Summary
Magnetic resonance imaging (MRI) depicts infectious foci in the perianal region better than any other imaging modality. MRI allows definition of the fistula, associated abscess formation and its secondary extensions. Accurate information is necessary for surgical treatment and to obtain a decrease in the incidence of recurrence and complications. Radiologists should be familiar with anatomical and pathological findings of perianal fistulas and classify them using the MRI – based grading system. The purpose of this article was to provide an overview for evaluation of perianal fistulas, examples of various fistula types and their classification.
MeSH Keywords: Classification, Magnetic Resonance Imaging, Rectal Fistula
Background
A fistula can be defined as an abnormal communication between two epithelial-lined surfaces. A fistula-in-ano is an abnormal tract or cavity communicating with the anal canal or rectum by an identifiable internal opening, usually between the anal canal and the perianal skin.
Anorectal fistulas have been the subject of medical literature for over 2,500 years [1]. The use of a seton (horsehair) in the treatment of anal fistulas was described by Hippocrates [2]. The true incidence of anal fistulas is unknown. The incidence and epidemiology of anal fistulas were studied during a 10-year period in a population of 510,000 people, from 1969 to 1978, by Sainio et al. [3]. The mean incidence of fistulas per 100,000 of the general population was established at 8.6, i.e. 12.3% for males, and 5.6% for females. Nelson et al. found in their meta-analysis that 20,000–25,000 fistulas were treated annually in the USA [4]. Anal fistula has its maximum incidence between the third and fifth decades. Men are affected two to four times more commonly [5] and in the study, all the patients younger than 15 years of age were male [3].
Anorectal Anatomy
To fully understand the role of imaging with regard to anal fistula, it is obligatory to understand its etiology and how various fistula types are defined by anatomical boundaries.
The anal canal extends from the anus to the rectal ampulla and is 2–5 centimeters long, and is shorter in women than in men. At approximately 2 cm in the anal canal lies the dentate line, where the epithelium becomes transitional, and there is histological junction between the anal squamous epithelium and the rectal columnar epithelium. Around the dentate line, there are anal glands that empty into anal sinuses. The glands are primarily within the intersphincteric space or the internal sphincter [6]. Anal glands are slightly more numerous in men than in women [6].
The anal canal is surrounded by two sphincter muscles. The smooth internal sphincter consists of a thickened circular muscle layer of the bowel wall. In most individuals, it can be divided without causing a loss of continence. The external sphincter is composed of striated muscle and is continuous superiorly with the puborectalis and levator ani muscles. A division of the external sphincter can lead to incontinence. The intersphincteric space is the surgical plane of dissection between the internal and external sphincters. It contains a sheet of fat with loose areolar tissue. The fat-filled ischioanal fossa lies lateral to the sphincter complex and is traversed by fibroelastic connective tissue fibers. The puborectalis is the lowermost part of the funnel-shaped levator ani muscles, which separate the perineum from the pelvic cavity [7]. The anal sphincter is surrounded by the fat containing ischioanal space.
Etiology
The majority of anal fistulas are of non-specific origin and result from inflammation of anal glands, and are usually termed as idiopathic or cryptoglandular [1]. The cryptoglandular hypothesis states that the infection arises in the anal glands at the dentate line when the draining duct becomes blocked by infected debris, as an intersphincteric infection, and from there may progress by different routes [8]. This abscess may resolve by means of spontaneous drainage into the anal canal or may progress to an acute anorectal abscess. Anal fistula develops when an intersphincteric infection continues. Perianal abscess is an acute manifestation and fistula-in-ano a chronic condition of the same disease.
Among the specific causes of anal fistula inflammatory bowel disease, especially Crohn’s disease is the major one. Other specific causes are trauma including surgery, specific infections such as tuberculosis, pelvic inflammatory processes, and foreign bodies and malignant diseases. The nonspecific fistulae accounted for 90.4%, the tuberculous fistulae for 0.2%, fistulae associated with Crohn’s disease for 1.3%, the postoperative and traumatic fistulae for 3.3%, and fistulae originating in the anal fissure for 3.3% [3].
Diagnosis
An anorectal abscess usually presents with classic symptoms of an abscess, such as pain, swelling and induration, tenderness, and often a raised temperature: it is usually evident at inspection of the perianal area. If the abscess is low-lying, the pain is often associated with other complaints, including swelling and redness. High abscesses are less likely to present with swelling or redness. These abscesses are more likely to be associated with systemic symptoms, such as fever and malaise [9].
An anal fistula, during the chronic phase of the infection, typically gives symptoms with intermittent discharge of pus or a little bleeding. If the external opening temporarily closes, there may be swelling and pain until it opens spontaneously or a new abscess forms. The differential diagnosis for anal abscesses includes anal fissure, thrombosed external hemorrhoids, malignancy, sexually transmitted diseases, proctitis, cellulitis, and levator muscle spasm [9]. Most patients can be diagnosed clinically on physical examination, by tenderness, induration, and fluctuation, which are the most common physical findings [10]. Patients with these findings often may not tolerate a rectal examination. For that reason, clinical examination under general anesthesia is the method for diagnosing the disease or determining its extent [11].
Management of idiopathic perianal fistulas is primarily surgical and involves a fistulotomy or fistulectomy of the tracts, combined with drainage of an associated abscess [12]. The primary objectives are to eradicate the tract and drain all associated sites of infection while simultaneously preserving anal continence. Recurrence after surgical therapy is the most common problem. To maximize success, the surgeon must assess the relationship of the fistula to the sphincter complex to best preserve anal continence and to identify secondary tracts or abscesses, which are the primary source of recurrence [13]. Therefore, preoperative imaging is very important.
Imaging
Fistulography had been the only imaging technique available for demonstrating the anatomy of an anal fistula. In comparison with operative findings, fistulography was unreliable, with only 16% concordance and 12% of false positive findings of high extensions and rectal openings [14]. The anal sphincters and their relationship to the fistula cannot be visualized with fistulography [12]. Computed tomography (CT) with rectal and intravenous contrast administration can be used to analyze anal fistulas, but has a limited value to define fistulas and abscesses because of the poor resolution of soft tissues [15]. With the development of endosonography and MRI, the use of other techniques has diminished.
Endorectal ultrasound (EUS) can provide detailed imaging information on the rectal wall, anal sphincter and intersphincteric fistulas. The limited field of view, operator dependence, and suboptimal patient tolerance are the major disadvantages. EUS was compared with digital rectal evaluation and MR imaging regarding correct detection of the primary fistulas, with modality of each method being as follows: 61% with digital examination, 81% with anal endosonography, and 91% with MR imaging [16].
MRI Technique
In recent years, MRI has emerged as the leading imaging modality for preoperative classification of perianal fistulas. The first studies on cryptoglandular fistulas were performed with body-coil MRI and the true potential of MRI in detection of fistulas became evident [17,18]. The success of MRI in preoperative classification of perianal fistulas is a direct visualization of the tracts and abscesses combined with high soft tissue resolution. The ability of MRI to help not only accurately classify tracts but also identify the extention of the disease that otherwise would have been missed, could affect the patient outcome. Buchanan et al. [19] showed that surgery guided by MRI reduced further recurrence by 75% in patients with recurrent anal fistula. MRI is now considered by many to be the golden standard in assessing and classifying anal fistulas, and is equal or superior to examination under anesthesia [12,16,18,20].
The endoluminal anal coil and the body phased-array coils can be used. A good spatial resolution can be achieved by using the endoluminal anal coil, but these coils are poorly tolerated in symptomatic patients and have a limited field of view [21]. MRI examinations performed with body phased-array coils require no special patient preparation and are well tolerated. Advantages of the body phased-array coils include a larger field of view, which prevents fistula extensions from being overlooked.
An important advantage of MRI is the multiplanar capability. The imaged volume should extend to the levators, include the whole presacral space and the entire perineum, which are common sites for extensions. The imaging planes are correctly aligned with respect to the anal canal. A sequence in the sagittal plane is first performed. The transverse and coronal sequences must be aligned with the anal canal at the sagittal sequence.
T2-weighted sequences with or without fat suppression are essential in evaluation because they provide excellent soft-tissue contrast, and pathological processes including fistulas, secondary fistulous tracks and fluid collections can be clearly depicted. They appear as areas of high signal intensity in contrast to the sphincters, muscles, and fat, i.e. structures of lower signal intensity. To assess fistulous tracts within an acute abscess may be difficult due to high signal intensity on T2-weighted images of pus and edema, which may obscure the underlying fistula tracks. Unenhanced T1-weighted images provide an excellent anatomic overview of the sphincter complex, and the ischiorectal fossae. Fistulous tracks, inflammation, and abscesses, appear as areas of low to intermediate signal intensity and may not be distinguished from normal structures. T1-weighted contrast-enhanced fat-suppressed MRI sequences are used to distinguish inflamed tissues from normal perineal tissues and help to differentiate fluid and scarring/granulation tissue, which is important in distinguishing abscesses (Figures 1–9).
Figure 1.
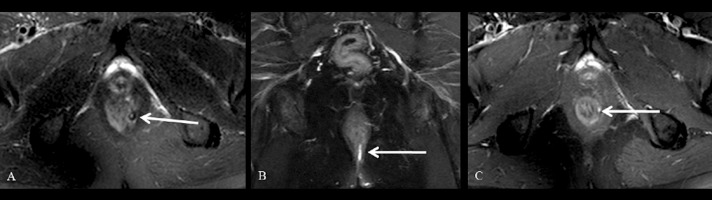
Grade 1 perianal fistula; linear intersphincteric. Axial (A) and coronal (B) fat-suppressed T2-weighted, axial contrast-enhanced fat-suppressed T1-weighted (C) MR images show the left intersphincteric fistula (arrows) surrounded by the external sphincter without an abscess or secondary branch. Hyperintense fistula tract on fat-supressed T2-weighted MR images (A, B arrows). Tract enhancement on contrast-enhanced fat-suppressed T1-weighted image (C arrow).
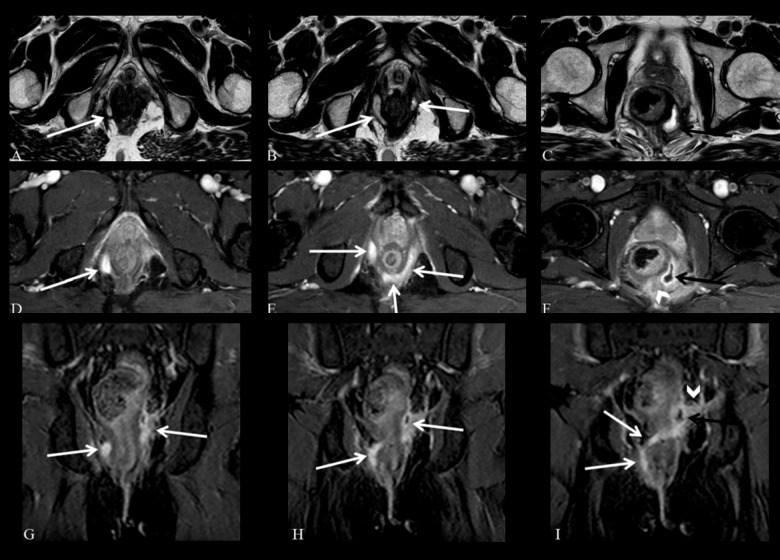
Figure 9.
Grade 5 perianal fistula; supralevator and translevator disease. Axial T2-weighted (A–C) and fat-suppressed T2-weighted (D–F), coronal contrast-enhanced fat-suppressed T1-weighted (G–I) MR images show a right translevator fistula (arrows) crossing the ischiorectal fossa which enters the intersphicteric space posterior to the anal canal and continues with a left supralevator abscess (black arrows) with inflammatory changes surrounding the rectum (arrowheads).
In theory, higher-field-strength MRI provides a better signal-to-noise ratio, which can be used to achieve increased temporal resolution, decreased imaging time, and increased spatial resolution. The increased spatial resolution has the potential to improve lesion visibility. Comparative studies with 1.5-T or 3.0-T have not been reported on [21]. The use of diffusion-weighted sequence for evaluating perianal fistulas has been reported on [22]. Because inflammatory tissues usually have high signal intensity at diffusion-weighted imaging [23], this technique is used as an adjunct to T2-weighted imaging for diagnosing anal fistulas.
Classification of Perianal Fistulas
The classification system, described by Parks et al. in 1976 [24], was primarily based on surgical findings and developed for surgical treatment. The principal finding in the classification was the primary tract’s relation to the external and internal sphincters and the levator ani muscle. They described the course and relationship of perianal fistulas to the sphincter mechanism with reference to the coronal plane [24]. There were four categories of fistulas distinguished: intersphincteric, transsphincteric, suprasphincteric and extrasphincteric. Superficial fistulas were not included in the original classification as they were considered to have a different etiology. The extension of the fistula was not included in the Parks’ classification. They may course in various directions and in different anatomical compartments.
As relevant findings of MRI could not be included in the Parks’ classification system, an MRI-based system was proposed. The St. James’s University Hospital classification for MRI [25] is an MRI-based grading system for perianal fistulas that was validated by surgically proved cases. The St James’s University Hospital classification, relates to the anatomy seen at MR images in both axial and coronal plane. This grading system deals not only with the primary tract but also with secondary tracts (branches) and associated abscesses, which is needed in pre-operative medical imaging.
Grade 1
Simple Linear Intersphincteric Fistula
In a simple linear intersphincteric fistula, the fistulous track extends from the skin of the perineum or the natal cleft. No secondary extentions or abscesses are detected within the sphincter complex. The enhancing track is seen in the plane between the sphincters and is entirely confined by the external sphincter (Figures 1 and 2).
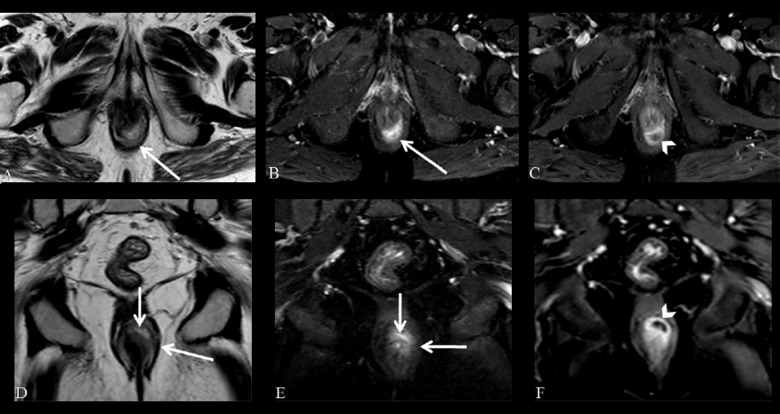
Figure 2.
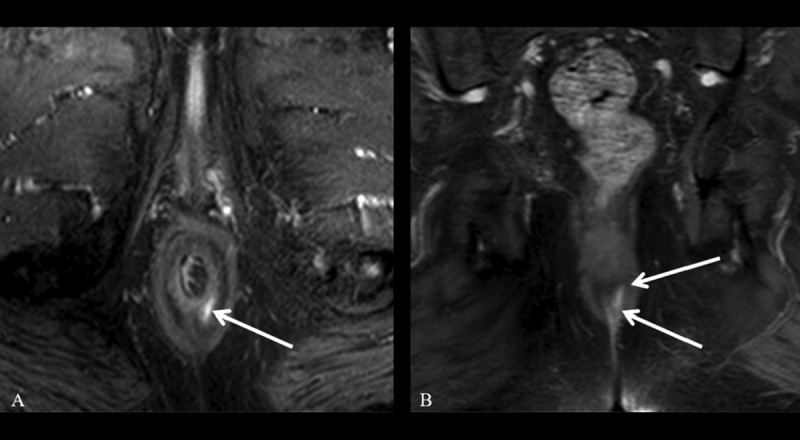
Grade 1 perianal fistula; linear intersphincteric. Axial (A) and coronal (B) contrast-enhanced fat-suppressed T1-weighted MR images show contrast enhancement of the left posterolateral intersphincteric fistula (arrows) surrounded by the external sphincter without an abscess.
Grade 2
Intersphincteric Fistula with Abscess or Secondary Track
Intersphincteric fistulas with an abscess or secondary track occur within the intersphincteric space. Secondary fistulous tracks may be of the horseshoe type, crossing the midline, or they may branch in the ipsilateral intersphincteric plane. This process is confined within the sphincter complex (Figures 3 and 4).
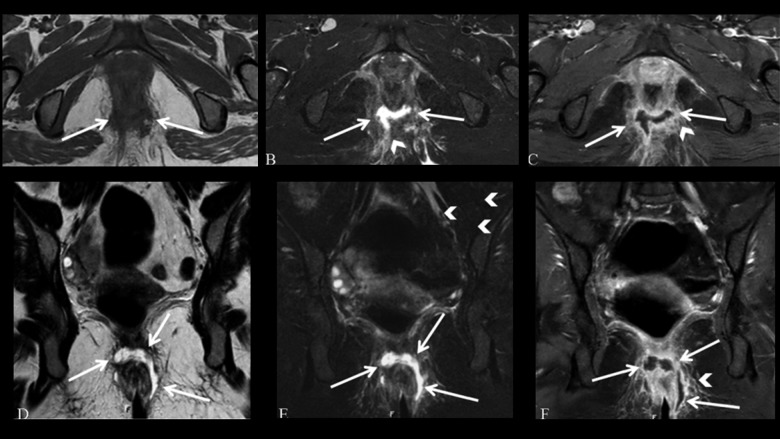
Figure 3.
Grade 2 perianal fistula; intersphincteric fistula with an abscess. Axial (A) and coronal (D) T2-weighted, axial (B) and coronal (E) fat-suppressed T2-weighted MR images show high-intensity fluid collection along the right posterolateral aspect of the anal canal (arrows). Axial (C) and coronal (F) contrast-enhanced fat-suppressed T1-weighted MR images show a peripherally enhancing abscess in the intersphincteric space (arrowheads).
Figure 4.
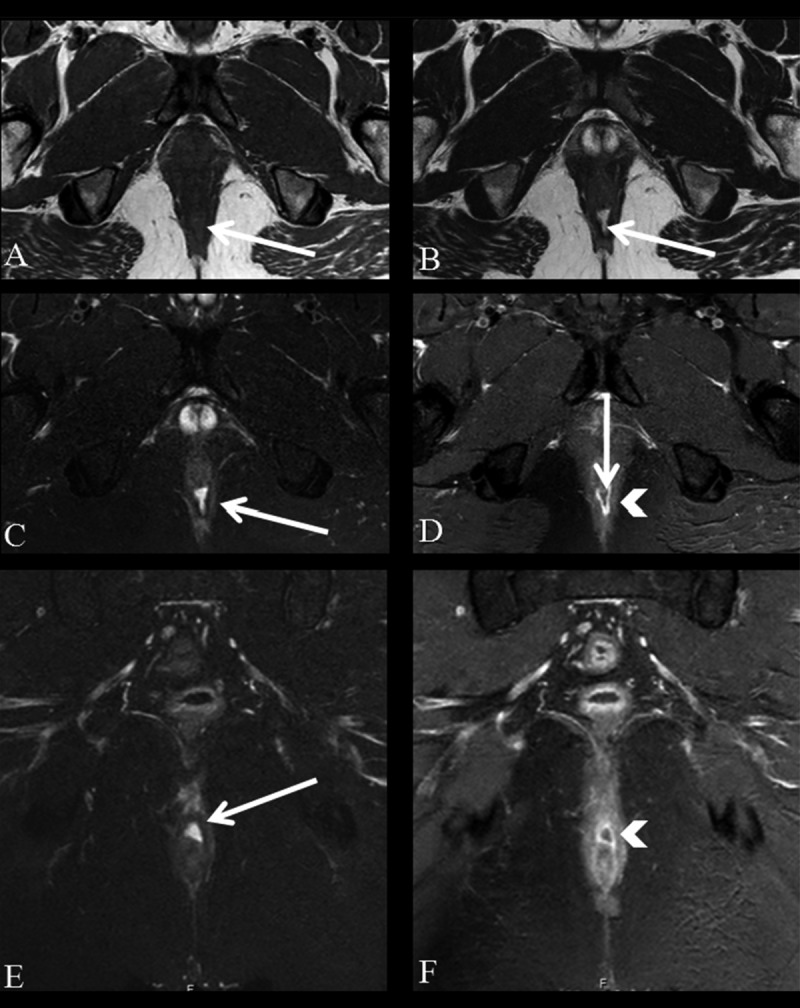
Grade 2 perianal fistula; intersphincteric fistula with an abscess. Fistulous tracks, inflammation and abscesses appear as areas of low to intermediate signal intensity and may not be distinguished from normal structures on T1-weighted images (A, arrow). Axial T2-weighted (B), axial (C) and coronal (E) fat-suppressed T2-weighted MR images show high-intensity fluid collection along the posterior wall of the anal canal (arrows). Axial (D) and coronal (F) contrast-enhanced fat-suppressed T1-weighted MR image shows an abscess which is peripherally enhanced (arrowheads) and contains a non-enhancing pus (D, arrow) in the intersphincteric space.
Grade 3
Trans-sphincteric Fistula
The trans-sphincteric fistula extends through both layers of the sphincter complex and reaches the skin through the ischiorectal and ischioanal fossae. These fistulas are not complicated by the secondary tracks or abscesses. Because these fistulas disrupt the integrity of the sphincter mechanism, their tracks must be excised by dividing both layers of the sphincter, thus risking fecal incontinence (Figures 5 and 6).
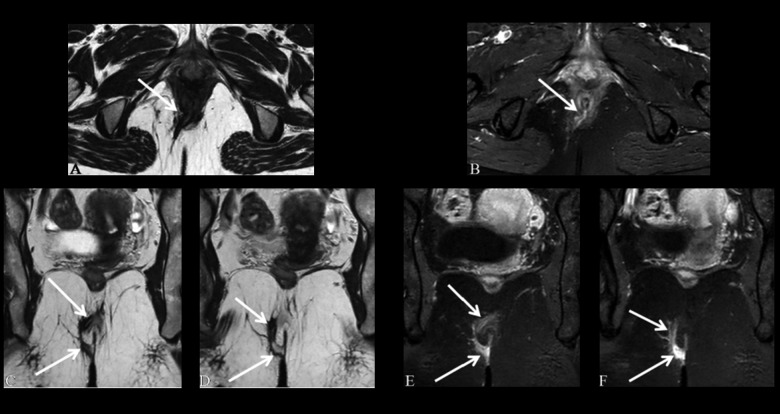
Figure 5.
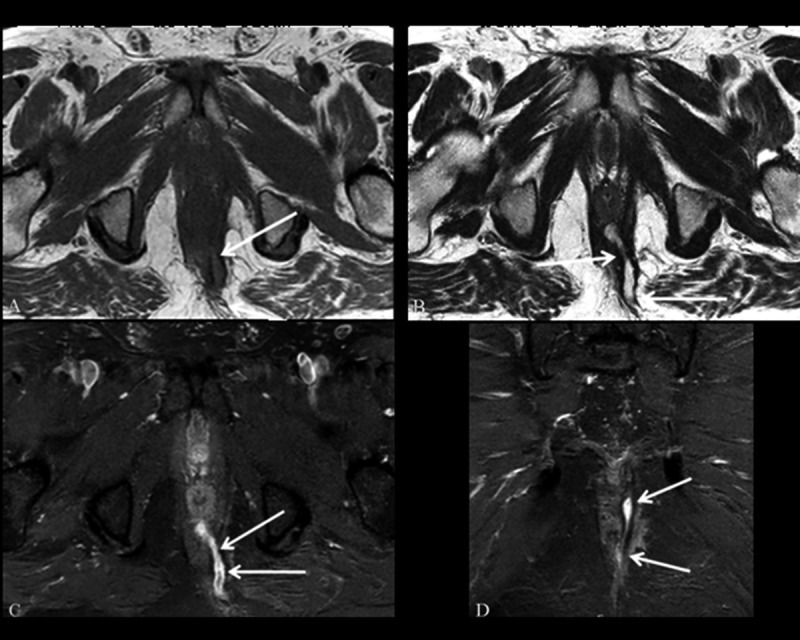
Grade 3 perianal fistula; transsphincteric fistula without an abscess. Axial T1-weighted (A) and T2-weighted (B), axial (C) and coronal (D) contrast-enhanced fat-suppressed T1-weighted MR images show the transsphincteric fistula (arrows) crossing the external sphincter. Axial (C), and coronal (D) contrast-enhanced fat-suppressed T1-weighted MR images show the highly enhancing transsphincteric fistula (arrows).
Figure 6.
Grade 3 perianal fistula; transsphincteric fistula without an abscess. Axial (A) and coronal (C, D) T2-weighted, axial (B) and coronal (E, F) contrast-enhanced fat-suppressed T1-weighted MR images show the transsphincteric fistula (arrows) crossing the external sphincter. Axial (B), and coronal (E, F) contrast-enhanced fat-suppressed T1-weighted MR images show the highly enhancing transsphincteric fistula (arrows).
Grade 4
Trans-sphincteric Fistula with Abscess or Secondary Track within the Ischiorectal Fossa
A trans-sphincteric fistula is complicated by an abscess or extention in the ischiorectal or ischioanal fossae (Figures 7 and 8).
Figure 7.
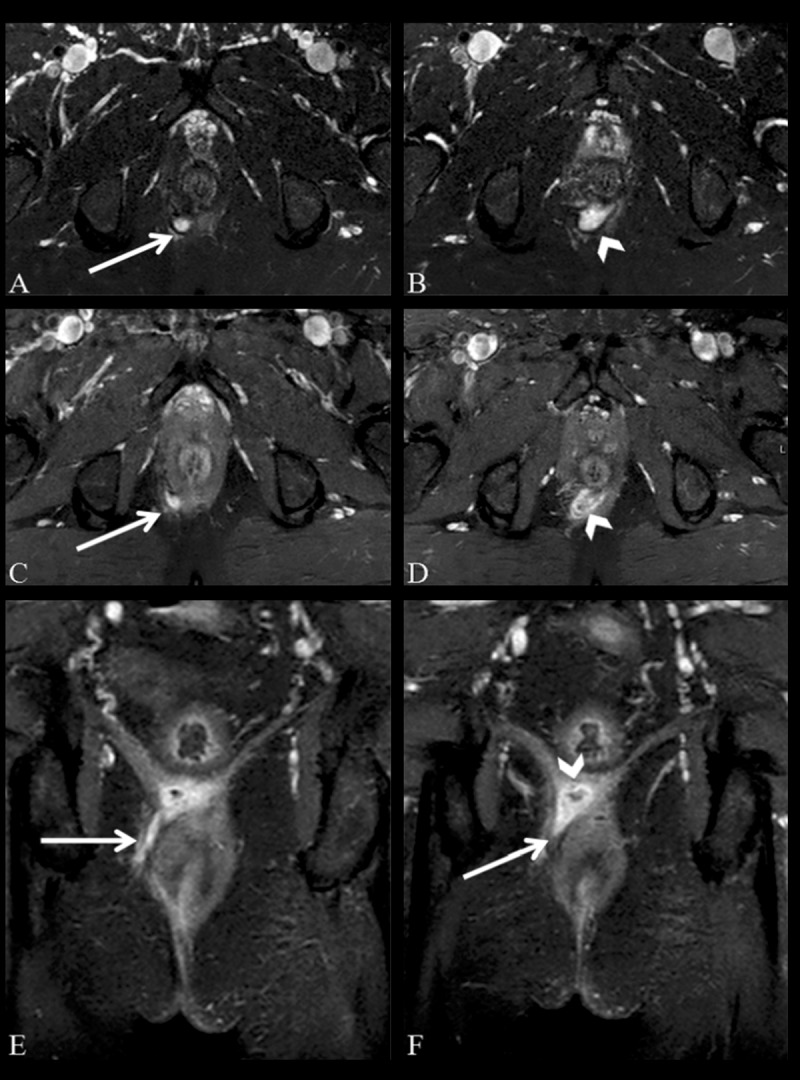
Grade 4 perianal fistula; transsphincteric fistula with an abscess. Axial fat-suppressed T2-weighted (A, B), axial (C, D) and coronal (E, F) contrast-enhanced fat-suppressed T1-weighted MR images show an abscess in the intersphincteric space, located posterior to the anal canal (arrowheads) and transsphincteric fistula (arrows).
Figure 8.
Grade 4 perianal fistula; transsphincteric fistula with an abscess. Axial T1-weighted (A) and fat-suppressed T2-weighted (B), coronal T2-weighted (D) and fat-suppressed T2-weighted (E), coronal and axial (C, F) contrast-enhanced fat-suppressed T1-weighted MR images show a horseshoe abscess in the intersphincteric space and extending to the ischioanal fossa (arrows) and prominent inflammation around the tract (arrowheads).
Grade 5
Supralevator and Translevator Disease
In rare cases, perianal fistula extends above the insertion of the levator ani muscle. Supralevator fistulas extend upward in the intersphincteric plane and over the top of the levator ani and then descend through the ischiorectal and ischioanal fossae to reach the skin. The translevator fistulas extend directly from their origin in the pelvis to the perineal skin through the ischiorectal and ischioanal fossae with no involvement of the anal canal (Figure 9).
MRI of Perianal Fistulas
The MRI examinations of the patients who were referred to two different university hospitals for imaging of perianal fistulas between 07.2012 and 03.2014 were reviewed retrospectively. MRIs were performed by using the Achieva 3T MRI and Ingenia 1.5 T MRI scanners (Philips Best, Netherlands) and Siemens Aera 1.5 T MRI scanners, (Siemens, Germany) with a body phased-array coil.
A total of 152 patients (115 men, 37 women) with a perianal fistula were detected. Perianal fistulas and infections were found in 13 patients who had malignancy (rectum Ca, prostate Ca etc.) or pelvic irradiation, and three patients had inflammatory bowel disease (i.e. 2 Crohn’s disease and 1 ulcerative colitis). A total of 136 patients (104 men, 32 women) with a perianal fistula were evaluated. Patients’ age was 18–80 years (mean 42.12 years). The most common complaints of the patients were anal pain and purulent discharge. The fistulas were classified with the St James’s University Hospital MR imaging – based grading system. Of the 136 patients, 70 (51.5%) had a grade 1 or simple linear intersphincteric fistula; 25 (18.4%) had a grade 2 or intersphincteric fistula with an abscess or secondary track; 16 (11.76%) had a grade 3 or transsphincteric fistula; 17 (12.3%) had a grade 4 or transsphincteric fistula with an abscess or secondary track in the ischiorectal or ischioanal fossa; and 2 (1.5%) had grade 5 or supralevator and translevator disease. Six fistulas, which were reported as a subsphincteric fistula (4.41%), were left unclassified according to the St James’s University Hospital classification system.
Morris et al. [26] mentioned that about 70% of all perianal fistulas were intersphincteric fistulas, while transsphincteric fistulas constituted 20% of the total. In another study, de Miguel Criado et al. [22], found that the transsphincteric type was the most common one. We found that 69.9% of all perianal fistulas were of intersphincteric type, whereas 24.2% were of transsphincteric type. Our results were consistent with those by Morris et al. [26]. MRI is the most commonly used imaging modality in patients with perianal fistulas in our institutions before treatment implementation. For that reason, we think that the simplier intersphincteric fistulas were the most common type in our patient group.
Conclusions
With MR imaging, perianal fistulas can be detected and classified accurately which is essential for appropriate surgical treatment, and decrease the incidence of recurrence and allows for side effects such as fecal incontinence to be avoided. Radiologists should be familiar with the anatomic and pathologic findings of perianal fistulas and classify them using MRI – based grading system, the St James’s University Hospital classification.
References
- 1.Nelson RL, Abcarian H. Epidemiology, Incidence and Prevalence of Fistula in Ano. In: Abcarian Herand., editor. Anal Fistula. Springer Science+Business Media; New York: 2014. pp. 1–7. [Google Scholar]
- 2.Corman ML. Classic articles in colon and rectal surgery. Hippocrates: on fistulae. Dis Colon Rectum. 1980;23(1):56–59. doi: 10.1007/BF02587204. [DOI] [PubMed] [Google Scholar]
- 3.Sainio P. Fistula in ano in a defined population. Incidence and epidemiologic aspects. Ann Chir Gynaecol. 1984;73:219–24. [PubMed] [Google Scholar]
- 4.Nelson RL. Anorectal Abscess fistulas. What do we know? SurgClin North Am. 2002;82:1139–51. doi: 10.1016/s0039-6109(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 5.Lunniss PJ, Jenkins PJ, Besser GM, et al. Gender differences in incidence of idiopathic fistula-in-ano are not explained by circulating sex hormones. Int J Colorectal Dis. 1995;10(1):25–28. doi: 10.1007/BF00337582. [DOI] [PubMed] [Google Scholar]
- 6.O’Donovan AN, Somers S, Farrow R, et al. MR imaging of anorectalCrohn’s disease: A pictorial essay. Radiographics. 1997;17:101–7. doi: 10.1148/radiographics.17.1.9017802. [DOI] [PubMed] [Google Scholar]
- 7.Stoker J, Wallner C. The anatomy of the pelvic floor and sphincters. In: Stoker J, Taylor S, DeLancey J, editors. Imaging Pelvic Floor Disorders. 2nd revised edition. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 1–29. [Google Scholar]
- 8.Cologne KG, Villanueva-Herrero AJ, Montaño-Torres E, Ortega EA. Clinical Assessment and Imaging Modalities of Fistula in Ano. In: Abcarian H, editor. Anal Fistula. Springer Science+Business Media; New York: 2014. pp. 31–37. [Google Scholar]
- 9.Parks AG. Pathogenesis and treatment of fistula-in-ano. BMJ. 1961;1(5224):463–69. doi: 10.1136/bmj.1.5224.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abcarian H. Anorectal infection: abscess-fistula. Clin Colon Rectal Surg. 2011;24(1):14–21. doi: 10.1055/s-0031-1272819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caliste X, Nazir S, Goode T, et al. Sensitivity of computed tomography in detection of perirectal abscess. Am Surg. 2011;77(2):166–68. [PubMed] [Google Scholar]
- 12.Berman L, Israel GM, McCarthy SM, et al. Utility of magnetic resonance imaging in anorectal disease. World J Gastroenterol. 2007;13:3153–58. doi: 10.3748/wjg.v13.i23.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006;239:18–33. doi: 10.1148/radiol.2391041043. [DOI] [PubMed] [Google Scholar]
- 14.Jordán J, Roig JV, García-Armengol J, et al. Risk factors for recurrence and incontinence after anal fistula surgery. Colorectal Dis. 2010;12:254–60. doi: 10.1111/j.1463-1318.2009.01806.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers HC, Schulpen T. Fistulography for fistula-in-ano: is it useful? Dis Colon Rectum. 1985;28(2):103–4. doi: 10.1007/BF02552656. [DOI] [PubMed] [Google Scholar]
- 16.Yousem DM, Fishman EK, Jones B. Crohn disease: perirectal and perianal findings at CT. Radiology. 1988;167(2):331–34. doi: 10.1148/radiology.167.2.3357940. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan GN, Halligan S, Bartram CI, et al. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233(3):674–81. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 18.Lunniss PJ, Armstrong P, Barker PG, et al. Magnetic resonance imaging of anal fistulae. Lancet. 1992;340(8816):394–96. doi: 10.1016/0140-6736(92)91472-k. [DOI] [PubMed] [Google Scholar]
- 19.Lunniss PJ, Barker PG, Sultan AH, et al. Magnetic resonance imaging of fistula-in-ano. Dis Colon Rectum. 1994;37(7):708–18. doi: 10.1007/BF02054416. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan G, Halligan S, Williams A, et al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet. 2002;360(9346):1661–62. doi: 10.1016/S0140-6736(02)11605-9. [DOI] [PubMed] [Google Scholar]
- 21.Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33(1):26–30. doi: 10.1007/s00261-007-9309-y. [DOI] [PubMed] [Google Scholar]
- 22.de Miguel Criado J, del Salto LG, Rivas PF, et al. MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics. 2011;32(1):175–94. doi: 10.1148/rg.321115040. [DOI] [PubMed] [Google Scholar]
- 23.Hori M, Oto A, Orrin S, et al. Diffusion-weighted MRI: a new tool for the diagnosis of fistula in ano. J Magn Reson Imaging. 2009;30(5):1021–26. doi: 10.1002/jmri.21934. [DOI] [PubMed] [Google Scholar]
- 24.Purwar A, Rathore RKS, Husain N, et al. Biological correlates of diffusivity in brain abscess. Magn Reson Med. 2005;54:878–85. doi: 10.1002/mrm.20645. [DOI] [PubMed] [Google Scholar]
- 25.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 26.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623–35. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]