Abstract
Intramolecular C-H insertion on diazosulfone and diazosulfonate substrates was used to prepare synthetically useful intermediates from easily available starting materials.
Keywords: sultones, sulfones, synthetic intermediates, C-H insertion
INTRODUCTION
We previously reported a modification of C-H insertion that leads to selective formation of six-membered sulfur containing heterocycles (δ-sultones or thiane-1,1-dioxides, depending on the substrate used).[1] More reports of this transformation by other research groups have appeared since then.[2,3] The most notable feature of this reaction is that the usual preference for formation of five-membered rings[4,5] is overturned in this case, permitting to target different C-H bonds in the molecule for insertion (Scheme 1). Using this reaction, easily available chiral starting materials can be transformed into useful synthetic intermediates. Earlier, we demonstrated the potential of this approach by converting the δ-sultone, obtained from (-)-citronellol by diazosulfonate C-H insertion,[2] to natural product bakuchiol.[7] We, thus, set out to explore preparation of other synthetic intermediates from common precursors using this reaction.
Scheme 1.
C-H insertion on diazosulfones and diazosulfonates
RESULTS AND DISCUSSION
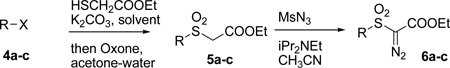
We started by exploring the diazosulfonate substrates. That required suitable alcohols substrates. We identified borneol (1a), isomenthol (1c), and 3-methylpentan-1-ol (1b, available from isoleucine[8]) as such compounds. The alcohols were converted to the corresponding diazosulfonates using our previously developed procedure, with a few modifications (Table 1).[1] We found it beneficial, as recommended by Du Bois,[2] to use pyridine instead of triethylamine in the sulfonation step, while use of mesyl azide instead of nosyl azide in diazotransfer step simplified purification. Additionally, the diazosulfone substrates were of particular interest to us. Cyclization of these substrates leads to cyclic sulfones (thiane-1,1-dioxides) that may have even greater synthetic potential. For example, we previously demonstrated facile alkylation of these compounds and rearrangement of the alkylated products. [9] The diazosulfone substrates were prepared by a common sequence of an SN2 substitution of alkyl sulfonates with ethyl mercaptoacetate, followed, without purification, by Oxone oxidation of the intermediate sulfide to sulfone. The obtained sulfones were then subjected to diazotransfer (Table 2). Tosylates of previously obtained 3-methylpentan-1-ol (4a), as well as of 2-methylpentan-1-ol (4b, easily available in chiral form via stereoselective methylation[10] using Myers chiral auxiliary[11], or asymmetric hydrogenation of (E)-2-methylpent-2-en-1-ol[12]), and mesylate of menthol (4c) were thus converted to the corresponding diazosulfones. In case of mesylate of menthol, the SN2 substitution step required harsher conditions (60 °, and DMSO as a solvent instead of acetone) and proceeded in a lower yield. The latter sulfone can also be more conveniently prepared by a reaction of neomenthylthiol[13] with ethyl bromoactate, followed by Oxone oxidation. The obtained diazosulfonates (3a-3c) and diazosulfones (6a-6c) were subjected to C-H insertion as previously described.[1,2] In case of borneol diazosulfonate (3a), unexpectedly, two products were obtained (7a and 7b, Entry 1, Table 3) – resulting from insertion into methyl group as well as into the methylene bridge, of which the former predominated. 3-Methylpentyl and isomenthyl diazosulfonates (3b and 3c) cleanly provided the expected C-H insertion products, as mixtures of diastereomers. C-H insertion on diazosulfones also proceeded readily. Notably, C-H insertion of diazosulfones 6a and 6b proceeded diastereoselectively, providing the trans-3,4- and cis-3,5- dimethyl thiane-1,1-dioxides (10 and 11) in good yields, effectively creating a new stereocenter in 1,2- and 1,3-positions relative to the existing one, respectively (small amounts of other diastereomers appeared to be present in the crude mixture, but were not isolated or identified). The relative stereochemistry of the observed products was confirmed by NOE studies (Figure 1). In case of neomenthyl diazosulfone, 6c, we hoped to direct C-H insertion into a different site than observed on a corresponding menthol diazosulfonate (Scheme 1). However, exclusive insertion into the same C-H site was observed, producing the five membered cyclic sulfone. The reaction was also slower than with other diazosulfones, requiring 5 hours of reflux in dichloromethane to complete, as opposed to 1 hour in other cases. Notably, two diastereomers of the product were observed in the crude reaction mixture by NMR, but only one was isolated, apparently due to equilibration during chromatography (similar equilibration was observed in case of other five membered cyclic sulfones[6]).
Table 1.
Preparation of diazosulfonates
 | |||
|---|---|---|---|
| Entry | R | Yield of 2a-c | Yield of 3a-c |
| 1 |  |
90% | 75% |
| 2 | 85% | 72% | |
| 3 |  |
85% | 77% |
Table 2.
Preparation of diazosulfones
 | |||
|---|---|---|---|
| Entry | R,X | Yield of 5a-c | Yield of 6a-c |
| 1 |  |
90% | 82% |
| 2 |  |
85% | 80% |
| 3 |  |
45% | 85% |
Table 3.
C-H insertion
| Entry | Substrate | Conditions | Product(s) | Yield |
|---|---|---|---|---|
| 1 | 3a | Rh2(esp)2, DCM rt, 10 h |  |
75% (~9:1) |
| 2 | 3b | Rh2(esp)2, DCM reflux, 1 h | 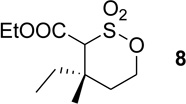 |
65% |
| 3 | 3c | Rh2(OAc)4, DCM reflux, 1 h |  |
85% |
| 4 | 6a | Rh2(OAc)4, DCM reflux, 1 h |  |
60% |
| 5 | 6b | Rh2(OAc)4, DCM reflux, 1 h |  |
65% |
| 6 | 6c | Rh2(OAc)4, DCM reflux, 5 h |  |
90% |
Figure 1.
Confirmation of configuration of 10 and 11
CONCLUSION
We thus prepared a series of synthetically useful intermediates from cheap and readily available starting materials using C-H insertion on sulfonyl substrates. Stereoselective formation of new stereocenters was observed in two cases. These intermediates may serve as precursors to natural products. Further studies will be reported in due course.
EXPERIMENTAL
General procedure for C-H insertion
To the suspension of the rhodium catalyst (Rh2(esp)2 or Rh2(OAc)4) (0.02 mmol) in CH2Cl2 (4 ml), a solution of the corresponding diazo compound (1 mmol) in CH2Cl2 (2 ml) was added either at rt over a period of 1 h using a syringe pump, or manually over 5 minutes at reflux. Upon completion of the addition, the reaction mixture was stirred at the specified temperature for the specified time (see Table 3). The volatiles were removed under reduced pressure and the crude reaction mixture was purified on silica column (Ethyl Acetate-Hexanes, 0 to 40%).
Sample physical data:
Ethyl (2S,3S,4R)-3,4-dimethylthiane-1,1-dioxide-2-carboxylate (10)
m.p. 99–100 °C. 1H NMR (500 MHz, CDCl3): δ 4.32 (dq, J = 7, 2 Hz, 2H), 3.60 (d, J = 12 Hz, 1H), 3.14 (dt, J = 14, 3.5 Hz, 1H), 2.97–3.05 (m, 1H), 2.16–2.26 (m, 1H), 1.94–2.06 (m, 2H), 1.33 (t, J = 7 Hz, 3H), 1.34–1.42 (m, 1H), 1.06 (d, J = 6.5 Hz, 3H), 1.01 (d, J = 6.5 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 164.0 (C), 71.9 (CH), 62.7 (CH2), 52.2 (CH2), 39.9 (CH), 36.9 (CH), 31.9 (CH2), 19.4 (CH3), 17.8 (CH3), 14.3 (CH3). HRMS (ESI) calcd for C10H19O4S [M+H]+ 235.1004, found 235.0992.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health under grant No. GM085645. We thank Alena Kubatova for HRMS analyses. The work on TOF MS was supported by the National Science Foundation under grant No. CHE-0216038.
Footnotes
Supporting Information: Full experimental detail, 1H and 13C NMR spectra can be accessed on the publisher’s website.
REFERENCES
- 1.John JP, Novikov AV. Selective Formation of Six Membered Cyclic Sulfones and Sulfonates by C-H Insertion. Org. Lett. 2007;9:61–63. doi: 10.1021/ol062592h. [DOI] [PubMed] [Google Scholar]
- 2.Wolckenhauer SA, Devlin AS, Du Bois J. δ-Sultone Formation Through Rh-Catalyzed C-H Insertion. Org. Lett. 2007;9:4363–4366. doi: 10.1021/ol701950d. [DOI] [PubMed] [Google Scholar]
- 3.Flynn CJ, Elcoate CJ, Lawrence SE, Maguire AR. Highly Enantioselective Intramolecular Copper Catalyzed C-H Insertion Reactions of α-Diazosulfones. J. Am. Chem. Soc. 2010;108:7686–7693. doi: 10.1021/ja909713a. [DOI] [PubMed] [Google Scholar]
- 4.Taber DF, Ruckle RE., Jr. Cyclopentane Construction by Dirhodium Tetraacetate-mediated Intramolecular C-H Insertion: Steric and Electronic Effects. J. Am. Chem. Soc. 1986;108:7686–7693. doi: 10.1021/ja00284a037. [DOI] [PubMed] [Google Scholar]
- 5.Doyle MP, Westrum LJ, Wolthuis WNE, See MM, Boone WP, Bagheri V, Pearson MM. Electronic and Steric Control in Carbon-hydrogen Insertion Reactions of Diazoacetoacetates Catalyzed by Dirhodium(II) Carboxylates and Carboxamides. J. Am Chem. Soc. 1993;115:958–964. [Google Scholar]
- 6.Jungong CJ, John JP, Novikov AV. Formation of Six- versus Five-Membered Cyclic Sulfones by C–H Insertion. Tetrahedron Lett. 2009;50:1954–1957. doi: 10.1016/j.tetlet.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bequette JP, Jungong CJ, Novikov AV. Synthesis of Bakuchiol Using C-H Insertion to Install the Quaternary Center. Tetrahedron Lett. 2009;50:6963–6964. doi: 10.1016/j.tetlet.2009.09.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schurig V, Leyrer U, Wistuba D. Simple Access to Highly Enantiomerically Enriched (S)-3-methyl-1-pentanol, (S)-3-methyl-1-pentene, (2R,3S)-2-deuterio-3-methyl-1-pentanol and (2S,3S)-3-methyl-2-pentanol from Natural (L)-isoleucine. J. Org. Chem. 1986;51:242–245. [Google Scholar]
- 9.Jungong CJ, John JP, Bequette JP, Novikov AV. Synthetically Useful Transformations of δ-Sultones and Thiane-1,1-dioxides Obtained by C-H Insertion. Heterocycles. 2009;78:2531–2539. [Google Scholar]
- 10.Chen Y-H, McDonald FE. New Chiral Synthons for Efficient Introduction of Bispropionates via Stereospecific Oxonia—Cope Rearrangements. J. Am. Chem. Soc. 2006;128:4568–4569. doi: 10.1021/ja061082f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. Pseudoephedrine as a Practical Chiral Auxiliary for the Synthesis of Highly Enantiomerically Enriched Carboxylic Acids, Alcohols, Aldehydes, and Ketones. J. Am. Chem. Soc. 1997;119:6496–6511. [Google Scholar]
- 12.Wang A, Fraga RPA, Hormann E, Pfaltz A. Iridium-Catalyzed Asymmetric Hydrogenation of Unfunctionalized, Trialkyl-Substituted Olefins. Chem. Asian J. 2011;6:599–606. doi: 10.1002/asia.201000595. [DOI] [PubMed] [Google Scholar]
- 13.Mikolajczyk M, Perlikowska W, Omelanczuk J. Synthesis of (+)-Neomenthanethiol and Some of its Derivatives. A New Example of Asymmetric Induction in the Sulfoxide Synthesis. Synthesis. 1987:1009–1012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




