrane signal indicative of MECA-32 staining. A minimum of 1000 endothelial cells were evaluated per point.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0. For endothelial apoptosis experiments, two-sided Chi Square test was employed to evaluate significance. For tumor growth studies, Kaplan–Meier survival analysis was used to evaluate progression free survival and two-sided Fisher’s exact t-test was used compare complete response rates. We considered p values <0.05 to be significant.
Results
Axitinib increases acute tumor endothelial cell apoptosis after SDRT
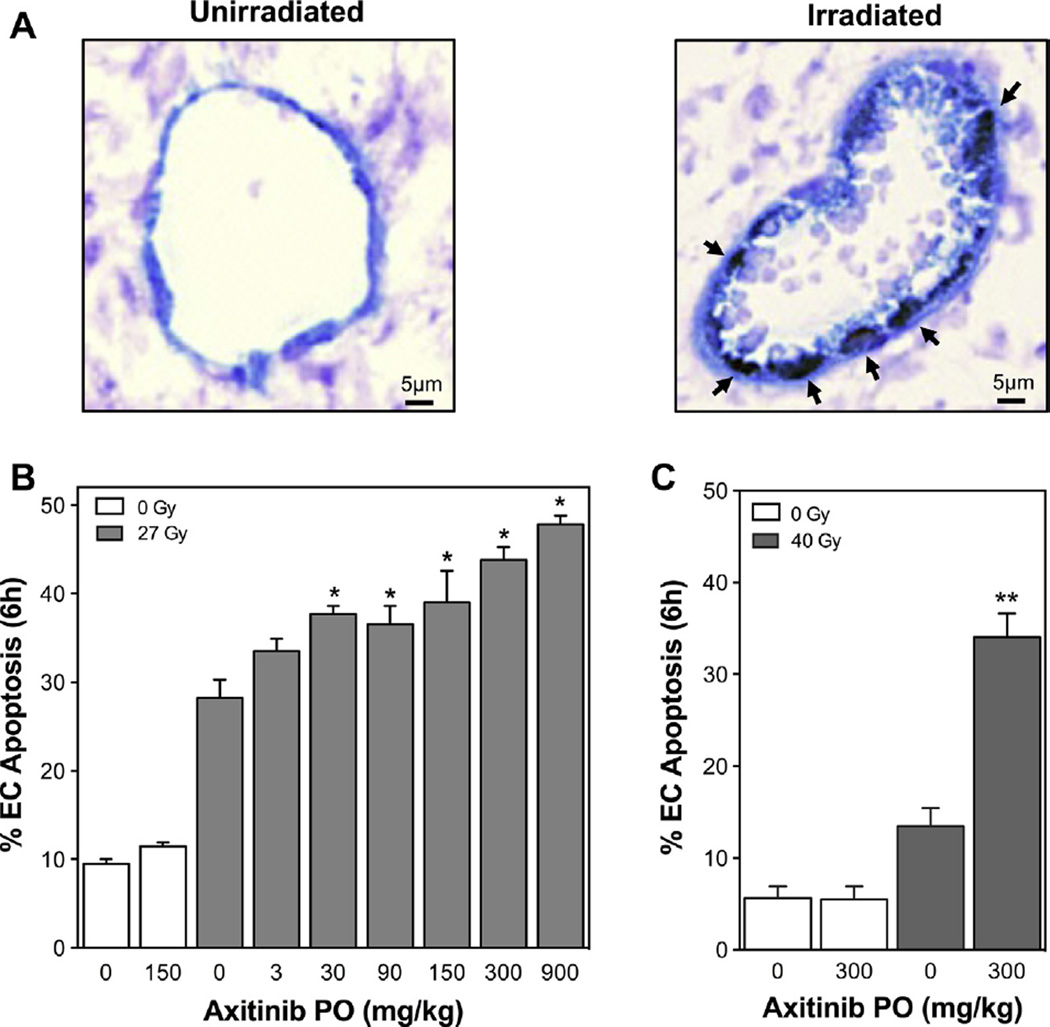
For initial in vitro studies we utilized BAEC, which manifest radiosensitivity similar to neo-angiogenic tumor endothelial cells [9, 15]. BAEC were exposed to escalating doses of axitinib for 60 min, and then treated with IR at 0–15 Gy. Apoptosis was assessed at 6 h, the peak time for apoptosis induction with SDRT, by bis-benzamide staining [15, 16]. Axitinib pre-treatment dose dependently increased IR-induced apoptosis (p < 0.001 each for axitinib ≥5 nM), while axitinib alone was without effect (Fig. S1). To confirm axitinib radiosensitization of endothelial apoptosis in vivo, Sv129/BL6 mice were implanted with MCA/129 mouse sarcoma cells to generate flank tumors. At 100–150 mm3, tumors were treated with 27 Gy SDRT. A cohort of mice was administered axitinib by oral gavage (0–900 mg/kg) and after one hour treated with SDRT. Six hours after SDRT, tumors were harvested, fixed and double-labeled using MECA-32 mAb to stain endothelial cell membranes and TUNEL assay to identify cells undergoing apoptosis. Fig. 1A shows a representative microvessel displaying multiple TUNEL positive endothelial nuclei post-IR. While tumor cells did not show acute apoptosis (<2%), tumor endothelial cells demonstrated moderate apoptosis with 27 Gy alone (Fig. 1B). Axitinib, when delivered prior to 27 Gy SDRT, dose-dependently enhanced acute tumor endothelial cell apoptosis (p < 0.001 for axitinib ≥30 mg/kg). As little as 30 mg/kg axitinib was effective and at 900 mg/kg, the maximal tolerated dose, axitinib demonstrated the greatest effect, increasing endothelial apoptosis with 27 Gy from 28 ± 2% to 48 ± 1%. These observations were confirmed in a second tumor type using B16F1 melanoma cells, which when implanted in Sv129/BL6 mice form tumors highly resistant to SDRT cure [17]. Mice were treated with 300 mg/kg axitinib and after one hour exposed to 40 Gy SDRT, a dose that alone does not yield tumor cure. While 40 Gy alone caused minimal, 13 ± 2%, endothelial apoptosis at 6 h (Fig. 1C), axitinib pre-treatment increased tumor endothelial cell apoptosis to 33 ± 2% (p < 0.001). Note that axitinib without radiation did not increase endothelial cell apoptosis above background levels for either tumor model.
Fig. 1.
Axitinib increases endothelial cell apoptosis after SDRT in vivo. Axitinib was administered to Sv129/BL6 mice bearing MCA/129 sarcoma or B16F1 melanoma flank tumors (100–150 mm3) and after 1 h tumors were treated with SDRT. Tumors were double-stained using TUNEL labeling and MECA-32 immunohistochemistry to identify apoptotic endothelial cells 6 h after SDRT. (A) Representative 5-µm sections of MCA/129 tumors untreated or at 6 h after 27 Gy SDRT plus axitinib. Apoptotic endothelium exhibits a brown TUNEL-positive nuclear signal surrounded by a dark blue plasma membrane signal for MECA-32 (indicated by arrows, magnification 400×). (B) Axitinib given to mice bearing MCA/129 tumors at 1 h preceding 27 Gy dose-dependently increases SDRT-induced endothelial cell apoptosis. (C) Axitinib administered 1 h preceding 40 Gy to mice bearing B16F1 tumors increases endothelial cell apoptosis. Data (mean ± SEM) are collated from 2 to 3 mice per dose with 1000–2000 endothelial cells evaluated. *p < 0.001 compared to 27 Gy alone. **p < 0.001 compared to 40 Gy alone.
Axitinib improves tumor response to SDRT
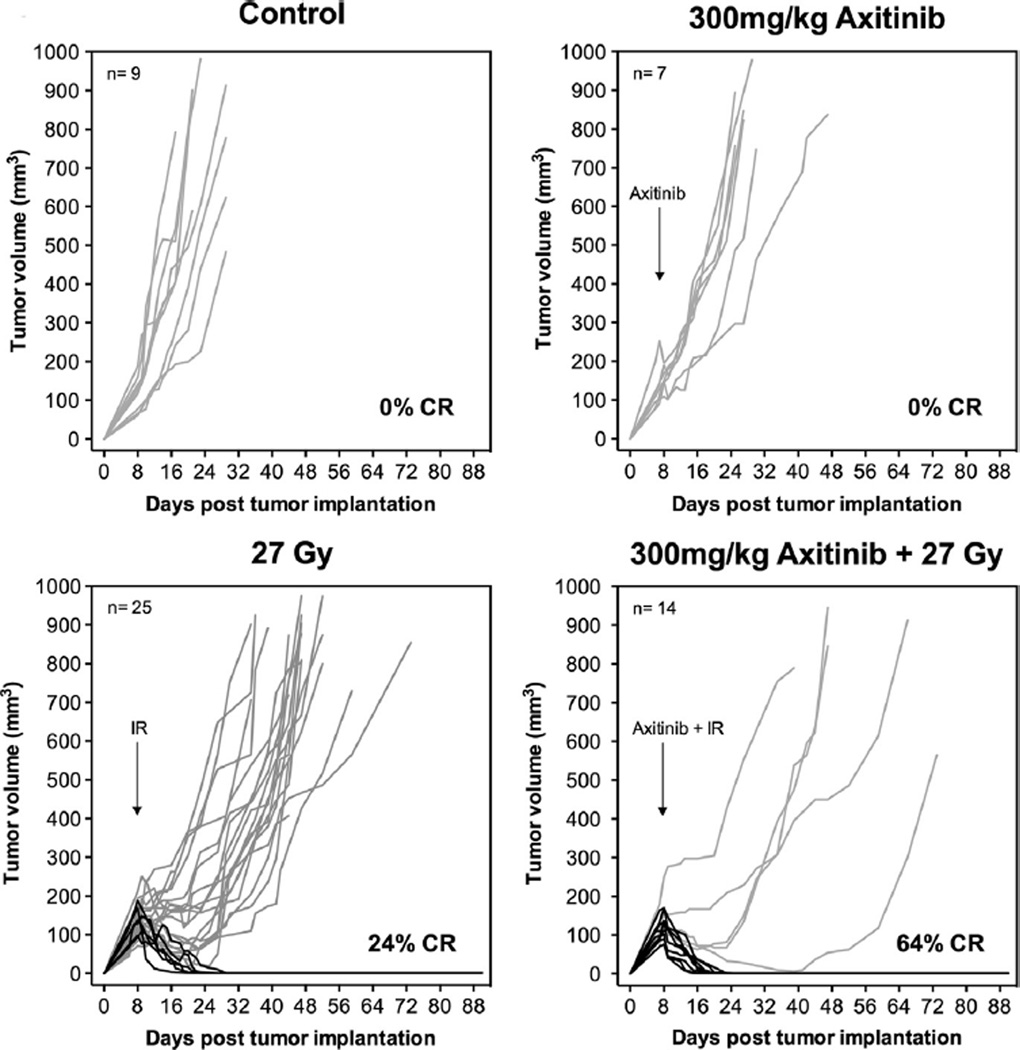
With confirmation that axitinib radiosensitizes tumor endothelial cell damage to SDRT in vivo, we evaluated the effect of axitinib on tumor response (Fig. 2). Sv129/BL6 mice bearing 100–150 mm3 MCA/129 sarcomas were treated with IR and/or axitinib, and tumor volumes were measured over 90 days (or until euthanized due to tumor burden). Single dose axitinib (300 mg/kg) provided minimal tumor growth delay in MCA/129 sarcomas (1.8 ± 0.7 days, mean ± SEM). SDRT alone (27 Gy) yielded a 24% complete response rate, while pretreatment with 300 mg/kg axitinib increased the complete response rate to 64% (p < 0.01). From individual tumor growth profiles, it is apparent that mice not demonstrating complete response nonetheless experienced an 11.7 ± 8.1 day delay (p < 0.05) in tumor growth progression with the addition of axitinib to 27 Gy SDRT. Therefore, Sv129/BL6 mice bearing MCA/129 sarcoma tumors were administered increasing doses of axitinib (0.3–300 mg/kg), treated with 27 Gy SDRT one hour later, and followed for 90 days. Tumor progression, defined as 25% increase over baseline, was calculated and progression free survival determined using Kaplan–Meier analysis. Fig. S2 shows that axitinib delivered immediately prior to SDRT dose-dependently increased progression free survival as assessed by Kaplan Meier actuarial analysis (p < 0.001). At 1-month post treatment, progression free survival was 28% with 27 Gy alone, 60% with 3 mg/kg axitinib pre-treatment prior to 27 Gy, and 72% with 300 mg/kg preceding 27 Gy.
Fig. 2.
Axitinib pre-treatment increases complete response rate of MCA/129 sarcomas to SDRT. Sv129/BL6 mice bearing MCA/129 flank tumors (100–150 mm3) were given a single PO dose of axitinib (300 mg/kg) 1 h prior to tumor treatment with 27 Gy. Tumor volumes were measured for 90 days. Arrows indicate day of treatment. Lines represent individual tumor responses measured daily for each tumor with complete responses shown in black.
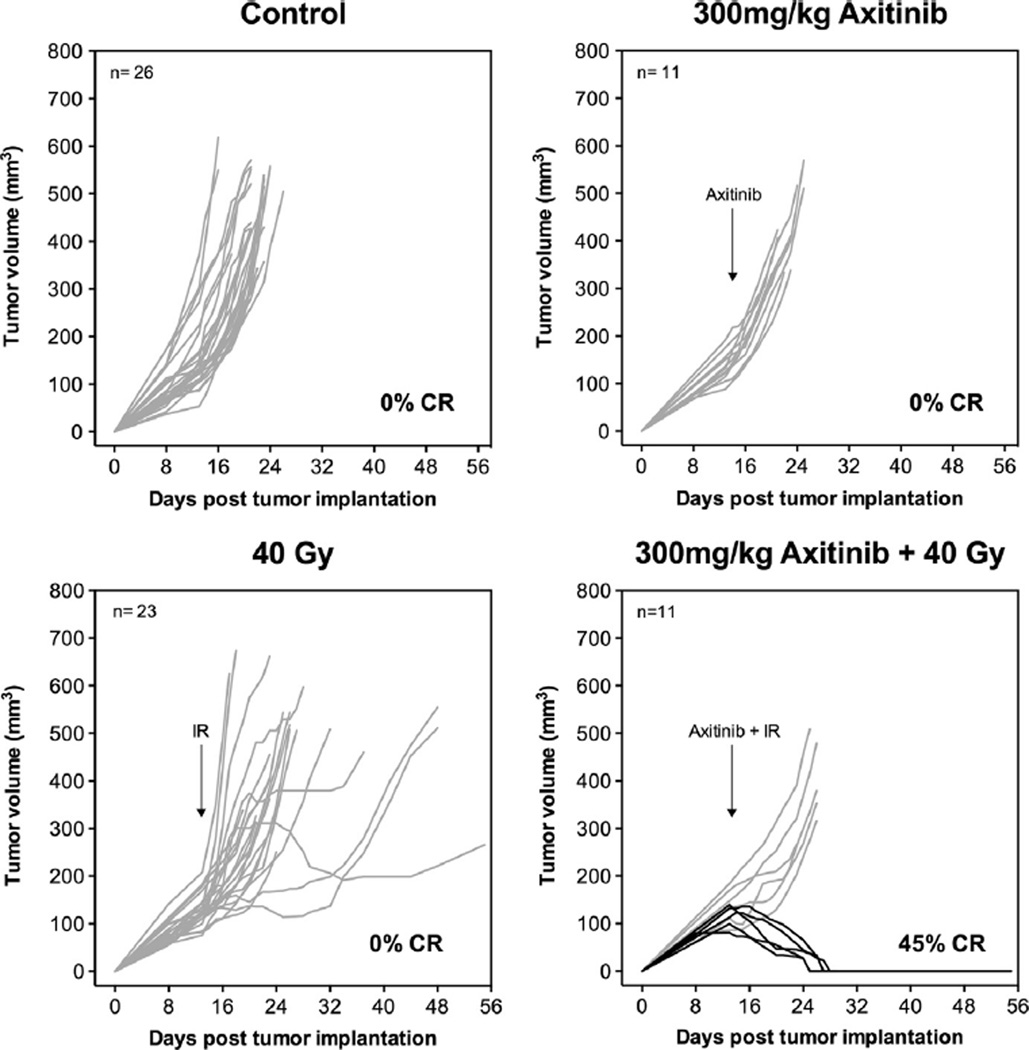
A radiosensitizing effect of axitinib on tumor SDRT was also demonstrated in radioresistant B16F1 melanomas. Fig. 3 shows that while 40 Gy SDRT alone or axitinib alone was insufficient to generate complete responses or statistically significant delay in tumor progression, axitinib administered prior to 40 Gy SDRT yielded a 45% complete response rate (p < 0.01).
Fig. 3.
Axitinib pre-treatment enables complete responses in radioresistant B16F1 melanomas after SDRT. Sv129/BL6 mice with B16F1 flank tumors were given axitinib 1 h prior to 40 Gy SDRT to the tumor. Arrows denote day of treatment. Lines represent individual tumor responses for each mouse with complete responses in black.
Time dependence of axitinib administration for radiosensitization with SDRT
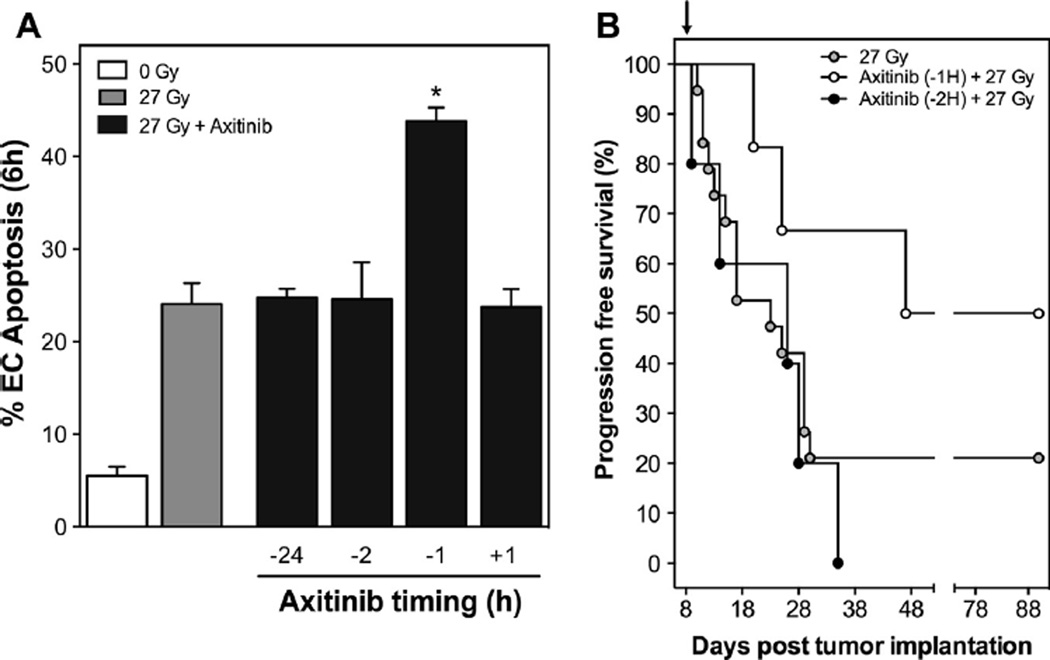
Our previously published studies indicated a strict time restriction for anti-angiogenic de-repression of acid sphingomyelinase-driven radiosensitization using antibodies to VEGF or VEGFR2 [9]. Here we show a similar time dependence of the axitinib effect on SDRT. Sv129/BL6 mice with flank MCA/129 sarcomas were treated with 300 mg/kg axitinib at 24, 2, and 1 h(s) preceding and at 1 h after 27 Gy SDRT. Six hours post-irradiation, tumors were harvested and double-stained for endothelial cells using MECA-32 immunohistochemistry and TUNEL assay. The optimal time for axitinib delivery to yield maximal apoptotic sensitization was one hour before SDRT, with minimal tumor endothelial radiosensitization at all other times (Fig. 4A). Furthermore, axitinib (90 mg/kg) at 1 h preceding 27 Gy SDRT yielded increased progression free survival by Kaplan Meier analysis while administration of axitinib only one hour earlier at 2 h preceding 27 Gy SDRT, failed to do so (p < 0.05) (Fig. 4B). Note that while axitinib given 1 h prior to SDRT resulted in 66% progression free survival at 1 month, axitinib at 2 h prior to 27 Gy was no better than 27 Gy alone (control). Furthermore, axitinib given 2 h prior to 27 Gy did not increase the complete response rate compared to 27 Gy alone (p = 0.55). Similarly, axitinib given 1 h after 27 Gy SDRT (Fig. S3), did not result in statistically significant changes in tumor growth progression or complete response rates compared to 27 Gy alone (31% vs. 24%, p = 0.72).
Fig. 4.
Time dependence of axitinib administration with SDRT. Sv129/BL6 mice bearing MCA/129 flank sarcomas were treated with axitinib at various time intervals before or after 27 Gy SDRT. (A) At 6 h after SDRT, tumor endothelial cells were examined for apoptosis. Data (mean ± SEM) are collated from 2 to 4 mice per dose with 1000–2000 endothelial cells evaluated. *p < 0.001. (B) Mice were given axitinib either 1 or 2 h prior to 27 Gy SDRT (black arrow) and tumor size was measured for 60 days. Graph depicts Kaplan–Meier survival analysis of tumor progression (defined as 25% growth over baseline).
Discussion
The present studies confirm our published model regarding the role of tissue VEGF in tumor radioresistance [9], and provide a basis for clinical trials of oral axitinib as an SDRT sensitizer to improve local tumor cure. We show that single dose axitinib mimics the anti-VEGF/VEGFR impact on SDRT, increasing endothelial dysfunction, and exhibiting critical dependence on timing relative to IR exposure, suggesting a mechanism of action identical to that demonstrated for anti-VEGF/VEGFR2 antibodies [9]. Furthermore we document axitinib can dramatically increase tumor cure rates with SDRT.
Several mechanisms have been posited by which VEGF confers resistance to therapy via tumor microvasculature, dictating different modes of application of anti-angiogenic agents in attempting to sensitize tumors to radiation or drugs. Continuous dosing of anti-VEGFR antibody has been employed with a goal of tonic suppression of neo-angiogenesis and tumor microvascular “normalization” [18]. This strategy hypothesizes that pruning of dysfunctional neo-angiogenic vessels can increase tumor perfusion and decrease hypoxia, thereby increasing efficacy of chemotherapy and radiation therapy. However, pre-clinical studies with continuous axitinib dosing did not demonstrate sustained normalization of vasculature [19, 20]. Additionally, while the combination of fractionated radiation and continuous axitinib dosing improved tumor response additively, timing studies suggested no difference whether drug was administered at 1 h before or 1 h after 2 Gy fractions repeated daily [20]. Hoang et al. similarly showed that while anti-VEGF antibody bevacizumab decreased mouse tumor growth in combination with fractionated radiation, results were additive, and occurred whether a 2.5-week course of bevacizumab was administered before, during or after the 2.5-week course of fractionated radiotherapy [21]. These observations contrast with the strict timing dependence of single dose anti-VEGF/VEGFR antibodies or axitinib relative to SDRT for synergistic tumor cure, supporting our concept that SDRT acts by a different biologic mechanism than fractionated radiotherapy, functioning via ASMase-mediated vascular dysfunction.
Another finding reported here is that axitinib dose escalation delivered with a fixed SDRT dose enhances tumor endothelial apoptosis and cure in two different tumor types. This observation holds high promise for a general approach to improve local cure of human tumors with axitinib plus SDRT. While early clinical experience provided proof-in-principle that 24 Gy SDRT alone can locally cure >90% of human cancer deposits regardless of tumor type or size, this curative effect has been accomplished in cases where critical normal tissues could be completely avoided. In reality, SDRT is limited in many clinical settings by close proximity of the tumor to critical normal tissue. For example, Cox et al. recently reported a 6.8% severe late esophageal toxicity (grade 3–4 including stenosis, ulceration, and trachea-esophageal fistula formation) in 204 patients treated with 24 Gy SDRT for spinal metastases, in whom targeted tumor abutted the esophagus [22]. Detailed analysis of this cohort yielded a set of guidelines on DLTs of spinal SDRT, constraining planned treatment dose to 12–22 Gy pending specification of esophagus volumes expected to be collaterally exposed. Similarly, DLT estimates and consequent treatment planning constraints have been established for most common clinical/normal tissue settings [23, 24]. In general, the critical DLT for SDRT in multiple clinical settings appears to be within the range of 14–16 Gy.
A potential approach to overcome this restriction on SDRT delivery might be via temporally-constrained use of anti-VEGFR radiosensitization [9]. As well-oxygenated normal tissues do not show elevations of VEGF observed within tumors [25, 26], preferential, or even selective, radiosensitization of tumor versus normal tissues might be reasonably expected. Such a configuration may enable dose de-escalation without reducing the SDRT local tumor control level achieved without sensitizer. Experimental data presented here strongly support this notion (Fig. 3).
As only transient VEGF inhibition with a single exposure of sensitizer is required in the SDRT model, and as the pharmacokinetic profile of single dose axitinib is favorable, the data presented here on the magnitude of SDRT sensitization indicate high promise for combined use in clinical studies. Axitinib is absorbed rapidly, reaching maximum observed plasma concentrations (Cmax) within 0.5–4 h of oral administration [11]. It has a relatively short effective plasma half-life (2–6 h) and is rapidly eliminated via hepatobiliary excretion [11]. This profile also implies the potential for avoidance of side effects that occur with longer acting inhibition, including bowel perforation and cerebral hemorrhage [12, 13].
Our data show linear dependence of oral axitinib dose and magnitude of SDRT sensitization, both in terms of microvascular apoptotic response and local tumor cure. An open question for clinical trials would be the extent of preferential SDRT dose de-escalation achievable while maintaining P90% local tumor cure. Provided that the current clinically-used PO formulation yields optimal serum concentration at 30–120 min, it will be necessary to perform axitinib dose escalation to dose limiting toxicities (DLT) concomitant with SDRT dose de-escalation to maximize potential for this combination.
In summary, recent understanding of the regulatory crosstalk between microvascular endothelium and tumor stem cells has provided new targets for improving local cure of human cancer with radiation. The magnitude of SDRT sensitization observed here with temporally-constrained single dose axitinib appears comparable or superior to other anti-VEGF/VEGFR radiosensitizers, and coupled with a favorable pharmacokinetic profile indicates high promise for use in clinical studies.
Supplementary Material
Acknowledgments
Funding source
This work was supported by an Investigator-initiated Research Award from Pfizer, axitinib was provided by Pfizer. The sponsors had no role in the study design, analysis, or interpretation.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2014.02.010.
Footnotes
Conflict of interest
None.
References
- 1.Budach W, et al. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. 1993;85:988–993. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]
- 2.Gerweck LE, et al. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–8355. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa K, et al. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–4021. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Barros M, et al. Impact of stromal sensitivity on radiation response of tumors implanted in scid hosts revisited. Cancer Res. 2010;70:8179–8186. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stancevic B, et al. Adenoviral transduction of human acid sphingomyelinase into neo-angiogenic endothelium radiosensitizes tumor cure. PLoS One. 2013;8:e69025. doi: 10.1371/journal.pone.0069025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo SS, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Truman JP, et al. Endothelial membrane remodeling is obligate for antiangiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santana P, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Clinical pharmacology of axitinib. Clin Pharmacokinet. 2013;52:712–725. doi: 10.1007/s40262-013-0068-3. [DOI] [PubMed] [Google Scholar]
- 12.Letarte N, Bressler LR, Villano JL. Bevacizumab and central nervous system (cns) hemorrhage. Cancer Chemother Pharmacol. 2013;71:1561–1565. doi: 10.1007/s00280-013-2155-4. [DOI] [PubMed] [Google Scholar]
- 13.Taugourdeau-Raymond S, et al. Bevacizumab-induced serious side-effects: a review of the french pharmacovigilance database. Eur J Clin Pharmacol. 2012;68:1103–1107. doi: 10.1007/s00228-012-1232-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, et al. Potentiation of radiation effects on two murine tumors by lonidamine. Cancer Res. 1986;46:1120–1123. [PubMed] [Google Scholar]
- 15.Haimovitz-Friedman A, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garzotto M, et al. 12-o-tetradecanoylphorbol-13-acetate-induced apoptosis in lncap cells is mediated through ceramide synthase. Cancer Res. 1998;58:2260–2264. [PubMed] [Google Scholar]
- 17.Stancevic B, et al. Adenoviral transduction of human acid sphingomyelinase into neoangiogenic endothelium radiosensitizes tumor cure. PLoS One. 2013;8:e69025. doi: 10.1371/journal.pone.0069025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 19.Fenton BM, Paoni SF. The addition of ag-013736 to fractionated radiation improves tumor response without functionally normalizing the tumor vasculature. Cancer Res. 2007;67:9921–9928. doi: 10.1158/0008-5472.CAN-07-1066. [DOI] [PubMed] [Google Scholar]
- 20.Fenton BM, Paoni SF. Alterations in daily sequencing of axitinib and fractionated radiotherapy do not affect tumor growth inhibition or pathophysiological response. Radiat Res. 2009;171:606–614. doi: 10.1667/RR1595.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoang T, et al. Enhancement of radiation response with bevacizumab. J Exp Clin Cancer Res. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedict SH, et al. Stereotactic body radiation therapy: the report of aapm task group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 24.Grimm J, et al. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12:3368. doi: 10.1120/jacmp.v12i2.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 26.Miebach S, et al. Isolation and culture of microvascular endothelial cells from gliomas of different who grades. J Neurooncol. 2006;76:39–48. doi: 10.1007/s11060-005-3674-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.