Abstract
Background
The goal of the Proton Priority System (PROPS) is to guide the allocation of proton therapy treatment at Blinded Institution. The PROPS score is a priority points framework that assigns higher scores to patients thought to more likely benefit from proton therapy.
Methods
We present the principles and rationale of PROPS and the distribution of weighted PROPS scores by patient characteristics. We perform multivariable logistic regression to evaluate the association between PROPS scores and receipt of proton therapy, adjusted for insurance status, gender, race, geography, and the domains that inform the PROPS score.
Results
Among 1,529 adult patients considered for proton therapy prioritization during our Center's ramp up phase of treatment availability, PROPS scores varied by age, diagnosis, site and other PROPS domains. In adjusted analyses, receipt of proton therapy was lower for patients with non-Medicare relative to Medicare health insurance [commercial vs. Medicare: adjusted odds ratio (OR) 0.47 95% CI (0.34 – 0.64); managed care vs. Medicare: OR 0.40 95% CI (0.28 – 0.56); Medicaid vs. Medicare: OR 0.24 95% CI (0.13 – 0.44)]. PROPS score and age were not significantly associated with receipt of proton therapy.
Conclusions
PROPS is a rationally designed and transparent system for allocation of proton therapy slots based on the best available evidence and expert opinion. Because the actual allocation of treatment slots depends mostly on insurance status, payers may consider incorporating PROPS, or its underlying principles, into proton therapy coverage policies.
INTRODUCTION
In 2013, 13 proton therapy centers were operational in the United States (US) with 13 more in development. Another 30 centers were operational in the rest of the world. In contrast, over 2,300 US facilities deliver photon (i.e., x-ray) radiation therapy to nearly 1 million patients per year.1 The primary reason for this imbalance is the recent development of clinical proton therapy accelerators and the capital investment required to establish facilities.2
The therapeutic advantages of proton over photon radiation therapy are more potential than demonstrated. Photon therapy passes through normal tissue on its way to and beyond a tumor, whereas proton therapy delivers radiation to tumors and their very close vicinity, decreasing integral radiation dose to normal tissues and potentially avoiding collateral damage. Efforts are being made to generate evidence in support of proton therapy for more common cancers. Nevertheless, the incremental clinical benefit of proton therapy, either in improved survival or reduced toxicity compared to alternative treatments, has yet to be shown except in a few pediatric or rare cancers.3,4
With limited proton therapy availability nationally, uncertainty regarding its incremental benefit, and variability in insurance coverage, we developed the Proton Priority System (PROPS) to guide the allocation of proton therapy treatment at Blinded Institution. We were unsure whether demand for proton therapy in the Blinded region would outstrip our capacity to provide treatment, especially during the Center's ramp up phase. We also speculated that circumstances might lead to allocation of scarce treatment time to patients with cancers that may be treated well with alternative therapies, thereby limiting treatment availability for patients whose conditions might plausibly derive more incremental benefit from proton therapy.
Our goal was to prepare for competing demands that might require us to balance evidence of effectiveness, equity, and the ability to generate new knowledge to advance the field. In this report, we present the underlying principles and rationale for PROPS and examine its application in treatment allocation among patients with a range of cancer diagnoses.
METHODS
Setting
The Blinded Proton Therapy Center at Blinded Institution opened in January, 2010. The Center has four gantries and one fixed beam room. Pencil beam scanning is available on two gantries and the fixed beam. As with other proton centers, the facility continues to expand treatment availability through a staged approach. During the study period, our Center's capacity to provide treatment remained greater than patient demand. In December, 2012, approximately 85 treatment slots were available daily.
Proton Priority Oversight and Advisory Board (POAB)
In September, 2009, we established the POAB to develop and oversee a multi-stakeholder process for proton treatment slot prioritization. The POAB comprises members from the clinical and University community, including two representatives from Radiation Oncology, a medical oncologist, a surgical oncologist, a medical ethicist, a nurse, and a patient representative. The POAB established principles of proton therapy prioritization and developed the Proton Priority System (PROPS) to guide the allocation of patient treatment slots.
Principles of Proton Therapy Prioritization
As noted, we anticipated the need to prioritize proton therapy based on comparative clinical need, a sense of justice over scarce resources, and a desire to advance our knowledge of the best uses of proton therapy. Accordingly, PROPS is based on five primary considerations that draw from prior work examining resource allocation in health care: incremental benefit, age (‘youngest first’), equity, contribution to medical knowledge, and transparency.5-8
First, the primary concern of PROPS is the incremental benefit of proton therapy for patients. Incremental benefit from proton therapy in this context can be defined as the extent to which an individual patient would benefit from proton treatment, as compared to alternative treatments. Defining incremental benefit for proton therapy is challenging because experience with the treatment is limited beyond select cancers and essentially no comparative trials have been performed. Therefore, the PROPS score reflects the expert opinions of the community of clinicians and other stakeholders at Blinded Institution.
Second, PROPS prioritizes younger patients, who are expected to accrue greater benefits from proton therapy relative to older patients. Proton therapy spares normal tissues from radiation dose, thereby decreasing the integral dose of radiation administered to the body. We prioritized younger patients first because they have more capacity to benefit from reductions in integral dose associated with proton radiation therapy than older patients—the value of normal tissue sparing is greater for those with more years of life ahead of them.
Third, the equitable distribution of health resources is essential. The etiology of a patient's cancer is not considered by PROPS. For example, lung cancers caused by smoking are not given lower priority than sporadic lung cancers. In situations in which proton therapy is felt to offer significant advantages, we explicitly charged PROPS to advocate on behalf of patients regardless of insurance or insurance type.
Fourth, as a research institution, Blinded Institution values and contributes to the advancement of medical knowledge. PROPS considers the extent to which patients are eligible and willing to participate in active clinical research protocols, as the benefit from treating such patients extends beyond the individual. While we did not want to make participation in research a condition of receiving proton therapy, we wanted to encourage research participation in order to grow the evidence base for future patients.
Fifth, the prioritization process should be transparent to patients and clinicians.10 To that end, the POAB is a peer-review board and encourages dissemination of the rationale for and processes of PROPS. This paper is part of that process.
The Proton Priority System (PROPS) Score
The purpose of the PROPS score is to guide the allocation of proton therapy through an objective priority points framework that assigns higher scores to patients who are more likely to benefit from proton therapy. The PROPS score consists of a weighted sum of seven domains: diagnosis, anatomic site, stage, performance status/comorbidities, age, urgency, and protocol participation. Within each domain is a set of factors used to evaluate patients. Factors within domains are given a priority score of 0 – 10, with more points given for factors in which proton therapy is thought to offer greater benefit (Table 1). Each domain is given a weight, with more weight given for domains in which proton therapy is thought to offer greater benefit as well (Table 2). The weighted sum allows for the incorporation of between-domain weights. A higher weighted PROPS score may indicate a particularly compelling case for proton therapy. Strict domain definitions were established to promote the greatest possible objectivity (Table 3).
Table 1.
Within-Domain Proton Prioritization System (PROPS) Scores
| ICD9 Diagnosis Code | Points | Diagnosis Code | Points | |
|---|---|---|---|---|
| Tongue | 141 | 0 | 59 different diagnoses | 0 - 10 |
| Other major salivary glands | 142 | 0 | ||
| Gum | 143 | 0 | Site | Points |
| Retromolar area | 145 | 0 | Base of skull | 10 |
| Oropharynx | 146 | 5 | Orbit | 10 |
| Nasopharynx | 147 | 10 | Spine | 10 |
| Pyriform sinus | 148 | 10 | Mediastinum | 10 |
| Sinus | 160 | 10 | Brain | 5 |
| Larynx | 161 | 5 | Retroperitoneum | 5 |
| Thyroid gland | 193 | 5 | Retreatment | 5 |
| Head, face, and neck | 195 | 0 | Other | 0 |
| Esophagus | 150 | 10 | ||
| Stomach | 151 | 5 | Stage | Points |
| Duodenum | 152 | 5 | Local | 10 |
| Colon | 153 | 5 | Regional | 5 |
| Rectum | 154 | 5 | Metastatic | 0 |
| Liver | 155 | 5 | ||
| Extrahepatic bile ducts | 156 | 5 | Performance Status | Points |
| Pancreas | 157 | 5 | Group I | 10 |
| Lung | 162 | 10 | Group II | 5 |
| Parietal pleura | 163 | 5 | Group III | 0 |
| Thymus | 164 | 5 | ||
| Bone | 170 | 5 | Age | Points |
| Soft tissue | 171 | 5 | <30 | 10 |
| Melanoma | 172 | 0 | 30 - 40 | 8 |
| Other skin | 173 | 0 | 30 - 50 | 6 |
| Breast | 174 | 5 | 50 - 60 | 3 |
| Carcinoma in situ of breast | 233 | 5 | > 60 | 0 |
| Breast, unspecified | 239 | 5 | ||
| Uterus | 179 | 5 | Urgency | Points |
| Cervix | 180 | 10 | Urgent | 5 |
| Other Uterine | 182 | 5 | Non urgent | 0 |
| Ovary | 183 | 0 | ||
| Vulva | 184 | 0 | Clinical Protocol | Points |
| Prostate | 185 | 5 | Protocol | 5 |
| Testis | 186 | 5 | Registry | 0 |
| Bladder | 188 | 5 | ||
| Kidney | 189 | 5 | ||
| Brain | 191 | 10 | ||
| GBM | 191.9 | 0 | ||
| Spinal cord | 192 | 10 | ||
| Pineal/adrenal gland | 194 | 10 | ||
| Paraganglia | 237 | 5 | ||
| Primary thrombocytopenia, unspecified | 287 | 0 | ||
| Other unspecified | 199 | 0 | ||
| Secondary neoplasm | 196, 197, 198 | 0 | ||
| Hodgkin's lymphoma | 201 | 5 | ||
| Other lymphoma | 202 | 5 | ||
| Multiple myeloma | 203 | 0 | ||
| Lymphoid leukemia | 204 | 0 | ||
| Myeloid leukemia | 205 | 0 | ||
| Unspecified leukemia | 208 | 0 | ||
| Lymphatic/hematopoetic tissue, uncertain | 238 | 0 | ||
| Salivary glands - benign | 210 | 0 | ||
| Thymus - benign | 212 | 0 | ||
| Cerebral - benign | 225 | 10 | ||
| Keloid scar | 701 | 0 | ||
| Postoperative heterotopic calcification | 728 | 0 | ||
Note: In 2013, based on clinical experience and evolution in techniques, head and neck was added to the site domain (10 points) and points for individual head and neck diagnoses were recalibrated in the diagnosis domain: oropharynx was increased to 10 points and other major salivary glands to 5 points. Nasopharynz, pyriform sinus, sinus, and larynx were reduced to 5 points. These changes occurred after the study period.
Table 2.
Between-Domain Proton Prioritization System (PROPS) Weights
| Domain | Weight |
|---|---|
| Diagnosis | 1 |
| Site | 2 |
| Stage | 1.5 |
| Performance Status/Comorbidity | 1.5 |
| Age | 2 |
| Urgency | 2 |
| Clinical Protocol | 1.5 |
Table 3.
Description of Proton Prioritization System (PROPS) Domains
| Diagnosis | Certain diagnoses are given priority based on anticipated clinical benefit or department research efforts |
|---|---|
| Site | Proton therapy offers particular benefits in certain anatomic sites (base of skull, orbit, spine, brain, retroperitoneum, mediastinum, reirradiation situations). |
| Stage | Proton therapy will provide greater incremental benefit for patients with local or regional cancers than those with metastatic cancer. |
| Performance status/co-moridity | The purpose of this domain is to capture the influence of performance status and co-morbid conditions on patient prognosis. Patients are grouped into 3 categories: 1) the healthiest patients with few comorbidities and excellent performance status (ECOG 0/1); 2) those in poor health with multiple serious comorbidities and poor performance status (ECOG ≥3); and 3) a middle group between these 2 extremes with moderate comorbidities. |
| Age | The patient's age at time of treatment. |
| Urgency | The time sensitivity under which radiation should commence. More urgent cases are prioritized higher. Several situations may require minimal delay in initiating treatment, including: 1) patients with gross disease causing symptoms; 2) diagnoses where medical literature has demonstrated detrimental outcomes associated with delayed or prolonged treatment (e.g. head and neck cancer, cervical cancer); 3) patients who will receive combined modality therapy and whose radiotherapy course must be integrated with a chemotherapy schedule. |
| Clinical Protocol | Most patients will be enrolled in clinical protocols. Preference will be given to patients who are enrolled in protocols of particular departmental priority. |
The priority scores and weights were proposed by the POAB based on the best available evidence and expert opinion, iteratively refined during a series of collaborative discussions with each faculty member of the Department of Radiation Oncology and an unaffiliated radiation oncology expert (Louis B. Harrison, MD, Chair of Radiation Oncology and Physician-in-Chief, Continuum Cancer Centers, New York, NY), and approved by the Chair of Radiation Oncology and the Dean of the Blinded School of Medicine at Blinded Institution.
The PROPS framework also allows for evolution over time, as evidence and experience accumulate. For example, in July, 2010, we increased the points for the diagnosis of thymoma from 5 to 10 and added mediastinum as a favorable anatomic site based on advantageous proton beam arrangements in the central thorax.
Proton Therapy for Pediatrics Cases
By contract, and consistent with the principles of PROPS, approximately 20% of treatment time is set aside for pediatric patients from the Blinded Institution. When greater demand for pediatric proton treatment slots exists than what has been allocated in advance, pediatric patients are considered with adult patients for proton therapy prioritization under the PROPS system.
PROPS Working Group
During weekly meetings, Radiation Oncology faculty are required to present the clinical rationale for proton therapy to the PROPS working group on behalf of any patient considering proton therapy. Three radiation oncologists, a nurse, and a social worker are voting members of the working group. A physicist, a dosimetrist or therapist, and a department administrator serve in an advisory capacity. As necessary, comparative proton/photon dose volume histograms are reviewed. Insurance status is not considered when making a treatment recommendation. For patients who present with diagnoses not yet considered by PROPS or for whom an appeal to the PROPS recommendation is requested, the POAB established an algorithm for exceptional cases (Appendix Figure 1).
Cohort Definition
Over the three year period from January, 2010, to December, 2012, faculty presented 1,912 patients to the PROPS working group. Pediatric patients comprised 322/1,912 (16.8%) and adult patients comprised 1,590/1,912 (83.2%). In total, 216/322 (67.1%) of pediatric patients and 864/1,590 (54.4%) of adult patients were treated with proton therapy. (All pediatric patients whose care plan involved proton therapy at Blinded Institution were treated with proton therapy; about one third of pediatric patients were presented but not treated with proton therapy when the care plan changed or when treatment was provided elsewhere.)
Of the 1,590 adult patients reviewed under the PROPS system, 57/1,590 (3.6%) were considered inappropriate for proton therapy prioritization for one of two reasons: 1) alternative modalities (e.g., photon-based palliative treatment) were sufficient (n=38); and 2) the normal tissue or target tumor anatomy was unfavorable (n=19). As our center gained experience, the vast majority of patients were considered appropriate for proton therapy prioritization because few palliative cases were presented and our delivery techniques included both scattered and scanning beams. We excluded 4 patients whose PROPS reviews were pending at the time of this analysis. Therefore, this report focuses on the 1,529 adult patients who were considered appropriate for proton therapy prioritization.
Analysis
We present summary descriptive data, including the mean and standard deviation (SD) of weighted PROPS scores by patient characteristics. Patient characteristics were categorized and analyzed according to the variables in Table 4.
Table 4.
Adults Considered for Proton Therapy, 2010 – 2012
| Patients Considered for Proton Therapy | ||||
|---|---|---|---|---|
| Characteristic | N | (%) | PROPS Score Mean (SD) | P Value |
| Total | 1,529 | (100.0%) | 48.6 (12.4) | |
| PROPS Scores | ||||
| ≤ Median Score of 44.5 | 766 | (50.1%) | 38.9 (5.0) | |
| > Median Score of 44.5 | 763 | (49.9%) | 58.3 (9.7) | <0.001 |
| Insurance | ||||
| Medicare | 548 | (35.8%) | 45.7 (11.1) | |
| Commercial | 572 | (37.4%) | 49.5 (12.6) | |
| Managed Care | 334 | (21.8%) | 50.5 (12.5) | |
| Medicaid | 65 | (4.3%) | 54.5 (14.4) | |
| Self Pay | 10 | (0.7%) | 55.3 (12.3) | <0.001 |
| Race | ||||
| White | 1,238 | (81.0%) | 48.9 (12.4) | |
| Black | 230 | (15.0%) | 46.4 (10.8) | |
| Asian | 39 | (2.6%) | 51.1 (15.7) | |
| Other/unknown | 22 | (1.4%) | 50.6 (16.6) | 0.034 |
| Gender | ||||
| Male | 1,111 | (72.7%) | 46.3 (11.1) | |
| Female | 418 | (27.3%) | 54.6 (13.5) | <0.001 |
| State of Residence | ||||
| Pennsylvania | 899 | (58.8%) | 48.5 (12.5) | |
| New Jersey | 388 | (25.4%) | 49.2 (12.3) | |
| Delaware | 59 | (3.9%) | 49.0 (10.8) | |
| Other | 183 | (12.0%) | 47.9 (12.1) | 0.718 |
| Domains that Comprise the PROPS Score | ||||
| Age | ||||
| >= 75 | 202 | (13.2%) | 45.4 (10.1) | |
| 65 – 74 | 469 | (30.7%) | 43.5 (9.3) | |
| 55 – 64 | 452 | (29.6%) | 45.8 (9.7) | |
| 44 – 54 | 197 | (12.9%) | 55.2 (12.2) | |
| 35 – 44 | 98 | (6.4%) | 62.4 (12.2) | |
| 18 – 35 | 111 | (7.3%) | 63.5 (12.3) | <0.001 |
| Diagnosis | ||||
| Prostate | 684 | (44.7%) | 42.1 (4.8) | |
| Thorax | 198 | (13.0%) | 53.5 (12.1) | |
| Gastrointestinal | 169 | (11.1%) | 45.9 (12.3) | |
| CNS | 163 | (10.7%) | 65.1 (9.8) | |
| Soft Tissue sarcoma/bone | 108 | (7.1%) | 58.2 (12.6) | |
| Head and Neck | 44 | (2.9%) | 51.9 (15.7) | |
| Lymphoma | 41 | (2.7%) | 47.7 (11.9) | |
| Other | 122 | (8.0%) | 49.3 (14.4) | <0.001 |
| Site | ||||
| Other | 1,037 | (67.8%) | 42.4 (7.4) | |
| Retreatment | 186 | (12.2%) | 60.7 (9.7) | |
| Brain | 130 | (8.5%) | 65.3 (9.5) | |
| Mediastinum | 92 | (6.0%) | 55.6 (9.3) | |
| Base of Skull | 38 | (2.5%) | 66.4 (8.1) | |
| Spine | 29 | (1.9%) | 68.5 (8.7) | |
| Retroperitoneum | 11 | (0.7%) | 49.4 (13.3) | |
| Orbit | 6 | (0.4%) | 66.2 (9.1) | <0.001 |
| Stage | ||||
| Local | 1,115 | (72.9%) | 47.7 (11.4) | |
| Regional | 194 | (12.7%) | 44.1 (11.8) | |
| Retreatment | 186 | (12.2%) | 60.7 (9.7) | |
| Metastatic | 34 | (2.2%) | 36.0 (15.3) | |
| Performance status/comorbidity | ||||
| Group I | 1,465 | (95.8%) | 48.6 (12.3) | |
| Group II or III | 64 | (4.2%) | 49.0 (13.2) | <0.001 |
| Urgency | ||||
| Not urgent | 1,158 | (75.7%) | 45.5 (10.7) | |
| Urgent | 371 | (24.3%) | 49.0 (12.3) | <0.001 |
| Clinical Protocol | ||||
| Experimental Protocol Eligible | 879 | (57.5%) | 51.3 (11.3) | |
| Registry Protocol Eligible | 650 | (42.5%) | 44.9 (12.8) | <0.001 |
To be consistent with the principles of incremental benefit, ‘youngest first,’ and equity, receipt of proton therapy should depend solely on the PROPS score, and not on other factors such as insurance status, race, gender, or geography. Therefore, we performed univariate and multivariable logistic regression to evaluate the association between PROPS scores and receipt of proton therapy, adjusted for insurance status, gender, race, state of residence, PROPS score and the domains that inform the PROPS score. Analyses were performed using SAS version 9.3 (Cary, NC). Reported P values are two sided. The study was approved by the University's IRB.
RESULTS
Among 1,529 adult patients, the most common diagnoses were prostate cancer [684 patients (44.7%)], thoracic cancers [198 patients (13.0%)], and gastrointestinal cancers [169 patients (11.1%)] (Table 4). The majority of patients had local disease, did not require proton therapy urgently, had excellent performance status and few comorbidities, and were eligible for a clinical protocol (either experimental or registry). There were 548 patients (35.8%) with Medicare, 572 patients (37.4%) with commercial insurance, and 334 patients (21.8%) with managed care health insurance.
Overall, the mean PROPS score was 48.6 (SD 12.4) (Table 4). PROPS scores varied by age, diagnosis, site and other PROPS domains, reflecting the points system that underlies the prioritization framework. Mean PROPS scores were higher for younger patients. Mean PROPS scores were higher for patients with diagnoses and sites thought to have greater incremental benefit from proton therapy. For example, the mean PROPS score for patients with brain/CNS tumors was 65.3 (SD 9.5) whereas the mean PROPS score for patients with prostate cancer was 42.1 (SD 4.8). The mean PROPS score for patients with Medicare was lower than for patients with other health insurance.
Table 5 identifies patient characteristics that were associated with receipt of proton therapy. In adjusted analyses, receipt of proton therapy was higher for patients with Medicare compared with other types of insurance [commercial vs. Medicare: 52.8% vs. 66.6%, adjusted OR 0.47 95% CI (0.34 – 0.64); managed care vs. Medicare: 42.9% vs. 66.6%, adjusted OR 0.40 95% CI (0.28 – 0.56); Medicaid vs. Medicare: 41.5% vs. 66.6%, adjusted OR 0.24 95% CI (0.13 – 0.44)]. PROPS score and age were not significantly associated with receipt of proton therapy, nor were minor differences in receipt of proton therapy by race, gender and state of residence.
Table 5.
Odds of Receiving Proton Therapv at Blinded Institution, 2010 – 2012
| Characteristic | N | % Receiving Proton Therapy | Unadjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Total | 1,529 | 56.5% | ||||
| PROPS Score | ||||||
| Per 1.0 increase in PROPS Score | 1.00 (1.00 — 1.01) | 0.34 | 1.01 (0.98 — 1.04) | 0.60 | ||
| Insurance | ||||||
| Medicare | 548 | 66.6% | Reference | Reference | ||
| Commercial | 572 | 52.8% | 0.56 (0.44 — 0.71) | <0.0001* | 0.47 (0.34 — 0.64) | <0.0001* |
| Managed Care | 334 | 49.4% | 0.49 (0.37 — 0.65) | <0.0001* | 0.40 (0.28 — 0.56) | <0.0001* |
| Medicaid | 65 | 41.5% | 0.36 (0.21 — 0.60) | <0.0001* | 0.24 (0.13 — 0.44) | <0.0001* |
| Self Pay | 10 | 50.0% | 0.50 (0.14 — 1.75) | 0.28 | 0.53 (0.14 — 2.01) | 0.35 |
| Race | ||||||
| White | 1,238 | 56.3% | Reference | Reference | ||
| Black | 230 | 57.4% | 1.05 (0.79 — 1.39) | 0.76 | 1.16 (0.85 — 1.58) | 0.36 |
| Asian | 39 | 64.1% | 1.39 (0.71 — 2.69) | 0.34 | 1.41 (0.70 — 2.82) | 0.34 |
| Other/unknown | 22 | 45.5% | 0.65 (0.28 — 1.51) | 0.31 | 0.63 (0.26 — 1.53) | 0.31 |
| Gender | ||||||
| Male | 1,111 | 57.3% | Reference | Reference | ||
| Female | 418 | 54.3% | 0.88 (0.71 — 1.11) | 0.29 | 0.85 (0.64 — 1.13) | 0.25 |
| State of Residence | ||||||
| Pennsylvania | 899 | 56.6% | Reference | Reference | ||
| New Jersey | 388 | 58.2% | 1.07 (0.84 — 1.36) | 0.59 | 1.02 (0.79 — 1.32) | 0.88 |
| Delaware | 59 | 55.9% | 0.97 (0.57 — 1.65) | 0.92 | 0.93 (0.54 — 1.60) | 0.78 |
| Other | 183 | 52.5% | 0.85 (0.61 — 1.16) | 0.30 | 0.75 (0.53 — 1.05) | 0.09 |
| Domains that Comprise the PROPS Score | ||||||
| Age | ||||||
| >= 75 | 202 | 59.9% | Reference | Reference | ||
| 65 - 74 | 469 | 60.8% | 1.04 (0.74 — 1.45) | 0.83 | 1.18 (0.83 — 1.68) | 0.36 |
| 55 - 64 | 452 | 51.8% | 0.72 (0.51 — 1.01) | 0.05 | 1.23 (0.82 — 1.86) | 0.32 |
| 44 - 54 | 197 | 50.3% | 0.68 (0.45 — 1.01) | 0.05 | 1.12 (0.67 — 1.88) | 0.67 |
| 35 - 44 | 98 | 56.1% | 0.86 (0.53 — 1.40) | 0.53 | 1.28 (0.63 — 2.58) | 0.50 |
| 18 - 35 | 111 | 63.1% | 1.14 (0.71 — 1.84) | 0.58 | 1.92 (0.89 — 4.17) | 0.10 |
| Diagnosis | ||||||
| Prostate | 684 | 57.0% | Reference | Reference | ||
| Thorax | 198 | 52.5% | 0.83 (0.61 — 1.15) | 0.26 | 0.95 (0.60 — 1.52) | 0.84 |
| Gastrointestinal | 169 | 56.2% | 0.97 (0.69 — 1.36) | 0.85 | 1.12 (0.74 — 1.71) | 0.59 |
| CNS | 163 | 68.7% | 1.66 (1.15 — 2.38) | <0.01* | 1.28 (0.63 — 2.60) | 0.49 |
| Soft Tissus sarcoma/bone | 108 | 60.2% | 1.14 (0.75 — 1.72) | 0.54 | 1.24 (0.72 — 2.15) | 0.44 |
| Head and Neck | 44 | 45.5% | 0.63 (0.34 — 1.16) | 0.14 | 0.61 (0.30 — 1.22) | 0.16 |
| Lymphoma | 41 | 51.2% | 0.79 (0.42 — 1.49) | 0.47 | 0.89 (0.39 — 2.03) | 0.78 |
| Other | 122 | 46.7% | 0.66 (0.45 — 0.97) | 0.04 | 0.83 (0.49 — 1.40) | 0.48 |
| Site | ||||||
| Other | 1,037 | 55.8% | Reference | Reference | ||
| Retreatment | 186 | 51.6% | 0.84 (0.62 — 1.15) | 0.29 | 0.87 (0.50 — 1.50) | 0.62 |
| Brain | 130 | 69.2% | 1.78 (1.20 — 2.63) | <0.01* | 1.49 (0.68 — 3.27) | 0.32 |
| Mediastinum | 92 | 53.3% | 0.90 (0.59 — 1.38) | 0.63 | 0.91 (0.50 — 1.67) | 0.76 |
| Base of Skull | 38 | 60.5% | 1.21 (0.63 — 2.35) | 0.57 | 0.94 (0.35 — 2.51) | 0.90 |
| Spine | 29 | 69.0% | 1.76 (0.79 — 3.90) | 0.17 | 1.49 (0.51 — 4.34) | 0.47 |
| Retroperitoneum | 11 | 36.4% | 0.45 (0.13 — 1.55) | 0.21 | 0.41 (0.11 — 1.52) | 0.18 |
| Orbit | 6 | 50.0% | 0.79 (0.16 — 3.94) | 0.77 | 1.10 (0.18 — 6.76) | 0.92 |
| Stage | ||||||
| Local | 1,115 | 58.1% | Reference | Excluded from adjusted model** | ||
| Regional | 194 | 51.6% | 0.77 (0.56 — 1.05) | 0.10 | ||
| Retreatment | 186 | 55.9% | 0.91 (0.46 — 1.82) | 0.79 | ||
| Metastatic | 34 | 52.1% | 0.78 (0.58 — 1.06) | 0.12 | ||
| Performance status/comorbidity | ||||||
| Group I | 1,465 | 56.7% | Reference | Reference | ||
| Group II or III | 64 | 51.6% | 0.81 (0.49 — 1.34) | 0.42 | 0.81 (0.45 — 1.46) | 0.49 |
| Urgency | ||||||
| Not Urgent | 1,158 | 57.9% | Reference | Reference | ||
| Urgent | 371 | 52.3% | 0.80 (0.63 — 1.01) | 0.06 | 0.79 (0.53 — 1.17) | 0.24 |
| Clinical trial | ||||||
| Experimental Protocol Eligible | 879 | 57.2% | Reference | Reference | ||
| Registry Protocol Eligible | 650 | 55.5% | 0.93 (0.76 — 1.15) | 0.51 | 1.01 (0.74 — 1.40) | 0.93 |
P<0.01
Stage excluded from adjusted model (collinear with site variable)
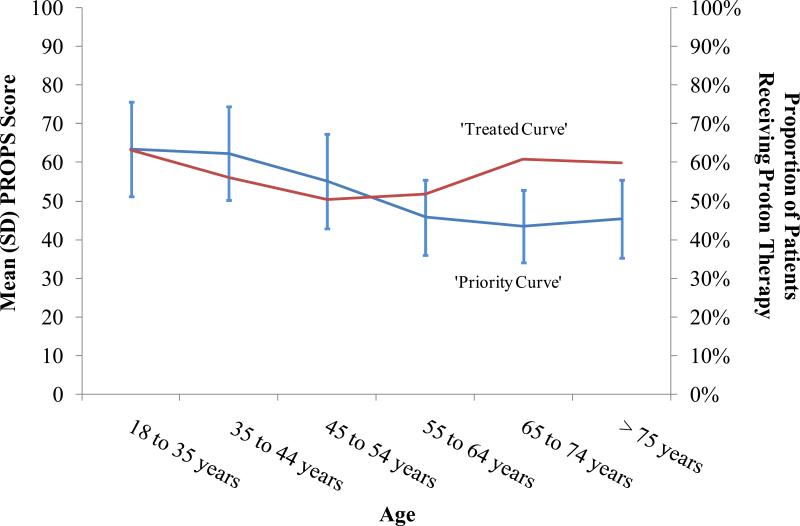
Figure 1 graphically compares the mean (SD) PROPS scores for patients considered for proton therapy prioritization to the proportion of patients who received proton therapy by 10 year increments. The ‘Priority Curve,’ derived by connecting mean PROPS scores across age increments of patients, is highest for younger patients and is diminished for older patients. However, the ‘Treated Curve,’ derived by connecting the proportion of patients who received proton therapy across age increments, shows that a greater proportion of older patients receive proton therapy despite lower mean PROPS scores.
Figure 1.
Discordance between ‘Priority Curve’ and ‘Treated Curve’ (among Patients Considered for Proton Therapy and Patients Treated with Proton Therapy)
DISCUSSION
We undertook this study to present the principles and rationale of PROPS and to examine its application in the allocation of treatment slots for proton therapy at Blinded Institution. Based on seven domains, PROPS assigns a score to each patient considered for proton therapy and provides a weekly forum for collaborative discussion through which recommendations are made for proton therapy or alternative therapies. The PROPS score guides the allocation of proton therapy slots by providing a transparent algorithm based on the best available evidence and expert opinion.
PROPS is consistent with and extends previous efforts at patient prioritization in other health care settings where medical resources are limited; namely, the allocation of organs for liver transplantation and intensive care unit (ICU) beds. The Model for End-stage Liver Disease (MELD) incorporates three laboratory variables to derive an objective score, validated as an accurate predictor of survival among patients with advanced liver disease. The adoption of the MELD system allowed for those with more severe cases of organ failure to be transplanted first and substantially reduced transplantation wait times.11,12
In ICUs, age, illness severity, intensity of care, and medical diagnosis factor into prioritization decisions.7,13-15 However, a survey revealed that instead of using formal policies, physicians often made decisions regarding who to admit on a per patient basis.13 Another survey noted that 86% of physicians report ICU admission errors, most commonly as a result of clinical doubt, limited decision time, error in assessment, pressure from superiors, and threat of legal action.14 Anticipating the possibility of similar challenges in proton therapy prioritization, PROPS was designed to minimize individual physician preferences and variability in physician decision-making through a formal prioritization process that applies to all patients.
During the 3-year study period, which spanned the ramp up phase of treatment availability at Blinded Institution, our Center's capacity to provide treatment remained greater than patient demand. In this context, and perhaps not surprisingly, we found that the PROPS score was not associated with receipt of proton therapy. Rather, insurance status was the only factor that significantly predicted for receipt of proton therapy: patients with Medicare insurance were substantially more likely to receive proton therapy than those with commercial, managed care, and Medicaid insurance.
Nearly 97% of American adults age 65 years and older are Medicare beneficiaries; elderly adults are more likely to receive proton therapy than younger patients, whose non-Medicare coverage policies may be more restrictive for proton therapy. This discordance is depicted in Figure 1, where the relationship between the ‘Priority Curve’ and the ‘Treated Curve’ is distorted among elderly patients.
Restrictive coverage policies are one of the tools by which payers limit utilization for costly cancer therapies that have uncertain clinical benefit.16 Absent comparative effectiveness evidence for proton therapy, many commercial payers and Medicaid carriers have restricted access to proton therapy; meanwhile, Medicare generally covers proton therapy without a requirement for effectiveness evidence (there remains some variation through local coverage determinations).17 The implication is that, at least when the supply of treatment slots exceeds demand, payer coverage policies may be the main determinant of proton therapy utilization, rather than disease, anatomic site, age, or other indicators of potential incremental clinical benefit.
What can be done to align receipt of proton therapy with its potential for incremental clinical benefit? High-quality comparative studies of clinical effectiveness are essential. Payers may consider regional or national evidence development policies pegged to reimbursement to promote comparative effectiveness research for proton therapy.18 Several federally-sponsored randomized trials comparing proton therapy to alternative radiotherapy treatments for prostate and lung cancer are accruing.19-21 Although unlikely to report results for years, these ongoing trials are important to direct evidence-based treatment decisions in the future.
As a complement to evidence generation for proton therapy, a priority framework like PROPS could help determine for whom proton therapy is best suited. PROPS relies on the treatment preferences of a community of stakeholders involved in cancer care – including radiation, medical, and surgical oncologists, nurses, social workers, comparative effectiveness researchers, health system leadership, and patients – and is grounded in the best available evidence and expert opinion to guide the assignment of points and weights. Payers could consider incorporating similar prioritization systems into coverage policies, though integrating such systems into payment policy would be a complex undertaking.
There are important limitations to our report. First, while evidence suggests public support for the concept of ‘youngest first,’ healthy debate continues in the bioethics community regarding its rationale and application to the allocation of medical resources.9
Second, unlike MELD, PROPS has not been validated against important patient outcomes, such as improvements in survival or reduced toxicity. PROPS uses objective criteria but also incorporates criteria that may be more subjective. For example, priority scores were delineated in increments of 0, 5, or 10 because integers were easy to remember and calculate; however, weights could be assigned empirically based on survey and modeling techniques to effect a more objective classification of subjects into priority categories. This is an area for further research.
Third, the urgency domain is defined as situations that may require minimal delay in initiating treatment (Table 3). More urgent cases are prioritized higher, because, other priorities being equal, more urgent cases should receive therapy sooner. In theory, a problem with this approach could manifest if points added for urgency place a less appropriate urgent case ahead of a more appropriate non-urgent case. In practice, we have not experienced this problem, though we may in the future. As we continue to iterate the PROPS framework, we are evaluating an alternative approach in which urgency among similarly scored cases is considered after prioritization.
Fourth, faculty may have refrained from presenting patients as they learned which clinical cases were appropriate for proton therapy or which payers were not covering various clinical indications.
Fifth, PROPS was designed in anticipation of the difficult decisions we might face with treatment slot scarcity, which did not materialize at our Center during the study period. Other centers have experienced limited proton therapy availability, and, more recently, with our center treating over 100 patients daily, we have utilized the PROPS system to allocate pencil beam scanning technology, a form of proton therapy with much more restricted availability.
Lastly, prioritization systems like PROPS that are based on expert opinion may unintentionally obstruct evidence generation – particularly if experts are incorrect in their beliefs regarding which tumors are incrementally better treated with proton therapy.
In conclusion, PROPS and its underlying principles could form the foundation for a prioritization framework that evolves as the technology and evidence matures. Established through a multi-stakeholder approach, PROPS has promoted objectivity and transparency for physicians and their patients. We have made the content and process of PROPS explicit and accessible for public discussion22 and have highlighted the difference between PROPS scores and actual treatment patterns. Our goal is to underscore the need for continued commitment to rational allocation of therapeutic resources like proton therapy, even as evidence develops to demonstrate its comparability, superiority, or inferiority to alternative treatments.
Supplementary Material
SUMMARY.
We present the principles and rationale for the Proton Priority System (PROPS), a priority points framework that assigns higher scores to patients thought to more likely benefit from proton therapy. The PROPS score guides the allocation of proton therapy slots for patients by providing a transparent algorithm. Because the actual allocation of treatment slots depends on insurance status, payers may consider incorporating PROPS, or its underlying principles, into proton therapy coverage policies.
ACKNOWLEDGEMENT
We acknowledge the efforts of the following members of and staff to the Proton Oversight and Advisory Board and the Proton Working Group: Karima Cooper; Jared Finley, PhD; Tracy Lautenbach, MSW, LCSW, OSW-C; Alexander Lin, MD; Lilie Lin, MD; Robert Lustig, MD; Marinela Masuki, BS; James McDonough, PhD; Susan Prendergast, RN, RN; Debbie Smith, BSN; and Patricia Velez, BA. We thank the anonymous reviewers for substantive comments on the underpinnings of the PROPS system that improved the manuscript.
Funding: The work was supported by National Cancer Institute 1K07CA16316-01 and the David and Leslie Clarke Outcomes Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Bekelman reports grants from National Cancer Institute during the conduct of the study. Dr. Friedberg reports serving on the medical advisory board for Varian Medical system (a position which was initiated after completion of the study). Dr. Vaughn reports serving as a consultant to Jannsen Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Conception and design: Bekelman, Asch, Tochner, Friedberg, Vaughn, Raksowski, Rash, Hahn
Administrative support: Bekelman, Hahn
Provision of study materials or patients: Bekelman, Rash
Collection and assembly of data: Bekelman, Rash
Data analysis and interpretation: Bekelman, Asch, Hahn
Manuscript writing: Bekelman, Asch, Tochner, Friedberg, Vaughn, Raksowski, Rash, Hahn
Final approval of manuscript: Bekelman, Asch, Tochner, Friedberg, Vaughn, Raksowski, Rash, Hahn
APPENDIX FIGURE
This online only appendix has been provided by the authors to give readers additional information about the study.
Appendix Figure 1. Proton Therapy Algorithm for Exceptional Cases
REFERENCES
- 1.Ballas LK, Elkin E, Schrag D, Minsky BD, Bach P. Radiation Therapy Facilities in the United States. International journal of radiation oncology, biology, physics. 2006;66(4):1205–1211. doi: 10.1016/j.ijrobp.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 2.Goozner M. The proton beam debate: are facilities outstripping the evidence? J Natl Cancer Inst. 2010 Apr 7;102(7):450–453. doi: 10.1093/jnci/djq112. [DOI] [PubMed] [Google Scholar]
- 3.Brada M, De Ruysscher D, Pijls-Johannesma M. Evidence for Proton Therapy. J Clin Oncol. 2008 Apr 28; [Google Scholar]
- 4.Olsen DR, Bruland OS, Frykholm G, Norderhaug IN. Proton therapy - a systematic review of clinical effectiveness. Radiother Oncol. 2007 May;83(2):123–132. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009 Jan 31;373(9661):423–431. doi: 10.1016/S0140-6736(09)60137-9. [DOI] [PubMed] [Google Scholar]
- 6.Jagsi R, DeLaney TF, Donelan K, Tarbell NJ. Real-time rationing of scarce resources: the Northeast Proton Therapy Center experience. J Clin Oncol. 2004 Jun 1;22(11):2246–2250. doi: 10.1200/JCO.2004.10.083. [DOI] [PubMed] [Google Scholar]
- 7.Sinuff T, Kahnamoui K, Cook DJ, et al. Rationing critical care beds: a systematic review. Crit Care Med. 2004 Jul;32(7):1588–1597. doi: 10.1097/01.ccm.0000130175.38521.9f. [DOI] [PubMed] [Google Scholar]
- 8.Kamath PS, Kim WR, Advanced Liver Disease Study G The model for end-stage liver disease (MELD). Hepatology. 2007 Mar;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya A. Age-related preferences and age weighting health benefits. Social Science & Medicine. 1999;48(2):267–276. doi: 10.1016/s0277-9536(98)00343-8. [DOI] [PubMed] [Google Scholar]
- 10.Daniels N. How to achieve fair distribution of ARTs in 3 by 5: fair process and legitimacy in patient selection. World Health Organization; Geneva: 2004. [Google Scholar]
- 1.Ahmad J, Downey KK, Akoad M, Cacciarelli TV. Impact of the MELD score on waiting time and disease severity in liver transplantation in United States veterans. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007 Nov;13(11):1564–1569. doi: 10.1002/lt.21262. [DOI] [PubMed] [Google Scholar]
- 12.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008 May;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Walter KL, Siegler M, Hall JB. How decisions are made to admit patients to medical intensive care units (MICUs): a survey of MICU directors at academic medical centers across the United States. Crit Care Med. 2008 Feb;36(2):414–420. doi: 10.1097/01.CCM.0000299738.26888.37. [DOI] [PubMed] [Google Scholar]
- 14.Giannini A, Consonni D. Physicians' perceptions and attitudes regarding inappropriate admissions and resource allocation in the intensive care setting. British journal of anaesthesia. 2006 Jan;96(1):57–62. doi: 10.1093/bja/aei276. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AB, Joglekar AS, Gibson J, Swota AH, Martin DK. Communication of bed allocation decisions in a critical care unit and accountability for reasonableness. BMC health services research. 2005;5:67. doi: 10.1186/1472-6963-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009 Feb 5;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 17.Jarosek SL, Elliott S, Virnig BA. Proton beam radiotherapy in the U.S. Medicare population: growth in use between 2006 and 2009. Data Points Publication Series. Agency for Healthcare Research and Quality; Rockville, MD: 2011. [PubMed] [Google Scholar]
- 18.Pearson SD, Bach PB. How Medicare could use comparative effectiveness research in deciding on new coverage and reimbursement. Health Aff (Millwood) 2010 Oct;29(10):1796–1804. doi: 10.1377/hlthaff.2010.0623. [DOI] [PubMed] [Google Scholar]
- 19. [July 8, 2013];Stereotactic Body Radiotherapy (SBRT) Versus Stereotactic Body Proton Therapy (SBPT) 2013 at http://www.clinicaltrials.gov/ct2/show/study/NCT01511081.
- 20. [July 8, 2013];PARTIQoL (Prostate Advanced Radiation Technologies Investigating Quality of Life) Trial: Phase III Randomized Clinical Trial of Proton Therapy vs. IMRT for Low or Low-Intermediate Risk Prostate Cancer. 2013 at http://clinicaltrials.gov/ct2/show/record/NCT01617161.
- 2. [July 8, 2013];Image-Guided Adaptive Conformal Photon Versus Proton Therapy. 2013 at http://www.clinicaltrials.gov/ct2/show/NCT00915005.
- 22.Cookson R, Dolan P. Principles of justice in health care rationing. Journal of medical ethics. 2000 Oct;26(5):323–329. doi: 10.1136/jme.26.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.