Abstract
Induced pluripotent stem cells (iPSCs) hold tremendous potential both as a biological tool to uncover the pathophysiology of disease by creating relevant cell models and as a source of stem cells for cell-based therapeutic applications. Typically, iPSCs have been derived by the transgenic overexpression of transcription factors associated with progenitor cell or stem cell function in fibroblasts derived from skin biopsies. However, the need for skin punch biopsies to derive fibroblasts for reprogramming can present a barrier to study participation among certain populations of individuals, including children with autism spectrum disorders (ASDs). In addition, the acquisition of skin punch biopsies in non-clinic settings presents a challenge. One potential mechanism to avoid these limitations would be the use of peripheral blood mononuclear cells (PBMCs) as the source of the cells for reprogramming. In this article we describe the derivation of iPSC lines from PBMCs isolated from the whole blood of autistic children, and their subsequent differentiation in GABAergic neurons.
Introduction
Autism spectrum disorders (ASD) comprise a complex and heterogeneous group of neurodevelopmental conditions including autism, Asperger syndromes, and pervasive developmental disorder-not otherwise specified (PDD-NOS)[37]. These conditions are characterized by impaired social interactions, deficits in verbal and nonverbal communication, and the presence of restricted and/or stereotyped repetitive behaviors[17]. The incidence of ASDs varies between 10 to 20 per 1000 children, making ASDs one of the most common neurodevelopmental disorders[2]. Despite their high prevalence, relatively little is known about the pathology underlying ASDs. The lack of widely available biological samples of relevant cell and tissue types has made studying the pathophysiology of many neurological conditions a challenging prospect. Although animal models have been used successfully to increase our understanding of the biology underlying a variety of disorders, the development of these models is often time consuming, expensive, and requires an understanding of the genetic underpinnings of the disease being studied[31]. Therefore, alternative model systems are needed. One potential model system that has recently emerged is induced pluripotent stem cells (iPSCs). The ectopic expression of transcription factors that control pluripotency and self-renewal in mouse and human stem cell population was shown to reprogram terminally differentiated somatic cells into a pluripotent state [18,23,35,42]. Most commonly this involves the expression of some combination of SOX2, KLF4, OCT3/4, LIN28, Nanog and c-MYC, using oncoretroviral or lentiviral-based vectors[42]. Additional mechanisms of reprogramming have been described, including purified recombinant proteins, in vitro transcribed mRNAs, non-integrating plasmid-based expression systems and the overexpression of the stem cell-specific miR-302-367 cluster[1,38,43]. IPSCs are morphologically identical to embryonic stem (ES) cells, display similar gene expression profiles and phenotypic markers, can self-renew, and retain the potential to be differentiated into all cell types in the body[40]. Therefore, iPSCs provide a powerful platform for studying the genetic and molecular underpinnings of complex diseases in the cell type(s) most relevant for that disease.
Fibroblasts derived from skin punch biopsies are predominantly used as the starting material for the derivation of iPSCs since they can be easily cultured and will continue to grow and propagate in vitro for extended periods of time prior to undergoing cellular senescence. However, skin punch biopsy samples are difficult to acquire outside of a clinic setting and can be restrictive in certain patient populations, such as individuals with ASD. On the other hand, the collection of whole blood is less invasive and is routinely performed on patients in the field. Therefore, the development of iPSCs from peripheral blood mononuclear cells (PBMCs) would be beneficial in the study of ASDs. Although derivation of iPSCs from blood cells has been recently reported, this technology has not been implemented in the creation of patient-specific iPSCs, despite its practicality for such use[7,16,32]. Furthermore, differentiation of reprogrammed PBMCs into cell types relevant for the study of human disease has not been shown. To address these issues, we developed iPSC lines from PBMCs derived from individuals with ASDs. These iPSCs efficiently formed embryoid bodies (EBs) expressing markers from the three different germ layers - endoderm, mesoderm and ectoderm. In addition, the ASD patient-specific iPSCs could be differentiated into GABAergic neurons, a cell type postulated to play a central role in the pathogenesis of ASDs. These studies lay the ground work for the development of relevant neuronal (or non-neuronal) cell models to begin to unravel the pathophysiology of ASDs.
Materials and Methods
Reprogramming of PBMCs into pluripotent stem cells
Lentiviral plasmids expressing OCT4, SOX2, KLF4, and c-MYC human cDNAs from tetracycline responsive promoters were obtained from Addgene. Production of lentiviral particles was carried out using standard protocols[12,33]. Peripheral blood was isolated from three ASD-affected males following informed consent under University of Miami guidelines and regulations. Prior to infection, PBMCs were cultured in α-MEM/10% FBS medium containing 10 ng/ml IL-7 (R&D Systems). One day before infection 0.5 μM thiazovivin and 10 μM Y27632 were added to the media and continually added thereafter. Starting 24 hours after transduction, 2mM valproic acid, 2 μM R(+)Bay K 86644, 1 μM BIX1294 were added to the medium for 8 days and 2 μg/mL doxycycline was added up to 5 weeks. On the third day post-infection, the PBMCs were transferred to MEF feeder plates. After 2–3 weeks, colonies exhibiting embryonic stem cell morphology were selected and expanded either manually or enzymatically with collagenase IV (Stemcell technologies). See the supporting online material for detailed protocols for differentiating EBs, neural progenitor cells (NPCs), and GABAergic neurons.
RT-PCR and qRT-PCR
Total RNA was extracted using TRIzol (Invitrogen) and reverse transcribed using SuperScript III First-Strand Synthesis (Invitrogen) and random hexamers. The resulting cDNA was amplified by PCR using an ABI Veriti Thermal Cycler with Platinum Taq (Invitrogen). Quantitative real-time PCR was performed on an ABI 7900HT using SYBR Green PCR Master Mix (ABI). See supplemental Table 1 for a complete list of primers used.
Immunocytochemistry and alkaline phosphatase staining
For immunocytochemistry cells were fixed with 4% formaldehyde in PBS, blocked with PBS containing 5% normal donkey serum (Jackson ImmunoResearch) and 0.1% Triton X-100, and incubated with the primary enzyme (in PBS containing 2% normal donkey serum). See Supplementary Table 2 for a complete list of antibodies. Alkaline phosphatase staining was performed using the Alkaline Phosphatase Staining Kit (Stemgent).
Results
Derivation of ASD-iPSC lines from PBMCs
Whole blood was acquired from three individuals with ASDS representative of the heterogeneity within this group of disorders (see Supplemetary Table 1). The IL-7 stimulated PBMCs were transduced with lentiviral vectors expressing human OCT3/4, KLF4, SOX2 and c-MYC from a doxycycline (DOX) inducible promoter and a constituitively active reverse tetracycline transactivator[12]. Following transduction, the cells were cultured on mitomycin-C treated mouse embryonic fibroblasts in the presence of DOX to induce pluripotent transgene expression. IPSC colonies with embryonic stem (ES) cell-like morphology began to be observed in culture approximately 9 days after DOX induction. By day 14 there were ≥ 200 colonies growing in each patient’s culture, with the exception of ASD13 which had ≤ 20 colonies. Interestingly, almost all of these initial colonies had a granulated morphology suggestive of partially reprogrammed cells. However, they rapidly self-renewed and were capable of doubling in cell number every 3 days. From each of the patient samples, 2 to 7 individual colonies that most closely resembled the morphology of ES cell colonies were picked and expanded into cell lines. See Figure 1 A for representative iPSC colonies from two of the patients, ASD49 and ASD26, compared to a well validated control iPSC line. After 2 to 3 weeks DOX was removed from the medium with the majority of the ASD-iPSCs independently sustaining pluripotency after an extended number of passages. Immunocytochemical staining of the ASD-iPSC lines showed uniform expression of the pluripotency markers NANOG, OCT3/4, SOX2, and SSEA4 (Figure 1 B). Furthermore, the ASD-iPSCs exhibited strong alkaline phosphatase activity (Figure 1 C), an early marker for pluripotency[21].
Figure 1.

Derivation of ASD-specific iPSCs from PBMCs. Transgenic overexpression of OCT3/4, SOX2, KLF4 and c-MYC from doxycycline-inducible lentiviral-based vectors reprogrammed PBMCs derived from individuals with an ASD into iPSC lines. These iPSC colonies adopted the characteristic spherical colonies when grown on mitomycin C-treated MEFs similar to a well characterized control iPSC line. (A) Brightfield images of representative colonies from iPSC derived from two ASD individuals and the control iPSC. The ASD-specific iPSC lines stained positive for several markers of pluripotency (Nanog, Oct3/4, Sox2 and SSEA4) (B) and were alkaline phosphatase positive (C). (D) Quantitative real-time PCR confirmed the expression of pluripotency markers (Nanog, OCT3/4, KLF4, SOX2) and stem cell-associated transcripts (DPPA5 and ZFP42) from representative iPSC lines derived from ASD13 (cl1), ASD26 (cl1) and ASD49 (cl1-3).
Quantitative real time-PCR (qRT-PCR) showed endogenous expression of NANOG, OCT3/4, SOX2, KLF4, DPPA5, and ZFP42 in all the ASD-iPSC lines tested (ASD26-iPS1, ASD49-iPS1 to iPS3) at levels comparable to those found in a control human iPSC line (Figure 1D). Although all of these genes were shown to be expressed in the ASD-iPSC lines, there was some heterogeneity in expression between the individual cell lines. This heterogeneity in expression levels of the pluripotency factors and stem cell markers is consistent with other published reports and reflects variability in the extent of reprogramming which requires a balance between the four reprogramming factors, as well as, stochastic differences resulting from the iPSC line reprogramming, selection and culturing.[6,19].
Embryoid body (EB) formation of ASD-iPSCs
When cultured in suspension in the absence of bFGF, human pluripotent stem cells and iPSCs form EBs which are spherical aggregates of cells that are differentiating into the three primary germ layers[14]. After 24 hours in suspension culture, the ASD iPSCs formed spherical EBs (Figure 2A). RT-PCR analysis of the EBs derived from ASD26- and ASD49-iPSCs confirmed that the differentiated cells expressed NESTIN (ectoderm), NCAM (ectoderm), RUNX1 (mesoderm), BRACHYURY (mesoderm), and GATA4 (endoderm) (Figure 2B).
Figure 2.

ASD iPSC-derived Embryoid Bodies from PBMCs express markers for all three germ layer. A) Phase contrast images of representative EBs derived from the ASD 26 iPSC and ASD49 iPSC. B) Gene expression analysis for ectodermal (Nestin and NCAM), mesodermal (RUNX1 and Brachyury), and endodermal markers (GATA4).
Derivation of GABAergic neurons from ASD-iPSCs
GABAergic neurons from the cerebral cortex are derived from neural progenitor cells (NPCs) in the ventral forebrain [46]. To determine the potential of ASD-iPSCs to differentiate into GABAergic neurons, we induced iPSC lines from two of the ASD individuals to first adopt a ventral forebrain cell fate before terminal differentiation was carried out by treatment with RA. To accomplish this, the ASD iPSC-derived EBs were cultured in the presence of Noggin (BMP antagonist), DKK1 (Dickkopf-related protein 1) (Wnt antagonist), and DAPT, an inhibitor of α-secretase (Notch antagonist).
Recent studies have implicated an important role for RA signaling in corticogenesis and differentiation of GABAergic neurons[8,14]. Terminal differentiation of ASD-NPCs into GABAergic neurons was initiated by the withdrawal of growth factors (bFGF and IGF1) from the medium and beginning treatment with RA with the addition of BDNF (brain-derived neurotrophic factor), and GDNF (glial-derived neurotrophic factor) to maintain the mature neurons. Final analysis of the neural cultures took place after 20 days of differentiation. Using this method, the ASD26- and ASD49-iPSCs were able to differentiate into cells having neuron morphology (i.e. rounded cell bodies, clear axonal and dendritic projections) and expressed specific markers of mature and GABA-synthesizing neurons (Figure 3).
Figure 3.
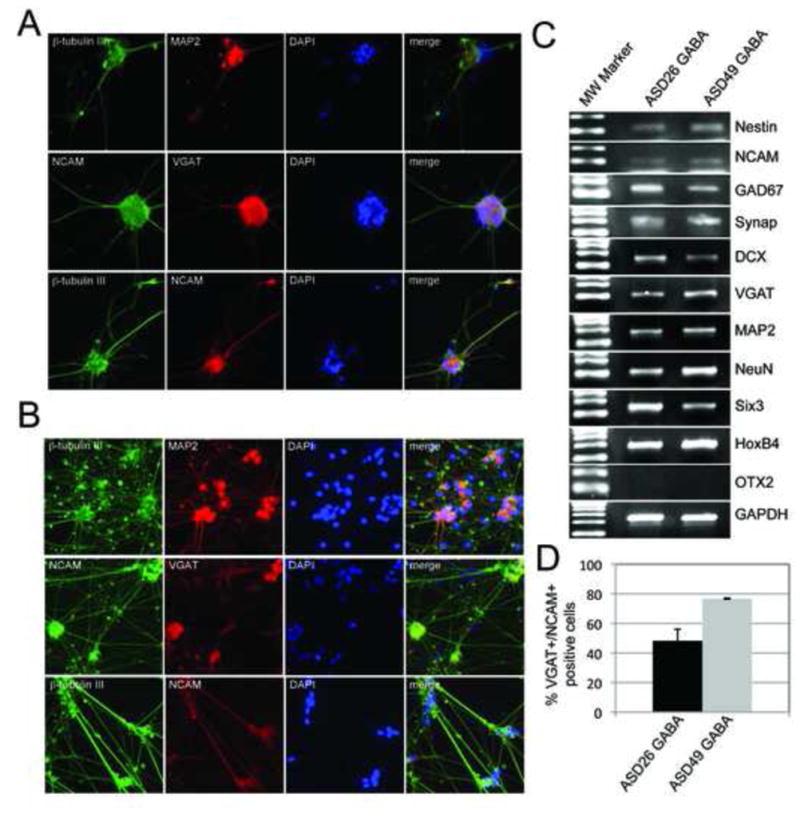
Differentiation of ASD-specific iPSCs into GABAergic neurons. ASD-specific GABAergic neurons were derived from ASD26 and ASD49-specific iPSC lines. The ASD-specific iPSC-derived GABAergic neurons stained positively for both neuronal- (NCAM, MAP2 and β-tubulin III) and GABAergic (VGAT)-specific markers. Representative images from GABA-ergic neurons derived from ASD26 (A) and ASD49 (B) iPSCs are shown. C) Reverse transcription PCR analysis from the ASD26 and ASD49-specific iPSC-derived GABAergic neuronal cultures showed strong expression of the neuronal markers (NCAM and Nestin), mature neuronal markers (NeuroN, Synapsin, and MAP2), GABAergic neuron-specific markers (GAD67 (posterior) and VGAT (anterior)) and the positional markers (SIX3 and HoxB4). The anterior positional marker OTX2 was not expressed in the culture suggesting its expression is unresponsive to RA stimulation. Heterogeneity of the neuronal culture was indicated by expression of the immature neuronal marker DCX. D) Quantitation of the VGAT/NCAM double positive cells in the ASD26 and ASD49-specific iPSC-derived GABAergic neuronal cultures relative to the total number of cells.
In mammals, the inhibitory transmitter GABA is synthesized from glutamate by two different glutamate decarboxylases, GAD67 and GAD65, and is transported by VGAT (vesicular GABA transporter) into synaptic vesicles. Co-expression of these genes is fundamental for differentiation into GABAergic neurons. The mature GABAergic neurons derived from two ASD-iPSC lines showed strong expression of VGAT by immunocytochemistry (Figure 3A and B) and both VGAT and GAD67 by RT-PCR (Figure 3C). In addition, both ASD26- and ASD49-neuronal cultures stained positively for the neuronal-specific markers NCAM and MAP2 (Figure 3A and B). The number of cells that stained VGAT and NCAM positive relative to the total number of cells in the culture was 48%(±7.8%) and 77%(±0.33%) in the ASD26- and ASD49-neuronal cultures, respectively. Not unexpectedly, although these cultures are enriched for GABAergic neurons, there are a variety of other cell types that are present, including other neuronal cell types (eg. dopaminergic neurons) and astrocytes (data not shown).
RT-PCR analysis showed that the ASD-GABAergic neuronal cultures expressed significant levels of the mature neuron markers - MAP2 (dendrite marker), NeuN (mature neuronal marker), Synapsin (synaptic activity marker) (Figure 3C). In addition, β-tubulin III and MAP2 were expressed and colocalized in the dendrites of ASD26- and ASD49-neurons as shown by immunocytochemical staining (Figure 3A and B). However, DCX (immature neuronal marker) was also observed in the ASD-neural cultures indicating that some of the cells were still mitotically active and in the process of maturation (Figure 3C).
In vivo, GABAergic neurons are expressed throughout all brain regions along the anterior-posterior axis. Upon exposure to RA, both ASD26- and ASD49-neurons expressed the anterior marker SIX3 and the posterior marker HOXB4 (Figure 3C). The anterior marker OTX2 was not seen expressed in the ASD neurons indicating that its expression is independent of RA.
Discussion/Conclusions
The main advantage of using PBMCs for the derivation of iPSC lines is the ease with which lymphocytes can be obtained from whole blood in a non-invasive manner that facilitates acquiring samples in non-clinic settings and among patient populations where the need for skin punch biopsies would deter participation. Despite these benefits, only a small number of studies have demonstrated the derivation of iPSCs from PBMCs or specific populations of lymphocytes [5,10,22,28]. This lack of studies using blood cells for iPSC derivation results principally from difficulties associated with manipulating PBMCs, their low transduction efficiencies and the challenges surrounding the selection methods for the establishment of iPSC lines[10,16,29,32]. We developed iPSC lines from PBMCs obtained from several children with ASDs. These iPSC expressed a panel of stem cell and pluripotency markers similar to a well validated human iPSC line. EBs were derived from two of the ASD-iPSCs lines. These EBs expressed markers from all three germ layers and could be differentiated into GABAergic neurons in culture demonstrating that the ASD-iPSCs were competent for the production of differentiated cell populations. GABAergic interneurons orchestrate the integration of projection neuron circuits into cerebral networks and harmonize oscillation rhythms and temporal synchrony between neural networks[9,11]. GABA-mediated calcium signaling regulates several key developmental processes including cellular proliferation, migration, differentiation, synapse maturation, and cell death. Therefore, defects in the circuitry, migration, and function of inhibitory GABAergic neurons in the developing brain could contribute to the pathogenesis of ASDs.
In recent reports, the use of neurons derived from patient-specific iPSCs has proven to be a useful tool in determining cellular phenotypes and gene expression profiles associated with neurophychiatric disorders[24,25]. Therefore, the ability to derive iPSC lines from the whole blood of ASD patients and the subsequent differentiation into neuronal (or non-neuronal) cell populations would provide the tools necessary to study the complex changes in cellular functionality that underlie ASDs. Like naturally occurring stem cell populations, iPSCs can self-renew and, therefore, provide an almost unlimited source of cells for differentiation into the relevant cell type to be studied. In addition, since iPSCs are pluripotent, they can be induced to differentiate into practically any cell type desired. This is an important feature of these cells since in many disorders multiple cell types are impacted and, in some cases such as ASDs, there is no definitive cell type linked to the disorder. As a result, this approach will allow researchers to generate large quantities of live human cells to elucidate the cellular and molecular defects that are contributing to the initiation and progression of ASDs. Although it would be beneficial to know the specific genetic variation associated with the disorder, given the genetic complexity of ASDs this is not always possible. Even in the absence of known causal genetic variations, these iPSCs can shed much needed light on the pathophysiology of ASDs.
Supplementary Material
Highlights.
Induced pluripotent stem cells (iPSCs) were generated from autistic patients
The iPSCs lines had traits similar to naturally occurring stem cells populations
Embryoid bodies with germ layer characteristics were derived from ASD-specific iPSC
Mature GABAergic neurons could be differentiated from the ASD-specific iPSC
iPSC provide valuable reagents for the characterization of the pathology of autism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autism and Developmental Disabilities Monitoring Network. Surveillance Year 2006 Principal Investigators, Centers for Disease Control and Prevention (CDC), Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 3.Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brustle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28(5):955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- 4.Bosch M, Pineda JR, Sunol C, Petriz J, Cattaneo E, Alberch J, Canals JM. Induction of GABAergic phenotype in a neural stem cell line for transplantation in an excitotoxic model of Huntington’s disease. Exp Neurol. 2004;190(1):42–58. doi: 10.1016/j.expneurol.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, Sloane-Stanley J, Silk KM, Davies TJ, Fairchild PJ, Enver T, Watt SM. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117(15):4008–4011. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- 6.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21(3):518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle DE, Li J, Baccei M. Regional differentiation of retinoic acid-induced human pluripotent embryonic carcinoma stem cell neurons. PLoS One. 2011;6(1):e16174. doi: 10.1371/journal.pone.0016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72(1):1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133(2):250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 12.Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3(3):346–53. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, Lensch MW, Park IH, Agarwal S, Church GM, Collins JJ, Irion S, Daley GQ. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7(1):15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 18.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, Yao M, Robbins RC, Wu JC. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nat R, Dechant G. Milestones of directed differentiation of mouse and human embryonic stem cells into telencephalic neurons based on neural development in vivo. Stem Cells Dev. 2011;20(6):947–958. doi: 10.1089/scd.2010.0417. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor MD, Kardel MD, Iosfina I, Youssef D, Lu M, Li MM, Vercauteren S, Nagy A, Eaves CJ. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells. 2008;26(5):1109–1116. doi: 10.1634/stemcells.2007-0801. [DOI] [PubMed] [Google Scholar]
- 22.Ohmine S, Dietz AB, Deeds MC, Hartjes KA, Miller DR, Thatava T, Sakuma T, Kudva YC, Ikeda Y. Induced pluripotent stem cells from GMP-grade hematopoietic progenitor cells and mononuclear myeloid cells. Stem Cell Res Ther. 2011;2(6):46. doi: 10.1186/scrt87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 24.Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, Bernstein JA, Hallmayer J, Geschwind DH, Dolmetsch RE. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17(12):1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedrosa E, Sandler V, Shah A, Carroll R, Chang C, Rockowitz S, Guo X, Zheng D, Lachman HM. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J Neurogenet. 2011;25(3):88–103. doi: 10.3109/01677063.2011.597908. [DOI] [PubMed] [Google Scholar]
- 26.Reimers D, Gonzalo-Gobernado R, Herranz AS, Osuna C, Asensio MJ, Baena S, Rodriguez M, Bazan E. Driving neural stem cells towards a desired phenotype. Curr Stem Cell Res Ther. 2008;3(4):247–253. doi: 10.2174/157488808786733980. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein JL. Annual Research Review: Development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52(4):339–355. doi: 10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki T, Yuasa S, Fukuda K. Derivation of induced pluripotent stem cells from human peripheral circulating T cells. Curr Protoc Stem Cell Biol. 2011;Chapter 4(Unit4A.3) doi: 10.1002/9780470151808.sc04a03s18. [DOI] [PubMed] [Google Scholar]
- 29.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N, Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7(1):11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7(1):20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svendsen CN, Caldwell MA, Ostenfeld T. Human neural stem cells: isolation, expansion and transplantation. Brain Pathol. 1999;9(3):499–513. doi: 10.1111/j.1750-3639.1999.tb00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 38.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465(7299):704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


