Abstract
Giant cell arteritis (GCA) is an important cause of preventable blindness, most commonly due to anterior ischemic optic neuropathy. Ischemic tissue injury is the end result of a process that begins within the walls of susceptible arteries in which local dendritic cells (DCs) recruit and activate CD4 T cells that, in turn, direct the activity of effector macrophages. In response to the granulomatous inflammation, the blood vessel forms lumen- stenosing intima. Multiple cascades of excessive T-cell reactivity contribute to the autoimmune features of giant cell arteritis with TH1 and TH17 immunity responsible for the early phase and TH1 immunity promoting chronic-smoldering inflammation. These cascades are only partially overlapping, supporting the concept that a multitude of instigators jeopardize the immune privilege of the vessel wall. The artery actively participates in the abnormal immune response through endogenous immune sentinels, so-called vascular DCs embedded in the adventitia. Advancing age, the strongest of all risk factors for GCA, likely contributes to the dysfunction of the immune system and the vascular system. Expansion of the therapeutic armamentarium for GCA needs to focus on approaches that mitigate the impact of the aging artery and adapt to the needs of the immunosenescent host.
Giant cell arteritis (GCA) is an autoinflammatory and autoimmune syndrome (1). Recognizing and managing GCA remain an ophthalmologic emergency because the disease can quickly progress to irreversible vision loss, dip- lopia, or stroke (2). If the underlying vasculitis is promptly treated, vision loss may be reversible and the fellow eye, also at high risk for involvement, can be protected.
The disease process underlying GCA is a granulomatous inflammation, which is typically positioned within the wall layers of medium and large arteries. Granulomatous infiltrates are composed of CD4 T cells and highly activated macrophages, often including multinucleated giant cells. The vascular wall, generally an immune-privileged site, responds to the attack with a response-to-injury program, which cul- minates in hyperplasia of the intimal layer, leading to luminal compromise and vessel occlusion.
In the majority of patients with GCA, the arteritis is associated with a syndrome of systemic inflammation, with constitutional symptoms and results in the well-described laboratory abnormalities, such as elevated acute phase markers (sedimentation rate, C-reactive protein). This systemic component is relatively easy to treat with currently available immunosuppressive regimens. In contrast, the vascular complications of GCA remain a major clinical challenge. Recent data suggest that wall-centered inflammation persists chronically. Luminal stenosis/occlusion results in ischemia, and involvement of different vessels supplying the eye, optic nerve, and brain leads to different ocular findings and patterns of vision loss. Extracranial vessel involvement can cause head- ache, jaw claudication, eye pain, scalp infarction, and other ischemic presentations. GCA aortitis leads to aneurysm formation with the associated risk for dissection and rupture.
While formerly suspected to represent a granulomatous reaction to a yet unidentified instigator, it is now clear that the immunopathogenesis of GCA reaches a much higher degree of complexity. Separable lineages of dysfunctional immune cells have been implicated in driving the disease, making it highly unlikely that a single etiologic agent induces GCA (3). At least 2 distinct immune processes govern early and late disease, emphasizing the need to search for a variety of inciting events. Probably the most important observation in GCA research has come from the recognition that the blood vessel regulates disease susceptibility and progression through immune-stromal communications.
A much improved understanding of the immunopathology of GCA, which has emerged over the last decade, is impacting the diagnostic approach to patients suspected to have GCA or diagnosed with GCA. Immunologic studies suggest a much more chronic course of the disease than previously appreciated (4). Accordingly, current therapeutic strategies, while successful in managing acute disease, need to be adapted to longer term goals. An overriding challenge is the advanced age of the affected patient population. While immune aging emerges as one of the underlying pathogenic principles in conferring risk for GCA, it also restricts the potential use of more aggressive means to immunosuppress vessel-wall centered chronic inflammation.
DISEASE RISK FACTORS IN GCA
Age
By far the strongest risk factor to develop GCA is the age of the patient (Table 1) (5). Individuals younger than 50 years seem to be almost completely protected, whereas the 7th and 8th decade of life are high-risk periods. Exceptions to this rule are patients diagnosed with Takayasu's arteritis that have a vasculitis similar to GCA. They are typically in the 2nd to 4th decade of life and current diagnostic criteria require that the disease onset before 40 years of age. Similar to fundamental differences in age of onset, the geographic regions of the world with high-risk populations for GCA or Takayasu's arteritis are almost mutually exclusive, supporting the concept that the 2 diseases affect two nonoverlapping host populations and thus must have critical differences in etiology and pathogenesis.
TABLE 1.
Diseases risk factor in giant cell arteritis
| Age | Older than 50 years |
|---|---|
| Sex | 3 women, 1 man |
| Genetic | HLA-DR4 haplotype; multiple immune response gene polymorphisms with low risk ratio |
| Geography | High incidence region: Iceland, Sweden, Norway, Denmark; intermediate incidence region: Middle Europe; low incidence region: Japan |
| Ethnic origin | High risk: Caucasian |
| Low risk: African American | |
| Immune aging | Remodeled adaptive immune system; hyperactive innate immune system |
| Vascular aging | ?Altered vascular dendritic cell function |
| ?Altered matrix composition | |
| ?Endothelial dysfunction | |
| ?Altered smooth muscle cell behavior |
Aging leads to a profound remodeling of the immune system with weakening of adaptive immunity because of thymic involution, abating ability to maintain tolerance, and resurgence of less sophisticated innate defense mechanisms (6,7). Also, progressive accumulation of chronic infections reshapes the immune system, detracts from the ability to devote immune responses toward new antigens, and promotes a progressive rearrangement of naive and memory immune cell populations.
Whether an aged immune system is more likely to generate granulomatous reactions and whether aged macrophages are more susceptible to fuse into giant cells are not entirely understood. Equally important is the inherent immune function of the blood vessel wall and the impact that age-related structural changes have on cell trafficking, cell survival, and cell–cell communications. Interactions between the aging immune system and the aging arterial wall rely on fundamentally different molecular networks. The coordinated senescence of the immune system and the vascular tree may be the critical factor in allowing GCA to occur (8).
Sex
Women are at much higher risk of developing granulomatous arteritis of the medium and large arteries with a 3:1 female-to-male risk ratio (Table 1). Hormonal and reproductive factors are suspected to participate in disease risk, but mechanistic concepts and data are lacking. Considering that almost all patients are postmenopausal, one would have to postulate a role for sex-dependent imprinting of either the immune system or the vascular system that renders the host susceptible many decades later.
Genetic Factors
Data from the early 1990s have established that GCA is an human leukocyte antigen (HLA)-associated disease implicating the strongest of all immune response genes in pathogenesis (Table 1) (9). The GCA risk haplotype is HLA-DR4—a haplotype also found enriched among patients with the autoimmune syndrome rheumatoid arthritis. Interestingly, despite this common genetic risk, GCA and rheumatoid arthritis seldom co-occur, suggesting that HLA-DR4 can serve more than one role in biasing immune responses toward inflammation and tissue injury. An extended list of possible non-HLA disease risk genes have been examined over the last decade. Some are present at higher frequency in patients than controls, but the overriding theme has been that such polymorphisms contribute only a relatively small risk. The described disease risk genes all seem to be connected to the host's ability to generate immune responses, emphasizing the central role of immunity in the pathogenesis of GCA.
Noninherited Risk Factors
In 1932, Horton (10), at the Mayo Clinic, obtained the first temporal artery biopsy and recognized the combination of headaches, fever, weakness, anemia, and painful tender scalp vessels as a novel disease entity. Early on, he was convinced that his first 2 patients had actinomycosis but later aban-doned this diagnosis (11). Since then, there has been intense interest in whether GCA is precipitated by an infectious agent. Episodically, reports surface that attract attention to a bacterial or viral pathogen as the etiologic agent. So far, none of such reports has been confirmed in subsequent studies and in independent cohorts (12,13). The explicit sensitivity of the disease to high doses of corticosteroids makes a classical infectious pathogenesis unlikely. Also, the very strict age dependence of GCA questions how an infection could exclusively occur in individuals older than 50 years. This does not exclude an indirect role of the infectious burden in disease pathogenesis. Accumulative infection of the host with multiple organisms could well reprogram the host's immune system such that subsequent noninfectious triggers would elicit tissue-damaging inflammatory reactions.
Deciphering the relationship between GCA and infectious organisms is complicated by the fact that chronic inflammatory lesions may serve as “waste depositories.” Phagocytic cells loaded with pathogen-derived materials may end up in chronic inflammatory infiltrates due to nonspecific recruitment signals; yet, their cargo has no contribution in initiating or sustaining disease (14).
The geographic variations of GCA across the world, with an impressively high prevalence in the Northern latitudes (Table 1) (15,16), raises the question how environmental factors affect disease risk. Sun exposure has been discussed as a potential risk factor. However, the observation that Scan-dinavians migrating from Sweden, Norway, and Denmark to Midwestern states of the United States brought with them the increased susceptibility to GCA is best compatible with the host immune system as the predominant risk determinant.
Dysfunctional Immunity in GCA
The immunopathogenesis of GCA is driven by 3 cell types: dendritic cells (DCs), CD4 T cells, and macrophages (17,18). While classical components of a granulomatous reaction, the distribution of these cell types is unique in that the dendritic cell is supplied by the blood vessel, determining the tropism of the disease for the transmural part of human arteries. In the translation of tissue inflammation into vascular disease, the immune system seems less important than the artery. Arterial wall cells respond to the immune attack with a vigorous remodeling program causing luminal stenosis and occlusion. This rule does not hold for the aorta where GCA aortitis almost always results in aneurysmal deformation, dissection, or even rupture. Pathogenic events underlying the systemic manifestations of GCA are less well understood. Polymyalgia rheumatica (PMR) has recently been associated with the accumulation of inflammatory cytokines in the interstitial fluid of painful muscles (19). Anemia, thrombocytosis, and elevated liver function tests point toward involvement of the bone marrow and the liver. Whether these are distant effects mediated by circulating inflammatory cytokines or reflect in situ inflammation is not yet known.
Initiation of Vascular Inflammation
GCA begins with the activation of DCs, so-called vasDC, that are embedded in the vessel wall (Fig. 1A) (20,21). They function as sentinels and monitor the tissue environment for danger signals. They are positioned at the media–adventitial junction, in close proximity to the vasa vasorum network. They are phagocytic and are equipped with surface receptors dedicated to the binding of danger signals. Such danger signals may arise from a variety of sources, including infectious agents, products of tissue breakdown, metabolic abnormalities, and deposition of irritating agents. VasDC functionally are more similar to myeloid DC but may represent a separate type of tissue-residing DC.
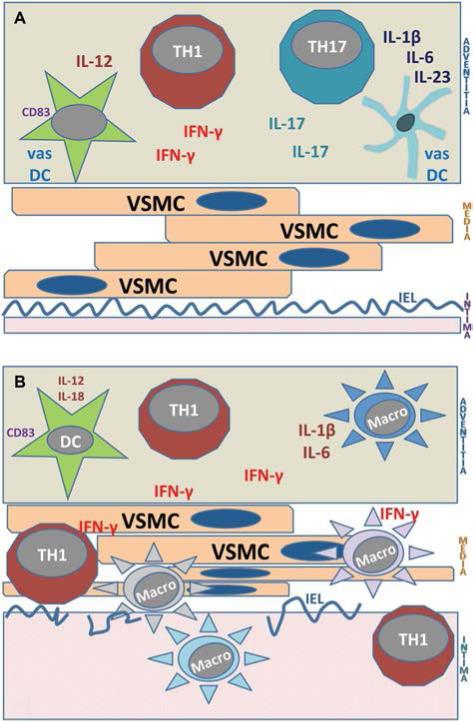
FIG. 1.
Immunopathways in giant cell arteritis (GCA). A. Early GCA. Initial steps in the immune pathogenesis of GCA center on the adventitia. Resident vascular dendritic cells (vasDC) are triggered by danger signals to recruit and acti- vate CD4 T cells. T cells committed to the TH1 lineage release their marker cytokine IFN-γ, whereas T cells committed to the TH17 lineage secret IL-17. Both T-cell lineages participate in the evolving granulomatous inflammation. B. Chronic GCA. Chronic GCA is dominated by TH1 immunity. TH17 cells are explicitly sensitive to corticosteroid therapy and are eliminated from the vascular infiltrates in treated patients. Through the secretion of IFN-γ, vasculitic T cells attract, activate, and guide macrophages. Granulomatous infiltrates move into the media and intima. The vessel wall responds to the immune injury with the formation of hyper- plastic intima that compromises the lumen and leading to ischemic complications. TH, T helper cell; macro, macro- phage; IL, interleukin; INF, interferon; VSMC, vascular smooth muscle cells; IEL, internal elastic lamina; DC, dendritic cells.
Once activated, vasDC produce chemokines to attract other immune cells that have access to the vessel wall through the vasa vasorum. VasDC upregulate the molecular machinery to stimulate T cells by upregulating CD40, CD83, CD80, CD86, and possibly many more receptors and ligands that ultimately shape the nature of the immune response.
VasDC in GCA-affected arteries display 2 abnormalities. They break the rule of DC tissue mobility in that they fail to leave the peripheral tissue site to migrate to lymph nodes but acquire a fully-developed phenotype of highly-activated DC while positioned in the artery (21). This phenomenon has been described as trapping. Also, they bring adaptive immunity to a tissue microenvironment that is normally protected from in situ immune activation. Considering the enormously high price the host pays for engaging the immune system in the arterial wall, healthy vasDC display a phenotype of suppressing immunity and participate in the artery's immune privilege.
VasDC have been implicated in defining the tissue tropism of GCA. In a study comparing the expression profile of Toll-like receptors (TLR), typically expressed by DC, in 6 different vascular territories (temporal, carotid, subclavian, aorta, mesenteric, iliac), each vascular region was found to have a typifying TLR signature (22). This has given rise to the novel concept that human arteries have immune sensing functions and that each vascular region specializes in particular danger signals. As a consequence, each human artery interacts with the immune system in a specific fashion making it susceptible to vessel-specific immune abnormalities.
In a model system of GCA, which relies on human arteries being engrafted into immune-compromised mice, the required components of vascular inflammation have been defined (20,22–24). Arteries lacking vasDC are protected from inflammatory attack. VasDC require activation. Pathogen-derived molecular patterns serve this purpose through distant activation, and infection is not necessary. The activation conditions of the vasDC dictate the architecture and the aggressiveness of the subsequent vasculitis. Not all human T cells and macrophages can cause vasculitis in this model. Those initiating vasculitis are functionally selected. The molecular determinants of that functional selection are currently unknown but could represent ideal biomarkers of disease risk and inflammatory burden.
Distinct T-Cell Lineages in Early and Chronic GCA
T cells are a condicio sine qua non in GCA (25). The majority of T cells arise from the CD4 T-cell subpopulation and displays a memory phenotype in the transmural lesion. Experimental evidence strongly supports that T cells undergo clonal proliferation in temporal artery lesions and that only selected T cells do so—by far the best evidence that this process is antigen specific (26).
Recent data advance the idea that T-cell biology allows the dissection of the arteritis into early and late GCA (Fig. 1A, B). In patients undergoing repeated temporal artery biopsy, one harvested at diagnosis and one while on chronic treatment, there is fundamental change in the composition of the T-cell infiltrate (4). Early GCA is characterized by the presence of 2 T-cell lineages, TH1 cells and TH17 cells. These T-cell lineages are defined through the production of marker cytokines (interferon [IFN]-γ and interleukin [IL]-17, respectively). IFN-γ– and IL-17–releasing T cells have distinct immu- noregulatory potential and interact with distinct partner cells. In late GCA, with the second biopsy taken 3, 6, 9, or 12 months after the initiation of corticosteroid therapy, TH17 cells are no longer present. Instead, chronic GCA is a “poor” TH1 disease. These findings strongly endorse the concept that GCA: 1) has several disease phases; 2) multiple molecular networks sustain vasculitis; 3) is not self-limited; and 4) the therapeutic management needs to adapt to the disease stage.
Little is known about the tissue injury that is mediated by IL-17–secreting T cells. IL-17 receptors are broadly distributed on a variety of cell types, but vascular smooth muscle cells (VSMC), fibroblasts, and endothelial cells are likely affected by IL-17 stimulation. In contrast, substantial infor- mation has been collected on the action of IFN-γ in GCA. The prime source of IFN-γ is CD4 T cells positioned in the adventitia, close to the vasDC. Temporal arteries with high tissue IFN-γ are found in patients with ischemic symptoms, including vision loss, whereas patients with predominance of PMR or fever, favor production of IL-2 (27,28). IFN-γ levels in the tissue correlate with neoangiogenesis, which is part of vessel wall remodeling, and supports the outgrowth of the hyperplastic intima. Tissue IFN-γ production is relatively resistant to several clinically applied and experimental therapies, including corticosteroids, salicylic acid, and NOTCH pathway inhibitors (23,29). Persistence of IFN-γ production in the mural lesions signifies the chronicity of GCA and emphasizes the central position of activated T cells in the disease process. Major cellular targets of IFN-γ are monocytes and macrophages, and giant cell formation is mediated by IFN-γ. Inflammation-caused tissue damage results from activation of macrophages that mediate cellular injury and matrix destruction and regulate the formation of new microvessels.
Temporal artery specimens may show a classical panarteritis but in a subset of cases the inflammatory infiltrate is mostly arranged around the vasa vasorum in the adventitia (30). In experimental GCA, these 2 types of arteritis—panarteritis and periarteritis—can be induced according to the initial activation that triggers vasDC (24). TLR4 ligands consistently cause panarteritis. Periarteritis emerges after stimulating vasDC with TLR5 ligands. Whether differences in the microarchitecture of vasculitis are a result of distinct T cells mediating the disease or are a consequence of differen- tial activation programs in vasDC remains to be determined.
Vasculitic Macrophages in GCA
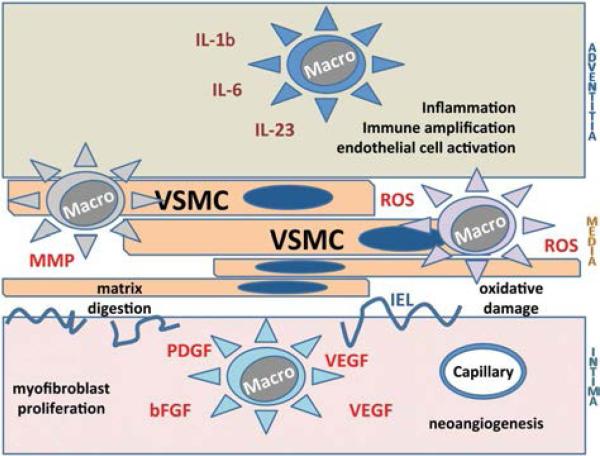
Granulomas are typically composed of activated T cells and macrophages. Multinucleated giant cells are derived from highly activated macrophages that fuse together. The spectrum of macrophage products and functions relevant in the arteritis is broad (Fig. 2), but some correlation exists] between the physical location of the macrophages and their functional contribution (31,32). Different macrophage sub-types may be recruited to the artery. Alternatively, specifics of the microenvironment in different regions of the vessel wall, such as matrix proteins and 3-dimensional configura- tions, may guide macrophage differentiation. Macrophages in the adventitia have been reported to be engaged in the production of classical proinflammatory cytokines, such as IL-6 and IL-1β. Macrophages in the medial layer are mostly devoted to the digestion of cellular structures and elastic membranes (33). Production of metalloproteinases has been assigned to such macrophages. They have also been implicated in the local production of reactive oxygen species (34,35). Nitrotyrosine has been described, mostly colocating with endothelial cells in newly formed microvessels (36).
FIG. 2.
Vasculitic macrophages in giant cell arteritis (GCA). A multitude of macrophages contributes to the inflammatory infiltrates in GCA. Location within the vascular wall layers is associated with functional specialization. Adventitial macrophages produce proinflammatory cytokines (e.g., interleukin [IL]-6, IL-1). Macrophages caught in the media drive matrix and fiber destruction by the release of metal-loproteinases and expose the tissue to reactive oxygen and nitrogen species. Macrophages recruited to the intima provide growth factors (PDGF, VEGF, FGF) needed to support myofibroblast outgrowth and the development of a capillary system to supply oxygen, metabolites and cells for expanding intima. bFGF, Basic fibroblast growth factor; IEL, internal elastic lamina; MMP, matrix metalloproteinases; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Macrophages recruited to the expanding intimal layer are focused on cell growth–promoting actions. They produce platelet-derived growth factor and basic fibroblast growth factor, needed to stimulate the dedifferentiation, migration, and proliferation of medial smooth muscle cells (37). Macrophages residing at the media–intima junction also supply vascular endothelial growth factor, enabling the sprouting of new microvessels and securing oxygen supply for the wall remodeling process (38).
Recent progress in macrophage biology has established that monocytes differentiate along different pathways to generate M1 and M2 macrophages. M1 macrophages specialize in proinflammatory actions, whereas M2 macrophages are responsible for the removal of debris and repair activity in the tissue site. It is unknown whether macrophages in the GCA lesions differentiate in situ, which signals regulate their functional commitment, how long they survive, and how they contribute to the distinct phases of the disease. Results from the second-side temporal artery biopsy study (4) reveal that IL-1β and IL-6 production are explicitly susceptible to corticosteroid therapy. In contrast, production of the T-cell polarizing cytokine IL-12 is almost unaffected by steroids, suggesting that macrophages display similar differences in steroid responsiveness and resistance as T cells.
Dysfunction of Vascular Cells in GCA
The strict tissue tropism of GCA strongly suggests that the immune system is only one component in the disease process and that the disease microenvironment is equally important. The arteries targeted by the vasculitic reaction contribute through several pathways. The artery's role is nonredundant, and it is likely that the permissibility of the vascular tissues is the ultimate checkpoint in GCA.
Endothelial Cells
The immune reaction leading to GCA enters the blood vessel wall through the vasa vasorum, with no evidence of participation of the endothelium. T-cell activation occurs exclusively in the adventitia, and human arteries stripped of the adventitial layer fail to sense danger signals (22). To enter the vessel wall, immune cells need to migrate from the capillary network of the vasa vasorum tree. How this process is enabled by endothelial cells is currently unknown. Once the remodeling program of the vascular wall is initiated, new capillaries are formed. They can serve as an entrance port for T cells and macrophages and thus accelerate granuloma formation (39).
Vascular Dendritic Cells
Through their vessel-specific distribution and their selective portfolio of danger signals to which they respond, vasDC are prime candidates to control tissue tropism of GCA. They are localized between the vasa vasorum and the media, at the junction of the adventitial and media and thus lie in the path of incoming T cells and macrophages. They are critically involved in chemoattracting immune cells and no vessel wall inflammation can be initiated without them. There are numerous questions regarding their origin, survival times, turnover, and age-related changes that await investigation.
Vascular Smooth Muscle Cells
VSMC are active participants in the granulomatous reactions in that they serve as signal-sending and signal-receiving cells (40). Their major involvement lies in a phenotypic switch from a contractile into migratory and secretory cells. The formation of the lumen-compromising intimal hyperplasia requires myofibroblasts, a dedif-ferentiated form of VSMC. Previous studies have implicated them in regulating the response of the arterial wall to oxidative stress. Specifically, they upregulate the enzyme aldose reductase to deal with oxidative alterations of cellular proteins (34). In a recent study, another VSMC-centered pathway, the NOTCH–NOTCH ligand pathway, was described as an amplification loop to sustain vasculitis (41). Components of the NOTCH signaling pathway regulate critical aspects of vascular formation and morphogenesis in development. Notch1, Notch4, Jagged1, Delta-like1, and Delta-like4 participate in arterial cell fate induction and determine the selection of endothelial tip and stalk cells during sprouting angiogenesis. CD4 T cells from patients with GCA spontaneously express the Notch1 receptor, enabling them to communicate with Notch ligand-positive cells. Vascular cells, in particular VSMC, are a rich source of Notch receptors and ligands. Developing VSMC express Notch3 receptors (Notch3 mutations cause CADASIL, a cerebral arteriopathy with subcortical infarcts and leukoencephalopathy), and mature VSMC have the ligand Jagged1 and the receptor Notch2 on their surface. This provides ideal conditions for immune–stromal communications between T cells and VSMC. Gene expression profiling has demonstrated that Notch1, Jagged1, and Delta-like1 are abundantly expressed in temporal arteries affected by GCA, and molecular studies support the notion that the Notch pathway is actively signaling in the inflamed arteries. Blockade of Notch signaling through an enzyme blocker relevant for a cleavage event that liberates the Notch intracellular domain effectively suppressed adaptive and innate immunity in vasculitis.
Therapies of the Future
While corticosteroids are highly effective in treating patients with GCA, it would be preferable to have a more targeted approach (Table 2; Tables 3–6, Supplemental Digital Content, http://links.lww.com/WNO/A47). Steroids promptly suppress IL-6, IL-1, IL-23, and IL-17, but they fail to control the production of IL-12 and IFN-γ, which seem critical in supporting chronic vasculitis. New treatment approaches are required that can safely target TH1 immunity without compromising protective immunity to a dangerous degree. Inflammatory cascades that derive from communication between the vessel wall and the immune system should be addressed. Preclinical studies suggest that inhibition of such immune–stromal interactions can successfully disrupt vasculitis (41). Equally unexplored are the response-to-injury pathways that the artery launches in an attempt to repair wall damage. Interfering with the formation of intimal hyperplasia, blocking the formation of new microvessels in the injured wall, and suppressing oxidative damage all have the potential to counteract the deleterious consequences of vascular inflammation without further compromising the immune system of the elderly patient. Ultimately, an attempt needs to be made to reestablish the immune privilege of the arterial wall. Here, vasDC emerge as the therapeutic target. Understanding how they protect arteries from detrimental immune attack could open an entirely new paradigm in the management of GCA.
TABLE 2.
Typical clinical features of giant cell arteritis
| Cranium | Headache, scalp tenderness, jaw claudication |
| Eye | Vision loss; painless, sudden, partial, or complete diplopia; ophthalmoplegia |
| Systemic | Fever, malaise, anorexia, depression,weight loss, night sweats |
| Musculoskeletal | Polymyalgia rheumatica |
| Central nervous system | Stroke, dizziness, vertebrobasilar insufficiency; central vision loss |
| Extremities | Upper extremity claudication; pulselessness; asymmetric blood pressure |
| Aorta | Aortic insufficiency; aortic aneurysm, dissection, rupture |
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI 44142, AR 42527, EY 11916, HL 58000, AI 57266, and AI 90019) and a pilot grant from the North American Neuro-Ophthalmology Society.
Footnotes
The authors have no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www.jneuro-ophthalmology.com).
REFERENCES
- 1.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Younge BR, Goronzy JJ. IFN-g and IL-17: the two faces of T-cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23:43–49. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki A, Purvin V. Giant cell arteritis: an updated review. Acta Ophthalmol. 2009;87:13–32. doi: 10.1111/j.1755-3768.2008.01314.x. [DOI] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13:231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. The HLA-DRB1 locus as a genetic component in giant cell arteritis. Mapping of a disease-linked sequence motif to the antigen binding site of the HLA-DR molecule. J Clin Invest. 1992;90:2355–2361. doi: 10.1172/JCI116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton BT. Temporal arteritis; report of 39 cases. Proc Annu Meet Cent Soc Clin Res U S. 1946;19:78. [PubMed] [Google Scholar]
- 11.Boes CJ. Bayard Horton's clinicopathological description of giant cell (temporal) arteritis. Cephalalgia. 2007;27:68–75. doi: 10.1111/j.1468-2982.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt J, Duhaut P, Bourgeois AM, Salle V, Smail A, Chatelain D, Betsou F, Mazière JC, Ducroix JP. Procalcitonin at the onset of giant cell arteritis and polymyalgia rheumatica: the GRACG prospective study. Rheumatology (Oxford) 2009;48:158–159. doi: 10.1093/rheumatology/ken437. [DOI] [PubMed] [Google Scholar]
- 13.Duhaut P, Bosshard S, Ducroix JP. Is giant cell arteritis an infectious disease? Biological and epidemiological evidence. Presse Med. 2004;33:1403–1408. doi: 10.1016/s0755-4982(04)98939-7. [DOI] [PubMed] [Google Scholar]
- 14.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alba MA, Mena-Madrazo JA, Reyes E, Flores-Suárez LF. Giant cell arteritis in Mexican patients. J Clin Rheumatol. 2012;18:1–7. doi: 10.1097/RHU.0b013e31823e2e35. [DOI] [PubMed] [Google Scholar]
- 16.Pereira LS, Yoon MK, Hwang TN, Hong JE, Ray K, Porco T, McCulley TJ. Giant cell arteritis in Asians: a comparative study. Br J Ophthalmol. 2011;95:214–216. doi: 10.1136/bjo.2009.177220. [DOI] [PubMed] [Google Scholar]
- 17.Weyand CM, Ma-Krupa W, Pryshchep O, Gröschel S, Bernardino R, Goronzy JJ. Vascular dendritic cells in giant cell arteritis. Ann N Y Acad Sci. 2005;1062:195–208. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- 18.Weyand CM, Younge BR, Goronzy JJ. T cells in arteritis and atherosclerosis. Curr Opin Lipidol. 2008;19:469–477. doi: 10.1097/mol.0b013e32830bfdc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiner F, Langberg H, Galbo H. Increased muscle interstitial levels of inflammatory cytokines in polymyalgia rheumatica. Arthritis Rheum. 2010;62:3768–3775. doi: 10.1002/art.27728. [DOI] [PubMed] [Google Scholar]
- 20.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupa WM, Dewan M, Jeon MS, Kurtin PJ, Younge BR, Goronzy JJ, Weyand CM. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–1823. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piggott K, Biousse V, Newman NJ, Goronzy JJ, Weyand CM. Vascular damage in giant cell arteritis. Autoimmunity. 2009;42:596–604. doi: 10.1080/08916930903002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner AD, Björnsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 26.Weyand CM, Schönberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994;179:951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42:311–317. doi: 10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Weyand CM, Tetzlaff N, Björnsson J, Brack A, Younge B, Goronzy JJ. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 29.Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum. 2002;46:457–466. doi: 10.1002/art.10071. [DOI] [PubMed] [Google Scholar]
- 30.Chatelain D, Duhaut P, Loire R, Bosshard S, Pellet H, Piette JC, Sevestre H, Ducroix JP. Small-vessel vasculitis surrounding an uninflamed temporal artery: a new diagnostic criterion for polymyalgia rheumatica? Arthritis Rheum. 2008;58:2565–2573. doi: 10.1002/art.23700. [DOI] [PubMed] [Google Scholar]
- 31.Weyand CM, Wagner AD, Björnsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner AD, Goronzy JJ, Weyand CM. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest. 1994;94:1134–1140. doi: 10.1172/JCI117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Pla A, Bosch-Gil JA, Rosselló-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–269. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- 34.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–1013. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 36.Borkowski A, Younge BR, Szweda L, Mock B, Björnsson J, Moeller K, Goronzy JJ, Weyand CM. Reactive nitrogen intermediates in giant cell arteritis: selective nitration of neocapillaries. Am J Pathol. 2002;161:115–123. doi: 10.1016/S0002-9440(10)64163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser M, Weyand CM, Björnsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–633. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser M, Younge B, Björnsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitrijevic I, Andersson C, Rissler P, Edvinsson L. Increased tissue endothelin-1 and endothelin-B receptor expression in temporal arteries from patients with giant cell arteritis. Ophthalmology. 2010;117:628–636. doi: 10.1016/j.ophtha.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 40.Régent A, Dib H, Ly KH, Agard C, Tamby MC, Tamas N, Weksler B, Federici C, Broussard C, Guillevin L, Mouthon L. Identification of target antigens of anti-endothelial cell and anti-vascular smooth muscle cell antibodies in patients with giant cell arteritis: a proteomic approach. Arthritis Res Ther. 2011;13:R107. doi: 10.1186/ar3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piggott K, Deng J, Warrington K, Younge B, Kubo JT, Desai M, Goronzy JJ, Weyand CM. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123:309–318. doi: 10.1161/CIRCULATIONAHA.110.936203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.