Abstract
Introduction
Prenatal diagnosis provides valuable information regarding a variety of congenital heart defects. Some defects occur early in gestation with little change throughout pregnancy, whereas others evolve during mid and late gestation. Fetal cardiac intervention (FCI) affords the opportunity to interrupt progression of disease in this latter category, resulting in improved perinatal and lifelong outcomes.
Aim
This chapter addresses three lesions for which percutaneous FCI can be utilized: (1) aortic stenosis with evolving hypoplastic left heart syndrome, for which aortic valvuloplasty may prevent left ventricular hypoplasia and has yielded a biventricular circulation in approximately one third of cases; (2) hypoplastic left heart syndrome with intact atrial septum, for which relief of atrial restriction has potential to improve perinatal survival; and (3) pulmonary atresia with intact ventricular septum and evolving right ventricular hypoplasia, for which pulmonary valvuloplasty has resulted in a biventricular circulation in the majority of patients. The pathophysiology, rationale for intervention, patient selection criteria, procedural technique, and outcomes for each lesion will be reviewed. This chapter will also review complications of FCI and their treatment, and maternal and fetal anesthesia specific to FCI. The importance of a specialized center with experience managing infants delivered after FCI will also be addressed.
Keywords: congenital heart disease, fetal cardiac intervention, aortic stenosis, hypoplastic left heart syndrome, pulmonary atresia with intact ventricular septum
Prenatal diagnosis provides valuable information regarding the evolution of congenital heart defects during gestation. Certain defects clearly form early in gestation with relatively little change throughout pregnancy. Other disease, however, evolves during fetal life from mid though late gestation. As progression of disease has become characterized, alteration in the natural course of congenital heart disease using a variety of fetal interventions has become an increasingly attractive goal.
Intrauterine catheter-based intervention for fetal cardiac disease was first reported in 1991. These reports described fetal aortic valvuloplasty for aortic stenosis (AS).[1] Since that time, catheter-based intervention for fetal AS has become the quintessential example of percutaneous fetal cardiac intervention (FCI) for evolving congenital heart disease. Since its initial descriptions, FCI has further evolved to address a variety of lesions.
This chapter will specifically address three lesions for which in utero percutaneous FCI is utilized: (1) AS with evolving hypoplastic left heart syndrome (eHLHS), (2) hypoplastic left heart syndrome (HLHS) with intact (or highly restrictive) atrial septum (IAS), and (3) pulmonary atresia (PA) with intact ventricular septum (IVS). Pathophysiology of each lesion, rationale for intervention, technique, and outcomes will be discussed. This chapter will also briefly review maternal and fetal anesthesia relevant to FCI and the risks and complications of the procedure. Finally, the importance of a delivery center experienced with patients having undergone fetal intervention will also be addressed.
Severe Aortic Stenosis with Evolving Hypoplastic Left Heart Syndrome
Pathophysiology and Rationale for Intervention
HLHS comprises a spectrum of disease in which the unifying feature is the inability of the left-sided heart structures to support the systemic circulation in part or in full. In its most severe form, there is mitral and aortic valve atresia and no identifiable left ventricular cavity. Often, however, there is varying underdevelopment of left-sided heart structures, and the degree of hypoplasia has important implications for postnatal outcomes. Most forms of HLHS occur in early gestation and are not amenable to fetal intervention. However, a subgroup of fetuses appears to suffer an in utero insult resulting in the initiation and evolution of HLHS during mid and late gestation. The number of such patients and the distribution of gestational ages at the time of inciting insult not entirely clear. Nonetheless, some of these patients have potential to benefit from timely FCI.
In cases of eHLHS, some insult to the aortic valve, likely a combination of environmental factors and genetic susceptibility, causes abnormal underdevelopment, dysplasia, and thickening of the valve, resulting in AS. If the stenosis is severe, a relatively predictable series of events follows.[2] Initially, the left ventricle pumps against increasing afterload, resulting in left ventricular dilation, dysfunction, and mitral regurgitation. As myocardial perfusion diminishes, myocardial cell death and scarring ensue. Left ventricular growth slows and eventually ceases.[3]
Left ventricular hypoplasia is tolerated well in utero. Umbilical, systemic, and pulmonary venous blood enters the right ventricle (RV). In contrast to the usual fetal circulation, the coronary, head and neck circulations are supplied in varying degree via retrograde flow from the ductus arteriosus. After delivery, however, ductal closure results in systemic hypoperfusion, acidemia, and death, typically in a period of days. Infants born with HLHS require a prostaglandin infusion to maintain patency of the ductus arteriosus and preserve systemic perfusion until definitive therapy can be undertaken.
Although therapies for HLHS have evolved remarkably during the last three decades, treatment alternatives are limited and include surgical palliation, transplant, or comfort care. In the United States, a common approach is for the infant to undergo three palliative surgeries.[4] In brief, the three palliative surgeries, Norwood, Glenn, and Fontan, are designed to redirect blood flow such that the RV becomes the systemic blood flow pump. The systemic venous return is redirected largely or entirely outside the heart to the pulmonary circulation through superior (Glenn) and inferior (Fontan) cavopulmonary anastomoses.
It is important to understand the attendant morbidity and mortality associated with palliative surgery for HLHS. Mortality among patients who undergo staged palliation for HLHS approaches 30% by age 5 years. Among patients who survive to discharge after Norwood surgery, a conservative estimate of the mortality between the first and second surgeries alone is 10%. Furthermore, there are attendant issues with morbidity and complex medical care.[5] The Glenn and Fontan procedures are associated with less mortality, but ongoing morbidity and impaired quality of life remain highly problematic, with ventricular and valvar dysfunction, protein-losing enteropathy, thromboembolism, and arrhythmia being common.[6] [7] [8] Because of these and other problems, a growing number of patients require heart transplant as survivors of staged palliation enter late childhood, adolescence, and adulthood. Given these attendant issues with a palliative approach to HLHS, seeking alternatives to this strategy is attractive.
The goal of fetal intervention for AS/eHLHS is to avoid a univentricular circulation. If the aortic valve can be dilated before the development of irreversible left ventricular dysfunction and cessation of growth, recovery of function and resumed growth are possible. Relief of AS and restoration of robust anterograde left ventricular flow relieves the afterload on the left ventricle, diminishes left ventricular diastolic pressure, alleviates mitral regurgitation, and allows for resumed left ventricular growth.
Patient Selection
In this era, it is possible to predict which fetus with AS will evolve into HLHS. These features include retrograde flow in the transverse aortic arch, left-to-right flow across the foramen ovale, a monophasic mitral inflow pattern, and significant left ventricular dysfunction.[2] [9] Although such fetuses can be readily identified using standard fetal echocardiographic techniques, the diagnosis of eHLHS alone is insufficient to recommend intervention. Patient selection is crucial, as only patients likely to receive benefit from the procedure should be subjected to its attendant risks. A threshold scoring system to estimate potential for biventricular outcome in midgestation fetuses with AS and eHLHS has been developed ([Table 1]). A score of ≥ 4 reliably identifies those patients with little chance of achieving a biventricular outcome. Additional refinements to this threshold scoring system are ongoing and may confer additional likelihood of a biventricular outcome.
Table 1.
Threshold Scoring system to estimate potential for biventricular outcome in midgestation fetuses with AS and evolving HLHS
| One point for each of the following |
| LV long-axis Z-score > 0 |
| LV short-axis Z-score > 0 |
| Aortic annulus Z-score > 3.5 |
| MV annulus Z-score > 2 |
| MV or AS maximum gradient ≥ 20 |
Abbreviations: AS, aortic stenosis; HLHS, hypoplastic left heart syndrome; LV, left ventricle; MV mitral valve.
Note: Threshold score ≥ 4 had 100% sensitivity, 53% specificity, 38% positive predictive value, and 100% negative predictive value for identifying fetuses that had a biventricular outcome from the time of birth without intermediate univentricular palliation.
Source: Reproduced with permission from McElhinney et al.[9]
Technique
The basic principle of fetal aortic valvuloplasty is the same as that for infants, children, and adults with valvar AS. Access to the aortic valve is achieved, a guide wire is threaded via the left ventricular outflow tract into the proximal ascending aorta, and a balloon of the desired size is inflated across the valve, with a goal of relieving stenosis while incurring as little valve insufficiency as possible. The differences attendant to fetal intervention are largely related to access to the fetus and the associated maternal and fetal risks.
Essential to gaining access to the fetus is attaining a fetal position in which the thorax is anterior and ideally left-side superior. This position can be attained through a combination of the following: (1) left lateral positioning of the mother before initiating the procedure; (2) fetal version, with gentle manipulation of the fetus through the maternal abdominal wall to attain the correct position; and (3) fetal traction, including final manipulation and stabilization of the fetus in an ideal position using a 19-gauge styleted needle. An intramuscular paralytic delivered to the fetus assures cessation of spontaneous movement, although this does not preclude buoyant movement within the amniotic space. Occlusion of the mother’s urinary catheter to allow passive filling of the bladder compresses the lower uterine space, restricting the floating movement of the fetus. In some instances, physically securing the fetus by direct pressure from an operator’s hand on the maternal abdomen is needed. Laparotomy to assist with fetal positioning and imaging resolution is possible but infrequently needed.
Percutaneous access to the fetal heart is guided using two-dimensional ultrasound. The optimal trajectory allows the cannula to traverse the following structures: maternal abdominal wall, uterine wall, amniotic fluid, fetal chest wall, and fetal heart coming to rest at the aortic valve. It is desirable for the cannula to traverse as few layers as possible, as more crossed layers will limit repositioning of the cannula. The placenta should be avoided if possible, but a transplacental approach may be used if absolutely necessary.
A maternal fetal medicine specialist with experience in ultrasound-guided needle procedures is responsible for achieving access to the fetal heart, using a cannula with a solid sharp-tipped stylet of the lowest profile that can accept the desired balloon. Once the cannula reaches the chest wall, the tip is guided through the fetal intercostal space toward the left ventricular outflow tract. The left ventricular apex is punctured with a rapid thrust to minimize cardiac distortion and to pass through what is often fibrotic endomyocardial tissue. The cannula is directed toward the left ventricular outflow tract and upon removal of the stylet, blood return is confirmed.
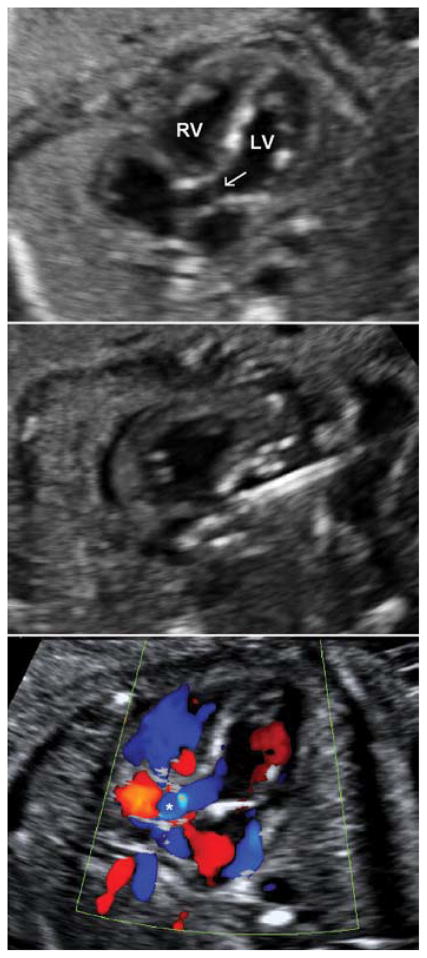
A pediatric cardiologist with expertise in interventional catheterization then performs the valvuloplasty. A guidewire with a preloaded coronary artery balloon dilation catheter is inserted and advanced out of the needle. With subtle repositioning, the wire traverses the valve, is identified in the ascending aorta, and the balloon is advanced across the aortic annulus. The balloon is then inflated in successive dilations, retracted against the cannula, and upon deflation, the cannula, balloon, and wire are all removed from the patient.[10] [11] [12] Antegrade flow is typically demonstrated immediately, frequently accompanied by a prompt, appreciable improvement in left ventricular function (Fig. 1).
Fig. 1.
Top panel: Aortic stenosis and evolving hypoplastic left heart syndrome at 25 weeks gestational age. Left ventricular size is preserved in midgestation. Areas of left ventricular echo brightness consistent with endocardial fibroelastosis and myocardial scarring are evident. The thickened, doming, and stenotic aortic valve is visible. Middle panel: The cannula is directed into the left ventricular outflow tract. A guidewire is advanced into the proximal ascending aorta, and a coronary angioplasty balloon is inflated across the aortic valve. Bottom panel: Still-frame color Doppler image demonstrating a broad jet of anterograde flow (asterisk) across the left ventricular outflow tract and aortic valve. LV, left ventricle; RV, right ventricle; arrow, stenotic aortic valve.
Outcomes
Proper patient selection as described above yields a higher likelihood of a technically successful procedure.[2] Aortic regurgitation is common after intervention, although in most patients this improves by the time of delivery. Postnatal outcomes are highly variable, and to a large extent depend on the extent of left ventricular damage before intervention. The most recent published experience indicates that 30% of fetuses experiencing a technically successful intervention will go on to become a child with a biventricular circulation.[13] It is important to note, however, that the majority of children will require additional catheter-based and surgical interventions to achieve this outcome. An additional 8% of patients underwent initial-staged surgical palliation, but they were later converted to a biventricular circulation.[9] More recent efforts toward revising selection criteria may confer additional benefit in terms of achieving a higher proportion of biventricular outcomes. Of note, left ventricular diastolic dysfunction after FCI, likely due to prior in utero myocardial scarring, is particularly common and can occur even if left ventricular size is preserved. This can be particularly debilitating for patients, as therapies for diastolic dysfunction are limited.[13] [14]
It is crucial for both families and providers alike to understand that FCI alone is often insufficient to result in a biventricular circulation. The neonatal management of these patients is beyond the scope of this chapter, but patients should deliver at a center familiar with the challenges and management of this unique group of patients. In the ideal scenario, no postnatal intervention is necessary. Frequently, however, a combination of multiple catheter-based and surgical interventions during the first several months and years of life is necessary to achieve a biventricular circulation.[15] Some patients with left ventricular hypoplasia who do not achieve a biventricular circulation may still benefit from the diminutive ventricle’s contribution to systemic output in a single-ventricle type palliation. Studies regarding longer term outcomes in these patients are ongoing.
Hypoplastic Left Heart Syndrome with Highly Restrictive or Intact Atrial Septum
Pathophysiology and Rationale for Intervention
HLHS with IAS is a variant of HLHS in which the flow of blood across the foramen ovale is restricted or absent due to abnormal foramen closure, abnormal thickening of the atrial septum, or an abnormally large septum primum. A restrictive septum complicates up to 22% of all HLHS cases, with a frankly IAS occurring in an additional 6% of cases.[16] [17] [18] The result of this association has serious deleterious consequences on postnatal physiology, even outside the spectrum of HLHS without IAS.
HLHS/IAS is relatively well tolerated during fetal life. As in the normal fetus, flow to the fetal pulmonary vasculature is generally low. However, with an intact or restrictive atrial septum, there is in utero left atrial hypertension resulting in ongoing damage to the pulmonary vasculature, lung parenchyma, and lymphatic system. After delivery, pulmonary vascular resistance drops and blood flow into the pulmonary vascular bed increases. The pulmonary venous return has little or no means of egress from the left atrium. Oxygenated pulmonary venous blood cannot enter the systemic circulation via the foramen ovale and RV. Simultaneously, left atrial pressure increases dramatically, resulting in pulmonary venous congestion and respiratory insufficiency. The combined result is hypoxemia, respiratory and metabolic acidosis, and rapid demise.
Treatment typically requires urgent postnatal catheterization for balloon dilation and/or stenting of the atrial septum. Urgent Norwood palliation is sometimes required, and is preferred by some centers. Despite adequate catheter-based or surgical relief of atrial obstruction, mortality remains as high as 38% in this group, likely due to irreversible pathologic lung changes in utero.[16] [19] [20] Relief of atrial restriction in utero provides a means to interrupt or ameliorate lung damage and provide a more favorable postnatal course.
Patient Selection
Because of the relative infrequency of HLHS/IAS, strict patient selection criteria for septoplasty have not been developed. Characteristics suggesting atrial restriction or the need for urgent postnatal intervention on the atrial septum have been previously described by other authors (Table 2).[21] [22] [23] Of these factors, pulmonary vein flow reversal remains a highly sensitive indicator of the need for urgent atrial decompression after birth.
Table 2.
Characteristics suggesting atrial restriction in HLHS
| Intact atrial septum |
| ≤1-mm atrial septal defect |
| Prominent pulmonary vein flow reversal |
| Forward: reverse pulmonary vein velocity time integral ratio < 5 |
Abbreviation: HLHS, hypoplastic left heart syndrome.
Fetuses undergoing this procedure at our institution have done so at gestational ages ranging from 24 to 34 weeks. Unlike eHLHS, which is unlikely to see benefit from intervention at late gestational age, FCI for HLHS/IAS has a theoretical benefit across a spectrum of gestational ages. At earlier gestational age, intervention may arrest or ameliorate the pulmonary vascular changes described previously. At a later gestational age, in utero septoplasty may obviate the need for urgent postnatal surgical or catheter-based intervention to relieve left atrial hypertension and may modify the remaining postnatal course.
Technique
Using ultrasound guidance, an unobstructed approach from the maternal abdomen to the fetal right atrium is visualized. If access to the right atrium is limited, the left atrium can also be accessed. An 18- or 19-gauge introducer cannula is advanced in similar fashion as described for eHLHS. The introducer is advanced to the right atrial epicardial surface and into the right atrium. The right atrium is either punctured by advancing the tip of the introducer or by puncturing the septum with a 22-gauge Chiba needle, through which a 0.014″ wire is introduced into the left atrium or a pulmonary vein. The Chiba needle is then exchanged for a balloon angioplasty catheter. The balloon is inflated across the atrial septum. If so desired, a stent may also be placed in the atrial septum in an effort to maintain patency of the newly created atrial communication (Fig. 2).
Fig. 2.
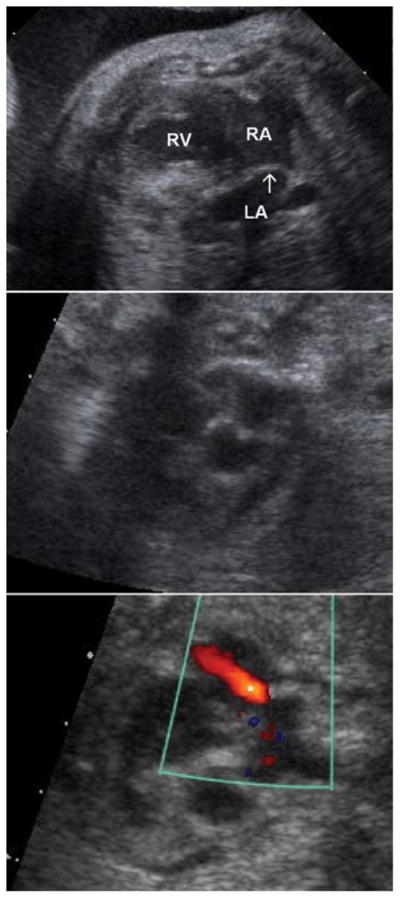
Top panel: Hypoplastic left heart syndrome with intact atrial septum at 31 weeks gestational age. Severe left ventricular hypoplasia is noted. The left atrium is markedly dilated, and the thickened, hypertrophied septum primum (arrow) bulges into the right atrium. Middle panel: An inflated angioplasty balloon traverses the right atrium, atrial septum and into the left atrium. Bottom panel: Still-frame color Doppler image demonstrating a jet of left-to-right flow (asterisk) across the newly created atrial septal defect. LA, left atrium; RA, right atrium; RV, right ventricle.
Outcomes
In a series of 21 fetuses with HLHS/IAS undergoing atrial septoplasty at our institution, 13 of 21 patients achieved a medium (or larger)-sized atrial septal defect (ASD), defined as ≥2.5 mm or more than one small defect. Among those patients, antegrade pulmonary vein flow improved. In eight patients, the atrial communication was unchanged. There were two cases of interventional fetal loss related to hemopericardium. A large (≥3 mm) ASD on follow-up fetal echocardiogram was associated with higher postnatal oxygen saturation and decreased need for emergent postnatal catheterization for left atrial decompression.[24]
The 19 patients who survived to delivery all underwent a Stage 1 Norwood procedure. In this group, surgical survival among those who underwent urgent left atrial decompression before surgery was 42%, compared with 86% among patients who underwent routine Stage 1 palliation only. This difference, however, did not achieve statistical significance. The overall survival of this group, including fetal losses related to intervention, was 52% (11/21).[24] Further experience is required to identify the ideal candidate for atrial septoplasty to maximize postnatal benefit. A significant limitation of fetal atrial septoplasty is that the defect can close or become smaller during the remainder of gestation. As noted above, this has prompted fetal atrial stenting in some patients.
Pulmonary Atresia with Intact Ventricular Septum
Pathophysiology and Rationale for Intervention
PA with IVS encompasses a broad spectrum of pathology; the common unifying feature is atresia of the pulmonary valve and absence of a ventricular communication. At the least severe end of the spectrum, there is membranous pulmonary valve atresia with leaflet fusion and a normally sized RV. In its most severe form, there is atresia of the right ventricular infundibulum (outflow tract), no pulmonary valve, severe right ventricular hypoplasia, and abnormal fistulous communications between the diminutive right ventricular cavity and the coronary circulation. In certain cases, the coronary circulation may depend in part or entirely on these abnormal communications, and coronary ostial atresia may develop. This scenario carries a particularly poor prognosis.[25] [26] [27] [28] The pulmonary arteries are usually of normal size and are supplied in fetal life in a retrograde fashion via the ductus arteriosus. Tricuspid regurgitation is a common feature of this lesion and may actually provide favorable right ventricular growth due to right ventricular volume loading.
Postnatal treatment for PA/IVS depends primarily on the size of the RV, the presence of right ventricular coronary dependence, and to a much lesser degree, the pulmonary valve itself. Treatment may be limited to catheterization in the perinatal period to perforate and dilate the pulmonary valve. Conversely, transplant is not infrequently recommended for patients with PA/IVS with right-ventricular coronary dependence and coronary ostial atresia.[27] [28] The approach to care for newborns between these two extremes is highly variable. However, patients with severe right-ventricular hypoplasia require staged palliation resulting in a Fontan circulation and its attendant short- and long-term risks.
Like eHLHS, PA/IVS evolves during pregnancy with progressive right ventricular hypoplasia. As fetal aortic valve dilation for eHLHS affords the potential to resume left ventricular growth, fetal pulmonary valvuloplasty in PA/IVS analogously has the potential to result in resumed right ventricular growth. Right ventricular outflow obstruction is relieved, antegrade blood flow through the right heart is restored, growth of the RV resumes, and a biventricular circulation can be achieved.
Patient Selection
Experience with fetal intervention for PA/IVS is considerably more limited than for eHLHS, mainly due to the relative rarity of this lesion. Patient selection is equally critical, however, and perhaps more challenging in this population. Postnatal care for PA/IVS with a good-sized RV and pulmonary valve is well-established and safe. Similarly, at the opposite end of the spectrum, patients with severe RV hypoplasia and RV coronary dependence are unlikely to benefit from intervention. Only patients with the potential to restore ventricular growth and obviate the need for univentricular palliation should be considered for intervention. In our institution, criteria for intervention for PA/IVS include: (1) membranous PA, (2) an intact or highly restrictive ventricular septum, and (3) right heart hypoplasia with a tricuspid valve annulus Z-score below 2.5 and an identifiable but qualitatively small RV.[29]
Technique
Ultrasound guidance is utilized to visualize the fetus and angle of approach. The right ventricular geometry is somewhat more complex than in eHLHS. In eHLHS, the left ventricle is often dilated at the time of intervention, and there is a direct trajectory of the needle toward the left ventricular outflow. In contrast, the right heart in PA/IVS is often small and has marked hypertrophy and irregular and thick muscle trabeculations. This is further complicated by the curved nature of the right ventricular outflow and its anterior position, making the angle of entry a challenge.
The trajectory of access is equivalent to a subcostal approach or through an intercostal space adjacent to the sternum. The atretic pulmonary valve is perforated with the stylet of a 19-gauge cannula or an ultrasharp 22-gauge Chiba needle introduced through the cannula. A 0.014″ floppy-tipped guidewire is positioned in the pulmonary artery or the ductus arteriosus, and a coronary angioplasty balloon is inflated across the valve (Fig. 3).
Fig. 3.
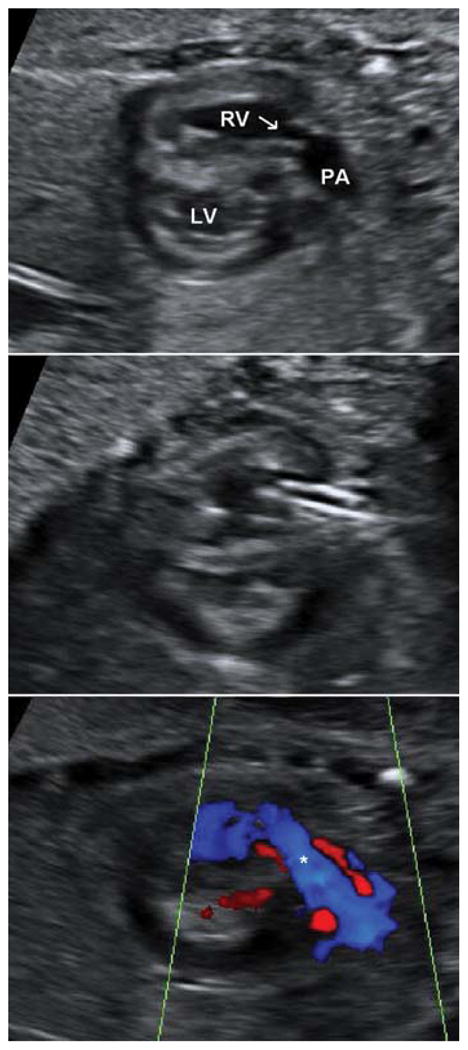
Top panel: Pulmonary atresia with intact ventricular septum at 29 weeks gestational age. The right ventricle is hypertrophied, and the thickened, doming, and atretic pulmonary valve (arrow) is visible. A normally sized right pulmonary artery is visualized. Middle panel: After perforation of the pulmonary valve with a cannula, an angioplasty balloon is inflated in the right ventricular outflow tract and across the pulmonary valve. Bottom panel: Still-frame color Doppler image after pulmonary valve perforation and balloon angioplasty in the same patient. There is a broad jet of anterograde flow (asterisk) into the pulmonary artery. LV, left ventricle; RV, right ventricle; PA, main pulmonary artery.
Outcomes
Experience with FCI for PA/IVS is limited due to the relative rarity of the lesion. The most recently published series from our institution described 10 such patients. A small number of other centers worldwide have also performed the procedure.[30] [31] [32] [33] Of the 10 patients, 6 patients described in the series from our institution had a technically successful pulmonary valvuloplasty. When comparing the intervention group with a control group of fetuses, those with intervention had maintenance of growth of right-sided heart structures and stable Z-scores, whereas those without intervention had arrest of growth, and the Z-scores declined throughout the pregnancy.[29]
Among the six patients who had a technically successful fetal intervention for PA/IVS at our center, five went on to have a biventricular circulation with or without surgical neonatal RV outflow tract augmentation and/or systemic-to-pulmonary artery shunting. Overall, however, the number of fetuses having undergone this procedure is very small and additional experience is required to further refine patient selection and to draw conclusions about the procedure.
Additional Considerations
Maternal and Fetal Anesthesia Relevant to Fetal Cardiac Intervention
Currently, procedures are performed percutaneously with maternal epidural neuroaxial analgesia and fetal intramuscular narcotic and paralytic. While the majority of procedures (118/150, 78.7%) were performed with the patients under general anesthesia to achieve uterine relaxation and facilitate fetal positioning, a change to epidural analgesia has not altered the rate of technically successful interventions. A minority of patients (19/150; 12.7%) during our early experience underwent a limited laparotomy to facilitate imaging and access. Subsequent attention to preoperative maternal positioning and earlier placement of fetal paralytic agent has facilitated fatal positioning without the need for maternal laparotomy. Use of a completely percutaneous approach affords the advantage of neuroaxial anesthesia with an improved maternal safety profile, and minimizes the risk of preterm labor and physiologic stress to the fetus.
Fetal paralysis is of paramount importance for positioning the fetus and facilitating access to desired heart structures. Typically, a combination fetal intramuscular fentanyl and vecuronium or pancuronium is utilized.[12] [34] Preprocedure allocation of resuscitation medications is paramount to assuring a smooth resuscitation effort. Epinephrine is particularly important given the propensity to fetal bradycardia as described below. Additional resuscitation medications may include atropine, sodium bicarbonate, calcium gluconate, and packed red blood cells. In the setting of percutaneous FCIs, medication is typically delivered via the intramuscular or intracardiac route. Care should be utilized when delivering medications via the intracardiac route, so as not to inadvertently distend the left ventricle and precipitate ischemia.[35]
Complications
The overall safety profile of FCIs is quite good, although several fetuses experience short-term complications of intervention. Bradycardia and ventricular dysfunction occur commonly in fetuses undergoing ventricular puncture. As a prophylactic measure, epinephrine and bicarbonate can be used through the balloon catheter at the time of intervention. If dysfunction is sustained, the intracardiac or intramuscular resuscitation medications noted above may be administered. Hemopericardium can also be a complication. When smaller than 2 mm, it is unlikely to be of hemodynamic consequence. When larger, hemodynamic instability may develop, and percutaneous drainage can be attempted, often with successful removal of fluid. Only one known instance of fetal demise has been attributed directly to a large hemopericardium, which was documented at postmortem examination.[9] [34]
Fetal loss both during the procedure and in the 72 hours following the procedure is uncommon. Early in the learning curve with this procedure, the rate of in utero fetal demise was reported as 15%.[36] More recently, published data indicate that IUFD (excluding elective terminations of pregnancy) following attempted aortic valvuloplasty occurred in less than 10% of fetuses.[9] In the most recently described data, only three fetuses were delivered prematurely after FCI. Alterations in blood flow to end organs and end-organ damage due to FCI have not been studied in detail, although one study did demonstrate that cerebral blood flow patterns did not change meaningfully after FCI.[37] There have been no maternal complications described in the eHLHS cohort.[9]
Postnatal Management
The complete range of postnatal management of these patients is complex and varied. Patients having undergone FCI are a unique population. As noted earlier in this chapter, in the case of eHLHS or PA/IVS, the feasibility of a biventricular circulation may not be readily discernible upon the first postnatal evaluation of the heart. There is often temptation to direct care toward staged palliation. Nonetheless, particularly in the case of eHLHS, it is often prudent to challenge the left ventricle to sustain systemic output. Even if a biventricular circulation is not possible at birth, modified palliative procedures can be utilized to direct care toward an eventual biventricular circulation. Some patients that do not achieve a biventricular circulation may benefit from some contribution of a smaller left ventricle to systemic output. Therefore, delivery at a center with expertise in caring for this unique group of patients, as well as a thoughtful, multidisciplinary approach, is critical in affording the best possible outcomes for these patients.
Acknowledgments
This article was supported in part by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991;65(5):256–258. doi: 10.1136/hrt.65.5.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäkikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113(11):1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77(3):205–210. doi: 10.1136/hrt.77.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18-year period. J Thorac Cardiovasc Surg. 2010;139(1):119–126. doi: 10.1016/j.jtcvs.2009.04.061. discussion 126–127. [DOI] [PubMed] [Google Scholar]
- 5.Schidlow DN, Anderson JB, Klitzner TS, et al. JCCHD National Pediatric Cardiology Quality Improvement Collaborative. Variation in interstage outpatient care after the Norwood procedure: a report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit Heart Dis. 2011;6(2):98–107. doi: 10.1111/j.1747-0803.2011.00509.x. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart HM, Dearani JA, Mair DD, et al. The modified Fontan procedure: early and late results in 132 adult patients. J Thorac Cardiovasc Surg. 2003;125(6):1252–1259. doi: 10.1016/s0022-5223(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 7.Mair DD, Puga FJ, Danielson GK. The Fontan procedure for tricuspid atresia: early and late results of a 25-year experience with 216 patients. J Am Coll Cardiol. 2001;37(3):933–939. doi: 10.1016/s0735-1097(00)01164-5. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg DJ, Shaddy RE, Ravishankar C, Rychik J. The failing Fontan: etiology, diagnosis and management. Expert Rev Cardiovasc Ther. 2011;9(6):785–793. doi: 10.1586/erc.11.75. [DOI] [PubMed] [Google Scholar]
- 9.McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation. 2010;121(10):1256–1263. doi: 10.1161/CIRCULATIONAHA.109.870246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins-Haug LE, Tworetzky W, Benson CB, Marshall AC, Jennings RW, Lock JE. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. 2006;28(1):47–52. doi: 10.1002/uog.2732. [DOI] [PubMed] [Google Scholar]
- 11.Tworetzky W, Marshall AC. Balloon valvuloplasty for congenital heart disease in the fetus. Clin Perinatol. 2003;30(3):541–550. doi: 10.1016/s0095-5108(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 12.Tworetzky W, Marshall AC. Fetal interventions for cardiac defects. Pediatr Clin North Am. 2004;51(6):1503–1513. vii. doi: 10.1016/j.pcl.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.McElhinney DB, Vogel M, Benson CB, et al. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol. 2010;106(12):1792–1797. doi: 10.1016/j.amjcard.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Friedman KG, Margossian R, Graham DA, et al. Postnatal left ventricular diastolic function after fetal aortic valvuloplasty. Am J Cardiol. 2011;108(4):556–560. doi: 10.1016/j.amjcard.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElhinney DB, Marshall AC, Wilkins-Haug LE, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120(15):1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34(2):554–560. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 17.Forbess JM, Cook N, Roth SJ, Serraf A, Mayer JE, Jr, Jonas RA. Ten-year institutional experience with palliative surgery for hypoplastic left heart syndrome. Risk factors related to stage I mortality. Circulation. 1995;92(9):II262–II266. doi: 10.1161/01.cir.92.9.262. [DOI] [PubMed] [Google Scholar]
- 18.Forbess JM. Pre-stage II mortality after the Norwood operation: addressing the next challenge. J Thorac Cardiovasc Surg. 2003;126(5):1257–1258. doi: 10.1016/s0022-5223(03)01040-7. [DOI] [PubMed] [Google Scholar]
- 19.Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation. 2004;109(19):2326–2330. doi: 10.1161/01.CIR.0000128690.35860.C5. [DOI] [PubMed] [Google Scholar]
- 20.Atz AM, Feinstein JA, Jonas RA, Perry SB, Wessel DL. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. Am J Cardiol. 1999;83(8):1224–1228. doi: 10.1016/s0002-9149(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 21.Taketazu M, Barrea C, Smallhorn JF, Wilson GJ, Hornberger LK. Intrauterine pulmonary venous flow and restrictive foramen ovale in fetal hypoplastic left heart syndrome. J Am Coll Cardiol. 2004;43(10):1902–1907. doi: 10.1016/j.jacc.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Michelfelder E, Gomez C, Border W, Gottliebson W, Franklin C. Predictive value of fetal pulmonary venous flow patterns in identifying the need for atrial septoplasty in the newborn with hypoplastic left ventricle. Circulation. 2005;112(19):2974–2979. doi: 10.1161/CIRCULATIONAHA.105.534180. [DOI] [PubMed] [Google Scholar]
- 23.Glatz JA, Tabbutt S, Gaynor JW, et al. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg. 2007;84(5):1633–1638. doi: 10.1016/j.athoracsur.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Marshall AC, Levine J, Morash D, et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28(11):1023–1028. doi: 10.1002/pd.2114. [DOI] [PubMed] [Google Scholar]
- 25.Sandor GG, Cook AC, Sharland GK, Ho SY, Potts JE, Anderson RH. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum diagnosed during fetal life. Cardiol Young. 2002;12(5):436–444. doi: 10.1017/s1047951102000756. [DOI] [PubMed] [Google Scholar]
- 26.Selamet SE, Hsu DT, Thaker HM, Gersony WM. Complete atresia of coronary ostia in pulmonary atresia and intact ventricular septum. Pediatr Cardiol. 2004;25(1):67–69. doi: 10.1007/s00246-003-0517-0. [DOI] [PubMed] [Google Scholar]
- 27.Kipps AK, Powell AJ, Levine JC. Muscular infundibular atresia is associated with coronary ostial atresia in pulmonary atresia with intact ventricular septum. Congenit Heart Dis. 2011;6(5):444–450. doi: 10.1111/j.1747-0803.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- 28.Wald RM, Juraszek AL, Pigula FA, Geva T. Echocardiographic diagnosis and management of bilateral coronary ostial atresia in a patient with pulmonary atresia and intact ventricular septum. J Am Soc Echocardiogr. 2006;19(7):e1–e3. doi: 10.1016/j.echo.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Tworetzky W, McElhinney DB, Marx GR, et al. In utero valvuloplasty for pulmonary atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics. 2009;124(3):e510–e518. doi: 10.1542/peds.2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedra SR, Peralta CF, Crema L, Jatene IB, da Costa RN, Pedra CA. Fetal interventions for congenital heart disease in Brazil. Pediatr Cardiol. 2014;35(3):399–405. doi: 10.1007/s00246-013-0792-3. [DOI] [PubMed] [Google Scholar]
- 31.Tulzer G, Arzt W, Franklin RC, Loughna PV, Mair R, Gardiner HM. Fetal pulmonary valvuloplasty for critical pulmonary stenosis or atresia with intact septum. Lancet. 2002;360(9345):1567–1568. doi: 10.1016/S0140-6736(02)11531-5. [DOI] [PubMed] [Google Scholar]
- 32.Galindo A, Gutiérrez-Larraya F, Velasco JM, de la Fuente P. Pulmonary balloon valvuloplasty in a fetus with critical pulmonary stenosis/atresia with intact ventricular septum and heart failure. Fetal Diagn Ther. 2006;21(1):100–104. doi: 10.1159/000089058. [DOI] [PubMed] [Google Scholar]
- 33.Arzt W, Tulzer G, Aigner M, Mair R, Hafner E. Invasive intrauterine treatment of pulmonary atresia/intact ventricular septum with heart failure. Ultrasound Obstet Gynecol. 2003;21(2):186–188. doi: 10.1002/uog.48. [DOI] [PubMed] [Google Scholar]
- 34.Mizrahi-Arnaud A, Tworetzky W, Bulich LA, et al. Pathophysiology, management, and outcomes of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res. 2007;62(3):325–330. doi: 10.1203/PDR.0b013e318123fd3a. [DOI] [PubMed] [Google Scholar]
- 35.Brusseau R, Mizrahi-Arnaud A. Fetal anesthesia and pain management for intrauterine therapy. Clin Perinatol. 2013;40(3):429–442. doi: 10.1016/j.clp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110(15):2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 37.McElhinney DB, Benson CB, Brown DW, et al. Cerebral blood flow characteristics and biometry in fetuses undergoing prenatal intervention for aortic stenosis with evolving hypoplastic left heart syndrome. Ultrasound Med Biol. 2010;36(1):29–37. doi: 10.1016/j.ultrasmedbio.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]