Abstract
Group C streptococci have been reported to cause invasive disease similar to that classically associated with group A streptococcus (GAS). We describe a fatal case of toxic shock-like syndrome due to Streptococcus equi subsp. zooepidemicus. The causative organism did not possess any known GAS superantigen exotoxin genes but did show evidence of superantigen production.
CASE REPORT
A 63-year-old man developed left thigh pain and swelling while on an airplane flight. Two hours later, he developed fever, rigors, and a rapidly progressing skin rash on his trunk and limbs. Two days earlier, after presenting with vertigo and vomiting, he had received an intramuscular injection of prochlorperazine into the left thigh for presumed acute labyrinthitis.
Treatment was commenced for presumed meningococcal sepsis with intravenous (i.v.) benzylpenicillin and ceftriaxone at his local hospital. A cranial computerized tomography scan showed no abnormalities. He was intubated for a reduced conscious state and transferred to the intensive care unit at our institution.
Examination revealed a temperature 39.5°C, a pulse of 120/min, a blood pressure of 100/60 mmHg, and a confluent erythematous skin rash on his trunk and limbs, with petechiae on his legs. His left thigh was tender, swollen, and erythematous. Hypotension developed requiring inotropic support, and there were no other clinical features of toxic shock syndrome (TSS).
Initial investigations revealed a leukocyte count of 5.1 × 109/liter (61% neutrophils, 18% band neutrophils) and a platelet count of 25 × 109/liter; the blood film showed neutrophilia with a left shift and toxic granulations. The C-reactive protein level was 239 mg/liter (normal range, <5 mg/liter). Serum urea was 12.8 mmol/liter (normal range, 2.5 to 9.6 mmol/liter), creatinine was 194 μmol/liter (normal range, 40 to 120 μmol/liter), calcium was 1.84 mmol/liter (normal range, 2.2 to 2.6 mmol/liter), and lactate was 9.2 mmol/liter (normal range, 0.5 to 2.0 mmol/liter). Creatine kinase was 14,790 U/liter (normal range, 25 to 200 U/liter), and troponin was 4.63 μg/liter (normal range, 0 to 0.4 μg/liter). Liver function tests were abnormal: bilirubin, 29 μmol/liter (normal range, <17 μmol/liter); alanine transferase, 97 U/liter (normal range, 7 to 56 U/liter); albumin, 19 g/liter (normal range, 35 to 45 g/liter). He had a coagulopathy: activated partial thromoplastin time, 41 s (normal range, 23 to 34 s); fibrinogen, 6.0 g/liter (normal range, 1.5 to 4.0 g/liter); D-dimer, 6.7 mg/liter (normal range, <0.20 mg/liter).
An ultrasound scan revealed generalized edema of the anterolateral musculature of the left thigh with no abscess. At operation, there was marked subcutaneous and muscle edema but no obvious necrosis. Biopsies demonstrated muscle necrosis and gram-positive cocci. Treatment was continued for presumed group A streptococcus-associated soft-tissue infection and TSS with benzylpenicillin and clindamycin plus i.v. immunoglobulin (IVIG) at 1.5 g/kg.
Postoperatively, he had a persistent fever exceeding 40°C and developed progressive multisystem organ failure. Repeat hemoglobin was 7.9 g/dl, leukocytes were 26.0 × 109/liter, and platelets were 14 × 109/liter. He had circulatory failure, requiring high-dose i.v. adrenaline and noradrenaline infusions, and ventricular tachycardia, requiring cardioversion. A transthoracic echocardiogram revealed severe global hypokinesis with no evidence of endocarditis. Respiratory failure developed with increasing hypoxia and ventilatory requirements. Chest radiograph revealed bilateral diffuse pulmonary alveolar opacities. Anuric renal failure (serum creatinine, 402 μmol/liter; urea, 18 mmol/liter) required continuous venovenous hemodiafiltration. Liver dysfunction (bilirubin, 77 μmol/liter; alanine transferase, 913 U/liter; albumin, 13 g/liter) and coagulopathy (international normalized ratio, 3.5) worsened. Despite maximal support, the patient died just over 48 h after arriving at our institution.
Microbiology.
Streptococcus equi subsp. zooepidemicus sensitive to penicillin was isolated from multiple muscle biopsies and knee joint fluid and from one of six blood culture bottles taken on admission. 16S rRNA sequencing (results not shown) confirmed that the patient's isolate had 100% sequence homology with S. equi subsp. zooepidemicus.
A fully characterized superantigen toxin-producing (including streptococcal pyrogenic exotoxin A) group A streptococcus (GAS) positive control strain and a non-toxin-producing group C streptococcus (GCS) negative control strain were obtained from clinical isolates. Bacterial strains were subcultured on horse blood agar plates at 37°C in 5% CO2 before use to ensure viability and purity. Supernatants for proliferation experiments were prepared by inoculating individual colonies in 50 ml of nutrient broth (brain heart infusion) and culturing overnight with shaking at 37°C. Broth cultures were centrifuged at 4,000 × g and passed through a 0.2-μm-pore-size filter before use.
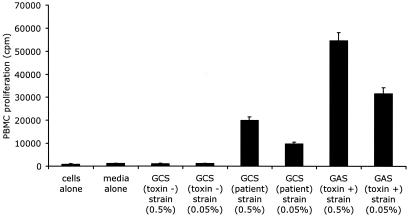
A proliferation assay specifically developed and validated to distinguish superantigen from conventional antigen activity was performed (15). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood on a Ficoll-Paque gradient. After washing, healthy donor PBMCs were adjusted to 5 × 105/ml in RPMI medium supplemented with antibiotics and 3% heat-inactivated fetal bovine serum. PBMCs were plated in 96-well round-bottom tissue culture plates at 105/200 μl of medium. Cells were stimulated in triplicate with 100 μl of 0.5% (1/100) or 0.05% (1/1,000) sterile supernatant in RPMI medium for 72 h. Tritiated thymidine was added 16 h before the cells were harvested, and radiation emission was detected with a beta counter. Sterile culture supernatant from the patient's isolate stimulated a dose-dependent proliferation. This effect was reproducible with PBMCs from multiple healthy donors. IVIG did not inhibit the proliferation. Proliferation data derived from three experiments with one donor are presented in Fig. 1.
FIG. 1.
Proliferation of PBMCs after stimulation for 3 days with culture supernatants from a non-toxin-producing GCS negative control strain, the patient's GCS isolate, and a toxin-producing GAS positive control strain. Each bar represents the mean and standard deviation of three assays. Note the marked dose-dependent proliferation produced by the patient's GCS and GAS supernatants compared to the negative controls.
Genomic DNA was isolated and purified by standard techniques following mechanical agitation with glass beads and lysis solution in a FASTPrep machine. PCR amplicons specific for the streptococcal superantigen exotoxins SpeA, SpeB, SpeC, SpeF, SpeG, SpeH, SpeI, SpeJ, SmeZ/2 and SSA were detected with AmpliTaq Gold (Applied Biosystems). The PCR primers used in this study were based on previously published sequences (11, 13, 17, 19). Samples were placed in a GeneAmp PCR System 9700 thermocycler (Applied Biosystems), and the following cycle parameters were used: preincubation at 95°C for 10 min; 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 5 min. Products were stored at 4°C until they were analyzed by electrophoresis. Each PCR was performed in duplicate, and positive controls (known superantigen gene-possessing GAS) were run in each experiment. Despite the mitogenic activity detected in the proliferation assay, no PCR product was detected for SpeB or any of the other nine GAS superantigen exotoxins tested (data not shown).
Discussion.
TSS due to GAS has been reported with increasing frequency since the mid 1980s. Several streptococcal pyrogenic exotoxins (SPEs) produced by GAS implicated in the pathogenesis of invasive infections have been identified. The SPEs act as superantigens and interact simultaneously with the major histocompatibility complex class II antigens on antigen-presenting cells and specific Vb regions of T-lymphocyte receptors to cause massive T-cell proliferation and cytokine production.
Reports are also emerging of toxic shock-like syndrome associated with group G streptococci (GGS) and GCS (10, 14, 16, 18). The role of superantigen exotoxins in GCS and GGS pathogenesis has also been investigated (1, 2, 21).
GCS are uncommon human pathogens and account for less than 1% of all bacteremias (6). Most infections are community acquired and occur in patients with significant underlying conditions. Clinical manifestations include endocarditis, meningitis, and primary bacteremia, and the mortality rate is high (20 to 30%) (12, 24). In one study, S. equi subsp. zooepidemicus represented only 1.4% of 214 GCS isolates from a variety of clinical specimens (3). S. equi subsp. zooepidemicus may cause a higher proportion of aggressive infections than would be expected from its rare occurrence at superficial sites (12).
Five well-documented reports of TSS associated with GCS bacteremia, including the present case, are summarized in Table 1 (10, 14, 16, 18). One case was due to Streptococcus equisimilis, one was due to S. equi subsp. zooepidemicus, and in three cases the GCS was not identified to the species level. Four cases had necrotizing fasciitis or myositis. Two patients died within 48 h. Production of superantigen exotoxins or induction of a mitogenic response was not demonstrated in one case (18). In another report, four GCS strains isolated from patients with “toxic shock-like syndrome” were analyzed for pyrogenic toxin superantigens, but no clinical details were provided (22). Two other cases of overwhelming fatal GCS infection were also postulated to be toxin mediated (4).
TABLE 1.
Summary of data from reported cases of GCS-associated TSS
| Case no. (reference) | Age (yr)/sexa | Causative species | Site(s) of culture | Underlying conditions | Clinical features | Complications | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 (14) | 41/F | S. equisimilis | Blood, leg lesion | None | Hemodynamic shock, confusion, necrotizing fasciitis | Thrombocytopenia, raised transaminase, hypocalcemia | Penicillin | Recovery |
| 2 (16) | 46/M | GCS | Blood, throat | None | Hypotension, myalgia, macular rash | Thrombocytopenia, renal impairment, raised transaminase and CKb cardiac failure | Ampicillin, teicoplanin, amikacin | Recovery |
| 3 (10) | 54/M | Large-colony-forming GCS | Blood, thigh blister | Liver cirrhosis, hepatoma | Leg pain and swelling, acute brain syndrome, hypotension | Thrombocytopenia, raised transaminase and CK, hypocalcemia, renal impairment | Inotropes | Death in 4 h |
| 4 (18) | 22/M | GCS | Blood | None | Pyomyositis, myonecrosis | Thrombocytopenia, coagulopathy, raised transaminase and CK, ARDSc | Penicillin, gentamicin, IVIG | Recovery |
| 5 (PR)d | 63/M | S. equi subsp. zooepidemicus | Blood, thigh muscle, knee joint fluid | None | Myositis, hemodynamic shock | Thrombocytopenia, coagulopathy, renal impairment, raised transaminase and CK, cardiac failure | Penicillin, clindamycin, ceftriaxone, IVIG | Death in 48 h |
F, female; M, male.
CK, creatine phosphokinase.
ARDS, acute respiratory distress syndrome.
PR, present report.
We describe a fatal infection that demonstrates the clinical and laboratory features of streptococcal TSS (24), with early onset of shock and organ failure and isolation of GCS from normally sterile sites. The onset of the illness occurred 2 days after an intramuscular injection, with pain at the site as a major presenting symptom. Muscle biopsies revealed myonecrosis and streptococci on Gram stain and culture. There was no obvious portal of entry noted in previous cases of GCS toxic shock-like syndrome, but there is a report of necrotizing fasciitis due to GCS following a podiatry procedure (8).
Interestingly, our patient had frequent contact with horses. Prior exposure to animals or animal products was documented in 24% of the cases in one series of GCS bacteremia (5), but population-based studies have found a lower rate (7). S. equi subsp. zooepidemicus is not considered part of the normal human flora and is a cause of infections in domestic animals, including horses, cattle, sheep, and pigs. Many cases of S. equi subsp. zooepidemicus infection can be traced to an animal source (12). S. equi subsp. zooepidemicus isolates from humans with septicemia were shown to be identical to strains from local pigs in Hong Kong (25).
Although S. equi subsp. zooepidemicus equi has over 92% DNA homology with S. equi subsp. equi, they have very different biological behaviors in the horse. S. equi subsp. zooepidemicus is a commensal of the horse nasopharynx and external genitalia but can cause wound, respiratory, and uterine infections in susceptible horses. In contrast, S. equi subsp. equi causes strangles, a highly contagious and invasive respiratory disease, in young horses (9).
Anzai et al. demonstrated that supernatants of equine clinical isolates of S. equi subsp. equi, but not of S. equi subsp. zooepidemicus, elicited potent mitogenic responses from PBMCs (1). Artiushin et al. detected mitogen responses and sepe-I and sepe-H genes encoding pyrogenic mitogens in S. equi subsp. equi, but not S. equi subsp. zooepidemicus, equine isolates (2). Sachse et al. detected speG in 15 of 24 human clinical isolates of S. dysgalactiae subsp. equisimilis belonging to GCS and GGS (21). Proft et al. recently identified two novel streptococcal superantigen genes (speLSe and speMSe) from the S. equi genome database, which were detected in clinical S. pyogenes isolates but not in eight S. equi isolates analyzed (20).
We did not detect the genes encoding GAS SPEs in the S. equi subsp. zooepidemicus isolated from this case of toxic shock-like syndrome. However, it is not clear that all of the genes encoding SPEs in the GAS genomes have been identified. The ability to stimulate PBMC proliferation is the most sensitive test of superantigen activity. The degree of mitogenic activity detected in the supernatant from the organism from our patient was characteristic of superantigen activity in an assay specifically developed and validated to distinguish superantigen from conventional antigen activity. This strongly suggests the presence of an unidentified novel superantigen exotoxin produced by S. equi subsp. zooepidemicus that could be implicated in the pathogenesis of the fatal infection in our patient.
The overall mortality rate due to GAS TSS remains high. Our patient died despite treatment with surgery, penicillin, clindamycin, and IVIG. Penicillin remains the treatment of choice for GAS and GCS infections, but the addition of clindamycin provided greater efficacy in experimental and retrospective clinical series of GAS TSS. Early aggressive surgical exploration and debridement of a suspected deep-seated infection are essential components of treatment. IVIG therapy enhances the ability of patient plasma to inhibit bacterial mitogenicity, reduces T-cell production of proinflammatory cytokines, and improved survival in GAS TSS studies. Therapy directed toward superantigens may be an efficient strategy against invasive infections due to GAS and other streptococci (23).
This fatal case of toxic shock-like syndrome due to S. equi subsp. zooepidemicus with evidence of superantigen toxin production highlights the need for further studies investigating the role of superantigen exotoxins in the pathogenesis of invasive disease due to GCS.
REFERENCES
- 1.Anzai, T., A. S. Sheoran, Y. Kuwamoto, T. Kondo, R. Wada, T. Inoue, and J. F. Timoney. 1999. Streptococcus equi but not Streptococcus zooepidemicus produces potent mitogenic responses from equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 67:235-246. [DOI] [PubMed] [Google Scholar]
- 2.Artiushin, S. C., J. F. Timoney, A. S. Sheoran, and S. K. Muthupalani. 2002. Characterization and immunogenicity of pyrogenic mitogens SePE-H and SePE-I of Streptococcus equi. Microb. Pathog. 32:71-85. [DOI] [PubMed] [Google Scholar]
- 3.Barnham, M., J. Kerby, R. S. Chandler, and M. R. Millar. 1989. Group C streptococci in human infection: a study of 308 isolates with clinical correlations. Epidemiol. Infect. 102:379-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman, A. C., A. D. Ramsay, and A. P. Pallett. 1993. Fatal infection associated with group C streptococci. J. Clin. Pathol. 46:965-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley, S. F., J. J. Gordon, D. D. Baumgartner, W. A. Marasco, and C. A. Kauffman. 1991. Group C streptococcal bacteremia: analysis of 88 cases. Rev. Infect. Dis. 13:270-280. [DOI] [PubMed] [Google Scholar]
- 6.Carmeli, Y., and K. L. Ruoff. 1995. Report of cases of and taxonomic considerations for large-colony-forming Lancefield group C streptococcal bacteremia. J. Clin. Microbiol. 33:2114-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeli, Y., J. M. Schapiro, D. Neeman, A. M. Yinnon, and M. Alkan. 1995. Streptococcal group C bacteremia: survey in Israel and analytic review. Arch. Intern. Med. 155:1170-1176. [DOI] [PubMed] [Google Scholar]
- 8.Gaunt, N., K. Rogers, D. Seal, M. Denham, and J. Lewis. 1984. Necrotising fasciitis due to group C and G haemolytic streptococcus after chiropody. Lancet i:516. [DOI] [PubMed] [Google Scholar]
- 9.Harrington, D. J., I. C. Sutcliffe, and N. Chanter. 2002. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 4:501-510. [DOI] [PubMed] [Google Scholar]
- 10.Hirose, Y., K. Yagi, H. Honda, H. Shibuya, and E. Okazaki. 1997. Toxic shock-like syndrome caused by non-group A beta-hemolytic streptococci. Arch. Intern. Med. 157:1891-1894. [PubMed] [Google Scholar]
- 11.Iwasaki, M., H. Igarashi, Y. Hinuma, and T. Yutsudo. 1993. Cloning, characterization and overexpression of a Streptococcus pyogenes gene encoding a new type of mitogenic factor. FEBS Lett. 331:187-192. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, C. C., and A. R. Tunkel. 2000. Viridans streptococci and groups C and G streptococci, p. 2167-2182. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Ltd., Edinburgh, Scotland.
- 13.Kapur, V., S. Topouzis, M. W. Majesky, L. L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 14.Keiser, P., and W. Campbell. 1992. ‘Toxic strep syndrome’ associated with group C Streptococcus. Arch. Intern. Med. 152:882-884. [DOI] [PubMed] [Google Scholar]
- 15.MacIssac, C. M., J. M. Cade, N. Curtis, and K. Visvanathan. 2003. Rapid analysis of the Vβ repertoire of CD4 and CD8 T lymphocytes in whole blood. J. Immunol. Methods 283:9-15. [DOI] [PubMed] [Google Scholar]
- 16.Natoli, S., C. Fimiani, N. Faglieri, L. Laurenzi, A. Calamaro, A. M. Frasca, and E. Arcuri. 1996. Toxic shock syndrome due to group C streptococci: a case report. Intensive Care Med. 22:985-989. [DOI] [PubMed] [Google Scholar]
- 17.Nelson, K., P. M. Schlievert, R. K. Selander, and J. M. Musser. 1991. Characterization and clonal distribution of four alleles of the speA gene encoding pyrogenic exotoxin A (scarlet fever toxin) in Streptococcus pyogenes. J. Exp. Med. 174:1271-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojukwu, I. C., D. W. Newton, A. E. Luque, M. Y. Kotb, and M. Menegus. 2001. Invasive group C Streptococcus infection associated with rhabdomyolysis and disseminated intravascular coagulation in a previously healthy adult. Scand. J. Infect. Dis. 33:227-229. [DOI] [PubMed] [Google Scholar]
- 19.Proft, T., S. L. Moffatt, C. J. Berkahn, and J. D. Fraser. 1999. Identification and characterization of novel superantigens from Streptococcus pyogenes. J. Exp. Med. 189:89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proft, T., P. D. Webb, V. Handley, and J. D. Fraser. 2003. Two novel superantigens found in both group A and group C Streptococcus. Infect. Immun. 71:1361-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachse, S., P. Seidel, D. Gerlach, E. Gunther, J. Rodel, E. Straube, and K. H. Schmidt. 2002. Superantigen-like gene(s) in human pathogenic Streptococcus dysgalactiae, subsp. equisimilis: genomic localisation of the gene encoding streptococcal pyrogenic exotoxin G (speG [dys]). FEMS Immunol. Med. Microbiol. 34:159-167. [DOI] [PubMed] [Google Scholar]
- 22.Schlievert, P. M. 1993. Role of superantigens in human disease. J. Infect. Dis. 167:997-1002. [DOI] [PubMed] [Google Scholar]
- 23.Visvanathan, K., A. Charles, J. Bannan, P. Pugach, K. Kashfi, and J. B. Zabriskie. 2001. Inhibition of bacterial superantigens by peptides and antibodies. Infect. Immun. 69:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA 269:390-391. [PubMed] [Google Scholar]
- 25.Yuen, K. Y., W. H. Seto, C. H. Choi, W. Ng, S. W. Ho, and P. Y. Chau. 1990. Streptococcus zooepidemicus (Lancefield group C) septicaemia in Hong Kong. J. Infect. 21:241-250. [DOI] [PubMed] [Google Scholar]