Abstract
Nanotheranostics is a relatively new, fast-growing field that combines the advantages of treatment and diagnosis via a single nanoscale carrier. The ability to bundle both therapeutic and diagnostic capabilities into one package offers exciting prospects for the development of novel nanomedicine. Nanotheranostics can deliver treatment while simultaneously monitoring therapy response in real-time, thereby decreasing the potential of over- or under-dosing patients. Polymer-based nanomaterials, in particular, have been used extensively as carriers for both therapeutic and bioimaging agents and thus hold great promise for the construction of multifunctional theranostic formulations. Herein, we review recent advances in polymer-based systems for nanotheranostics, with a particular focus on their applications in cancer research. We summarize the use of polymer nanomaterials for drug delivery, gene delivery, and photodynamic therapy, combined with imaging agents for magnetic resonance imaging, radionuclide imaging, and fluorescence imaging.
Keywords: polymer, nanoparticle, cancer, theranostics, drug delivery, imaging
1. Introduction
Originally introduced by Funkhouser in 2002, the term “theranostics” describes any “material that combines the modalities of therapy and diagnostic imaging” into a single package.1 More recently, theranostics has become commonly used to describe image-guided therapy, or therapeutic agents that concomitantly possess imaging capabilities. The traditional approach to treating patients involves first diagnosing a patient and then subsequently utilizing a certain known therapy to treat the disease or disorder. As a result, medical research has focused heavily on characterizing a type of disease and then developing a drug or standard treatment regimen for that general disease.2 While this approach is still typically the clinical standard, many of the most vexing diseases, such as cancer, are heterogeneous in their expression,3,4 necessitating more individualized and tailored methods of treatment.5 Toward this end, the field of nanotheranostics has emerged, which aims to allow physicians to simultaneously monitor drug distribution and release and evaluate therapeutic efficacy noninvasively and in real-time. This valuable information would enable physicians to better tailor treatment plans based on each patient’s individual responses and needs, thereby lowering the chances of the patient experiencing adverse side effects due to over- or under-dosing.6
A number of nanosized delivery vehicles have been studied for theranostic applications. For example, chemotherapeutic agents have been conjugated onto gold7,8 and iron oxide nanoparticles.9,10 Quantum dots (QDs) with their inherent fluorescence have also been utilized for image-guided therapies.11−13 Carbon nanotubes, another widely studied inorganic material, have also been presented as a potential candidate for concurrent optical imaging and drug/gene delivery.14,15 While these platforms have shown promising results in animal models, their inorganic or metallic nature has raised concerns of toxicity, immunogenicity, and slow excretion kinetics from the body, which need to be thoroughly examined prior to clinical test in human. Silver-based nanoparticles, for example, can cause adenosine 5′-triphosphate depletion and mitochondrial damage,16 while single-walled carbon nanotubes can cause oxidative stress and trigger apoptosis;17 magnetic nanoparticles can induce cell death through membrane damage.18 In this review, we will focus on the theranostic potential of polymer-based nanomaterials, which possess excellent biocompatibility, biodegradability, and structural versatility.19,20 Biopolymers naturally degrade into safe materials (i.e., carbon dioxide and water) over time in the body, and are typically nontoxic except at extremely high concentrations. Polymers such as polyethylene glycol (PEG), poly(d,l-lactic acid), poly(d,l-glycolic acid), and poly(ε-caprolactone) have already been approved for clinical use in macroformulations. Polymer-based platforms have been studied extensively for cancer therapy and offer many advantages.21 In particular, polymeric nanoparticles are able to enhance drug efficacy compared with free drugs via improved drug encapsulation and delivery, prolonged circulation half-life, and sustained or triggered drug release.22,23 Polymeric nanoparticles are also able to accumulate at specific disease sites through passive targeting by the enhanced permeability and retention (EPR) effect, or through active targeting by the incorporation of targeting moieties specific for a receptor or cell surface ligand at the region of interest.24−26
Building on the progress made in developing therapeutic polymeric nanoparticles, researchers are now incorporating clinically used imaging modalities into therapeutic nanocarriers.27 Among these are magnetic resonance imaging (MRI) contrast agents, radioactive agents for radionuclide imaging via positron emission tomography (PET) or single photon emission computed tomography (SPECT), fluorescent agents for fluorescent imaging, and nano/microbubbles for ultrasound imaging. Each modality has its own advantages and disadvantages (Table 1), which need to be weighed to determine the most appropriate imaging technique for the desired outcome. MRI offers high spatial resolution and soft tissue contrast without tissue-penetrating limitations and is widely used in hospitals, but is expensive and time-consuming. Radionuclide imaging is also commonly used in the clinic and offers high sensitivity with unlimited tissue penetration. On the other hand, it is also high cost and offers limited spatial resolution compared with MRI. Fluorescence imaging provides a means for high-throughput screening for target confirmation and compound optimization along with high sensitivity and multicolor imaging, but has low tissue penetration and spatial resolution.28 Ultrasound imaging offers high resolution at much lower cost than MRI or PET/SPECT, but has low depth penetration.
Table 1. Overview of Commonly Used Imaging Modalities.
| imaging modality | imaging agent | spatial resolution | advantages | disadvantages |
|---|---|---|---|---|
| MRI | gadolinium, iron oxide, manganese oxide, 19F-labeled compounds | 10–100 μm | clinical translation; high resolution; no radiation; no depth limit; quantitative results | high cost; long imaging time; limited to patients with no metal implants or tattoos |
| PET | 18F, 64Cu, 11C, 15O-labeled compounds | 1–2 mm | clinical translation; high sensitivity; unlimited penetration; image biochemical processes; quantitative results | high cost; radiation; low resolution |
| SPECT | 99 mTc, 111In chelates | 1–2 mm | clinical translation; high sensitivity; unlimited penetration; relatively lower cost; quantitative results | radiation; low resolution |
| optical imaging | fluorochrome, photoproteins | 1–5 mm | high sensitivity; no radiation; high-throughput screening for target confirmation and compound optimization; multichannel imaging | limited clinical translation; low resolution; low depth penetration |
| ultrasound | nano/microbubbles | 50 μm | high resolution; low cost; ease of operation; no radiation; quantitative results | low depth penetration |
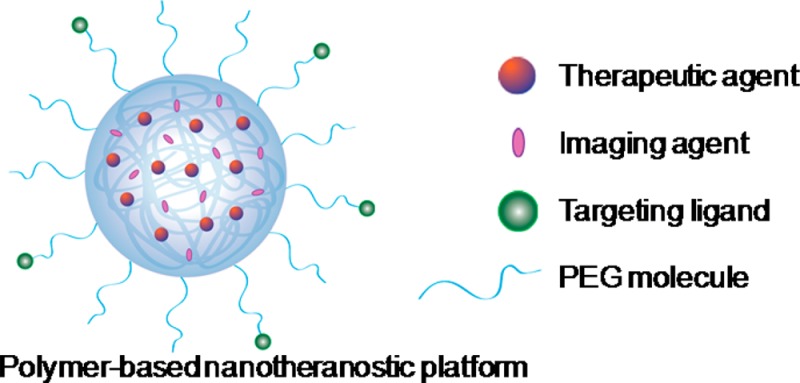
In general, a polymer-based theranostic material is comprised of at least three main components: (i) a polymer component that offers stabilization and biocompatibility, (ii) a therapeutic agent (i.e., small-molecule drug, siRNA, etc.), and (iii) an imaging agent (i.e., MRI contrast agent, radionuclide, fluorophore, etc.) (Figure 1). In some cases, the therapeutic component can also act as the imaging component, such as doxorubicin, which possesses an inherent fluorescence.29 These components can be arranged in different ways depending on the specific delivery platform. Many formulations now also incorporate targeting ligands as a fourth component to further enhance specific delivery to the tumor site. In this article, we will provide a review on current advances in using polymeric nanoparticles for drug delivery, gene delivery, and photodynamic therapy, combined with imaging agents for magnetic resonance imaging, radionuclide imaging, and fluorescence imaging.
Figure 1.
Schematic illustration of a polymer-based nanotheranostic platform, consisting of three major components: a biocompatible polymeric nanocarrier component, a therapeutic component, and an imaging component. Some platforms also incorporate targeting ligands for specific delivery.
2. Combined Drug Delivery and Imaging
Prior to the development of nanomedicine, traditional drug delivery has faced many obstacles, primarily related to poor pharmacokinetics and undesirable biodistribution of drug. In particular, typical chemotherapy drugs suffer from low solubility, in vivo degradation, accelerated in vivo clearance rate, and inability to cross biological barriers.30,31 Polymer-based nanostructures provide an attractive solution to overcome these problems by enabling the ability to precisely control the location, dose, and time of delivering therapeutics.
A variety of polymeric nanoparticles—including polymer conjugate complexes,32,33 nanospheres,34−36 micelles,37−39 and dendrimers40−42—have been developed to aid in the delivery of drugs to cancerous sites and have shown great efficacy against various types of cancers. Conjugation of drug molecules to the polymer backbone allows for precise drug loading and control over release kinetics.43 Self-assembled nanospheres, micelles, and dendrimers loaded with therapeutic agents offer sustained and controlled release through surface or bulk erosion, drug diffusion through the polymer matrix, or environmental activation or stimulation.44 By combining an imaging agent along with the encapsulated drug within a polymeric nanoparticle, researchers have been able to achieve analysis of drug distribution and release at the target site in real time. The live evaluation of drug distribution provides assistance in predicting drug response and can better facilitate treatment regimens to be specifically tailored for each individual.
2.1. Drug Delivery and MRI
MRI is a commonly used radiology technique to analyze tissues, and it offers high spatial resolution without the danger of ionizing radiation. In MRI, a magnetic field is applied at an appropriate resonant frequency that excites hydrogen atoms in the tissues. The excited hydrogen atoms give off a radiofrequency signal as they return to their equilibrium state, which is detected and transformed into an image. Each tissue’s hydrogen atoms relax at different rates, which provide contrast between different tissues. A variety of paramagnetic and superparamagnetic metals are commonly used as contrast agents for MRI. Among these, superparamagnetic iron oxide (SPIO) and gadolinium (Gd) are most often utilized. SPIO and Gd interact with an external magnetic field to improve the visibility of internal structures by altering the relaxation times of atoms in tissues where they are present. Polymeric nanoparticles have been shown to be effective carriers of both SPIO and Gd.45−47
Superparamagnetic iron oxide is used as a T2 (spin–spin) contrast enhancement MRI agent.48 To create T2-weighted images, the magnetization is allowed to decay for different amounts before measuring the MR signal by changing the echo time. T2-weighted images are often used to study pathology as fluid appears bright against the darker normal tissue. Various polymer-based SPIO-containing drug delivery systems have been developed in the form of nanospheres, micelles, nanogels, and polymersomes (Table 2). These systems offer narrow particle size distribution, biocompatibility, good stability, prolonged blood circulation times, high drug loading, and control over drug release rate, in addition to superparamagnetic behavior for MRI contrast. For example, SPIO and chemotherapy drug doxorubicin (DOX) can both be directly encapsulated by using an amphiphilic block copolymer composed of maleimide–PEG–poly(lactic acid). This block copolymer self-assembles into nanoparticles with functionalizable maleimide groups on the surface, which allows for further conjugation of targeting peptides.49 In tumor-bearing mice, these targeted theranostic nanoparticles showed increased tumor-specific accumulation and enhanced inhibition of tumor growth. In another example, biodegradable poly(lactic-co-glycolic acid) (PLGA) was used to encapsulate docetaxel and SPIO by Ling et al. (Figure 2).50 Drug-release experiments showed sustained release with no initial burst effect, and PC3 prostate cancer cells treated with targeted docetaxel- and SPIO-loaded PLGA nanoparticles showed high intracellular iron concentration with strong contrast in T2-weighted MRI. Yang and colleagues demonstrated the ability to target breast cancer in vivo using a HER2-targeted PLGA–PEG block copolymer nanoparticle encapsulating MnFe2O4 and DOX.51 These targeted nanoparticles exhibited not only ultrasensitive detection by MRI, but also excellent tumor growth retardation both in vitro and in vivo. Other polymers such as Pluronic F-127 have also been used to create stable nanotheranostic formulations, indicating the applicability of encapsulating SPIO-drug mixtures in existing polymeric nanocarrier systems.52
Table 2. Polymer-Based Theranostic Systems Containing SPIO for Anticancer Drug Delivery.
| polymer | drug | reference |
|---|---|---|
| pluronic-based polymers | curcumin, DOX, paclitaxel | (134−136) |
| PEG–PMAA–poly(glycerol monomethacrylate) block copolymers | ADR | (137,138) |
| PEG–PGA–PEG–acrylate | DOX | (139) |
| PEG–PLA–PEG–acrylate | DOX | (140) |
| PVA | 5-FU, DOX, ceftriaxone | (141−143) |
| poly(NIPAAm) | DOX | (144) |
| PLA, PLGA | IFNα-2b | (145) |
| PEO-b-PLGA-b-PEO | DOX | (146) |
| PEG–PCL | DOX | (147) |
Figure 2.
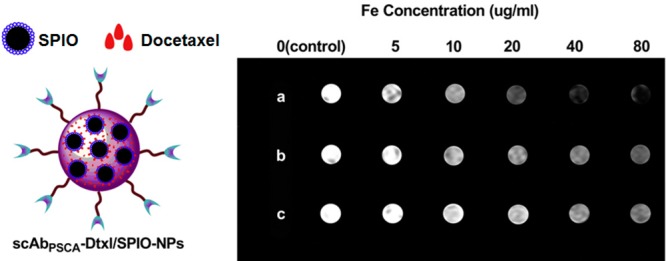
Targeted theranostic PLGA nanoparticles dual-loaded with SPIO and docetaxel for concurrent MR imaging and cancer therapy. (left) A schematic of the nanotheranostic formulation. (right) T2-weighted imaging of PC3 cells (1 × 106) after 2 h of incubation with (a) targeted SPIO/docetaxel-loaded nanoparticles, (b) nontargeted SPIO/docetaxel-loaded nanoparticles, and (c) Endorem (a commercially available MRI contrast agent) at Fe concentrations of 0, 5, 10, 20, 40, and 80 μg/mL; cells were then mixed with 2% agarose solution in PBS and scanned under a 1.5 T MRI scanner at room temperature. Reproduced with permission from reference (50).
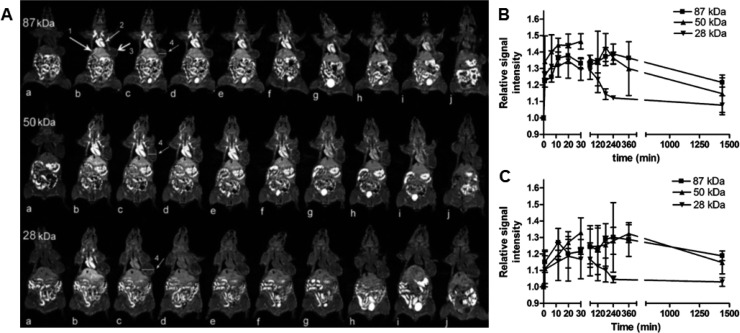
The most commonly used T1 (spin–lattice) MRI contrast agent, gadolinium(III), in imaging systems generates positive image contrast by increasing the longitudinal relaxation rate of the surrounding water protons. In T1-weighted images, fluid appears dark, water-based tissues appear midgray, and fat-based tissues appear bright. Typically, Gd(III)-diethylenetriaminepentaacetic acid (Gd-DTPA) or Gd-tetraazacyclododecanetetraacetic acid (Gd-DOTA) is used in the formulation of a polymeric nanocarrier for Gd-based MRI contrast.53 Ye et al. have conjugated Gd-DOTA to the side chains of poly(l-glutamic acid) (PGA) and demonstrated MRI signal enhancement in a breast tumor model in vivo (Figure 3).54 Ye and colleagues have also examined the effect of varying molecular weights of polymer, finding that conjugates with higher molecular weights (87 kDa) exhibited more prolonged blood circulation and increased tumor accumulation over lower molecular weight conjugates (28 kDa). In another study, Liao et al. synthesized a hybrid nanoparticle system consisting of a hydrophobic PLGA core and a hydrophilic Gd-DTPA folate-coated PEGylated liposome shell for MRI and targeted drug delivery.55 The nanoparticles showed high DOX loading efficiency and sustained release, while simultaneously offering high T1 relaxivities for high-resolution MRI via the paramagnetic Gd-DTPA chelated to the liposomal shell layer. Hong et al. have also taken a hybrid approach to nanotheranostics, combining a DOX-loaded liposomal core with an acid-sensitive cholesterol-terminated poly(acrylic acid) polymer shell functionalized with Herceptin and Gd(III).56 Their nanoparticle formulation revealed a 120-fold increase in cellular uptake of Gd in comparison with the clinically approved and commercially available Gd-DOTA, leading to significant T1 MRI contrast enhancement. Among other polymers utilized as theranostics vehicles for simultaneous MR imaging and drug delivery in cancer applications are N-(2-hydroxypropyl)methacrylamide (HPMA)-based copolymers, conjugated with Gd and therapeutic drugs,57 and multiarm star block copolymers.58
Figure 3.
(A) Coronal MR images of tumor bearing mice (a) before and at (b) 1, (c) 11, (d) 20, (e) 30, (f) 60, (g) 120, (h) 180, (i) 240 min, and (j) 24 h after injection with PGA–1,6-hexanediamine–(Gd-DO3A) conjugates of different molecular weights. Higher molecular weights demonstrated increased tumor accumulation. Arrows point to the (1) liver, (2) heart, and (3) tumor tissue. (B, C) Relative signal intensity in (B) tumor periphery and (C) tumor interstitium before and at various time points after the injection of the polymer conjugates. Reproduced with permission from reference (54).
2.2. Drug Delivery and Radionuclide Imaging
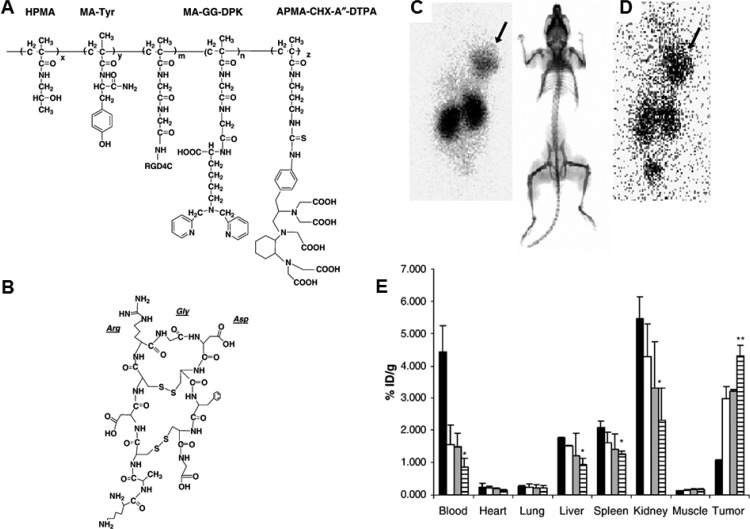
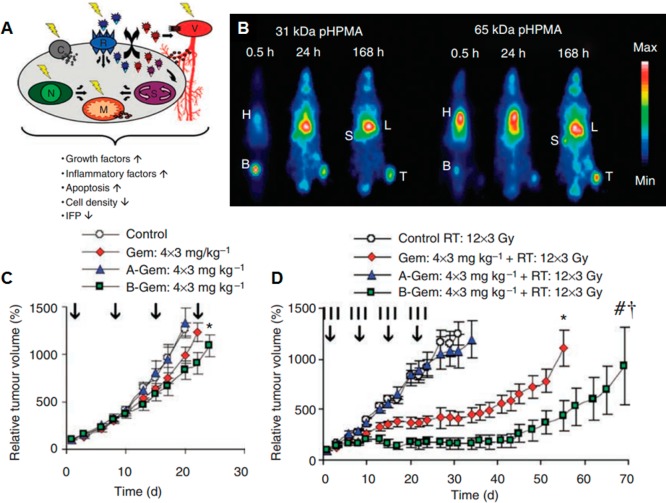
Radionuclide imaging is used in medicine to image the extent of disease development based on cellular metabolism and physiology within the body, rather than relying on physical changes in tissues like MRI. Similar to MRI, radionuclide imaging has high sensitivity with no tissue-penetration limitations. Radioisotopes such as 11C, 18F, 64Cu, 76Br, 99mTc, 111In, and 90Y are administered intravenously or orally. Gamma cameras are then used to capture and create images from the radiation emitted by the internalized radionuclides. Many such radionuclide compounds have been extensively explored along with a variety of copolymers with the goal of formulating a robust nanodelivery system.59−61 In one example, Mitra and colleagues have conjugated 99mTc and 90Y to HPMA, which was further conjugated to the αvβ3 targeting peptide RGD4C.62 These targeted polymer–radionuclide conjugates demonstrated enhanced cell adhesion to αvβ3 expressing endothelial cells as well as antitumor efficacy against a SCID mouse xenograft model of human prostate carcinoma (Figure 4). Lammers et al. have also taken advantage of HPMA to load 131I, along with antitumor agent doxorubicin or gemcitabine, to study the dual imaging and therapeutic capabilities of drug- and radionuclide-loaded polymeric nanocarriers.63 These polymeric drug carriers demonstrated prolonged circulation time and selective accumulation in the tumor site. The two components acted synergistically to increase the therapeutic efficacy against the tumor (Figure 5). As such, combining both chemotherapy and radiotherapy into a single nanocarrier can be an effective method to combat solid tumors.
Figure 4.
Structure of targeted HPMA–RGD4C conjugate and its biodistribution. (A) Chemical structure of HPMA conjugate. The side chains of MA-GG-DPK and APMA-CHX-A″-DTPA may be used to label the conjugate with 99mTc and 90Y, respectively. MA-Tyr may be used to couple iodine isotopes for biodistribution or radiotherapy (123I, 131I). (B) Structure of RGD4C peptide. The peptide has a doubly cyclized structure via two disulfide bridges and is conjugated to the polymer backbone via the ε-amino group of the terminal lysine residue. (C, D) Scintigraphic images of 99mTc-labeled HPMA copolymer–RGD4C conjugate in SCID mice bearing DU145 human prostate tumor xenografts (C) 24 and (D) 48 h post intravenous injection showed marked tumor accumulation. (E) Residual radioactivity in % injected dose per gram of organ tissue 1, 24, 48, and 72 h after administration of the 99mTc-labeled HPMA copolymer–RGD4C conjugate. Reproduced with permission from reference (62).
Figure 5.
(A) Potential physiological mechanisms by which radiotherapy increases tumor accumulation of drug targeting systems. Radiotherapy can affect the integrity and function of the tumor vasculature (V), the expression of certain cell receptors (R), several cell membrane-related (C), nuclear (N), mitochondrial (M), and signaling (S) processes. (B) Scintigraphic analysis of the biodistribution of two differently sized iodine-131-labeled HPMA copolymers in Copenhagen rats bearing subcutaneously transplanted Dunning AT1 tumors, demonstrating prolonged circulation and effective tumor accumulation (H: heart (blood), B: bladder, S: spleen, L: liver, T: tumor). (C) Growth inhibition of Dunning AT1 tumors induced by four intravenous injections (days 1, 8, 15, and 22; vertical arrows) of saline, free gemcitabine and HPMA copolymer-bound gemcitabine. A-Gem: pHPMA-AH-Gem (20 kDa); B-Gem: pHPMA-GFLG-Gem (24 kDa). (D) Tumor growth inhibition induced by four intravenous injections of the above-mentioned chemotherapeutic agents in combination with a clinically relevant regimen of fractionated radiotherapy (12 × 3 Gy; vertical lines). Reproduced with permission from reference (63).
2.3. Drug Delivery and Fluorescence Imaging
Fluorescence spectroscopy has been a powerful tool utilized throughout the years in many different fields, such as biochemistry, molecular biology, engineering, and nanomedicine. Many biomolecules in nature, including amino acids, proteins, and lipids, possess the inherent ability to fluoresce when excited with UV–vis light.64 The photons emitted from naturally fluorescing biomolecules or from externally administered fluorescent probes can be harnessed for imaging. Fluorescence imaging offers a low-cost technique with good spatial resolution in the UV–near-infrared (NIR) wavelength range and is on par with the sensitivity of radioisotopes used in PET and SPECT.65,66 Its weaknesses include limited tissue penetration, potentially high noise and background from tissue scattering of photons in the visible region, tissue autofluorescence, light absorption by proteins, and interference from water molecules.67 However, the use of NIR light for in vivo imaging overcomes some of these challenges, offering tissue penetration up to several centimeters and reduced autofluorescence and tissue scattering.68,69
NIR fluorescent probes have been incorporated into hyperbranched polyhydroxyl polymeric nanoparticles along with apoptosis-initiating protein cytochrome c by Santra et al.70 These nanoparticles were targeted using folic acid ligand, and they demonstrated enhanced uptake and therapeutic effect against various human carcinoma cells in vitro while also emitting photons for imaging via excitation of encapsulated indocyanine green (ICG). Also using a indocyanine-based dye along with Nile red, Quadir and co-workers have prepared dendritic core–multishell nanoparticle composed of hyperbranched polyethylenimine (PEI) conjugated to monomethyl PEG to encapsulate and transport three different antitumor drugs (DOX, methotrexate, and sodium ibandronate) (Figure 6).71 When injected into F9 teratocarcinoma bearing mice, the core–multishell nanoparticles demonstrated a strong contrast within the tumor tissues compared to free dye 6 h after administration. Hu et al. have successfully synthesized a multifunctional micelle with fluorescent imaging and drug delivery capabilities.72 Multifunctional micelles were prepared via the coassembly of DOX-conjugated monomethoxyl PEG-block-poly(l-lactide-co-mercaptoethanol) copolymer, rhodamine B-conjugated mPEG-b-p(LA-co-ME), and folic acid-conjugated PEG-b-PLA copolymer. In vivo fluorescence imaging experiments in mice with hepatocarcinomas demonstrated that the folic acid-conjugated micelles accumulated for longer periods in tumor tissues and exhibited enhanced antitumor efficacy compared with either free DOX or a nontargeted micelle formulation. Many researchers also take advantage of the inherent fluorescence of doxorubicin for therapeutic studies and have combined multiple imaging modalities together in conjunction with drug therapy.73,74
Figure 6.
(A) Dendritic core–multishell architecture with PEI core, inner hydrophobic segment, and terminal mPEG chain. Dynamics between the nanoparticle unimers and their aggregates are responsible for encapsulation of therapeutic drugs. (B) Strong contrast was observed 6 h after administration of ITCC dye-loaded nanoparticles to F9 teratocarcinoma-bearing mice. (C) Much less contrast was observed using free dye. Reproduced with permission from reference (71).
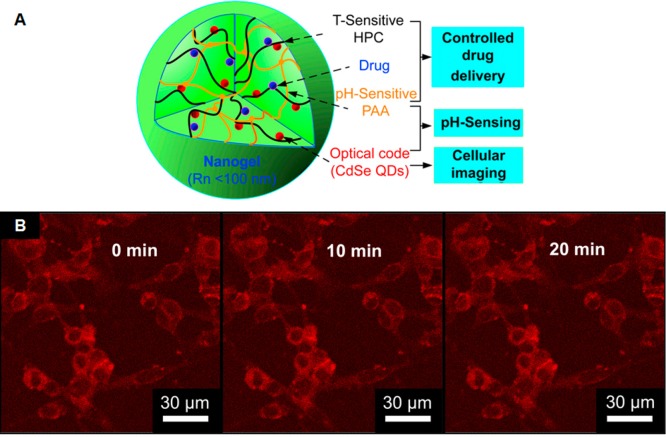
QDs have also been used as a potential fluorescent agent for theranostic applications.75,76 QDs are luminescent semiconductor nanocrystals that are typically composed of periodic groups II–VI (i.e., CdSe and CdTe) or III–V (i.e., InP and InAs) semiconductor materials. QDs have a narrow, symmetric, and size-tunable emission spectra and broad excitation spectra, making them particularly valuable for multicolor fluorescent applications. The benefits of using QDs are that they typically exhibit much stronger fluorescence as well as higher fluorescence stability against photobleaching compared with organic fluorophores or fluorescent proteins.77,78 This robust stability allows QDs to be used for prolonged fluorescence monitoring in living organisms. Song et al. have reported the fabrication of HIF-1α (hypoxia inducible factor-1α) antibody-conjugated pluronic triblock copolymer micelles loaded with paclitaxel and CdTe QDs.79 This targeted micelle formulation selectively killed HIF-1α overexpressing stomach cancer cells, with internalization of the nanoparticles easily visualized via fluorescence microscopy. CdTe QDs have also been incorporated, along with anticancer drug temozolomide, into pH and temperature dual-responsive polysaccharide-based nanogels composed of hydroxypropylcellulose-poly(acrylic acid).80 The pH- and temperature-triggered nanogels laden with temozolomide and QDs were effective against B16–F10 mouse melanoma in vivo, demonstrating sustained drug release at different pH values and prolonged intense stable photoluminescence (Figure 7). Other polymer-based theranostic systems that utilize fluorescent agents are summarized in Table 3.
Figure 7.
Schematic and fluorescence microscopy images of QD-loaded nanogels. (A) Design of hydroxypropylcellulose-poly(acrylic acid) hybrid nanogels loaded with CdSe QDs for pH and temperature dual-responsive multifunctional applications in biomedicine. (B) Scanning confocal fluorescence images of mouse melanoma B16–F10 cells after incubation with QD-loaded nanogel. Reproduced with permission from reference (80).
Table 3. Polymer-Based Theranostic Systems Containing Fluorescent Probes for Anticancer Drug Delivery.
| polymer | therapeutic component | fluorescent component | reference |
|---|---|---|---|
| poly(ethylene oxide)-b-poly(allyl glycidyl ether) | DOX | Dyomics 615 | (148) |
| chitosan | DTX | fluorescein | (149) |
| poly(ethylene glycol)-b-poly(l-lysine)-b-poly(l-phenylalanine) | DTX | Cy5.5 | (150) |
| amphiphilic star hyperbranched block copolymers | DOX | cascade blue | (151) |
| PVA | Temozolomide | Bi2O3 quantum dots | (152) |
| PEG-pentacosydonic acid | Herceptin | CdTe/CdSe QDs | (153) |
| chitosan | Camptothecin, DOX | CdTe, ZnO QDs | (154), (155) |
2.4. Drug Delivery and Ultrasound and Photoacoustic Imaging
Ultrasound imaging and acoustic microscopy are commonly used diagnostic imaging techniques in the clinic. Ultrasound imaging makes use of sound waves at frequencies of 2 MHz or higher, with shorter wavelength allowing for the resolution of small internal details in tissues and organs. Acoustic imaging utilizes high and ultrahigh ultrasound waves with frequencies up to 4 GHz. The two modalities are used to visualize muscles, tendons, and many internal organs to investigate their size, structure, and any pathological lesions in real time. Ultrasound offers high resolution and ease of operation with relatively low costs. As a drug delivery modality, ultrasound can be targeted in precise energy deposition patterns and can be performed noninvasively or minimally invasively.
Microbubbles and nanobubbles are the most commonly used contrast agents in ultrasound for imaging inflammation, angiogenesis, intravascular thrombi, and tumors. Gao et al. have used PEG–poly(l-lactic acid) and PEG–polycaprolactone block copolymers to form micelles that encapsulated doxorubicin. Perfluoropentane (PFP) was added, and the solution was sonicated, resulting in a mixture of doxorubicin-loaded micelles and doxorubicin-loaded, PFP-encapsulating nanobubbles.81 This mixture was shown to accumulate in an in vivo model of breast cancer. Upon accumulation, the mixture formed microbubbles, which cavitated and collapsed upon tumor-directed ultrasound. This led to localized drug release and tumor regression while simultaneously enabling molecular imaging of the nanobubbles.
Photoacoustic imaging technology has recently emerged as a novel method that allows for the visualization of molecular imaging probes with high performance. Upon absorption of light pulses of ultrashort duration, photoacoustic contrast agents in the tissue allow the absorbed energy to undergo thermoelastic expansion that emits mechanical waves at ultrasonic frequencies. These mechanical waves can then be detected by ultrasonic transducers to form images. Pu and colleagues have recently reported the use of NIR light-absorbing semiconducting polymer nanoparticles as a new class of contrast agents for photoacoustic molecular imaging.82 The nanoparticles produced a stronger signal than the commonly used gold nanorods and single-walled carbon nanotubes, providing real-time in vivo imaging of reactive oxygen species. Combined with a therapeutic agent, this platform would be a powerful theranostic tool.
3. Combined Gene Therapy and Imaging
Gene therapy is the transfer of specific genetic material to target cells in a patient for the ultimate purpose of preventing or altering a particular disease state. Studied extensively in the past few decades, gene therapy has the potential to treat a variety of acquired and inherited genetic disorders such as diabetes, blindness, Parkinson’s disease, and cancer.83 The goal of gene therapy is to replace, repair, regulate, or silence a defective gene through the administration of defined genetic material (i.e., DNA or siRNA). Currently, however, there are a number of technical challenges underlying gene therapy. First, gene therapy as of now is short-lived. Genetic material introduced into the target cell can be enzymatically degraded, preventing long-term benefits and necessitating multiple administrations of gene therapy. Second, the existing delivery mechanisms (typically viral vectors) oftentimes induce an immune response in patients, making it difficult for gene therapy to be repeated in patients. Third, there is a possibility of inducing a tumor if administered DNA is integrated into the wrong location in the genome, though the recent development of clustered regularly interspaced short palindromic repeats (CRISPR) technology significantly decreases this possibility.84,85 By inserting a plasmid containing cas genes and precisely designed CRISPRs (which are evolutionarily conserved), an organism’s genome can be edited via addition or deletion of DNA base pairs at any desired location. Fourth, the longer the therapeutic DNA strand is, the more difficult it is to efficiently incorporate it into cell genomes. Lastly, the current cost of gene therapy is extremely high. To address these concerns, researchers have begun to use polymer-based systems for the delivery of genetic material because polymers are more cost-effective, safer, and relatively easy to tailor.86 Generally, there are two types of polymer systems currently in use for gene delivery. In the first, the polymer carries the genetic material (i.e., is loaded within the nanoparticle). In the second, a cationic polymer is complexed with the genetic material to form a polyplex. Ultimately, the genetic material must be able to cross the cell membrane barrier and be transported into the nucleus to have a therapeutic effect.
3.1. Gene Delivery and MRI
Much like in drug delivery, paramagnetic and superparamagnetic metals have also been used as MRI contrast agents in combination with gene therapy. For example, Wu et al. have prepared a formulation in which DNA bound to a cationic methacrylamide-based polymer that was chelated with Eu3+.87 Without DNA bound, the Eu3+-polymer compound provided strong contrast in MRI; however, this contrast was diminished upon binding of DNA to the complex. This difference in contrast provided gene delivery information in real time. Bryson et al. utilized another polycation containing either three or four repeating ethyleneamines to package DNA into nanoparticle carriers, thereby protecting DNA from nuclease damage.88 Gd3+ was incorporated into the nanoparticle as well (Figure 8). These polymeric delivery vehicles were found to be taken up in vitro by human cervix adenocarcinoma (HeLa) cells, and provided contrast at the nanometers/micrometers scale via microscopy and submillimeters scale for MRI.
Figure 8.
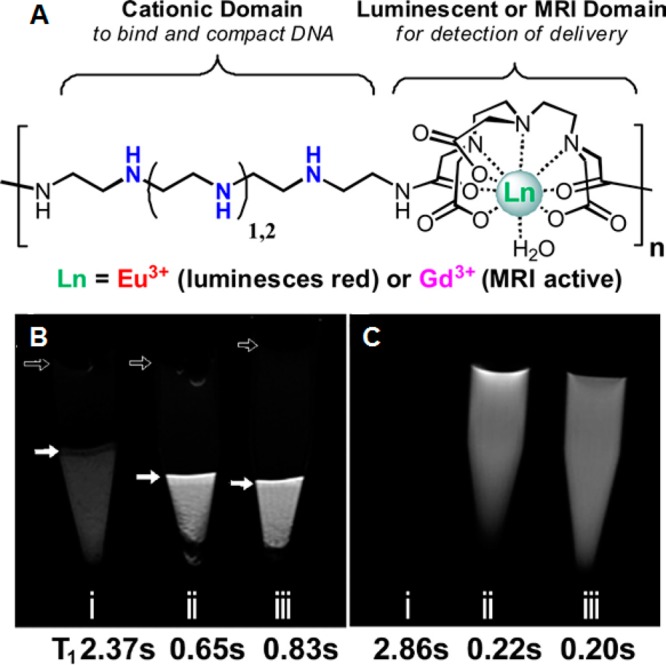
(A) Schematic representation of oligoethyleneamine polymeric beacons conjugated with MRI contrast agents. (B) HeLa cell pellets transfected with Gd3 polyplexes: (i) pellet of untreated HeLa cells; (ii) cells transfected with Gd3a polyplexes; and (iii) cells transfected with Gd3b polyplexes. Solid arrows indicate the buffer-cell interface in each sample. Open arrows indicate perturbations due to bubbles at the buffer-air interface. The darker spots in the cell pellet are due to cell density gradients. (C) MR images of controls without cells: (i) PBS buffer only, (ii) Gd3a only, and (iii) Gd3b only. Gd3a and Gd3b contain three and four ethyleneamines, respectively. Reproduced with permission from reference (88).
More commonly used for MRI contrast in theranostics for gene delivery are superparamagnetic systems. Superparamagnetic iron oxide has been used to track PEI-based,89−92 poly((2-dimethylamino)ethyl acrylate)-based,93 or poly(propyleneimine) dendrimer-based94,95 polymeric nanocarriers complexed with DNA or siRNA. Many of these platforms have shown efficacy both in vitro and in vivo in delivering genetic components to cells and altering gene expression, demonstrating the potential for eventual clinical translation. Combining MRI contrast agents with gene delivery, researchers can better understand the delivery and trafficking mechanisms of gene delivery polyplexes.
3.2. Gene Delivery and Radionuclide Imaging
Radionuclide imaging and gene delivery have not been typically studied together as a theranostic platform. However, Grunwald et al. have demonstrated transfection efficiency using a poly(amidoamine) dendrimer-coated adenovirus to deliver the theranostic sodium iodide symporter (NIS) gene.96 The dendrimer-coated adenovirus demonstrated an enhanced oncolytic effect following systemic administration. In addition, when expressed by the target cells, NIS causes the accumulation of iodine, thereby providing a means for imaging using both two-dimensional 123I scintigraphy and three-dimensional high-resolution 124I PET imaging. In this example, the gene itself acts as the theranostic agent, providing both oncolytic potential and contrast for radionuclide imaging. Gene transfection efficiency can be easily tracked and quantified using this method.
3.3. Gene Delivery and Fluorescence Imaging
The most common approach for image-guided gene delivery is to use fluorescence to track the delivery of genetic material. There are four main categories of fluorescent polymer-based gene delivery systems: (i) DNA complexed with fluorescently labeled polymer, (ii) fluorescent nanoparticle coated with polymer, to which DNA is then complexed, (iii) polymer complexed with fluorescent DNA, and (iv) fluorescently labeled polymer complexed with fluorescently labeled DNA.
In the first category, the polymer itself can be photoluminescent or it can be stained with a fluorescent dye.97−99 Typically a cationic polymer such as PEI is used to take advantage of electrostatic attraction between the polymer and DNA. In one example, however, Pangburn et al. have developed a noncationic polymersome composed of poly(1,2-butadiene)-b-poly(ethylene glycol) diblock copolymers with carboxyfluorescein incorporated.100 This system was used to deliver siRNA for the knockdown of the Orai3 gene and treatment of breast cancer. By integrating the fluorescent compound into the system, Pangburn and colleagues were able to observe that the polymersome formulation primarily released their cargo in the early endosomal intracellular compartment, and their data suggests that siRNA may be released into the cytosol. This offers a promising start for targeted theranostic delivery of siRNA.
In the second approach, a fluorescent particle is coated with polymer that is associated with DNA or siRNA. QDs are often used for this, and QD-based polymeric delivery is the most common approach for fluorescent polyplex gene therapy. As many other polymer-based gene delivery approaches, cationic polymers are usually used. QDs are coated with amino-containing polymers (i.e., PEI),101,102 or with a noncationic polymer that has been modified to be positively charged via the attachment of another cationic polymer103 or tertiary amine groups.104,105 These new types of siRNA carriers have superior transfection efficiency compared with the traditionally used Lipofectamine, while presenting reduced toxicity. The intrinsic fluorescence of QDs also provides a mechanism for real-time imaging of siRNA delivery both in vitro and in vivo.
The third method involves labeling of the therapeutic siRNA or DNA component with a fluorescent probe. The siRNA or DNA is typically labeled with a Cy5 dye. Several different polymeric carriers have been investigated in this potential platform, including PAMAM dendrimers,106,107 PEI,108,109 poly(glycoamidoamine),110 and chitosan.111 By associating fluorescent dye directly with the genetic material cargo, researchers are able to track exactly where DNA or siRNA is localized intracellularly in real time.
In the last category, both the carrier and the therapeutic cargo are labeled with fluorescent agents.112−116 The two components are typically labeled with probes that fluoresce at different wavelengths. With this method, it is possible to track the fates of each component of the theranostic delivery platform individually upon cellular uptake, thus gaining a better understanding of gene delivery mechanisms and cellular uptake of genetic material-loaded nanocarriers.
4. Combined Photodynamic Therapy and Imaging
Photodynamic therapy (PDT) achieves its therapeutic effect via a different mechanism from either drug or gene delivery. Rather than delivering an anticancer drug or protein or altering gene expression, nanotheranostic particles used for PDT directly destroy their targets. PDT is a minimally invasive technique that kills target cancer cells in the presence of oxygen via the release of reactive oxygen species upon light activation of a photosensitizer. This destroys cancer cells through direct cellular damage, vascular shutdown, and induction of the host immune response against the target cells.117 The importance of targeted delivery of the photosensitizer cannot be understated, as the photosensitizer will be activated when exposed to light regardless of its location, leading to potential off-target toxicity. As a result, it is of the utmost importance to be able to monitor PDT agents in real time to reduce the release of reactive oxygen species in healthy tissue areas.
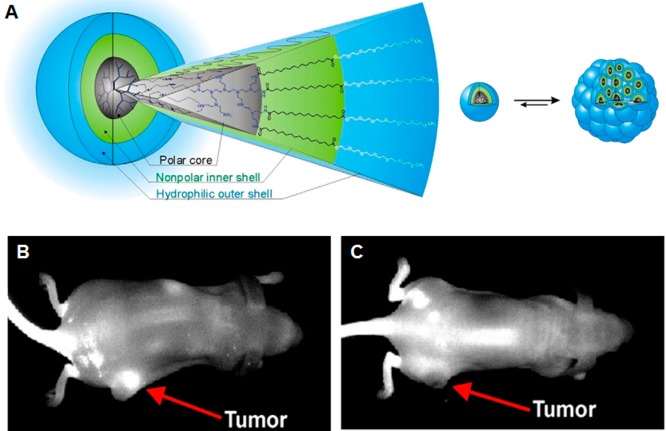
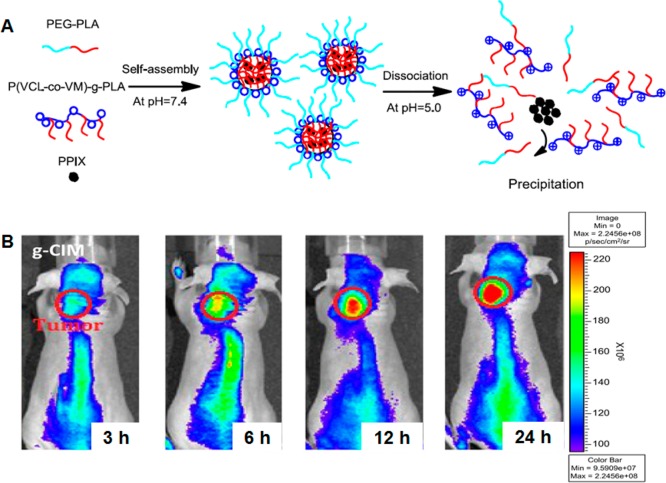
Fortunately, in most cases the photosensitizer used for PDT is also fluorescent, enabling researchers to track the location of the polymeric conjugate. For example, Peng and colleagues have developed a multifunctional polymeric nanoparticle composed of PEG-polycaprolactone diblock copolymer loaded with IR-780, which functions as both a NIR fluorescent dye and a photosensitizer.118 In BALB/c athymic nude mice bearing HCT-116 colorectal carcinoma, these micellar nanoparticles demonstrated enhanced tumor accumulation and tumor growth inhibition. Chlorin e6 (Ce6) has also been commonly used as a photosensitizer in theranostic applications.119−121 Lee et al. have used glycol chitosan nanoparticles to load Ce6. Compared with physical encapsulation, chemical conjugation of Ce6 to amphiphilic glycol chitosan-5β-cholanic acid resulted in more sustained release of Ce6, longer circulation half-life, and increased tumor accumulation.122 NIR imaging of mice bearing HT-29 human colon adenocarcinoma clearly showed strong tumor localization. The subsequent activation of the photosensitizer caused severe tumor necrosis and decreased tumor mass. Porphyrins and their derivatives are another class of photosensitizers that are commonly used.123−125 Tsai and co-workers have utilized poly(N-vinyl caprolactam)-g-PLA, a pH-sensitive copolymer with endosomolytic ability, and poly(N-vinyl caprolactam-co-N-vinyl imidazole)-g-PLA, a non-pH-responsive copolymer, to encapsulate protoporphyrin IX (PPIX) for in vitro and in vivo PDT studies (Figure 9).126 They found that PPIX accumulated in the nucleus of cancer cells when delivered by pH-sensitive particles but was largely trapped in lysosomes when delivered by non-pH-sensitive particles. In an in vivo model with mice bearing A549 xenografts, the pH-responsive particles demonstrated better tumor growth inhibition as well, indicating that the interaction between the photosensitizer and tumor cell also affects the efficacy of PDT.
Figure 9.
(A) Self-assembly of protoporphyrin IX with poly(N-vinyl caprolactam)-g-PLA graft copolymer. The polymers self-assemble into micelles at pH 7.4 and encapsulate PPIX, and they disassociate at pH 5.0 to release PPIX. (B) In vivo noninvasive fluorescence imaging of A549 tumor xenografted nude mice treated with PPIX-loaded pH-sensitive micelles. The micelles demonstrated high tumor accumulation. Reproduced with permission from reference (126).
5. Conclusions
With the ability to provide concurrent therapy and imaging, nanotheranostics have great potential and applicability in medicine and biomedical research. Polymers offer many benefits in their use, including biocompatibility, tailorability, and low cost. They can be used to encapsulate normally insoluble compounds, and they protect their cargo from degradation until they have reached their target location. Of course, there could be concerns about nanoparticle toxicity, as still not much is known about how nanoscale entities behave in human systems. The size and surface properties of nanoparticles, for example, can affect biodistribution and circulation via mechanisms such as nonspecific protein adsorption, macrophage interaction, and disturbance of biological barriers.127 As an example of the latter phenomenon, highly positively or negatively charged nanoparticles altered the integrity of the blood-brain barrier in rats, while neutral or slightly negatively charged nanoparticles did not.128 Polymeric nanoparticle formulations are also often relatively polydisperse, making it difficult to thoroughly characterize them to meet regulatory requirements. Additionally, it is possible for the long circulation of polymeric nanocarriers to induce toxicity or hypersensitivity reactions.129 As a result, careful toxicological testing and analysis is necessary for each new nanostructure. However, researchers are currently engineering increasingly sophisticated architectures with multiple therapeutic and imaging modalities to overcome these limitations.130
While theranostic nanoparticles have yet to be utilized in a clinical setting, the considerable advances made in cancer nanotheranostics will likely have far-reaching applications in other important fields such as cardiology131,132 and tissue engineering.133 Multifunctional polymeric nanoparticles can enable targeted cancer therapy and imaging and can also facilitate monitoring of the therapeutic effect. However, further in vivo work will be required to thoroughly investigate the safety and efficacy of these novel theranostic platforms prior to clinical application. With extensive multidisciplinary cooperation among nanoengineers, bioengineers, materials scientists, biologists, and clinicians, new theranostic nanostructures possess the potential to be translated into the clinic. This new and exciting field has a bright future ahead, offering the unique ability for more personalized medical treatment via simultaneous treatment and monitoring of a plethora of disorders and diseases.
Acknowledgments
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award No. R01DK095168. B.L. is supported by a National Institutes of Health R25CA153915 training grant from the National Cancer Institute.
Glossary
Abbreviations
- ADR
Adriamycin
- Ce6
chlorin e6
- CRISPR
clustered regularly interspaced short palindromic repeats
- DNA
deoxyribonucleic acid
- DOX
doxorubicin
- DTX
docetaxel
- Gd
gadolinium
- Gd-DOTA
gadolinium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- Gd-DTPA
diethylenetriaminepentacetate
- HPMA
N-(2-hydroxypropyl)methacrylamide
- ICG
indocyanine green
- mPEG-b-p(LA-co-ME)
monomethyl-polyethylene glycol-block-poly(lactic acid-co-mercaptoethanol)
- MRI
magnetic resonance imaging
- NIPAAm
N-isopropylacrylamide
- NIR
near-infrared
- NP
nanoparticle
- PAMAM
poly(amidoamine)
- PCL
polycaprolactone
- PDEAEMA
poly(2-(diethylamino)ethyl methacrylate)
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PEO
poly(ethylene oxide)
- PET
positron emission tomography
- PGA
poly(L-glutamic acid)
- PGMA
poly(glycidyl methacrylate)
- PLA
polylactic acid
- PLGA
poly(lactic-co-glycolic acid)
- PMAA
poly(methacrylic acid)
- PVA
poly(vinyl alcohol)
- QD
quantum dot
- siRNA
small interfering ribonucleic acid
- SPECT
single-photon emission computed tomography
- SPIO
super paramagnetic iron oxide
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Funkhouser J. Reinventing Pharma: The Theranostic Revolution. Curr. Drug Discovery 2002, 2, 17–19. [Google Scholar]
- Nie S. M.; Xing Y.; Kim G. J.; Simons J. W. Nanotechnology Applications in Cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288. [DOI] [PubMed] [Google Scholar]
- Marte B. Tumour Heterogeneity. Nature 2013, 501, 327–327. [DOI] [PubMed] [Google Scholar]
- Meacham C. E.; Morrison S. J. Tumour Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.; Lee S.; Chen X. Y. Nanoparticle-Based Theranostic Agents. Adv. Drug Delivery Rev. 2010, 62, 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers T.; Kiessling F.; Hennink W. E.; Storm G. Nanotheranostics and Image-Guided Drug Delivery: Current Concepts and Future Directions. Mol. Pharmaceutics 2010, 7, 1899–1912. [DOI] [PubMed] [Google Scholar]
- Gibson J. D.; Khanal B. P.; Zubarev E. R. Paclitaxel-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [DOI] [PubMed] [Google Scholar]
- Xiao Y. L.; Hong H.; Matson V. Z.; Javadi A.; Xu W.; Yang Y. A.; Zhang Y.; Engle J. W.; Nickles R. J.; Cai W. B.; Steeber D. A.; Gong S. Q. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and Pet Imaging. Theranostics 2012, 2, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler N.; Sun C.; Fichtenholtz A.; Gunn J.; Fang C.; Zhang M. Q. Methotrexate-Immobilized Poly(Ethylene Glycol) Magnetic Nanoparticles for MR Imaging and Drug Delivery. Small 2006, 2, 785–792. [DOI] [PubMed] [Google Scholar]
- Yu M. K.; Park J.; Jon S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagalkot V.; Zhang L.; Levy-Nissenbaum E.; Jon S.; Kantoff P. W.; Langer R.; Farokhzad O. C. Quantum Dot-Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007, 7, 3065–3070. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kulkarni A.; Nagesha D. K.; Sridhar S. In Vitro Evaluation of Theranostic Polymeric Micelles for Imaging and Drug Delivery in Cancer. Theranostics 2012, 2, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K. T.; Wang Y. C.; Roy I.; Rui H.; Swihart M. T.; Law W. C.; Kwak S. K.; Ye L.; Liu J. W.; Mahajan S. D.; Reynolds J. L. Preparation of Quantum Dot/Drug Nanoparticle Formulations for Traceable Targeted Delivery and Therapy. Theranostics 2012, 2, 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Liang X. J. Nano-Carbons as Theranostics. Theranostics 2012, 2, 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. T.; Luo J. B.; Zhou Q. H.; Wang H. F. Pharmacokinetics, Metabolism and Toxicity of Carbon Nanotubes for Biomedical Purposes. Theranostics 2012, 2, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AshaRani P. V.; Low Kah Mun G.; Hande M. P.; Valiyaveettil S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [DOI] [PubMed] [Google Scholar]
- Lison D.; Muller J. Lung and Systemic Responses to Carbon Nanotubes (CNT) in Mice. Toxicol. Sci. 2008, 101, 179–180author reply, pp 181–172. [DOI] [PubMed] [Google Scholar]
- Choi S. J.; Oh J. M.; Choy J. H. Toxicological Effects of Inorganic Nanoparticles on Human Lung Cancer A549 Cells. J. Inorg. Biochem. 2009, 103, 463–471. [DOI] [PubMed] [Google Scholar]
- Clawson C.; Ton L.; Aryal S.; Fu V.; Esener S.; Zhang L. Synthesis and Characterization of Lipid-Polymer Hybrid Nanoparticles with pH-Triggered Poly(Ethylene Glycol) Shedding. Langmuir 2011, 27, 10556–10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. M. J.; Kaushal S.; Cao H. S. T.; Aryal S.; Sartor M.; Esener S.; Bouvet M.; Zhang L. Half-Antibody Functionalized Lipid-Polymer Hybrid Nanoparticles for Targeted Drug Delivery to Carcinoembryonic Antigen Presenting Pancreatic Cancer Cells. Mol. Pharmaceutics 2010, 7, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. M.; Fang R. H.; Luk B. T.; Zhang L. Polymeric Nanotherapeutics: Clinical Development and Advances in Stealth Functionalization Strategies. Nanoscale 2014, 6, 65–75. [DOI] [PubMed] [Google Scholar]
- Petros R. A.; DeSimone J. M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discovery 2010, 9, 615–627. [DOI] [PubMed] [Google Scholar]
- Wang A. Z.; Langer R.; Farokhzad O. C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [DOI] [PubMed] [Google Scholar]
- Farokhzad O. C.; Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [DOI] [PubMed] [Google Scholar]
- Timko B. P.; Whitehead K.; Gao W. W.; Kohane D. S.; Farokhzad O.; Anderson D.; Langer R. Advances in Drug Delivery. Annu. Rev. Mater. Res. 2011, 41, 1–20. [Google Scholar]
- Fang R. H.; Hu C. M.; Chen K. N.; Luk B. T.; Carpenter C. W.; Gao W.; Li S.; Zhang D. E.; Lu W.; Zhang L. Lipid-Insertion Enables Targeting Functionalization of Erythrocyte Membrane-Cloaked Nanoparticles. Nanoscale 2013, 5, 8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasia-Christoforou T.; Georgiou T. K. Polymeric Theranostics: Using Polymer-Based Systems for Simultaneous Imaging and Therapy. J. Mater. Chem. B 2013, 1, 3002–3025. [DOI] [PubMed] [Google Scholar]
- Luk B. T.; Fang R. H.; Zhang L. Lipid- and Polymer-Based Nanostructures for Cancer Theranostics. Theranostics 2012, 2, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan P.; Rapoport N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharmaceutics 2010, 7, 1959–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juillerat-Jeanneret L. The Targeted Delivery of Cancer Drugs across the Blood-Brain Barrier: Chemical Modifications of Drugs or Drug-Nanoparticles?. Drug Discovery Today 2008, 13, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Lammers T.; Subr V.; Ulbrich K.; Hennink W. E.; Storm G.; Kiessling F. Polymeric Nanomedicines for Image-Guided Drug Delivery and Tumor-Targeted Combination Therapy. Nano Today 2010, 5, 197–212. [Google Scholar]
- Bogdanov A. A.; Mazzanti M.; Castillo G.; Bolotin E. Protected Graft Copolymer (PGC) in Imaging and Therapy: A Platform for the Delivery of Covalently and Non-Covalently Bound Drugs. Theranostics 2012, 2, 553–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. Drug Polymer Conjugates - Potential for Improved Chemotherapy. Anti-Cancer Drug 1992, 3, 175–210. [DOI] [PubMed] [Google Scholar]
- McCarthy J. R.; Perez J. M.; Bruckner C.; Weissleder R. Polymeric Nanoparticle Preparation That Eradicates Tumors. Nano Lett. 2005, 5, 2552–2556. [DOI] [PubMed] [Google Scholar]
- Hu C. M. J.; Zhang L.; Aryal S.; Cheung C.; Fang R. H.; Zhang L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S. D.; Sartor M.; Hu C. M.; Zhang W.; Zhang L.; Jin S. Magnetic Field Activated Lipid-Polymer Hybrid Nanoparticles for Stimuli-Responsive Drug Release. Acta Biomater. 2013, 9, 5447–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. W.; Kim S. Y.; Kim H. K.; Kim S. W.; Shin S. W.; Kim J. S.; Park K.; Lee M. Y.; Heo D. S. Multicenter Phase II Trial of Genexol-PM, a Novel Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, with Cisplatin in Patients with Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2007, 18, 2009–2014. [DOI] [PubMed] [Google Scholar]
- Trivedi R.; Kompella U. B. Nanomicellar Formulations for Sustained Drug Delivery: Strategies and Underlying Principles. Nanomedicine (London, U.K.) 2010, 5, 485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H. L.; Chen L. C.; Gu J. J.; Ren X. Q.; Wei Z.; Luo J. Q.; Chen Y. Z.; Jiang X. Y.; Sha X. Y.; Fang X. L. Enhanced Anti-Glioblastoma Efficacy by PTX-Loaded PEGylated Poly(ε-caprolactone) Nanoparticles: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2010, 402, 238–247. [DOI] [PubMed] [Google Scholar]
- Al-Jamal K. T.; Al-Jamal W. T.; Akerman S.; Podesta J. E.; Yilmazer A.; Turton J. A.; Bianco A.; Vargesson N.; Kanthou C.; Florence A. T.; Tozer G. M.; Kostarelos K. Systemic Antiangiogenic Activity of Cationic Poly-L-Lysine Dendrimer Delays Tumor Growth. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 3966–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros I. J.; Myc A.; Thomas T.; Mehta C. B.; Baker J. R. PAMAM Dendrimer-Based Multifunctional Conjugate for Cancer Therapy: Synthesis, Characterization, and Functionality. Biomacromolecules 2006, 7, 572–579. [DOI] [PubMed] [Google Scholar]
- Rupp R.; Rosenthal S. L.; Stanberry L. R. Vivagel (TM) (SPL7013 Gel): A Candidate Dendrimer Microbicide for the Prevention of HIV and HSV Infection. Int. J. Nanomed. 2007, 2, 561–566. [PMC free article] [PubMed] [Google Scholar]
- Aryal S.; Hu C. M. J.; Zhang L. Polymeric Nanoparticles with Precise Ratiometric Control over Drug Loading for Combination Therapy. Mol. Pharmaceutics 2011, 8, 1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D.; Karp J. M.; Hong S.; Farokhzad O. C.; Margalit R.; Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S.; Kayandan S.; Lin Y. N.; Kelly D. F.; House M. J.; Woodward R. C.; St Pierre T. G.; Riffle J. S.; Davis R. M. Toward Design of Magnetic Nanoparticle Clusters Stabilized by Biocompatible Diblock Copolymers for T-2-Weighted MRI Contrast. Langmuir 2014, 30, 1580–1587. [DOI] [PubMed] [Google Scholar]
- Mi P.; Kokuryo D.; Cabral H.; Kumagai M.; Nomoto T.; Aoki I.; Terada Y.; Kishimura A.; Nishiyama N.; Kataoka K. Hydrothermally Synthesized PEGylated Calcium Phosphate Nanoparticles Incorporating Gd-DTPA for Contrast Enhanced MRI Diagnosis of Solid Tumors. J. Controlled Release 2014, 174, 63–71. [DOI] [PubMed] [Google Scholar]
- Kim K. S.; Park W.; Hu J.; Bae Y. H.; Na K. A Cancer-Recognizable MRI Contrast Agents Using pH-Responsive Polymeric Micelle. Biomaterials 2014, 35, 337–343. [DOI] [PubMed] [Google Scholar]
- Wang Y. X. J.; Hussain S. M.; Krestin G. P. Superparamagnetic Iron Oxide Contrast Agents: Physicochemical Characteristics and Applications in MR Imaging. Eur. J. Radiol. 2001, 11, 2319–2331. [DOI] [PubMed] [Google Scholar]
- Guthi J. S.; Yang S. G.; Huang G.; Li S. Z.; Khemtong C.; Kessinger C. W.; Peyton M.; Minna J. D.; Brown K. C.; Gao J. M. MRI-Visible Micellar Nanomedicine for Targeted Drug Delivery to Lung Cancer Cells. Mol. Pharmaceutics 2010, 7, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y.; Wei K.; Luo Y.; Gao X.; Zhong S. Z. Dual Docetaxel/Superparamagnetic Iron Oxide Loaded Nanoparticles for Both Targeting Magnetic Resonance Imaging and Cancer Therapy. Biomaterials 2011, 32, 7139–7150. [DOI] [PubMed] [Google Scholar]
- Yang J.; Lee C. H.; Ko H. J.; Suh J. S.; Yoon H. G.; Lee K.; Huh Y. M.; Haam S. Multifunctional Magneto-Polymeric Nanohybrids for Targeted Detection and Synergistic Therapeutic Effects on Breast Cancer. Angew. Chem., Int. Ed. 2007, 46, 8836–8839. [DOI] [PubMed] [Google Scholar]
- Lai J. R.; Chang Y. W.; Yen H. C.; Yuan N. Y.; Liao M. Y.; Hsu C. Y.; Tsai J. L.; Lai P. S. Multifunctional Doxorubicin/Superparamagnetic Iron Oxide-Encapsulated Pluronic F127 Micelles Used for Chemotherapy/Magnetic Resonance Imaging. J. Appl. Phys. 2010, 107, 09B318. [Google Scholar]
- Liu Y. J.; Zhang N. Gadolinium Loaded Nanoparticles in Theranostic Magnetic Resonance Imaging. Biomaterials 2012, 33, 5363–5375. [DOI] [PubMed] [Google Scholar]
- Ye F. R.; Ke T. Y.; Jeong E. K.; Wang X. L.; Sung Y. G.; Johnson M.; Lu Z. R. Noninvasive Visualization of in Vivo Drug Delivery of Poly(L-glutamic acid) Using Contrast-Enhanced MRI. Mol. Pharmaceutics 2006, 3, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z. Y.; Wang H. J.; Wang X. D.; Zhao P. Q.; Wang S.; Su W. Y.; Chang J. Multifunctional Nanoparticles Composed of a Poly(DL-lactide-co-glycolide) Core and a Paramagnetic Liposome Shell for Simultaneous Magnetic Resonance Imaging and Targeted Therapeutics. Adv. Funct. Mater. 2011, 21, 1179–1186. [Google Scholar]
- Hong B. J.; Swindell E. P.; MacRenaris K. W.; Hankins P. L.; Chipre A. J.; Mastarone D. J.; Ahn R. W.; Meade T. J.; O’Halloran T. V.; Nguyen S. T. pH-Responsive Theranostic Polymer-Caged Nanobins: Enhanced Cytotoxicity and T-1 MRI Contrast by Her2 Targeting. Part. Part. Syst. Charact. 2013, 30, 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarabi B.; Nan A. J.; Zhuo J. C.; Gullapalli R.; Ghandehari H. HPMA Copolymer-Doxorubicin-Gadolinium Conjugates: Synthesis, Characterization, and in Vitro Evaluation. Macromol. Biosci. 2008, 8, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. J.; Qian Y. F.; Liu T.; Hu X. L.; Zhang G. Y.; You Y. Z.; Liu S. Y. Amphiphilic Multiarm Star Block Copolymer-Based Multifunctional Unimolecular Micelles for Cancer Targeted Drug Delivery and MR Imaging. Biomaterials 2011, 32, 6595–6605. [DOI] [PubMed] [Google Scholar]
- Mitra A.; Nan A.; Line B. R.; Ghandehari H. Nanocarriers for Nuclear Imaging and Radiotherapy of Cancer. Curr. Pharm. Des. 2006, 12, 4729–4749. [DOI] [PubMed] [Google Scholar]
- Cho Y. W.; Park S. A.; Han T. H.; Son D. H.; Park J. S.; Oh S. J.; Moon D. H.; Cho K. J.; Ahn C. H.; Byun Y.; Kim I. S.; Kwon I. C.; Kim S. Y. In Vivo Tumor Targeting and Radionuclide Imaging with Self-Assembled Nanoparticles: Mechanisms, Key Factors, and Their Implications. Biomaterials 2007, 28, 1236–1247. [DOI] [PubMed] [Google Scholar]
- Yamamoto F.; Yamahara R.; Makino A.; Kurihara K.; Tsukada H.; Hara E.; Hara I.; Kizaka-Kondoh S.; Ohkubo Y.; Ozeki E.; Kimura S. Radiosynthesis and Initial Evaluation of F-18 Labeled Nanocarrier Composed of Poly(L-lactic acid)-block-poly(sarcosine) Amphiphilic Polydepsipeptide. Nucl. Med. Biol. 2013, 40, 387–394. [DOI] [PubMed] [Google Scholar]
- Mitra A.; Nan A.; Papadimitriou J. C.; Ghandehari H.; Line B. R. Polymer-Peptide Conjugates for Angiogenesis Targeted Tumor Radiotherapy. Nucl. Med. Biol. 2006, 33, 43–52. [DOI] [PubMed] [Google Scholar]
- Lammers T.; Subr V.; Peschke P.; Kuhnlein P.; Hennink W. E.; Ulbrich K.; Kiessling F.; Heilmann M.; Debus J.; Huber P. E.; Storm G. Image-Guided and Passively Tumour-Targeted Polymeric Nanomedicines for Radiochemotherapy. Br. J. Cancer 2008, 99, 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu R.; Comelli D.; D’Andrea C.; Taroni P.; Valentini G. Time-Resolved Fluorescence Imaging in Biology and Medicine. J. Phys. D: Appl. Phys. 2002, 35, R61–R76. [Google Scholar]
- Basu S.; Alavi A. SPECT-CT and PET-CT in Oncology—An Overview. Curr. Med. Imaging Rev. 2011, 7, 202–209. [Google Scholar]
- Chopra A.; Shan L.; Eckelman W. C.; Leung K.; Menkens A. E. Important Parameters to Consider for the Characterization of PET and SPECT Imaging Probes. Nucl. Med. Biol. 2011, 38, 1079–1084. [DOI] [PubMed] [Google Scholar]
- Janib S. M.; Moses A. S.; MacKay J. A. Imaging and Drug Delivery Using Theranostic Nanoparticles. Adv. Drug Delivery Rev. 2010, 62, 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V. In Vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V.; Bremer C.; Weissleder R. Fluorescence Imaging with Near-Infrared Light: New Technological Advances That Enable In Vivo Molecular Imaging. Eur. J. Radiol. 2003, 13, 195–208. [DOI] [PubMed] [Google Scholar]
- Santra S.; Kaittanis C.; Perez J. M. Cytochrome C Encapsulating Theranostic Nanoparticles: A Novel Bifunctional System for Targeted Delivery of Therapeutic Membrane-Impermeable Proteins to Tumors and Imaging of Cancer Therapy. Mol. Pharmaceutics 2010, 7, 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir M. A.; Radowski M. R.; Kratz F.; Licha K.; Hauff P.; Haag R. Dendritic Multishell Architectures for Drug and Dye Transport. J. Controlled Release 2008, 132, 289–294. [DOI] [PubMed] [Google Scholar]
- Hu X. L.; Wang R.; Yue J.; Liu S.; Xie Z. G.; Jing X. B. Targeting and Anti-Tumor Effect of Folic Acid-Labeled Polymer-Doxorubicin Conjugates with pH-Sensitive Hydrazone Linker. J. Mater. Chem. 2012, 22, 13303–13310. [Google Scholar]
- Li D.; Zhang Y. T.; Yang P.; Yu M.; Guo J.; Lu J. Q.; Wang C. C. An Optical Sensing Strategy Leading to in Situ Monitoring of the Degradation of Mesoporous Magnetic Supraparticles in Cells. ACS Appl. Mater. Interfaces 2013, 5, 12329–12339. [DOI] [PubMed] [Google Scholar]
- Li D. A.; Zhang Y. T.; Yu M.; Guo J.; Chaudhary D.; Wang C. C. Cancer Therapy and Fluorescence Imaging Using the Active Release of Doxorubicin from MSPs/Ni-LDH Folate Targeting Nanoparticles. Biomaterials 2013, 34, 7913–7922. [DOI] [PubMed] [Google Scholar]
- Ho Y. P.; Leong K. W. Quantum Dot-Based Theranostics. Nanoscale 2010, 2, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F.; Barrefelt A.; Asem H.; Abedi-Valugerdi M.; El-Serafi I.; Saghafian M.; Abu-Salah K.; Alrokayan S.; Muhammed M.; Hassan M. Biodegradable Polymeric Vesicles Containing Magnetic Nanoparticles, Quantum Dots and Anticancer Drugs for Drug Delivery and Imaging. Biomaterials 2014, 35, 3885–3894. [DOI] [PubMed] [Google Scholar]
- Bruchez M.; Moronne M.; Gin P.; Weiss S.; Alivisatos A. P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [DOI] [PubMed] [Google Scholar]
- Resch-Genger U.; Grabolle M.; Cavaliere-Jaricot S.; Nitschke R.; Nann T. Quantum Dots Versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [DOI] [PubMed] [Google Scholar]
- Song H.; He R.; Wang K.; Ruan J.; Bao C. C.; Li N.; Ji J. J.; Cui D. X. Anti-HIF-1 Alpha Antibody-Conjugated Pluronic Triblock Copolymers Encapsulated with Paclitaxel for Tumor Targeting Therapy. Biomaterials 2010, 31, 2302–2312. [DOI] [PubMed] [Google Scholar]
- Wu W. T.; Aiello M.; Zhou T.; Berliner A.; Banerjee P.; Zhou S. Q. In-situ Immobilization of Quantum Dots in Polysaccharide-Based Nanogels for Integration of Optical pH-Sensing, Tumor Cell Imaging, and Drug Delivery. Biomaterials 2010, 31, 3023–3031. [DOI] [PubMed] [Google Scholar]
- Gao Z.; Kennedy A. M.; Christensen D. A.; Rapoport N. Y. Drug-Loaded Nano/Microbubbles for Combining Ultrasonography and Targeted Chemotherapy. Ultrasonics 2008, 48, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu K.; Shuhendler A. J.; Jokerst J. V.; Mei J.; Gambhir S. S.; Bao Z.; Rao J. Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotechnol. 2014, 9, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby C. L.; Leong K. W. Balancing Protection and Release of DNA: Tools to Address a Bottleneck of Non-Viral Gene Delivery. J. R. Soc., Interface 2010, 7, S67–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L.; Ran F. A.; Cox D.; Lin S. L.; Barretto R.; Habib N.; Hsu P. D.; Wu X. B.; Jiang W. Y.; Marraffini L. A.; Zhang F. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P.; Esvelt K. M.; Church G. M. Cas9 as a Versatile Tool for Engineering Biology. Nat. Methods 2013, 10, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdan T.; Kopecek J.; Kissel T. Prospects for Cationic Polymers in Gene and Oligonucleotide Therapy against Cancer. Adv. Drug Delivery Rev. 2002, 54, 715–758. [DOI] [PubMed] [Google Scholar]
- Wu Y. K.; Carney C. E.; Denton M.; Hart E.; Zhao P.; Streblow D. N.; Sherry A. D.; Woods M. Polymeric Paracest MRI Contrast Agents as Potential Reporters for Gene Therapy. Org. Biomol. Chem. 2010, 8, 5333–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson J. M.; Fichter K. M.; Chu W. J.; Lee J. H.; Li J.; Madsen L. A.; McLendon P. M.; Reineke T. M. Polymer Beacons for Luminescence and Magnetic Resonance Imaging of DNA Delivery. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit F. M.; Veiseh O.; Fang C.; Bhattarai N.; Lee D.; Ellenbogen R. G.; Zhang M. Q. Chlorotoxin Labeled Magnetic Nanovectors for Targeted Gene Delivery to Glioma. ACS Nano 2010, 4, 4587–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J.; Muthiah M.; Lee H. J.; Lee H. J.; Moon M. J.; Che H. L.; Heo S. U.; Lee H. C.; Jeong Y. Y.; Park I. K. Synthesis and Characterization of Magnetic Nanoparticle-Embedded Multi-Functional Polymeric Micelles for MRI-Guided Gene Delivery. Macromol. Res. 2012, 20, 188–196. [Google Scholar]
- Wan Q.; Xie L. S.; Gao L.; Wang Z. Y.; Nan X.; Lei H. L.; Long X. J.; Chen Z. Y.; He C. Y.; Liu G.; Liu X.; Qiu B. S. Self-Assembled Magnetic Theranostic Nanoparticles for Highly Sensitive MRI of Minicircle DNA Delivery. Nanoscale 2013, 5, 744–752. [DOI] [PubMed] [Google Scholar]
- Duan J.; Dong J.; Zhang T.; Su Z.; Ding J.; Zhang Y.; Mao X. Polyethyleneimine-Functionalized Iron Oxide Nanoparticles for Systemic siRNA Delivery in Experimental Arthritis. Nanomedicine (London, U.K.) 2014, 96789–801. [DOI] [PubMed] [Google Scholar]
- Boyer C.; Priyanto P.; Davis T. P.; Pissuwan D.; Bulmus V.; Kavallaris M.; Teoh W. Y.; Amal R.; Carroll M.; Woodward R.; St Pierre T. Anti-Fouling Magnetic Nanoparticles for siRNA Delivery. J. Mater. Chem. 2010, 20, 255–265. [Google Scholar]
- Taratula O.; Garbuzenko O.; Savla R.; Wang Y. A.; He H. X.; Minko T. Multifunctional Nanomedicine Platform for Cancer Specific Delivery of siRNA by Superparamagnetic Iron Oxide Nanoparticles-Dendrimer Complexes. Curr. Drug Delivery 2011, 8, 59–69. [DOI] [PubMed] [Google Scholar]
- Taratula O.; Garbuzenko O. B.; Chen A. M.; Minko T. Innovative Strategy for Treatment of Lung Cancer: Targeted Nanotechnology-Based Inhalation Co-Delivery of Anticancer Drugs and siRNA. J. Drug Targeting 2011, 19, 900–914. [DOI] [PubMed] [Google Scholar]
- Grunwald G. K.; Vetter A.; Klutz K.; Willhauck M. J.; Schwenk N.; Senekowitsch-Schmidtke R.; Schwaiger M.; Zach C.; Wagner E.; Goke B.; Holm P. S.; Ogris M.; Spitzweg C. EGFR-Targeted Adenovirus Dendrimer Coating for Improved Systemic Delivery of the Theranostic NIS Gene. Mol. Ther.—Nucleic Acids 2013, 2, e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Zhou L. Z.; Pang Y.; Huang W.; Qiu F.; Jiang X. L.; Zhu X. Y.; Yan D. Y.; Chen Q. Photoluminescent Hyperbranched Poly(Amido Amine) Containing β-Cyclodextrin as a Nonviral Gene Delivery Vector. Bioconjugate Chem. 2011, 22, 1162–1170. [DOI] [PubMed] [Google Scholar]
- Feng X. L.; Tang Y. L.; Duan X. R.; Liu L. B.; Wang S. Lipid-Modified Conjugated Polymer Nanoparticles for Cell Imaging and Transfection. J. Mater. Chem. 2010, 20, 1312–1316. [Google Scholar]
- Lin S. Y.; Lin F. S.; Chen M. K.; Tsai L. R.; Jao Y. C.; Lin H. Y.; Wang C. L.; Hwu Y. K.; Yang C. S. One-Pot Synthesis of Linear-Like and Photoluminescent Polyethylenimines for Intracellular Imaging and siRNA Delivery. Chem. Commun. 2010, 46, 5554–5556. [DOI] [PubMed] [Google Scholar]
- Pangburn T. O.; Georgiou K.; Bates F. S.; Kokkoli E. Targeted Polymersome Delivery of siRNA Induces Cell Death of Breast Cancer Cells Dependent Upon Orai3 Protein Expression. Langmuir 2012, 28, 12816–12830. [DOI] [PubMed] [Google Scholar]
- Tan W. B.; Jiang S.; Zhang Y. Quantum-Dot Based Nanoparticles for Targeted Silencing of HER2/neu Gene Via RNA Interference. Biomaterials 2007, 28, 1565–1571. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Liu W. G. ZnO QD@PMAA-co-PDMAEMA Nonviral Vector for Plasmid DNA Delivery and Bioimaging. Biomaterials 2010, 31, 3087–3094. [DOI] [PubMed] [Google Scholar]
- Lee P. W.; Hsu S. H.; Tsai J. S.; Chen F. R.; Huang P. J.; Ke C. J.; Liao Z. X.; Hsiao C. W.; Lin H. J.; Sung H. W. Multifunctional Core-Shell Polymeric Nanoparticles for Transdermal DNA Delivery and Epidermal Langerhans Cells Tracking. Biomaterials 2010, 31, 2425–2434. [DOI] [PubMed] [Google Scholar]
- Qi L. F.; Gao X. H. Quantum Dot-Amphipol Nanocomplex for Intracellular Delivery and Real-Time Imaging of siRNA. ACS Nano 2008, 2, 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezhelyev M. V.; Qi L. F.; O’Regan R. M.; Nie S.; Gao X. H. Proton-Sponge Coated Quantum Dots for siRNA Delivery and Intracellular Imaging. J. Am. Chem. Soc. 2008, 130, 9006–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y. B.; Luo T.; Peng C.; Sheng R. L.; Cao A. M.; Cao X. Y.; Shen M. W.; Guo R.; Tomas H.; Shi X. Y. Gene Delivery Using Dendrimer-Entrapped Gold Nanoparticles as Nonviral Vectors. Biomaterials 2012, 33, 3025–3035. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Li Y. B.; Wang B.; Lin R. Y.; van Dongen M.; Zurcher D. M.; Gu X. Y.; Holl M. M. B.; Liu G.; Qi R. Efficient In Vitro siRNA Delivery and Intramuscular Gene Silencing Using PEG-Modified PAMAM Dendrimers. Mol. Pharmaceutics 2012, 9, 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Z.; Zheng M.; Meng F. H.; Zhong Z. Y. Non-Viral Gene Transfection in Vitro Using Endosomal pH-Sensitive Reversibly Hydrophobilized Polyethylenimine. Biomaterials 2011, 32, 9109–9119. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Zhong Z. H.; Zhou L.; Meng F. H.; Peng R.; Zhong Z. Y. Poly(Ethylene Oxide) Grafted with Short Polyethylenimine Gives DNA Polyplexes with Superior Colloidal Stability, Low Cytotoxicity, and Potent in Vitro Gene Transfection under Serum Conditions. Biomacromolecules 2012, 13, 881–888. [DOI] [PubMed] [Google Scholar]
- Taori V. P.; Lu H.; Reineke T. M. Structure-Activity Examination of Poly(glycoamidoguanidine)s: Glycopolycations Containing Guanidine Units for Nucleic Acid Delivery. Biomacromolecules 2011, 12, 2055–2063. [DOI] [PubMed] [Google Scholar]
- Malhotra M.; Lane C.; Tomaro-Duchesneau C.; Saha S.; Prakash S. A Novel Method for Synthesizing PEGylated Chitosan Nanoparticles: Strategy, Preparation, and In Vitro Analysis. Int. J. Nanomed. 2011, 6, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuzard G.; Tertil M.; Goncalves C.; Cheradame H.; Geguan P.; Pichon C.; Midoux P.. Nuclear Delivery of N kappa B-Assisted DNA/Polymer Complexes: Plasmid DNA Quantitation by Confocal Laser Scanning Microscopy and Evidence of Nuclear Polyplexes by FRET Imaging. Nucleic Acids Res. 2008, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H.; Ho Y. P.; Jiang X.; Mao H. Q.; Wang T. H.; Leong K. W. Quantitative Comparison of Intracellular Unpacking Kinetics of Polyplexes by a Model Constructed from Quantum Dot-FRET. Mol. Ther. 2008, 16, 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin K. G.; Fella C.; Ogris M.; Wagner E.; Ruthardt N.; Brauchle C. Dynamics of Photoinduced Endosomal Release of Polyplexes. J. Controlled Release 2008, 130, 175–182. [DOI] [PubMed] [Google Scholar]
- Hashimoto M.; Morimoto M.; Saimoto H.; Shigemasa Y.; Sato T. Lactosylated Chitosan for DNA Delivery into Hepatocytes: The Effect of Lactosylation on the Physicochemical Properties and Intracellular Trafficking of pDNA/Chitosan Complexes. Bioconjugate Chem. 2006, 17, 309–316. [DOI] [PubMed] [Google Scholar]
- Ho Y. P.; Chen H. H.; Leong K. W.; Wang T. H. Evaluating the Intracellular Stability and Unpacking of DNA Nanocomplexes by Quantum Dots-FRET. J. Controlled Release 2006, 116, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald I. J.; Dougherty T. J. Basic Principles of Photodynamic Therapy. J. Porphyrins Phthalocyanines 2001, 5, 105–129. [Google Scholar]
- Peng C. L.; Shih Y. H.; Lee P. C.; Hsieh T. M. H.; Luo T. Y.; Shieh M. J. Multimodal Image-Guided Photothermal Therapy Mediated by Re-188-Labeled Micelles Containing a Cyanine-Type Photosensitizer. ACS Nano 2011, 5, 5594–5607. [DOI] [PubMed] [Google Scholar]
- Kim H.; Mun S.; Choi Y. Photosensitizer-Conjugated Polymeric Nanoparticles for Redox-Responsive Fluorescence Imaging and Photodynamic Therapy. J. Mater. Chem. B 2013, 1, 429–431. [DOI] [PubMed] [Google Scholar]
- Yoon H. Y.; Koo H.; Choi K. Y.; Lee S. J.; Kim K.; Kwon I. C.; Leary J. F.; Park K.; Yuk S. H.; Park J. H.; Choi K. Tumor-Targeting Hyaluronic Acid Nanoparticles for Photodynamic Imaging and Therapy. Biomaterials 2012, 33, 3980–3989. [DOI] [PubMed] [Google Scholar]
- Wang C.; Cheng L.; Liu Y. M.; Wang X. J.; Ma X. X.; Deng Z. Y.; Li Y. G.; Liu Z. Imaging-Guided pH-Sensitive Photodynamic Therapy Using Charge Reversible Upconversion Nanoparticles under Near-Infrared Light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar]
- Lee S. J.; Koo H.; Jeong H.; Huh M. S.; Choi Y.; Jeong S. Y.; Byun Y.; Choi K.; Kim K.; Kwon I. C. Comparative Study of Photosensitizer Loaded and Conjugated Glycol Chitosan Nanoparticles for Cancer Therapy. J. Controlled Release 2011, 152, 21–29. [DOI] [PubMed] [Google Scholar]
- Shen X. Q.; Li L.; Wu H.; Yao S. Q.; Xu Q. H. Photosensitizer-Doped Conjugated Polymer Nanoparticles for Simultaneous Two-Photon Imaging and Two-Photon Photodynamic Therapy in Living Cells. Nanoscale 2011, 3, 5140–5146. [DOI] [PubMed] [Google Scholar]
- Kano A.; Taniwaki Y.; Nakamura I.; Shimada N.; Moriyama K.; Maruyama A. Tumor Delivery of Photofrin(R) by PLL-g-PEG for Photodynamic Therapy. J. Controlled Release 2013, 167, 315–321. [DOI] [PubMed] [Google Scholar]
- Tian J. W.; Ding L.; Xu H. J.; Shen Z.; Ju H. X.; Jia L.; Bao L.; Yu J. S. Cell-Specific and pH-Activatable Rubyrin-Loaded Nanoparticles for Highly Selective Near-Infrared Photodynamic Therapy against Cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [DOI] [PubMed] [Google Scholar]
- Tsai H. C.; Tsai C. H.; Lin S. A. Y.; Jhang C. R.; Chiang Y. S.; Hsiue G. H. Stimulated Release of Photosensitizers from Graft and Diblock Micelles for Photodynamic Therapy. Biomaterials 2012, 33, 1827–1837. [DOI] [PubMed] [Google Scholar]
- Davis M. E.; Chen Z.; Shin D. M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discovery 2008, 7, 771–782. [DOI] [PubMed] [Google Scholar]
- Lockman P. R.; Koziara J. M.; Mumper R. J.; Allen D. D. Nanoparticle Surface Charges Alter Blood-Brain Barrier Integrity and Permeability. J. Drug Targeting 2004, 12, 635–641. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Lee S.; Kim J. H.; Park K.; Kim K.; Kwon I. C. Polymeric Nanomedicine for Cancer Therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar]
- Park J. H.; von Maltzahn G.; Ruoslahti E.; Bhatia S. N.; Sailor M. J. Micellar Hybrid Nanoparticles for Simultaneous Magnetofluorescent Imaging and Drug Delivery. Angew. Chem., Int. Ed. 2008, 47, 7284–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. M.; Rhee J. W.; Drum C. L.; Bronson R. T.; Golomb G.; Langer R.; Farokhzad O. C. In Vivo Prevention of Arterial Restenosis with Paclitaxel-Encapsulated Targeted Lipid-Polymeric Nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 19347–19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobatto M. E.; Fuster V.; Fayad Z. A.; Mulder W. J. M. Perspectives and Opportunities for Nanomedicine in the Management of Atherosclerosis. Nat. Rev. Drug Discovery 2011, 10, 835–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T.; Timko B. P.; Kohane D. S.; Langer R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Li Y.; Guo C.; Wang J.; Ma J. H.; Liang X. F.; Yang L. R.; Liu H. Z. Temperature-Responsive Magnetite/PEO-PP-PEO Block Copolymer Nanoparticles for Controlled Drug Targeting Delivery. Langmuir 2007, 23, 12669–12676. [DOI] [PubMed] [Google Scholar]
- Jain T. K.; Richey J.; Strand M.; Leslie-Pelecky D. L.; Flask C. A.; Labhasetwar V. Magnetic Nanoparticles with Dual Functional Properties: Drug Delivery and Magnetic Resonance Imaging. Biomaterials 2008, 29, 4012–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu M. M.; Othman S. F.; Curtis E. T.; Gupta B. K.; Jaggi M.; Chauhan S. C. Multi-Functional Magnetic Nanoparticles for Magnetic Resonance Imaging and Cancer Therapy. Biomaterials 2011, 32, 1890–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.; Yan Y.; Zhang H. K.; Yan H. S.; Cao Y. J.; Liu K. L.; Wan S. R.; Huang J. S.; Yue W. Magnetic and pH-Responsive Nanocarriers with Multilayer Core-Shell Architecture for Anticancer Drug Delivery. J. Mater. Chem. 2008, 18, 5104–5112. [Google Scholar]
- Guo M. A.; Que C. L.; Wang C. H.; Liu X. Z.; Yan H. S.; Liu K. L. Multifunctional Superparamagnetic Nanocarriers with Folate-Mediated and pH-Responsive Targeting Properties for Anticancer Drug Delivery. Biomaterials 2011, 32, 185–194. [DOI] [PubMed] [Google Scholar]
- Yang X. Q.; Grailer J. J.; Rowland I. J.; Javadi A.; Hurley S. A.; Matson V. Z.; Steeber D. A.; Gong S. Q. Multifunctional Stable and pH-Responsive Polymer Vesicles Formed by Heterofunctional Triblock Copolymer for Targeted Anticancer Drug Delivery and Ultrasensitive MR Imaging. ACS Nano 2010, 4, 6805–6817. [DOI] [PubMed] [Google Scholar]
- Yang X. Q.; Grailer J. J.; Rowland I. J.; Javadi A.; Hurley S. A.; Steeber D. A.; Gong S. Q. Multifunctional SPIO/DOX-Loaded Wormlike Polymer Vesicles for Cancer Therapy and MR Imaging. Biomaterials 2010, 31, 9065–9073. [DOI] [PubMed] [Google Scholar]
- Hanessian S.; Grzyb J. A.; Cengelli F.; Juillerat-Jeanneret L. Synthesis of Chemically Functionalized Superparamagnetic Nanoparticles as Delivery Vectors for Chemotherapeutic Drugs. Bioorg. Med. Chem. 2008, 16, 2921–2931. [DOI] [PubMed] [Google Scholar]
- Kayal S.; Ramanujan R. V. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng., C 2010, 30, 484–490. [Google Scholar]
- Nayar S.; Mir A.; Ashok A.; Guha A.; Sharma V. Bovine Serum Albumin Binding and Drug Delivery Studies with PVA-Ferrofluid. J. Bionic. Eng. 2010, 7, 29–34. [Google Scholar]
- Purushotham S.; Chang P. E. J.; Rumpel H.; Kee I. H. C.; Ng R. T. H.; Chow P. K. H.; Tan C. K.; Ramanujan R. V. Thermoresponsive Core-Shell Magnetic Nanoparticles for Combined Modalities of Cancer Therapy. Nanotechnology 2009, 20, 305101. [DOI] [PubMed] [Google Scholar]
- Zhou S. B.; Sun J.; Sun L.; Dai Y. Q.; Liu L. P.; Li X. H.; Wang J. X.; Weng J.; Jia W. X.; Zhang Z. R. Preparation and Characterization of Interferon-Loaded Magnetic Biodegradable Microspheres. J. Biomed. Mater. Res., Part B 2008, 87B, 189–196. [DOI] [PubMed] [Google Scholar]
- Andhariya N.; Chudasama B.; Mehta R. V.; Upadhyay R. V. Biodegradable Thermoresponsive Polymeric Magnetic Nanoparticles: A New Drug Delivery Platform for Doxorubicin. J. Nanopart. Res. 2011, 13, 1677–1688. [Google Scholar]
- Yang X. Q.; Chen Y. H.; Yuan R. X.; Chen G. H.; Blanco E.; Gao J. M.; Shuai X. T. Folate-Encoded and Fe3O4-Loaded Polymeric Micelles for Dual Targeting of Cancer Cells. Polymer 2008, 49, 3477–3485. [Google Scholar]
- Vetvicka D.; Hruby M.; Hovorka O.; Etrych T.; Vetrik M.; Kovar L.; Kovar M.; Ulbrich K.; Rihova B. Biological Evaluation of Polymeric Micelles with Covalently Bound Doxorubicin. Bioconjugate Chem. 2009, 20, 2090–2097. [DOI] [PubMed] [Google Scholar]
- Lozano M. V.; Torrecilla D.; Torres D.; Vidal A.; Dominguez F.; Alonso M. J. Highly Efficient System to Deliver Taxanes into Tumor Cells: Docetaxel-Loaded Chitosan Oligomer Colloidal Carriers. Biomacromolecules 2008, 9, 2186–2193. [DOI] [PubMed] [Google Scholar]
- Koo A. N.; Min K. H.; Lee H. J.; Lee S. U.; Kim K.; Kwon I. C.; Cho S. H.; Jeong S. Y.; Lee S. C. Tumor Accumulation and Antitumor Efficacy of Docetaxel-Loaded Core-Shell-Corona Micelles with Shell-Specific Redox-Responsive Cross-Links. Biomaterials 2012, 33, 1489–1499. [DOI] [PubMed] [Google Scholar]
- Du W. J.; Xu Z. Q.; Nystrom A. M.; Zhang K.; Leonard J. R.; Wooley K. L. F-19- and Fluorescently Labeled Micelles as Nanoscopic Assemblies for Chemotherapeutic Delivery. Bioconjugate Chem. 2008, 19, 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. B.; Li Y. X.; Qiu R. Q.; Shi L.; Wu W. T.; Zhou S. Q. Responsive Fluorescent Bi2O3@PVA Hybrid Nanogels for Temperature-Sensing, Dual-Modal Imaging, and Drug Delivery. Biomaterials 2012, 33, 3058–3069. [DOI] [PubMed] [Google Scholar]
- Nurunnabi M.; Cho K. J.; Choi J. S.; Huh K. M.; Lee Y. H. Targeted Near-IR QDs-Loaded Micelles for Cancer Therapy and Imaging. Biomaterials 2010, 31, 5436–5444. [DOI] [PubMed] [Google Scholar]
- Shen J. M.; Guan X. M.; Liu X. Y.; Lan J. F.; Cheng T.; Zhang H. X. Luminescent/Magnetic Hybrid Nanoparticles with Folate-Conjugated Peptide Composites for Tumor-Targeted Drug Delivery. Bioconjugate Chem. 2012, 23, 1010–1021. [DOI] [PubMed] [Google Scholar]
- Yuan Q.; Hein S.; Misra R. D. K. New Generation of Chitosan-Encapsulated ZnO Quantum Dots Loaded with Drug: Synthesis, Characterization and In Vitro Drug Delivery Response. Acta Biomater. 2010, 6, 2732–2739. [DOI] [PubMed] [Google Scholar]