Abstract
Background
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease. To search for glomerular proteins associated with early-stage DN, glomeruli of spontaneous type 2 diabetic KKAy mice were analyzed by 2-dimensional differential gel electrophoresis (2D-DIGE).
Material/Methods
Glomeruli of 20-week spontaneous type 2 diabetic KKAy mice and age-matched C57BL/6 mice were isolated by kidney perfusion with magnetic beads. Proteomic profiles of glomeruli were investigated by using 2D-DIGE and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Western blot analysis was used to confirm the results of proteomics. Immunohistochemical and semi-quantitative analysis were used to confirm the differential expression of prohibitin and annexin A2 in glomeruli.
Results
We identified 19 differentially expressed proteins – 17 proteins were significantly up-regulated and 2 proteins were significantly down-regulated in glomeruli of diabetic KKAy mice. Among them, prohibitin and annexin A2 were up-regulated and Western blot analysis validated the same result in proteomics. Immunohistochemical analysis also revealed up-regulation of prohibitin and annexin A2 in glomeruli of KKAy mice.
Conclusions
Our findings suggest that prohibitin and annexin A2 may be associated with early-stage DN. Further functional research might help to reveal the pathogenesis of DN.
MeSH Keywords: Diabetic Nephropathies, Kidney Glomerulus, Proteomics, Two-Dimensional Difference Gel Electrophoresis
Background
Diabetic nephropathy (DN) is a leading cause of end-stage renal disease and is the major cause for diabetic disability and mortality [1–6]. It also negatively affects patient’s quality of life and their social environment, and it poses a significant burden on national healthcare budgets [7].
DN is characterized by a long period of clinical silence without significant signs or symptoms. Once diabetic patients develop persistent proteinuria, the condition often becomes irreversible, usually leading to endstage renal failure [8]. Therefore, pathogenesis and diagnosis of early stage DN remain key concerns for basic and clinical research.
Multiple factors, including glucose metabolism, oxidative stress, renal hemodynamic changes, cytokines, and genetic predisposition, may contribute to the development of DN [9–11]. To more fully understand the mechanisms involved in the pathogenesis of DN, various proteomic strategies have been applied to the pathophysiological study [12–14]. Since most studies only focus on urine or renal cortex, to obtain global proteomic profile in glomeruli, which are the functional units of the kidney, we investigated the expression of glomerular proteins in spontaneous type-2 diabetic KKAy and C57BL/6 control mice by using 2-dimensional differential gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometric analyses. The changes in glomerular proteins during early-stage DN were observed.
Material and Methods
Animals
Male KKAy mice (8 weeks of age, n=20) were purchased from the Laboratory Animal Science Institute, Chinese Academy of Medical Sciences. Male C57BL/6 mice (8 weeks of age, n=20), which made up the study control group, were purchased from the Laboratory Animal Center, China Medical University. Mice were individually housed in plastic cages and were fed a high-fat diet (58% fat, 25.6% carbohydrate, 16.4% protein). Mice had free access to food and tap water throughout the experimental period. All mice were maintained in a temperature – (23±3°C) and humidity – (50±20%) controlled room (China medical university, Laboratory Animal Center SPF rodent housing facility) with a regular 12-h light/dark cycle according to the Chinese National Standard (GB 14925-2001). All experiments were approved by the local animal research ethics committee.
Reagents
Collagenase A was purchased from Roche (Roche Diagnostics, Germany). The 2-D Clean-Up Kit and Ettan TM 2-D Quant Kit were purchased from GE (GE Healthcare, USA). Dynabeads M-450 Tosylactivated and magnetic particle concentrator were purchased from Dynal (Dynal A.S., Norway). Cell strainers (100-μm) were purchased from BD (BD, USA). Mouse Albumin and Creatinine ELISA Kits were purchased from RB (RB, USA). Immobilized non-linear pH gradient (IPG) strips, Cyanine dyes Cy2, Cy3, Cy5, and Amersham™ Deep purple total protein stain were purchased from GE. Rabbit polyclonal antibody to Prohibitin (ab28172) and Rabbit polyclonal antibody to Annexin A2 (ab75932) were purchased from Abcam.
Phenotypic characterization
At 20 weeks of age, body weight and random glucose levels of KKAy mice and C57BL/6 mice were measured. An automatic biochemical analyzer was used to detect serum creatinine and blood urea nitrogen levels, and an ELISA kit was used to measure urine albumin and urine creatinine levels.
Isolation of mouse glomeruli and sample preparation
Glomeruli of mice were isolated from KKAy mice and C57BL/6 mice at 20 weeks of age. Briefly, kidneys were perfused with ice-cold PBS via abdominal or thoracic aorta to remove any remaining blood from the blood vessels. Next, Dynabeads in a concentration of 4×106/ml PBS were perfused into the kidney at a constant rate of 7.4 ml/min/g kidney. Kidneys were removed, minced, and digested in collagenase A (1 mg/ml) for 30 min at 37°C with gentle agitation. The digested tissue was pressed through a 100-μm cell strainer, followed by intermittent flushing using ice-cold PBS. The filtered suspension was centrifuged at 200×g for 5 min under a stable temperature of 4°C. After the supernatant was discarded, the pellet was dissolved in 2 ml of PBS, and transferred to a 2-ml tube. Glomeruli that contained Dynabeads were separated from renal tubules by a magnetic particle concentrator. To remove the Dynabeads, the extracted glomeruli were lysed in a 2-DE lysis buffer (7M urea, 2M thiourea, 4% [w/v]CHAPS, 2% [v/v] immobilized pH gradient [IPG] buffer, 40 mM dithiothreitol [DTT]), and sonicated (30 Hz, 4×5 s pulses on ice). The lysates were subsequently centrifuged at 12 500×g for 10 min at a temperature of 4°C. The glomeruli protein was purified according to the instructions of the 2-D Clean-Up Kit, and protein concentration was determined using the Ettan TM 2-D Quant Kit. The samples were stored at −70°C. With the exception of the collagenase digestion, the entire procedure was performed on ice.
Fluorescence labeling (minimal labeling) with CyDyes
Protein samples were labeled with CyDyes (dissolved in N,N-dimethylformamide) according to the manufacturer’s instructions (GE Healthcare). Typically, 50 μg each of soluble protein samples from glomeruli of 20-week-old C57BL/6 mice and 20-week-old KKAy mice were labeled with 400 pmol of either Cy3 or Cy5, respectively. An internal standard (50 μg), which is the equivalent mixture of all glomerular protein samples in the study, was simultaneously labeled with 400 pmol of Cy2 on the same 2-D gel. Labeling reactions were performed on ice in the dark for 30 min. Subsequently, the reaction was quenched with 1 μl of 10 mM lysine for 10 min under the same conditions.
Two-dimensional differential gel electrophoresis (2D-DIGE)
Cy3-, Cy5-, and Cy2-labeled samples (total 150 μg) and preparative samples for later protein identification (600 μg) were pooled. An equal volume of 2×sample buffer (7M urea, 2M thiourea, 4% [w/v] CHAPS, 2% [v/v] IPG buffer, 40 mM DTT) and 1×hydrated fluid (7 M urea, 2 M thiourea, 2% [w/v] CHAPS, 2% [v/v] IPG buffer, 20 mM DTT) were added to the mixture to make a total volume of 450 μl. Subsequently, isoelectric focusing in Ettan IPGphor III (GE Healthcare) at 20°C (74510Vh) was performed with 24-cm immobilized non-linear pH gradient strips (pH 3–10).
After the first dimension, each strips was equilibrated in 10-ml equilibration solution-1 (6M urea, 75 mM Tris-HCl pH8.8, 29.3% glycerol, 2% sodium dodecyl sulphate[SDS], 0.002% bromphenol blue, 100 mg DTT) and 10-ml equilibration solution-2 (6M urea, 75 mM Tris-HCl pH8.8, 29.3% glycerol, 2% SDS, 0.002% bromphenol blue, 250 mg iodoacetamide) for 15 min at room temperature. After equilibration, the strips were individually overlaid on 12.5% polyacrylamide gels and immobilized with 0.5% agarose in a buffer of 1×Laemmli SDS buffer (25 mM Tris, 192 mM glycine, 0.1% [W/V] SDS) run at 2 W/gel using Ettan-DALTsix system (GE Healthcare).
Image analysis
After completion of 2-dimensional gel electrophoresis, the CyDyes-labeled images were scanned using a Typhoon TRIO scanner (GE Healthcare). Images were analyzed by using DeCyder™ 2-D Differential Analysis Software 6.5 (GE Healthcare). The DeCyder differential in-gel analysis (DIA) module was used for pairwise comparisons of each 20-week-old KKAy mice glomerular sample and 20-week C57BL/6 mice glomerular sample to the internal standard present in each gel and for simultaneous comparison of 20-week-old KKAy/C57BL/6 abundance. The DeCyder biological variation analysis (BVA) module was then used to simultaneously match all 9 protein-spot maps from 3 gels which dyed samples with crossed Cy3- and Cy5-dyes, and using the Cy3: Cy2 and Cy5: Cy2 DIA ratios. Statistical analysis of differences between the 2 groups was done using the paired t test and the level of statistical significance was set at p<0.05. The software automatically generated a list of differentially expressed proteins, including fold change, and these differentially expressed proteins were further identified by mass spectrometry.
Deep purple post-staining
Gels were fixed for 2 h in 7.5% (v/v) acetic acid and 10% (v/v) methanol, and washed twice in 35 mM NaHCO3 and 300 mM Na2CO3 for 15 min per cycle. The total protein was stained with deep purple dye for 1 h in the dark. Next, post-stained gels were washed twice in 7.5% (v/v) acetic acid and subsequently imaged by a Typhoon 9400 scanner. Post-stained images were matched with CyDyes-stained images using DeCyder™ 2-D Differential Analysis Software, and the chosen spots were picked by use of an Ettan spot picker (GE Healthcare).
In-gel tryptic digestion
The selected gel particles in 96-well plates were washed twice with deionized water, dehydrated with acetonitrile (ACN), extracted with 25 mM NH4HCO3, and dehydrated again with ACN. Subsequently, trypsin (Promega, USA) was added for at least 1 h while on ice, followed by an overnight addition of 25 mM NH4HCO3 at 37°C.
Mass spectrometric analysis for protein identification
Each trypsin-digested sample was spotted on an MTP Anchorchip™ target (Bruker Daltonics, Germany). Once the samples were completely dried, 1.1 μl of matrix (4 mg 4-hydroxy-alpha-cyanocinnamic acid dissolved in ACN: 0.1%TFA[7:3]) was also spotted. Tryptic peptides were identified by MALDI-TOF mass spectrometry (Bruker Daltonics) using peptide fragments of assorted lengths as the internal standard. Detailed analysis of peptide mapping fingerprint data was performed by flexAnalysis™ 3.0, and protein identification was performed by searching the Swiss Prot database using the MASCOT engine.
Western blot
Glomerular proteins isolated from 20-week-old KKAy and C57BL/6 mice were mixed with a 5×Laemmli sample buffer and boiled for 5 min. Subsequently, 30 μg of total proteins were loaded onto a 10% SDS-PAGE for separation. Proteins were transferred to a polyvinylidene fluoride membrane, which was treated with antibodies, and blocked with 5% milk/TBS. The antibody detection system consisted of a rabbit polyclonal antibody to prohibitin (1:1000 dilution), annexin A2 (1:1000 dilution), and HRP-conjugated anti-rabbit antibody. β-actin was used as internal control. Using MF-ChemiBIS 3.2 and ImageJ software, the Western blot images were captured and analyzed.
Immunohistochemistry
Paraffin sections were deparaffinized with xylene and rehydrated in a graded alcohol series. Tissue sections were treated with 3% H2O2 at room temperature for 10 min, followed by overnight incubation with anti-prohibitin (1:250) and anti-annexin A2 (1:250) antibodies at 4°C. Finally, the tissue sections were incubated with peroxidase-conjugated goat anti-rabbit antibody, developed with diaminobenzidine as the chromogen, and counterstained with hematoxylin. Twenty-five glomeruli in each group were observed, and semi-quantitative analysis of glomerular images was made by MetaMorph image analysis system (UIC, USA).
Statistical analysis
SPSS 15.0 statistic software was used for data processing. The results are expressed as average ± standard deviation. Analysis of variance (ANOVA) was used to compare differences between groups. A P≤0.05 was considered statistically significant.
Results
Phenotypic characterization
As shown in Table 1, body weight and random glucose levels at 20 weeks of age were both significantly higher in KKAy mice than in age-matched C57BL/6 control mice (P<0.05). The urinary albumin/creatinine ratio of KKAy mice at 20 weeks of age was significantly higher than that of the C57BL/6 control group (P<0.05). Blood creatinine and blood urea nitrogen levels were not statistically different between the 2 groups. Pathological lesions in 20-week-old KKAy mice were increasing in glomerular area under a light microscope, including glomerular basement membrane thickening, and segmental and diffuse mesangial expansion. Glomerular basement membrane thickening with fused foot processes were also observed under an electron microscope [15].
Table 1.
Mean phenotypic values at 20 weeks of age.
| KKAy | C57BL/6 | |
|---|---|---|
| Body weight (g) | 46.88±2.61* | 30.32±1.3 |
| Random blood sugar (mmol/L) | 29.62±3.99* | 11.07±1.26 |
| Blood creatinine (μmol/L) | 30.33±5.82 | 32.67±4.51 |
| Blood urea nitrogen (mmol/L) | 12.30±3.18 | 11.03±2.97 |
| Urinary albumin: creatinine ratio (mg/g) | 728.00±177.19* | 49.37±18.77 |
Data are expressed as mean ±SD.
P<0.05 vs. C57BL/6 control group.
Proteomic profile in glomeruli of diabetic KKAy mice
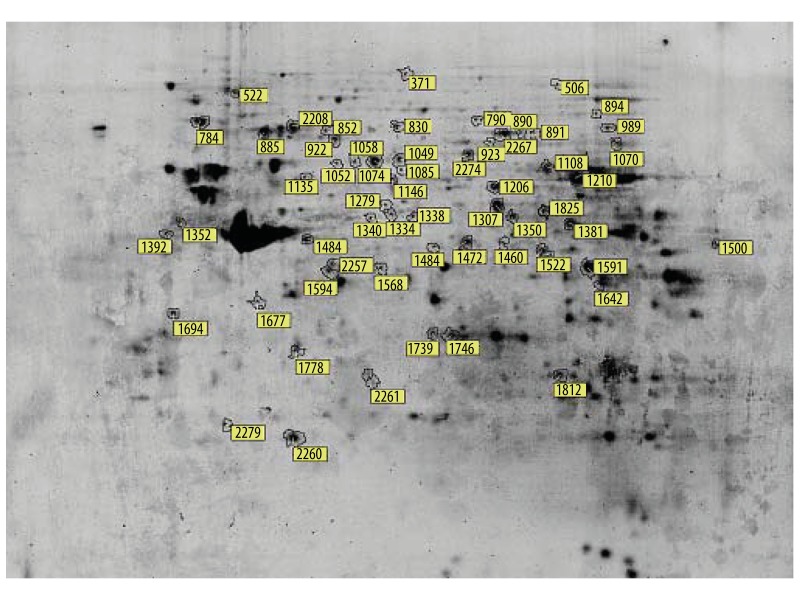
By using DeCyder™ 2-D Differential Analysis Software, 114 spots of differential expression of mouse glomeruli were extracted for further mass spectrometry analysis. By the individual analysis of MALDI-TOF mass spectrometry, 19 differential protein-spots of mice glomeruli were identified (Figures 1 and 2). Among the glomerular differential proteins, there were 17 proteins significantly up-regulated and 2 proteins significantly down-regulated in KKAy mice (Tables 2 and 3).
Figure 1.
Two-dimensional electrophoresis image of differentially expressed glomerular proteins in early-stage diabetic nephropathy. Glomerular proteins were separated by 2-dimensional gel electrophoresis (2-DE). The gel was stained with Deep Purple dye and 19 differentially expressed proteins were labeled on the map. Marked spot numbers represent differentially expressed proteins between 20-week-old spontaneous type-2 diabetic KKAy mice and 20-week-old C57BL/6 mice. The marked spot numbers refer to “Spot ID” in Tables 2 and 3.
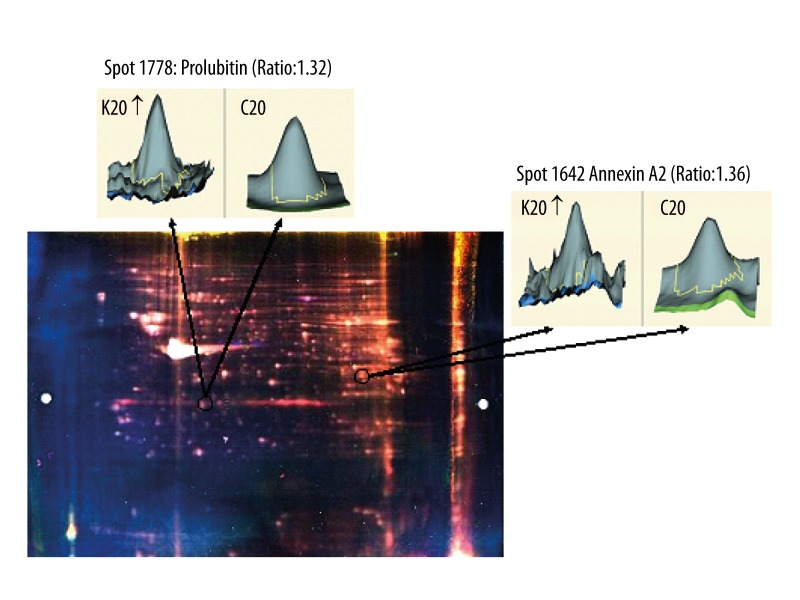
Figure 2.
Two-dimensional differential gel electrophoresis (2D-DIGE) image of mice glomeruli. Prohibitin and annexin A2 were up-regulated in glomeruli of 20-week spontaneous type-2 diabetic KKAy mice, with a differential expression rate of 1.32 and 1.36, respectively. The 3-dimensional images indicated by arrows were generated by DeCyder™ 2-D Differential Analysis software. K20 represents 20-week-old KKAy mice; C20 represents 20-week-old C57BL/6 mice.
Table 2.
Up-regulated glomerular proteins in KKAy mice.
| Spot ID | MW(Da) | pI | Protein name | Acc.no. SwissProt | Ratio (K20/C20) | Protein Score | Molecular function |
|---|---|---|---|---|---|---|---|
| 1392 | 32931 | 4.8 | 40S ribosomal protein SA | P14206 | 2.33 | 99 | Ribosomal protein |
| 1352 | 56265 | 5.19 | ATP synthase subunit beta, mitochondrial | P56480 | 1.77 | 184 | Hydrolase |
| 894 | 68272 | 7.23 | Transketolase | P40142 | 1.64 | 124 | Transferase |
| 1136 | 56857 | 5.57 | V-type proton ATPase subunit B, brain isoform | P62814 | 1.49 | 156 | Hydrolase |
| 923 | 62638 | 5.95 | Dihydropyrimidinase-related protein 2 | O08553 | 1.47 | 103 | Developmental protein |
| 790 | 80498 | 6.83 | Propionyl-CoA carboxylase alpha chain, mitochondrial | Q91ZA3 | 1.46 | 92 | Ligase |
| 1070 | 60013 | 7.72 | Catalase | P24270 | 1.41 | 98 | Oxidoreductase |
| 1500 | 47780 | 9.13 | Aspartate aminotransferase, mitochondrial | P05202 | 1.38 | 90 | Transferase |
| 1642 | 38937 | 7.55 | Annexin A2 | P07356 | 1.36 | 156 | Calcium ion binding |
| 1812 | 28928 | 6.67 | Phosphoglycerate mutase 1 | Q9DBJ1 | 1.36 | 115 | Glycolysis |
| 1049 | 63045 | 5.85 | Epoxide hydrolase 2 | P34914 | 1.35 | 99 | Hydrolase |
| 1350 | 49876 | 7.23 | Elongation factor Tu, mitochondrial | Q8BFR5 | 1.34 | 98 | Elongation factor |
| 865 | 71055 | 5.37 | Heat shock cognate 71 kDa protein | P63017 | 1.32 | 107 | Chaperone |
| 1778 | 29859 | 5.57 | Prohibitin | P67778 | 1.32 | 185 | DNA synthesis |
| 830 | 80501 | 6.25 | Heat shock protein 75 kDa, mitochondrial | Q9CQN1 | 1.24 | 147 | Chaperone |
| 1210 | 61640 | 8.05 | Glutamate dehydrogenase 1, mitochondrial | P26443 | 1.22 | 126 | Oxidoreductase |
| 1591 | 36792 | 6.9 | Alcohol dehydrogenase [NADP+] | Q9JII6 | 1.21 | 131 | Oxidoreductase |
“K20/C20” represents 20-week-old spontaneous type-2 diabetic KKAy mice vs. 20-week-old C57BL/6 mice. Protein score is −10*Log(P), where P is the probability that the observed match is a random event. Protein scores greater than 55 are significant (P<0.05).
Table 3.
Down-regulated glomerular proteins in KKAy mice.
| Spot ID | MW(Da) | pI | Protein name | Acc.no. SwissProt | Ratio (K20/C20) | Protein Score | Molecular function |
|---|---|---|---|---|---|---|---|
| 1338 | 52991 | 6.52 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial | Q91WD5 | −1.37 | 97 | Oxidoreductase |
| 1677 | 35720 | 5.3 | Aspartoacylase-2 | Q91XE4 | −1.29 | 88 | Hydrolase |
“K20/C20” represents 20-week-old spontaneous type-2 diabetic KKAy mice vs. 20-week-old C57BL/6 mice. Protein score is −10*Log(P), where P is the probability that the observed match is a random event. Protein scores greater than 55 are significant (P<0.05).
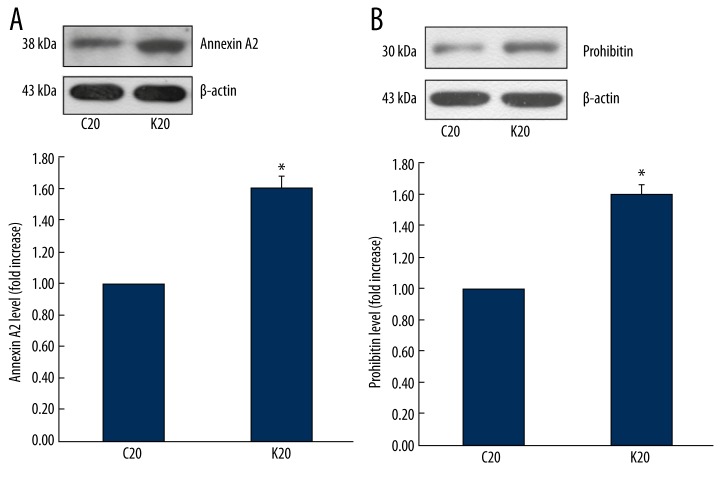
Western blot analysis of annexin A2 and prohibitin in glomeruli
Western blot analysis showed that the glomerular expression of annexin A2 and prohibitin were up-regulated in 20-week-old KKAy mice in comparison to C57BL/6 control mice (Figure 3).
Figure 3.
Western blot analysis of annexin A2 and prohibitin in glomeruli. (A) Annexin A2 was up-regulated in glomeruli of 20-week-old spontaneous type-2 diabetic KKAy mice, * P<0.05 compared to control. (B) Prohibitin was up-regulated in glomeruli of 20-week-old spontaneous type-2 diabetic KKAy mice, * P<0.05 compared to control. K20 represents 20-week-old KKAy mice; C20 represents 20-week-old C57BL/6 mice.
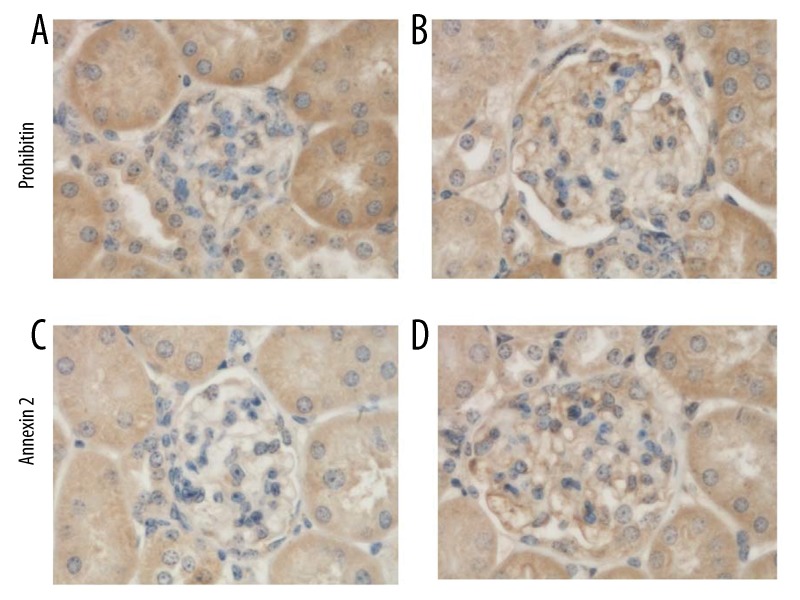
Immunohistochemical analysis of prohibitin and annexin A2 in glomeruli
Figures 4 shows immunostaining results of prohibitin and annexin A2 in kidneys of 20-week-old KKAy and C57BL/6 mice. In glomeruli, both prohibitin and annexin A2 were expressed at higher levels in KKAy mice than in C57BL/6 mice (Table 4).
Figure 4.
Immunohistochemical analysis of prohibitin and annexin A2 in glomeruli. In glomeruli, prohibitin and annexin A2 were observed in 20-week-old C57BL/6 control mice (A, C) and 20-week-old spontaneous type-2 diabetic KKAy mice (B, D). Both were expressed higher in KKAy mice than in C57BL/6 mice. Magnification power was 1000× in all panels.
Table 4.
Semi-quantitative analysis of glomerular images in mice (n=25).
| Image name | Optical density average | Integrated OD average |
|---|---|---|
| Annexin A2-C20 | 0.152±0.004 | 12.371±4.706 |
| Annexin A2-K20 | 0.247±0.004* | 29.688±16.329* |
| Prohibitin-C20 | 0.134±0.007 | 7.389±2.621 |
| Prohibitin-K20 | 0.228±0.004* | 25.881±7.178* |
Data are expressed as mean ±SD.
P<0.05 vs. C57BL/6 control group.
K20 represents 20-week-old spontaneous type-2 diabetic KKAy mice; C20 represents 20-week-old C57BL/6 mice.
Discussion
The purpose of this study was to identify proteins closely associated with early-stage DN. The KKAy mice, produced by the transfer of the yellow obese gene (Ay) into KK mice, become obese, hyperglycemic, hypertriglyceridemic, and hyperinsulinemic. Furthermore, after 16 weeks of age, these mice were found to develop typical proteinuria, glomerular mesangial matrix accumulation, and glomerular basement membrane thickening. Thus, the spontaneous type-2 diabetic KKAy mice are considered to be an ideal animal model for studying early-stage DN [16–20].
Glomeruli are the major target of injury in diabetic nephropathy [21]. However, because of the limitation in methodology and the demand of protein quantity, most proteomic studies about DN using mouse models only focused on whole kidney or renal cortex [22,23], and the glomerular proteome was rarely reported. In our study, by using Dynabeads perfusion, we successfully isolated mouse glomeruli with high purity [24]. By using 2D-DIGE and MALDI-TOF mass spectrometric analysis, we attained 19 differentially expressed glomerular proteins between KKAy mice and C57BL/6 mice. Out of these 19 proteins, 17 were up-regulated and 2 were down-regulated in KKAy mice. Among them, prohibitin and annexin A2 were up-regulated.
Prohibitin was named for its ability to inhibit cell proliferation. It is a pleiotropic protein in the cell, which has been implicated in the maintenance of mitochondrial function, protection against senescence, and also has been found to participate in the regulation of proliferation, apoptosis, transcription, and as a cell-surface receptor [25–28]. Shi et al. [29] analyzed the antiproliferative activity of prohibitin in children with nephritic syndrome, and results showed that serum prohibitin was significantly increased in children with kidney diseases and were positively correlated with the degrees of renal glomerular and interstitial damage. Research has identified that prohibitin may be a potential therapeutic option for treatment of diseases associated with increased oxidative stress and mitochondrial dysfunction, such as insulin resistance/type 2 diabetes, obesity, and cancer [30].
Prohibitin could interact with annexin A2, ie, annexin A2 was found with prohibitin as prey [31]. Annexin A2 belongs to a family of calcium-dependent, phospholipid-binding proteins [32,33]. It was first identified as an intracellular protein and attributed to intracellular functions, while extracellular annexin A2 has also been found in several tissues as both soluble and membrane-bound protein and has attracted increasing attention in recent years [34]. Extracellular annexin A2 is a co-receptor for plasminogen and tissue plasminogen activator on endothelial cells, and is one of the molecules required to maintain the anti-thrombogenic properties of endothelial cells. Ishii et al. [35] administered recombinant annexin-2 protein (rAN II) to KKAy mice; albuminuria and histological glomerular lesions were significantly milder without influencing the coagulation system. Cell surface annexin A2 is a high-affinity receptor for large Tenascin C splice variants, and may play a crucial role in the interaction between cells and the extracellular matrix [36].
In our study, we found that prohibitin and annexin A2 were up-regulated in glomeruli in diabetic KKAy mice. Prohibitin could interact with annexin A2, and the interactions between prohibitin, annexin A2 seem to be regulated in a Ca2+-dependent manner, and addition of Ca2+ blocked the association of the 2 proteins, which are both cell-surface receptors. Baran et al. [37] reported that annexin A2 may serve as a receptor for rapid actions of 1alpha, 25-dihydroxyvitamin D(3). Mooso et al. [38] demonstrated that prohibitin participates in the regulation of the reaction of vitamin D receptor to calcitriol (1,25(OH)(2)D3) in prostate cancer. Further studies on the up-regulation of prohibitin and annexin A2 participate in the mechanism relative to vitamin D insufficiency during early-stage DN might aid in understanding the pathophysiologic mechanism of DN. Although proteomic analysis is one of the most powerful and useful tools in the detection of novel proteins in kidney diseases, it requires further mechanical analysis of the results.
Conclusions
Our study identified 19 differentially expressed glomerular proteins in diabetic KKAy mice vs. C57BL/6 control mice. Prohibitin and annexin A2 were significantly up-regulated in glomeruli of KKAy mice. These results can be utilized as a database for analysis of protein expression during early-stage DN, and further studies on prohibitin and annexin A2 might yield new insights into the pathogenesis of DN.
Acknowledgement
The authors thank Weifan Yao, Yang Chen, Shuang Yu, Shuyan Du, and Dong Chen for their skillful technical support.
Footnotes
Disclosure
All the authors declared no competing interests.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 30700369) and Chinese Society of Nephrology (No. 13030320417)
References
- 1.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients-a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010;8:62. doi: 10.1186/1477-7525-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu XQ, Wei JL. Kidney disease in China: recent progress and prospects. Chin Med J (Engl) 2009;122(17):2048–53. [PubMed] [Google Scholar]
- 5.Stanton RC. Frontiers in diabetic kidney disease: introduction. Am J Kidney Dis. 2014;63(2 Suppl 2):S1–2. doi: 10.1053/j.ajkd.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Reddy MA, Tak Park J, Natarajan R. Epigenetic modifications in the pathogenesis of diabetic nephropathy. Semin Nephrol. 2013;33(4):341–53. doi: 10.1016/j.semnephrol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellemons ME, Kerschbaum J, Bakker SJ, et al. Validity of biomarkers predicting onset or progression of nephropathy in patients with Type 2 diabetes: a systematic review. Diabet Med. 2012;29(5):567–77. doi: 10.1111/j.1464-5491.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 8.Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38(4):252–60. doi: 10.4093/dmj.2014.38.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondeva T, Wolf G. Reactive oxygen species in diabetic nephropathy: friend or foe? Nephrol Dial Transplant. 2014;29(11):1998–2003. doi: 10.1093/ndt/gfu037. [DOI] [PubMed] [Google Scholar]
- 10.Dellamea BS, Leitão CB, Friedman R, Canani LH. Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndr. 2014;6(1):17. doi: 10.1186/1758-5996-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171(8):1917–42. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papale M, Di Paolo S, Vocino G, et al. Proteomics and diabetic nephropathy: what have we learned from a decade of clinical proteomics studies? J Nephrol. 2014;27(3):221–28. doi: 10.1007/s40620-014-0044-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2014 doi: 10.1007/s00467-014-2888-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhensdadia NM, Hunt KJ, Lopes-Virella MF, et al. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. 2013;83(6):1136–43. doi: 10.1038/ki.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan QL, Yang G, Liu XD, et al. Effect of losartan on the glomerular protein expression profile of type 2 diabetic KKAy mice. J Nephrol. 2013;26(3):517–26. doi: 10.5301/jn.5000176. [DOI] [PubMed] [Google Scholar]
- 16.Breyer MD, Böttinger E, Brosius FC, III, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki M, Saito Y, Udaka Y, et al. Diabetic nephropathy in KK and KK-Ay mice. Exp Anim. 2002;51(2):191–96. doi: 10.1538/expanim.51.191. [DOI] [PubMed] [Google Scholar]
- 18.Chen LM, Li XW, Huang LW, et al. The early pathological changes of KKAy mice with type 2 diabetes. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002;24(1):71–75. [PubMed] [Google Scholar]
- 19.Allen TJ, Cooper ME, Lan HY. Use of genetic mouse models in the study of diabetic nephropathy. Curr Diab Rep. 2004;4(6):435–40. doi: 10.1007/s11892-004-0053-1. [DOI] [PubMed] [Google Scholar]
- 20.Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970;17(1):23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- 21.Barati MT, Merchant ML, Kain AB, et al. Proteomic analysis defines altered cellular redox pathways and advanced glycation end-product metabolism in glomeruli of db/db diabetic mice. Am J Physiol Renal Physiol. 2007;293(4):F1157–65. doi: 10.1152/ajprenal.00411.2006. [DOI] [PubMed] [Google Scholar]
- 22.Thongboonkerd V, Zheng S, McLeish KR, et al. Proteomic identification and immunolocalization of increased renal calbindin-D28k expression inOVE26 diabetic mice. Rev Diabet Stud. 2005;2(1):19–26. doi: 10.1900/RDS.2005.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilton RG, Haidacher SJ, Lejeune WS, et al. Diabetes-induced changes in the renal cortical proteome assessed with two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2007;7(10):1729–42. doi: 10.1002/pmic.200700017. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Fan Q, Yang G, et al. Isolating glomeruli from mice: A practical approach for beginners. Exp Ther Med. 2013;5(5):1322–26. doi: 10.3892/etm.2013.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra S, Murphy LC, Nyomba BL, Murphy LJ. Prohibitin: a potential target for new therapeutics. Trends Mol Med. 2005;11(4):192–97. doi: 10.1016/j.molmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Mishra S, Murphy LC, Murphy LJ. The Prohibitins: emerging roles in diverse functions. J Cell Mol Med. 2006;10(2):353–63. doi: 10.1111/j.1582-4934.2006.tb00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793(1):27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Artal-Sanz M, Tavernarakis N. Prohibitin and mitochondrial biology. Trends Endocrinol Metab. 2009;20(8):394–401. doi: 10.1016/j.tem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, Huang WY, Xu H, et al. Serum levels of prohibitin in normal children and those with nephrotic syndrome. J Clin Pediatr. 2010;28:264–68. [Google Scholar]
- 30.Theiss AL, Sitaraman SV. The role and therapeutic potential of prohibitin in disease. Biochim Biophys Acta. 2011;1813(6):1137–43. doi: 10.1016/j.bbamcr.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacher S, Achatz G, Schmitz ML, Lamers MC. Prohibitin and prohibitone are contained in high-molecular weight complexes and interact with alpha-actinin and annexin A2. Biochimie. 2002;84(12):1207–20. doi: 10.1016/s0300-9084(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 32.Rescher U, Gerke V. Annexins – unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117(Pt 13):2631–39. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 33.Wang CY, Lin YS, Su WC, et al. Glycogen synthase kinase-3 and Omi/HtrA2 induce annexin A2 cleavage followed by cell cycle inhibition and apoptosis. Mol Biol Cell. 2009;20(19):4153–61. doi: 10.1091/mbc.E09-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siever DA, Erickson HP. Extracellular annexin II. Int J Biochem Cell Biol. 1997;29(11):1219–23. doi: 10.1016/s1357-2725(97)00057-5. [DOI] [PubMed] [Google Scholar]
- 35.Ishii H, Hiraoka M, Tanaka A, et al. Recombinant annexin-2 inhibits the progress of diabetic nephropathy in a diabetic mouse model via recovery of hypercoagulability. Thromb Haemost. 2007;97(1):124–28. [PubMed] [Google Scholar]
- 36.Esposito I, Penzel R, Chaib-Harrireche M, et al. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208(5):673–85. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 37.Baran DT, Quail JM, Ray R, Honeyman T. Binding of 1alpha,25-dihydroxyvitamin D(3) to annexin II: effect of vitamin D metabolites and calcium. J Cell Biochem. 2000;80(2):259–65. doi: 10.1002/1097-4644(20010201)80:2<259::aid-jcb150>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Mooso B, Madhav A, Johnson S, et al. Androgen Receptor regulation of Vitamin D receptor in response of castration-resistant prostate cancer cells to 1α-Hydroxyvitamin D5 – a calcitriol analog. Genes Cancer. 2010;1(9):927–40. doi: 10.1177/1947601910385450. [DOI] [PMC free article] [PubMed] [Google Scholar]