Abstract
The acute toxicity of cadmium, copper, and zinc to white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) were determined for 7 developmental life stages in flow-through water-only exposures. Metal toxicity varied by species and by life stage. Rainbow trout were more sensitive to cadmium than white sturgeon across all life stages, with median effect concentrations (hardness-normalized EC50s) ranging from 1.47 µg Cd/L to 2.62 µg Cd/L with sensitivity remaining consistent during later stages of development. Rainbow trout at 46 d posthatch (dph) ranked at the 2nd percentile of a compiled database for Cd species sensitivity distribution with an EC50 of 1.46 µg Cd/L and 72 dph sturgeon ranked at the 19th percentile (EC50 of 3.02 µg Cd/L). White sturgeon were more sensitive to copper than rainbow trout in 5 of the 7 life stages tested with biotic ligand model (BLM)-normalized EC50s ranging from 1.51 µg Cu/L to 21.9 µg Cu/L. In turn, rainbow trout at 74 dph and 95 dph were more sensitive to copper than white sturgeon at 72 dph and 89 dph, indicating sturgeon become more tolerant in older life stages, whereas older trout become more sensitive to copper exposure. White sturgeon at 2 dph, 16 dph, and 30 dph ranked in the lower percentiles of a compiled database for copper species sensitivity distribution, ranking at the 3rd (2 dph), 5th (16 dph), and 10th (30 dph) percentiles. White sturgeon were more sensitive to zinc than rainbow trout for 1 out of 7 life stages tested (2 dph with an biotic ligand model–normalized EC50 of 209 µg Zn/L) and ranked in the 1st percentile of a compiled database for zinc species sensitivity distribution. Environ Toxicol Chem 2014;33:2259–2272. © 2014. The Authors. This article is a US government work and, as such, is in the public domain in the United States of America. Environmental Toxicology and Chemistry published byWiley Periodicals, Inc. on behalf of SETAC. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Keywords: White sturgeon, Early life stages, Metals, Acute toxicity, Biotic ligand model
INTRODUCTION
White sturgeon (Acipenser transmontanus) in the trans-boundary reach of the Upper Columbia River (WA, USA) have experienced poor recruitment. Recruitment of white sturgeon in the Columbia River essentially has been nonexistent since the early 1980s 1. The Columbia River population of white sturgeon from the Grand Coulee Dam area upriver to Revelstoke, Canada is estimated to be approximately 1400 adults with a predicted population decline of 50% within the next 10 yr; white sturgeon are predicted to become functionally extinct within the next 40 yr as a result of sustained recruitment failure, senescence, and death of the adult population 2. Because embryos and early life stage larvae rarely are seen in the river, and older juveniles (9–10 mo old) that are released from hatcheries survive well and have good body condition factors, the recruitment failures may reflect sensitivity of 1 or more early life stages of white sturgeon to contaminants 3.
White sturgeon declines have been attributed to various factors such as habitat degradation, water quality impairment, genetic bottlenecks, and predation by introduced species 4–8. Additionally, historic and contemporary metal mining and smelting activities have resulted in the release of metals into the Upper Columbia River, leading to concerns that metal toxicity might also be a factor that could affect white sturgeon recovery 2. Contaminants accumulating at the sediment–water interface could potentially render them bioavailable for absorption, ingestion, and aqueous exposure 9. Because white sturgeon enter a negative phototaxis phase and begin hiding within the substrate around 5 d to 7 d after hatching 9,10, vulnerability to contaminant exposure increases while larval sturgeon are inhabiting the sediment-water interface. Limited toxicity data suggest that early life stages of white sturgeon are sensitive to copper 11–13. There is limited information available to define acute or chronic toxicity thresholds for white sturgeon for metals such as cadmium, copper, and zinc (H. Botcher, US Environmental Protection Agency, Seattle, WA, written communication) and data on other sturgeon species vary considerably in their sensitivity 14.
Recent studies evaluating the effects of cadmium, copper, and zinc to white sturgeon have shown that copper was more toxic to early life stage sturgeon compared with other aquatic species 11–13. The first objective of the present study was to determine the relative acute sensitivity of white sturgeon and rainbow trout (Oncorhynchus mykiss) at various stages of development to cadmium, copper, and zinc in aqueous exposures and identify the most sensitive age group for each species. A second objective was to determine if rainbow trout would be an effective surrogate species for white sturgeon based on their sensitivity to metals. The toxicity data generated from the present study were used to evaluate the level of protection of US Environmental Protection Agency (USEPA) acute water-quality criteria (AWQC) or Washington State water-quality standards (WQS) for cadmium, copper, or zinc to early life stage white sturgeon inhabiting the Upper Columbia River.
A companion study evaluated the chronic toxicity of cadmium, copper, zinc, or lead to early life stages of white sturgeon in water-only exposures and also tested rainbow trout under similar test conditions to determine the relative sensitivity between the 2 species 15. A report by Ingersoll and Mebane 16 provides additional supporting information regarding test methods and toxicity and chemistry data for both studies. Results of the present study will be used as part of a baseline ecological risk assessment being conducted at the Upper Columbia River in eastern Washington State.
MATERIALS AND METHODS
Culture of test organisms
White sturgeon were obtained from the Washington Department of Fish and Wildlife Program (Sherman Creek Hatchery, Kettle Falls, WA). Newly fertilized eggs were received at the US Geological Survey, Columbia Environmental Research Center (CERC; MO, USA) on 1 July 2010, approximately 36 h after fertilization took place. The embryos were products of 3 adult male and 3 adult female crosses, for a total of 9 combinations. In total, approximately 31 000 embryos were sampled impartially from all 9 combination crosses. The embryos were shipped overnight in plastic bags packed in coolers chilled to 10 °C using ice packs. On arrival at CERC, sturgeon embryos were maintained at 10 °C in their shipping bags until the water in the culture holding tank was chilled to 10 °C using in-line chiller units (Aquatic EcoSystems), and the embryos were then placed in 6-liter MacDonald hatching jars (Aquatic EcoSystems) with flowing well water diluted with deionized water to a hardness of approximately 100 mg/L as calcium carbonate (CaCO3). The temperature was adjusted on the chiller units and was increased 1 °C/d until the target temperature of 15 °C was reached. The 100 mg/L hardness culture and toxicity test water was prepared in 2 polypropylene tanks (7000-L tanks) by diluting well water of a hardness of approximately 300 mg/L as CaCO3 with deionized water to a hardness of approximately 100 mg/L as CaCO3 (alkalinity of approximately 90 mg/L as CaCO3, pH of approximately 8.0, and dissolved organic carbon [DOC] of approximately 0.4 mg C/L), which approximated the water-quality characteristics of the Upper Columbia River inhabited by white sturgeon 13,17). Water samples were collected weekly to measure water-quality characteristics of the culture water, including measures of dissolved oxygen, temperature, conductivity, pH, alkalinity, hardness, and total ammonia. The average water-quality characteristics for culture water were as follows: dissolved oxygen, 8.3 ± 1.4 mg/L; temperature, 15.7 ± 1.17 °C; conductivity, 260 ± 4.1 µS/cm2 at 25 °C; pH, 7.9 ± 0.09; alkalinity, 94 ± 2.6 mg/L as CaCO3; hardness, 104 ± 2.7 mg/L as CaCO3; and total ammonia, 0.18 ± 0.12 mg/L as nitrogen. The sturgeon culture was maintained with a 16:8-h light:dark photoperiod with an average light intensity ranging from 280 lux to 300 lux. Sturgeon started hatching 8 d after fertilization and continued for a period of 4 d. The date of hatch was established as the day when more than 50% of the eggs hatched (11 d postfertilization). The yolk sac larvae were then transferred to a 1850-L flow-through fiberglass tank for holding until testing. Larvae were fed 1-d-old brine shrimp (Artemia sp.) nauplii (Brine Shrimp Direct) starting 1 wk before the start of exogenous feeding. At 18 d posthatch (dph), they were transitioned to chopped and then whole live oligochaetes (Lumbriculus variegatus; California blackworm). Once larval sturgeon were feeding actively, BioDiet Starter #2 (Bio-Oregon) a semi-moist commercial food, also was provided every 3 h using an automated feeder.
More than 50% of the white sturgeon eggs were nonviable (received as non-fertilized eggs). Elevated mortality (17%) of larvae was observed during the transition phase to exogenous feeding, which was not unexpected because this is a critical developmental stage. As a precautionary measure to ensure adequate numbers of individuals for testing, a second batch of 2100 larval white sturgeon (24 dph) were obtained from Washington Department of Fish and Wildlife Program (Columbia Basin Hatchery). These larval white sturgeon were cohorts from the same adult crosses that previously were received as eggs and thus were of the same age and parentage. The second batch of white sturgeon was acclimated to culture conditions for at least 13 d before use in acute exposures. A side-by-side comparison of cohort sensitivity to acute copper exposure proved comparable 50% lethal concentration (LC50) and 50% effect concentration (EC50) estimates, indicating the 2 groups of sturgeon were similar in their sensitivity to copper 15.
The rainbow trout (Erwin/Arlee strain) were obtained from the US Fish and Wildlife Service, Ennis National Fish Hatchery (Ennis, MT). Approximately 10 000 trout eggs were received as eyed eggs and held in incubators at 12 °C in flowing well water at a hardness of 300 mg/L, then slowly transitioned to 100 mg/L hardness water for 48 h. Approximately 4% of the eggs did not hatch. Yolk sac larvae (17 dph) were then transferred out of the incubators into 500-L flow-through tanks immediately before yolk sac depletion and swim-up, where the trout were maintained at 12 °C under water and lighting conditions similar to those of the sturgeon cultures. Trout at exogenous feeding (20 dph) were fed 1-d-old brine shrimp (Artemia sp.) nauplii (Brine Shrimp Direct), transitioning to trout chow (Otohime B1–C1) and flake food (Worldwide Aquatics) as the larval trout developed.
Toxicity testing
Each metal exposure was performed using a modified Mount and Brungs 18 diluter following guidelines outlined by the ASTM International 19–21. The water used in testing was targeted for a hardness of 100 mg/L and DOC of 0.4 mg/L for comparability with earlier testing at the CERC laboratory 11 and approximated the water-quality characteristics of the Upper Columbia River inhabited by white sturgeon. Sturgeon tests were performed at 15 °C and trout tests at 12 °C. Three intermittent flow-through proportional diluters were used (1 for each metal) and provided a control and 5 concentrations of cadmium, copper, or zinc through 50% serial dilutions. The toxicants were delivered to the diluters using a Hamilton® syringe pump (Hamilton Company). In rounds 1 through 3 for the 3 youngest life stages, 4 glass replicate chambers were held in a temperature-controlled water bath (152.5 cm × 84 cm × 35.5 cm). An in-line 4-way flow splitter was attached to each delivery line to partition the water flow to each of 4 replicate chambers in the water bath. A photograph depicting the diluter and 4-way splitter setup is provided in Supplemental Data, Figure S1. Each test chamber (12 × 21.5-cm jar) had a hole (4-cm diameter) in the side covered with 30 mesh (0.5-mm opening) stainless steel screen containing 1 L of water. Test solution flowed directly into test chambers, and excess water overflowed to surrounding aquaria through the screen windows, so there was no exchange of test water among replicates. The diluter provided approximately 250 mL of water to each chamber every 30 min (resulting in 12 volume additions per day to each replicate test chamber). There was a similar setup in round 4, except larger test chambers (15 × 25.5-cm jar with a mesh covered hole cut in the side so the jar, which held 2 L of water) were used to maintain acceptable loading rates of sturgeon or trout. The diluter provided 250 mL of water to each chamber every 30 min (6 volume additions per day). In rounds 5 and 6, 4 glass replicate chambers were held in each of 6 rectangular 40-L glass aquaria in a temperature-controlled water bath. Each test chamber (28 cm × 13.5 cm × 25 cm) had a hole (4-cm diameter) in the side covered with 30 mesh (0.5-mm opening) stainless steel screen and contained 7 L of water. In rounds 5 and 6, the diluter also provided 250 mL of water to each chamber every 30 min (resulting in 2 volume additions per day). The same design was used for round 7, except that there were 4 replicate chambers in each of 12 40-L aquaria with a total of 8 replicate chambers for each metal concentration. Test conditions for performing the acute toxicity tests were adjusted by increasing or decreasing metal concentrations, increasing water turnover rate, decreasing the number of fish per replicate chamber, or increasing the number of replicates according to life stage. The number of fish, number of replicates, and mass of fish in each exposure chamber were established in accordance with guidance provided in ASTM International 21. The fish loading rate in test chambers did not exceed the ASTM International 19 guidelines of 1 g/L of solution passing through a chamber each day at the end of the exposures and did not exceed 10 g/L in the chamber at any time (at less than 17 °C). Moreover, water-quality conditions, measured metal concentrations, and ammonia concentrations were not affected by various loading densities throughout the exposures.
Metal salts were obtained from Sigma-Aldrich. A stock solution of each metal (copper II sulfate pentahydrate, CuSO4·5H2O; cadmium chloride hemi-pentahydrate, CdCl2·2 ½ H2O; and zinc chloride, ZnCl2) was prepared by adding the American Chemical Society reagent grade (>98% purity) metals to deionized water. Test stock solutions were prepared 2 d before the start of exposures in volumetric flasks and wrapped with aluminum foil to reduce exposure to ambient light. Stock solutions were then delivered to the diluters using a Hamilton Syringe Dispenser (MicroLab® 600 Series; Hamilton Company) and the diluters and test chemicals cycled for at least 2 d before starting the exposures. Fish were not fed 24 h before and during the acute exposures.
Water quality
Water temperature was monitored daily in select exposure chambers within each diluter at the control, low, medium, and high treatments. Water quality (dissolved oxygen, pH, conductivity, hardness, alkalinity, ammonia) was measured from a select replicate for each metal in the control, low, medium, and high concentrations on test day 0 and on test day 4 following standard methods 22.
Chemical analysis
A 20-mL filtered sample was collected for metal analyses from 1 random replicate chamber for each treatment on test day 0 (just before adding test organisms) and on test day 4 at the end of the exposures. One filtration blank (obtained using high-purity deionized water) was processed with each set of samples. In addition, a duplicate sample was collected from the medium treatment, and unfiltered samples were collected from a control and a medium treatment. The unfiltered control sample was collected to check for filter contamination specifically with a test water sample, whereas the unfiltered medium treatment was sampled to check for the presence of colloidal precipitates (>0.45 µm) that might be present as a result of the high exposure concentrations for some of the toxicity tests. Each sample was acidified to 1% v/v with high-purity, 16M nitric acid and stored for up to 3 mo before analysis was done by inductively coupled plasma mass spectrometry (USEPA method 6020a, ICP-MS 23).
Samples obtained for major cation (calcium, magnesium, sodium and strontium) and major anion (fluoride, chloride, nitrite, nitrate, and sulfate) analyses were collected, filtered, and preserved on test day 2 of the exposures in the same manner as those samples collected for metals. For the white sturgeon toxicity tests, 1 random replicate from the control and 1 random replicate from the medium treatment of each metal tested were sampled, whereas for rainbow trout toxicity tests, samples were collected only from the control and medium treatments of the Cu exposure. One filtration blank (obtained using high-purity deionized water) was processed with each set of samples. Analysis was done by inductively coupled plasma atomic emission spectroscopy (ICPAES) according to USEPA method 200.7 24 by Laboratory and Environmental Testing (LET) Labs.
Samples obtained for DOC analyses were each drawn using an oven-baked (450 °C) glass pipet and collected in a 60-mL, amber glass bottle fitted with a Teflon®-lined cap. Sample bottles were rinsed and then filled with high-purity deionized water to prevent potential airborne contamination until use. Filtration blanks were processed with each set of samples using commercially available total organic carbon (TOC) free water (<0.05 mg C/L; Ep Scientific Products, Thermo Fisher Scientific). Approximately 20 mL of sample was used to rinse each bottle before collection of a 60 mL sample. Samples were stored at 4 °C for up to 48 h before filtration and preservation to a pH <2 with 0.1 mL 9 molar sulfuric acid (H2SO4). Filtration was done with a vacuum applied to a 47-mm diameter, 0.45-µm pore size nylon membrane that was mounted on an all-glass filtration support mount. Before each sample filtration, a new membrane was inserted and the membrane and glass support apparatus were rinsed under vacuum with a minimal volume of TOC-free water. Preserved samples were stored at 4 °C for up to 28 d (but more typically 7 to 14 d) before analysis according to USEPA method 415.2 25. Filtration of the numerous blanks (TOC-free water) documented that DOC was not leached by the nylon membrane. Further documentation regarding absence of artifacts caused either by sorbtion or by leaching during filtration for either DOC or metals (filtered using PES membranes) can be found in Ingersoll and Mebane 16.
Data analysis
The mean measured concentrations of each metal were used to calculate 4-d effect concentrations for each species at each life stage. The Toxicity Relationship Analysis Program 26 was used to calculate the LC50 based on mortality and the EC50 based on mortality, loss of equilibrium, and immobilization along with 95% confidence intervals (CIs) for each life stage based on the measured metals concentrations. When LC50 or EC50 concentrations could not be estimated because the data did not meet the specific requirements for the model (insufficient mortality), LC50s or EC50s were reported as less than the highest test concentration when 100% mortality occurred or greater than the lowest test concentration when there was no significant (p > 0.05) difference from the controls. Effect concentrations for 10% and 20% reduction (EC10 and EC20, respectively) for mortality, loss of equilibrium, and immobilization also were estimated using the Toxicity Relationship Analysis Program and are provided in Ingersoll and Mebane 16. In addition to the LC50s or EC50s, TOXSTAT® 27 was used to determine the no-observed-effect concentration (NOEC) and the lowest-observed-effect concentration (LOEC) by analysis of variance with mean comparison made by one-tailed Dunnett 's test 28. Steel 's Many-One Rank test 28 was used when the data were not distributed normally or had heterogeneous variances. The level of statistical significance was set at p ≤ .05. These data are provided in Ingersoll and Mebane 16.
Water chemistry normalization of effect concentrations and species sensitivity
When comparing toxicity test results among different studies, the data should be normalized to account for differing water-quality characteristics. Similarly, the relationship between water-quality characteristics and toxicity can be used to extrapolate effects concentrations from laboratory test conditions to ambient conditions, such as from the laboratory waters tested in the study to the water-quality characteristics of the Upper Columbia River. For cadmium, this data normalization was done using hardness-toxicity relations described in Mebane 29. For copper and zinc, biotic ligand models (BLMs) were used to normalize effect concentrations to a common set of water-quality characteristics (USEPA 30 for copper; DeForest and VanGenderen, 31 for zinc). A summary of the water composition and original effects concentrations that were used in the BLM modeling with copper, the modeled critical effects accumulation (CA) values, and resulting effects concentrations extrapolated to a moderately hard BLM standard water are provided in Ingersoll and Mebane 16.
When evaluating the sensitivity of white sturgeon and rainbow trout relative to other species tested in other waters, effect concentrations were ranked in a species sensitivity distribution for each metal using databases used by the USEPA to derive AWQC. The EC50 concentrations for the most sensitive life stage for white sturgeon and for rainbow trout in the study were then compared with the final acute value to assess sensitivity in relation to the AWQC. When evaluating the protection of the AWQC (that is, criteria to protect against short-term exposures, also called criterion maximum concentration, or CMC, in USEPA criteria documents) to white sturgeon and rainbow trout, the EC50s obtained in the study were compared with 2 times the CMC. The reason for comparing test values to 2 times the CMC instead of directly to the CMC may not be intuitive, and takes some explanation. In USEPA criteria derivation, 2 times the CMC is the same as the final acute value (FAV). The FAV effectively is an EC50 that represents a hypothetical species with sensitivity equal to the 5th percentile of the species sensitivity distribution (SSD). The SSD, despite the common use of the word species, is calculated from the rank-ordered distribution of all available genus mean acute values 32. In the criteria development, the FAV is divided by 2 to extrapolate from a concentration that would likely be extremely harmful to sensitive species in short-term exposures (kill 50% of the population) to a concentration expected to kill few, if any, individuals 32. To maintain a consistent basis for comparison (test EC50s as compared with the EC50 representing the 5th percentile most sensitive species), test EC50s were compared with 2 times the CMC. Acute EC50s were compared with the FAVs used to develop AWQC or the FAVs used to develop the Washington State acute WQS to determine if effect concentrations for white sturgeon and rainbow trout were below the nationally recommended WQC or Washington State WQS.
RESULTS
Water temperature during the exposures was consistently 15 ± 1 °C during the white sturgeon exposures and 12 ± 1 °C during the rainbow trout exposures. Concentrations of ammonia and dissolved oxygen were within acceptable limits throughout all of the exposures for each metal at each life stage for each species 19–21. Total ammonia concentration was <0.34 mg nitrogen/L during all exposures for each species, and mean dissolved oxygen concentrations during all metal exposures ranged from 8.5 mg/L to 9.7 mg/L in the sturgeon tests and from 8.7 mg/L to 10.8 mg/L in the trout tests. Conductivity, pH, hardness, and alkalinity remained consistent throughout the duration of the individual tests for the sturgeon and trout exposures. The extensive water quality measurements associated with the exposures are provided in Supplemental Data, Table S1. The major cation and anion concentrations remained consistent throughout the exposures for all life stages tested for each species (Supplemental Data, Tables S2 and S3). The DOC concentration of the test waters in the study was based on an average concentration of 0.4 mg/L.
Chemical analyses for copper, cadmium, and zinc indicated that mean concentrations of each metal typically ranged from 80% to 120% of the nominal concentrations for the acute exposures for each species. The mean metal concentrations were calculated using test day 0 and test day 4 values for each exposure. During round 4 of the white sturgeon exposures and round 5 of the rainbow trout exposures, measured metal concentrations were below 80% of the nominal concentrations on test day 0; therefore, only the test day 4 measured concentrations were used for calculation of the acute effect concentrations for round 4 of the sturgeon exposures and round 5 of the trout exposures. Across all rounds of acute tests, the mean relative percent change between concentrations measured on test day 4 from that of test day 0 for rounds 1 through 5 ranged as follows: for white sturgeon exposures, Cu, +8% to +18%; Cd, +6% to +28%; Zn, +15% to +31%; for rainbow trout exposures, Cu, +4% to +17%; Cd, –3% to +3%; Zn, +7% to +12%.
White sturgeon exposures
There was no mortality in the control treatments during the 4-d exposures across each life stage for each metal exposure, except for the exposures started with 16 dph white sturgeon. Sturgeon mortality in the control treatments during exposures started with 16 dph fish was 20% for the cadmium exposure, 35% for the copper exposure, and 30% for the zinc exposure and thus greater than the test acceptability requirement of 10% 33. Therefore, the LC50 and EC50 for the 16 dph white sturgeon life stage were classified as non-definitive effect concentrations.
Metal toxicity varied across life stages as reflected by the mortality, loss of equilibrium, and immobilization toxicity endpoints (Figure 1A). The EC50s proved to be a more sensitive toxicity endpoint where loss of equilibrium and immobilization in addition to mortality were used to estimate effect concentrations (Figure 1A; Tables3). Of the surviving individuals from each metal exposure, loss of equilibrium or immobilization generally increased with increasing metal concentration by test day 4 (Figure 2).
Figure 1.
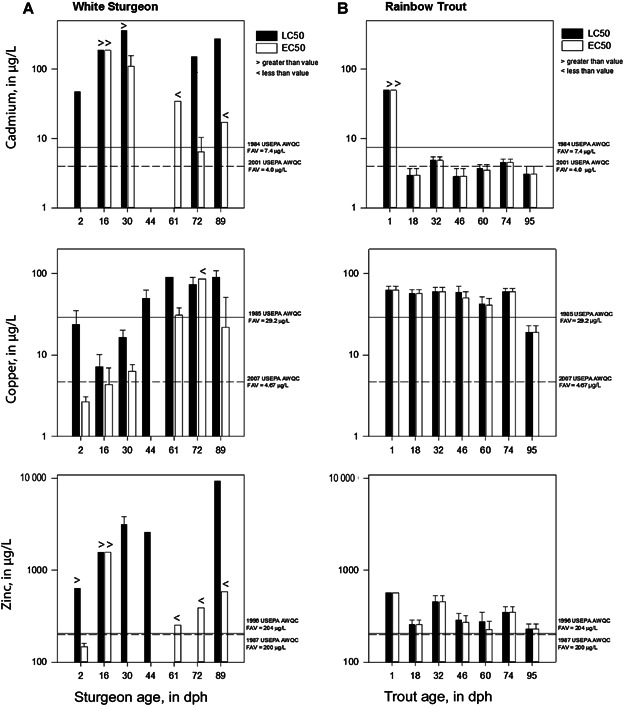
Acute 4-d median lethal concentration (LC50) or median effect concentration (EC50) for cadmium, copper, and zinc for (A) white sturgeon (Acipenser transmontanus) and (B) rainbow trout (Oncorhynchus mykiss) at various life stages, compared with US Environmental Protection Agency (USEPA) hardness-based ambient water-quality criteria (AWQC) adjusted to test water-quality characteristics. Error bars represent 95% confidence limts. FAV = final acute value; dph = days posthatch.
Table 3.
Zinc LC50 or EC50 4-d acute value estimates for white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss)
| Species | Life stage (dph) | Average fish weighta (g) | Average fish lengtha (cm) | LC50 (µg/L), mortality onlyb | EC50 (µg/L), mortality + LOE + immobilizationb> | BLM-normalizedc LC50 or EC50 (µg/L) |
|---|---|---|---|---|---|---|
| Sturgeon | ||||||
| 2d | 0.04 (0.0) | NM | >634 | 146.7 (134.4–160.1) | >928 / 209 | |
| 16e | 0.13 (0.17) | 21 (0.67) | >1575 | >1575 | >2161 | |
| 30 | 0.17 (0.04) | 30.6 (2.72) | 3109 (2499–3868) | 3109 (2499–3868) | 3793 | |
| 44 | 0.6 (0.15) | 49.3 (5.29) | >2610 | >2610 | >3088 | |
| 61 | 1.16 (0.41) | 62.4 (8.47) | <253 | <253 | <292 | |
| 72 | 2.02 (0.80) | 77.7 (11.08) | <391 | <391 | <450 | |
| 89 | 3.73 (1.06) | 97.57 (10.48) | <9330 | <586 | <10 487 / <657 | |
| Trout | ||||||
| 1 | 0.08 (0.12) | 14.3 (1.74) | >571 | >571 | >726 | |
| 18 | 0.10 (0.02) | 24.33 (1.06) | 253.2 (223.3–287.1) | 253.2 (223.3–287.1) | 306 | |
| 32 | 0.12 (0.03) | 26.67 (1.97) | 448.9 (381.3–528.7) | 448.9 (381.3–528.7) | 540 | |
| 46 | 0.22 (0.06) | 31.3 (3.02) | 282 (232.8–348.8) | 267.2 (220.9–323.2) | 409 / 389 | |
| 60 | 0.32 (0.07) | 36.8 (3.16) | 268.5 (206.7–348.8) | 223.8 (177.0–282.0) | 318 / 265 | |
| 74 | 0.39 (0.13) | 39.8 (3.55) | 345.9 (296.5–403.5) | 345.9 (296.5–403.5) | 436 | |
| 95d | 0.70 (0.18) | 45.43 (3.89) | 227.9 (200.5–259.2) | 227.9 (200.5–259.2) | 254 | |
Values in parentheses for average fish weight and average fish length (n = 10 for white sturgeon; n = 30 for rainbow trout) are the standard deviation.
Values in parentheses for LC50s and EC50s are the 95% confidence intervals.
USEPA 54 hardness-based FAV is 204 mg/L for moderately hard water, and DeForest and Van Genderen 31 revised BLM FAV is 261 µg/L for moderately hard water.
The most sensitive life stage for the respective species.
Control survival for the 16 dph test was less than 90 percent, thus the EC50 calculation is not definitive.
LC50 = median lethal concentration; EC50 = median effective concentration; dph = days posthach; LOE = loss of equilibrium; BLM = biotic ligand model; FAV = final acute value; NM = not measured; USEPA = US Environmental Protection Agency; AWQC = acute water-quality criteria.
Figure 2.
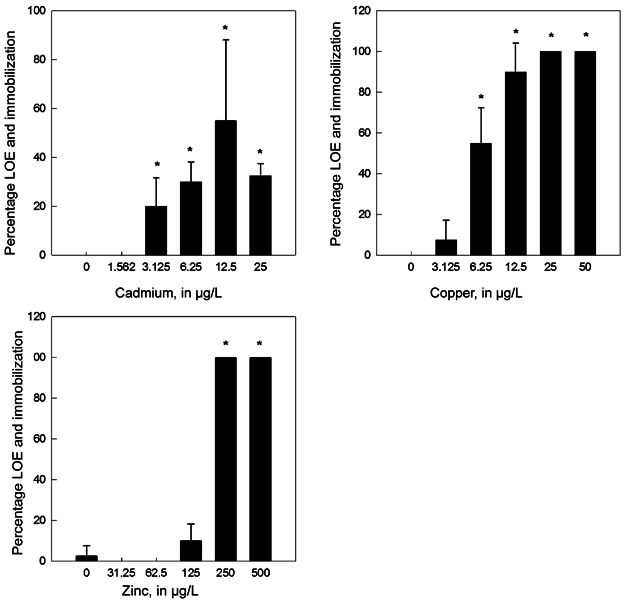
Mean percentage of surviving white sturgeon (Acipenser transmontanus) at 2 d posthatch exhibiting loss of equilibrium (LOE) and immobilization with increasing metal concentration following the 4-d exposure. Sturgeon were 6 d posthatch at the end of the 4-d exposures. Asterisk indicates significant difference from the control, and error bar represents standard deviation.
White sturgeon exposed to cadmium exhibited increased sensitivity at later stages of development, with the most sensitive life stage being 72 dph with an EC50 of 5.61 µg Cd/L, whereas the LC50 was estimated to be >149.5 µg Cd/L (Table1). The percentage of white sturgeon exhibiting the loss of equilibrium and immobilization tended to increase with increasing concentration (Figure 2). The most prevalent consequence of cadmium exposure was immobilization and loss of equilibrium. Immobilization was defined as fish lying without motion on their sides during the 5-min observation and having to be prodded to confirm they were still alive. Loss of equilibrium also was evident as fish swimming on their sides or upside down in the test chambers during 5-min observations (for example, see Supplemental Data, Video S1).
Table 1.
Cadmium LC50 or EC50 4-d acute value estimates for white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss)
| Species | Life stage (dph) | Average fish weighta (g) | Average fish lengtha (cm) | LC50 (µg/L), mortality onlyb | EC50 (µg/L), mortality + LOE + immobilizationb | Hardness-normalizedc LC50 or EC50 (µg/L) |
|---|---|---|---|---|---|---|
| Sturgeon | ||||||
| 2 | 0.03 (0.0) | NM | >47.2 | >47.2 | >25.72 | |
| 16d | 0.04 (0.01) | 21.1 (0.74) | >187 | >187 | >104.61 | |
| 30 | 0.17 (0.04) | 30.6 (1.58) | >355 | 102.72 (65.74–160.5) | 54.63 | |
| 44 | 0.48 (0.10) | 45.7 (4.45) | NE | NE | NE | |
| 61 | 1.15 (0.38) | 62.5 (7.69) | <34.4 | <34.4 | <18.08 | |
| 72e | 1.89 (0.54) | 75.6 (8.38) | >149.5 | 5.61 (2.96–10.64) | 3.02 | |
| 89 | 3.73 (1.06) | 97.57 (10.48) | >273.5 | <17 | <9.3 | |
| Trout | ||||||
| 1 | 0.08 (0.12) | 14.3 (1.74) | >49.40 | >49.40 | >26.91 | |
| 18 | 0.1 (0.02) | 24.33 (1.06) | 2.89 (2.22–3.75) | 2.89 (2.22–3.75) | 1.56 | |
| 32 | 0.12 (0.03) | 26.67 (1.97) | 4.83 (4.28–5.44) | 4.83 (4.28–5.44) | 2.55 | |
| 46e | 0.22 (0.06) | 32.1 (2.23) | 2.77 (2.05–3.73) | 2.77 (2.05–3.73) | 1.47 | |
| 60 | 0.33 (0.06) | 37.1 (2.08) | 3.71 (3.27–4.19) | 3.48 | 1.88 | |
| 74 | 0.42 (0.13) | 40.3 (3.47) | 4.54 (4.10–5.03) | 4.54 (4.10–5.03) | 2.62 | |
| 95 | 0.7 (0.18) | 45.43 (3.89) | 2.96 (2.19–4.01) | 2.96 (2.19–4.01) | 1.61 | |
Values in parentheses for average fish weight and average fish length (n = 10 for white sturgeon; n = 30 for rainbow trout) are the standard deviation.
Values in parentheses for LC50s and EC50s are the 95% confidence intervals.
The USEPA 52 AWQC FAV was 4.0 µg/L, and the USEPA 51 AWQC FAV was 7.4 µg/L for 100 mg/L hardness water. The Washington State Department of Ecology 50 water quality standards are based upon USEPA 51.
Control survival for the 16 dph test was less than 90 percent, thus the EC50 calculation is not definitive.
The most sensitive life stage for the respective species.
LC50 = median lethal concentration; EC50 = median effective concentration; dph = days posthach; LOE = loss of equilibrium; NM = not measured; NE = not estimated; USEPA = US Environmental Protection Agency; AWQC = acute water-quality criteria; FAV = final acute value.
The copper EC50s for white sturgeon at early life stages substantially were different than the copper LC50s based on mortality only (Table2). White sturgeon rapidly lost equilibrium or were immobile, quite often within the first 24 h of exposure. The 2 dph white sturgeon were the most sensitive life stage, with a, EC50 of 2.67 µg Cu/L (Table2). The greatest mortality (33%) was observed in the highest test concentration (23.6 µg Cu/L), so the LC50 estimate would be >23.6 µg Cu/L (Table2). The non-definitive EC50 and LC50 were 4.32 µg Cu/L and 7.14 µg Cu/L, respectively, for 16 dph white sturgeon. The EC50 for 30 dph white sturgeon was 6.31 µg Cu/L, and the LC50 was 16.4 µg Cu/L (Table2). The toxicity of copper primarily was evident as immobilization and loss of equilibrium among exposed white sturgeon. White sturgeon at later stages of development were less sensitive to copper exposure with EC50s ranging from 21.9 µg Cu/L to 30.8 µg Cu/L for 44 dph to 89 dph sturgeon (Table2).
Table 2.
Copper LC50 or EC50 4-d acute value estimates for white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss)
| Species | Life stage (dph) | Average fish weighta (g) | Average fish lengtha (cm) | LC50 (µg/L), mortality onlyb | EC50 (µg/L), mortality + LOE + immobilizationb | EC50 normalized for EPA 's BLM-standard waterc (µg/L) | Hardness-dependent acute FAVd for hardness of the test waters (µg/L) | BLM-based acute FAVe for test water chemistry (µg/L) |
|---|---|---|---|---|---|---|---|---|
| Sturgeon | ||||||||
| 2f | 0.03 (0.0) | NM | >23.6 | 2.67 (2.33–3.05) | 1.51 | 35.8 | 7.9 | |
| 16f | 0.04 (0.01) | 20.9 (0.57) | 7.14 (5.0–10.1) | 4.32 (2.69–6.94) | 2.59 | 35.4 | 7.6 | |
| 30 | 0.15 (0.04) | 29.2 (2.62) | 16.4 (13.4–20.2) | 6.31 (5.26–7.57) | 4.2 | 35.8 | 7.0 | |
| 44 | 0.46 (0.11) | 44.9 (3.90) | >49.8 | >49.8 | >34.1 | 34.6 | 7.0 | |
| 61 | 1.3 (0.33) | 64.2 (6.01) | <90 | 30.8 (25.0–37.9) | 21.98 | 35.0 | 7.0 | |
| 72 | 2.02 (0.80) | 77.7 (11.08) | 74 (60–90) | 74 (60–90) | 58.96 | 34.1 | 6.4 | |
| 89 | 3.73 (1.06) | 97.57 (10.48) | 90 (77–108) | 21.9 (15.9–30.3) | 17.25 | 32.4 | 6.4 | |
| Trout | ||||||||
| 1 | 0.08 (0.12) | 14.3 (1.74) | 62.9 (56.6–69.9) | 62.9 (56.6–69.9) | 47.8 | 32.4 | 6.4 | |
| 18 | 0.10 (0.02) | 24.33 (1.06) | 56.6 (50.6–63.4) | 56.6 (50.6–63.4) | 43.4 | 35.2 | 5.9 | |
| 32 | 0.12 (0.03) | 26.67 (1.97) | 59.9 (53.1–67.7) | 59.9 (53.1–67.7) | 42.42 | 35.8 | 6.6 | |
| 46 | 0.21 (0.03) | 31.1 (1.91) | 59 (49.2–70.9) | 50.1 (41.8–59.9) | 37.84 | 35.2 | 6.2 | |
| 60 | 0.37 (0.12) | 37 (3.50) | 42.4 (34.7–51.8) | 40.8 (33.5–49.8) | 32.21 | 34.8 | 6.0 | |
| 74 | 0.41 (0.12) | 40.2 (3.16) | 60.6 (54.9–66.2) | 60.6 (54.9–66.2) | 44.34 | 35.6 | 6.3 | |
| 95f | 0.70 (0.18) | 45.43 (3.89) | 19.1 (15.8–23.0) | 19.1 (15.8–23.0) | 15.24 | 34.4 | 6.2 | |
Values in parentheses for average fish weight and average fish length (n = 10 for white sturgeon; n = 30 for rainbow trout) are the standard deviation.
Values in parentheses for LC50s and EC50s are the 95% confidence intervals.
For BLM-normalized EC50s, the BLM was used to extrapolate the actual EC50s to those expected for the “BLM-standard” water conditions, which is a moderately hard water, with pH of 7.5 and 0.5 mg/L dissolved organic carbon, following the approach used by the USEPA 30. The FAV for BLM-standard water is 4.67 µg/L 30.
USEPA 53 FAV, calculated for the test hardness, which is equivalent to 2X the Washington acute water hardness standard for copper.
USEPA 30 FAV, calculated for the individual measured test water chemistries.
The most sensitive life stage for the respective species.
Control survival for the 16 dph test was less than 90 percent, thus the EC50 calculation is not definitive.
LC50 = median lethal concentration; EC50 = median effective concentration; dph = days posthach; LOE = loss of equilibrium; BLM = biotic ligand model; FAV = final acute value; NM = not measured; USEPA = US Environmental Protection Agency; AWQC = acute water-quality criteria.
White sturgeon at 2 dph were the most sensitive to zinc exposure, with an EC50 of 146.7 µg Zn/L (Table3). The percentage of 2 dph white sturgeon exhibiting a loss of equilibrium and immobilization increased with increasing zinc concentration (Figure 2). Other secondary effects consisted of initial hyperactivity of fish transitioning to lethargy (not included in the EC50 estimates) by the end of the exposure period. Physical abnormalities such as bloated abdomens were also observed at later life stages (61–89 dph) during exposure to concentrations of zinc greater than 500 µg Zn/L and therefore may have affected loss of equilibrium causing fish to swim upside down, or on their sides (see Supplemental Data, Video S2).
Rainbow trout exposures
There was no mortality in the control treatments during the 4-d exposures across each life stage tested for each metal. Loss of equilibrium and immobilization were not observed in rainbow trout during exposure to cadmium across all life stages, except at 60 dph; however, there were no substantial differences between the EC50s and LC50s, and therefore the reported effect concentrations at each life stage were LC50s based only on mortality. The sensitivity of rainbow trout to cadmium was consistent with LC50s ranging from 2.77 µg/L to 4.83 µg/L across the 6 life stages tested (Table1); 46 dph rainbow trout were the most sensitive life stage, with an LC50 of 2.77 µg Cd/L. Rainbow trout at 1 dph were less sensitive to cadmium, with an LC50 greater than 49.4 µg Cd/L (Table1).
Rainbow trout sensitivity to copper increased with later stages of development (Table2). Effect concentrations for each life stage were based on mortality only and reported as LC50s, given that trout generally did not exhibit loss of equilibrium or immobilization in the copper exposures. From 1 dph to 74 dph, the sensitivity of rainbow trout to copper was consistent with LC50s ranging from 42.4 µg Cu/L to 62.9 µg Cu/L (Table2). Rainbow trout at 95 dph were the most sensitive life stage, with an LC50 of 19.1 µg Cu/L (Table2). Rainbow trout exhibited some loss of equilibrium at 46 dph and 60 dph during exposure to copper within a concentration range of 13 µg Cu/L to 50 µg Cu/L; however, the effects were minimal or non-existent, with no substantial difference between the EC50 and LC50 at those life stages (Figure 1B; Table2).
The sensitivity of rainbow trout to zinc was relatively consistent across life stages tested. Effect concentrations for each life stage were based on mortality only and reported as LC50s. From 18 dph to 95 dph, LC50s ranged from 228 µg Zn/L to 449 µg Zn/L (Table3). Rainbow trout at 1 dph were less sensitive, with an LC50 greater than 571 µg Zn/L (Table3). Loss of equilibrium was observed at the 46 dph and 60 dph stages of development for zinc within a concentration range of 220 µg Zn/L to 350 µg Zn/L; however, the effects were minimal or non-existent with no substantial difference between the EC50 and LC50 at those life stages (Figure 1B and Table3). Other effects of zinc on rainbow trout at 46 and 60 dph were increased respiration and immobilization; however, effects on respiration were a secondary observation and were not included in the calculation of the EC50.
DISCUSSION
Effect of the developmental stage on acute sensitivity to metals
Acute sensitivity thresholds to cadmium, copper, and zinc were determined across 7 early life stages of development for white sturgeon and rainbow trout, and were compared between species at each life stage to determine the feasibility of using rainbow trout as a surrogate test species for white sturgeon. White sturgeon sensitivity to metal exposure varied with life stage, with the early life stages being more vulnerable, specifically to copper. During the toxicity tests and those reported by Little et al. 11, it became apparent that white sturgeon were severely impaired at concentrations that were not lethal. These impaired responses would result in death in the natural environment for white sturgeon and are referred to as being “ecologically dead” 34. Thus, EC50s were calculated based on mortality, immobilization, and loss of equilibrium, in addition to LC50s based on mortality only during the exposures. In some cases, particularly for white sturgeon copper exposures at the very early life stages, the inclusion of sublethal endpoints of immobilization and loss of equilibrium along with mortality in the determination of an EC50 proved to be considerably lower than just the LC50s based on mortality only. ASTM International 19 states “Death is the adverse effect most often used for the calculation of results of an acute toxicity test. … In order to account for the total severe acute adverse of the test material on test organisms, it is desirable to calculate an EC50 based on death plus immobilization, plus loss of equilibrium”. Our defined EC50s better reflect the total severe effect of the test material on white sturgeon than do LC50s or narrowly defined EC50s.
In 3 of the 7 life stages tested (2, 16, and 30 dph), white sturgeon were sensitive to copper exposure with EC50s ranging from 2.67 to 6.31 µg Cu/L compared with other life stages of sturgeon tested (the 16 dph EC50s for sturgeon were classified as non-definitive effect concentrations). Older (72 dph and 89 dph) sturgeon were relatively insensitive to copper at concentrations as great as 30 times higher than the threshold for early life stage white sturgeon. Early life stages of rainbow trout were less sensitive to copper at concentrations 30 times higher than the threshold for early life stages of white sturgeon. However, older life stages (74 dph and 95 dph) of trout were more sensitive to copper than the older life stages of sturgeon. For this reason, rainbow trout would not serve as a useful surrogate species for the protection of white sturgeon from exposure to copper.
Body size or developmental stage is an important factor modifying the toxicity of chemicals to aquatic organisms 35,36; however, unifying explanations for differences in response across species remain elusive. Juvenile organisms often are considered more susceptible to substances than adults of the same species. This has been presumed to be related to the greater ratio of body surface area to volume that in turn affects relative uptake and excretion rates, and incomplete development of detoxification mechanisms such as metallothionein 35,36. With copper, Grosell et al. 37 demonstrated situations in which smaller animals may be more sensitive than large animals, because these organisms exhibit higher sodium turnover rates. These principles and patterns suggest that smaller organisms also would have higher calcium turnover rates and in turn, smaller organisms would be more sensitive to calcium antagonists such as cadmium, lead, and zinc.
The results of the white sturgeon with copper exposures were consistent with this smaller and more sensitive rule of thumb, as were results of previous studies with other fish such as sculpin (Cottus spp.) and fathead minnow (Pimephales promelas) 38,39; however, for the rainbow trout tests, the older and larger fish tended to be more sensitive (Table2). This reversal in the general expectation that smaller fish would be more sensitive has been previously reported with rainbow trout as well as other species 40. At least with some salmonids, within the swim-up life stage, fish may initially lose resistance with increasing size. As the fish get older and larger, this pattern appears to reverse with the fish 41,42.
At present, mechanistic explanations for these differing metals sensitivity response patterns with size or early developmental stage of the different fish species are speculative. Juvenile fish undergo marked physiological and morphological changes as the fish metamorphose from larval to juvenile life stages. With white sturgeon, 2 concurrent changes during the early development are the transition from relying on the yolk sac for nourishment to external feeding and the transition from passive gas exchange through the body surface to active gill gas exchange 43. These changes in osmoregulation and respiration from dermal to gill likely increase oxygen supply to fish organs resulting in stronger swimming behaviors that allow larvae to inhabit changing environments 44. The transition from passive gas exchange through the body skin to gills in the early development of fish is preceded by a transition from ion regulation through the body skin to the gills 43. Because the toxicity of cadmium, copper, lead, and zinc are related to ion regulation disruption, the differing patterns of size and sensitivity to at least copper and zinc suggests possible connections with the timing of changes in ion regulation.
Rainbow trout are often considered to be a species that is sensitive to metal exposure and frequently considered as surrogates for developing ambient water-quality values 30,39. Because of the differences in sensitivity at the various life stages, rainbow trout would not serve as an appropriate surrogate for threatened or endangered species, such as the white sturgeon. The extent to which differences in tolerance to copper between trout and sturgeon were due solely to the difference in exposure temperatures (12 °C for rainbow trout and 15 °C for sturgeon) is unknown 11; however, there is a general trend that metal toxicity increases as temperature increases 45 which could potentially account for differences in sensitivity. However, species sensitivity distributions commonly compare species mean values obtained from acute toxicity tests conducted at different temperatures according to the test species.
Comparison of species sensitivity and species sensitivity distributions
Rainbow trout were more sensitive to cadmium exposure than white sturgeon for all life stages tested. As with white sturgeon, there was an increase in sensitivity with age for rainbow trout during the companion study by Wang et al. 15, in which acute toxicity was assessed during the first 4 d of the chronic exposure. In Wang et al. 15, the 1 dph 4-d EC50 was >12 µg Cd/L, whereas the 26 dph 4-d EC50 was 5.14 µg Cd/L, suggesting that older trout become more sensitive to exposure to cadmium. At 46 dph, Rainbow trout in the present study especially were sensitive to cadmium relative to other species and were in the 2nd percentile in a compiled cadmium acute toxicity database for the species sensitivity distribution, with a hardness normalized EC50 of 1.47 µg Cd/L (Figure 3 and Table1); however, white sturgeon were among the more sensitive of species at 72 dph (19th percentile; Figure 3), with a hardness normalized EC50 of 3.02 µg Cd/L (Table1). Based on these results, rainbow trout could serve as a surrogate species for the protection of white sturgeon in acute exposures to cadmium.
Figure 3.
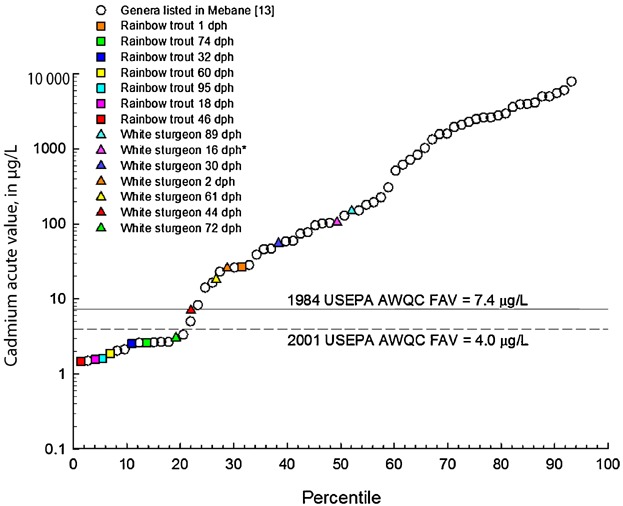
Ranks of various white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) life stages in species sensitivity distribution from an updated cadmium hardness–toxicity regression and effects database 29. Values are normalized to a water hardness of 100 mg/L for comparability with US Environmental Protection Agency (USEPA) criteria 51,52. AWQC = acute water-quality criteria; FAV = final acute value; dph = days posthatch.
White sturgeon were more sensitive than rainbow trout to copper at 2 dph, 16 dph, 30 dph, 44 dph, and 61 dph and less sensitive at 72 dph and 89 dph. The copper effect concentrations for older life stages of sturgeon were up to 30 times higher than the threshold for the earlier life stages of sturgeon. The effect was opposite for rainbow trout. Early life stages of rainbow trout (1–74 dph) were less sensitive to copper at concentrations as much as 30 times higher than the threshold for early life stage white sturgeon, and older life stages of rainbow trout (74 dph and 95 dph) were more sensitive to copper than the older life stage of white sturgeon. The 2 dph life stage for white sturgeon was the most sensitive life stage tested in the present study and ranked at the 3rd percentile in a compiled copper acute toxicity database for species sensitivity distribution, making it the most sensitive species with a BLM-normalized EC50 of 1.51 µg Cu/L (Figure 4 and Table2). Based on these findings, rainbow trout would not serve as a good surrogate species for protecting white sturgeon from acute copper exposure.
Figure 4.
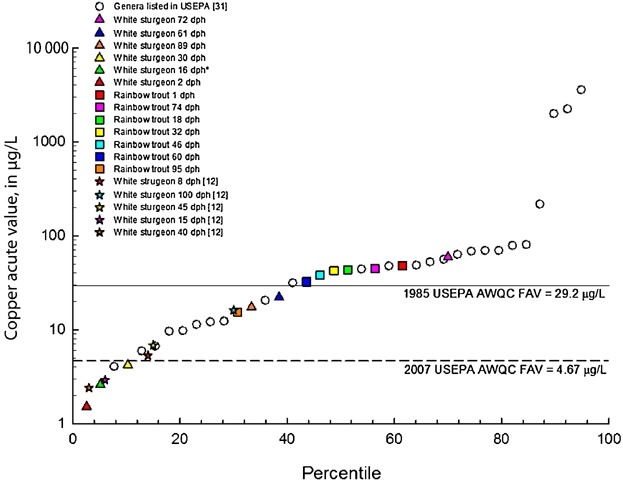
Ranks of various white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) life stages in species sensitivity distribution from US Environmental Protection Agency (USEPA) database for copper 30. Values are normalized to a moderately hard water composition with water hardness of 85 mg/L and dissolved organic carbon of 0.5 mg/L for comparability with USEPA values 30. Asterisk denotes that the control survival was less than 90% during this life stage exposure and the result should be interpreted with caution because of low control survival. AWQC = acute water-quality criteria; FAV = final acute value; dph = days posthatch.
Much like cadmium, zinc toxicity varied by life stage for white sturgeon and was somewhat consistent for rainbow trout across all life stages, except for 1 dph rainbow trout, which were less sensitive compared with the other 6 life stages of rainbow trout tested or compared with 2 dph white sturgeon (Table3). In Wang et al. 15, the 1 dph 4-d EC50 for rainbow trout at the start of the chronic exposures was >748 µg Zn/L, whereas at the 26 dph 4-d EC50 for rainbow trout at the start of the chronic exposures was 267 µg Zn/L, suggesting that older rainbow trout become more sensitive to exposure to zinc. White sturgeon and rainbow trout were ranked as highly sensitive in the species sensitivity distribution for zinc when compared with other species (Figure 5). White sturgeon at 2 dph were extremely sensitive to exposure to zinc, with a BLM-normalized EC50 of 209 µg Zn/L, ranked at the 1st percentile in a compiled zinc acute toxicity database for the species sensitivity distribution (Table3 and Figure 5), and were more sensitive than rainbow trout at 1 dph (>726 µg Zn/L), whereas rankings rose to the 35th percentile (Figure 5) for the older life stages of white sturgeon. Rainbow trout at 95 dph were the most sensitive life stage to zinc in the present study and ranked at the 4th percentile of the species sensitivity distribution (Table3 and Figure 5) with a BLM-normalized EC50 of 254 µg Zn/L.
Figure 5.
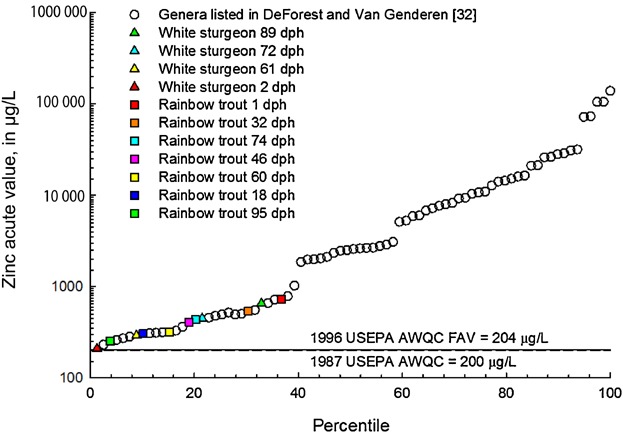
Ranks of various white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) life stages in species sensitivity distribution from updated databases for zinc 31. Values are normalized to a moderately hard water composition with water hardness of 85 mg/L and dissolved organic carbon of 0.5 mg/L for comparability with DeForest and Van Genderen 31. US Environmental Protection Agency (USEPA) aquatic life criteria for zinc are the bases of the currently recommended USEPA criteria and Washington State Department of Ecology 50 water quality standards, respectively. AWQC = acute water-quality criteria; FAV = final acute value; dph = days posthatch.
Comparison with other acute metal toxicity studies performed with white sturgeon
Evaluation of the toxicity of metals to aquatic organisms requires consideration of site-specific water quality variables (water hardness, pH, DOC, temperature, major anions, major cations) that can alter the availability and toxicity of metals, including copper and zinc 30,46. For instance, Entrix and University of Saskatchewan 47 performed a series of 4-d exposures with 8 dph to 10 dph white sturgeon using Columbia River water and laboratory water (dechlorinated City of Saskatoon water) adjusted to a hardness of 75 mg/L mixed with reverse osmosis water. The 4-d LC50s for copper, cadmium, and zinc varied between water type (river water vs laboratory water 47). The LC50s for the cadmium and zinc exposures performed in river water were much lower than the LC50s generated for laboratory water, whereas the opposite was reported for the copper exposures with an LC50 of 44 µg Cu/L for river water compared with an LC50 of 30 µg Cu/L for laboratory water suggesting differences in water quality variables can alter the toxicity of metals. Unfortunately, DOC was not reported in the Columbia River water used during testing 47.
Toxicity values for metals vary among studies because of water-quality characteristics of the exposures conditions. Certain variables, such as water hardness and DOC, have predictable effects on the bioavailability of metals and thus can be used to normalize toxicity for different exposure conditions. When comparing acute toxicity data from other studies with toxicity data in the present study, the data from all the studies were recalculated and normalized using the BLM 30 based on ASTM International standard water with hardness value of 85 mg/L and DOC concentration of 0.5 mg/L for comparability, specifically for copper exposures.
Vardy et al. 12 tested life stages of white sturgeon similar to the present study and the results were comparable (Table4). White sturgeon at 15 dph had a BLM-normalized 4-d LC50 of 2.9 µg Cu/L 12, compared with the non-definitive BLM-normalized LC50 of 4.4 µg Cu/L for 16 dph white sturgeon in the present study. The lowest copper effect concentration reported by Vardy et al. 12 was BLM-normalized 4-d LC50 of 2.4 µg Cu/L for 40 dph white sturgeon, whereas the most sensitive life stage in the present study was 2 dph white sturgeon with a BLM-normalized 4-d EC50 of 1.51 µg Cu/L (Tables2 and 4). Although the most sensitive life stage observed in Vardy et al. 12 for copper was white sturgeon at 40 dph, white sturgeon at 2 dph were more sensitive in the present study. The differences in sensitivity could be because of the different white sturgeon populations that were tested. We also cannot discount the possibility that copper may have not equilibrated with DOC in test exposure water. Notably, copper delivered by our test system to individual test treatments experienced relatively short equilibration times with DOC in the test water, which might have had an effect on the observed LC50s and EC50s 48,49. However, because the DOC concentration of our waters was quite low, we do not believe that increasing the equilibration time would have significantly affected these LC50 and EC50 values. Moreover, early life stages of white sturgeon in the age range of 2 dph to 40 dph proved to be sensitive to aqueous copper exposure in both the present study and Vardy et al. 12.
Table 4.
Comparison of 4-d acute LC50 values for white sturgeon (Acipenser transmontanus) to copper at various life stages
| Reference | Life stage (dph) | Reported 96-h LC50 (µg Cu/L) | BLM-adjusted LC50 to USEPA standard water (µg Cu/L)a | BLM-adjusted FAV based on test water chemistry (µg Cu/L)b |
|---|---|---|---|---|
| The present study | 2 | >23.6 | >16.2 | 7.9 |
| Wang et al. 15 | 2 | 8.06 | 4.9 | 7.7 |
| Vardy et al. 12c | 8 | 22 | 5.3 | 19.4 |
| M. Hecker, University of Saskatchewan, Saskatoon, Saskatchewon, Canada, personal communication | 8 | 17.2 | ||
| Entrix and U of S 47 | 8–10 | 30 | ||
| Vardy et al. 12c | 15 | 10 | 2.9 | 16 |
| The present study | 16d | 7.1 | 4.4 | 7.6 |
| Little et al. 11 | 26 | 4.5 | 2.2d | |
| Little et al. 11 | 27 | 6.8 | 8.2 | |
| The present study | 30 | 16.4 | 11.8 | 7 |
| Little et al. 11 | 38 | 4.1 | 2.3d | |
| M. Hecker, University of Saskatchewan, Saskatoon, Saskatchewon, Canada, personal communication | 40 | 11.7 | ||
| Vardy et al. 12c | 40 | 9 | 2.4 | 17.1 |
| Little et al. 11 | 40 | 4.7 | 5.5 | |
| The present study | 44 | >50 | >34.1 | 7 |
| Vardy et al. 12c | 45 | 17 | 6.8 | 11.6 |
| The present study | 61 | <90 | <56 | 7 |
| The present study | 72 | 74 | 51.3 | 6.4 |
| The present study | 89 | 90 | 62.9 | 6.4 |
| Vardy et al. 12c | 100 | 54 | 16.1 | 17.1 |
For BLM-normalized LC50s, the BLM was used to extrapolate the actual LC50s to those expected for the BLM-standard water conditions, which is a moderately-hard water with pH of 7.5 and 0.5 mg/L DOC, following the approach used by USEPA 30. The FAV for BLM-standard water is 4.67 µg/L 30.
USEPA 30 FAV, calculated for the individual measured test water chemistries.
Toxicity data for copper reported in Vardy et al. 12 were normalized using BLM based on moderately hard reconstituted water (hardness 85 mg/L, dissolved organic carbon 0.5 mg/L).
Control survival for the 16 dph test was less than 90 percent, thus the EC50 calculation is not definitive.
Value is at or below the USEPA 30 WQC for copper CMC = 2.3 µg/L.
LC50 = median lethal concentration; dph = days posthach; BLM = biotic ligand model; FAV = final acute value; USEPA = US Environmental Protection Agency; WQC = water-quality criteria.
Little et al. 11 also determined that endangered Kootenai white sturgeon at 38 dph were sensitive to copper exposure, with a BLM-normalized 4-d LC50 of 2.3 µg/L, which is similar to the USEPA AWQC for copper of 2.3 µg/L 30. The BLM-normalized LC50 for previously tested Columbia River white sturgeon at 26 dph was comparable at 2.2 µg/L 11, indicating that white sturgeon in the age range of 2 dph to 40 dph had copper sensitivity thresholds below the USEPA 30 BLM-based AWQC for copper of 2.3 µg/L. Loss of equilibrium or immobilization was observed but not an endpoint reported by Little et al. 11; however, sensitivity based on mortality only (LC50) indicated that the endangered Kootenai white sturgeon are more sensitive to copper during the early life stages. The 4-d EC50 (8.06 µg Cu/L) for 2 dph white sturgeon obtained from the companion chronic exposure 15 was substantially lower than the 4-d LC50 (>23.6 µg Cu/L) generated for the acute exposure during round 1, in which 2 dph white sturgeon were tested; when immobilization was included in the calculation of a 4-d EC50 for the chronic study, however, sensitivity of 2 dph white sturgeon at the start of the chronic copper exposures was more comparable to the acute exposure round 1 (Wang et al. 15 4-d EC50 of 5.29 µg Cu/L compared with a 4-d EC50 of 2.7 µg Cu/L for the present study). Interestingly, when the LC50s reported by Vardy et al. 12 and Little et al. 11 are BLM-normalized to moderately hard water with water hardness of 85 mg/L and DOC of 0.5 mg/L 30, the LC50s are similar to EC50s in the acute and chronic 15 parts of the present study, with BLM-normalized LC50s ranging from 2.4 µg Cu/L to 16.1 µg Cu/L for sturgeon at 8 dph to 100 dph (Table4).
White sturgeon at 2 dph were the most sensitive life stage tested relative to the sensitivity to copper of other species (3rd percentile in a compiled toxicity database; Figure 4), even when compared with other sturgeon species that were tested at older life stages 14. Rainbow trout at 95 dph, in contrast, were ranked at the 30th percentile in sensitivity to copper. This finding is consistent with those obtained in Wang et al. 15 chronic exposures in which acute sensitivity was examined in the first 4 d of a 53-d exposure initiated with 2 dph sturgeon.
An important caution when interpreting the ranked sensitivity of copper BLM-normalized tests is that uncertainties in DOC concentrations could lead to different interpretations of relative species sensitivity. Individual measurements of DOC in test chambers, as analyzed by Huffman Laboratory, ranged from 0.3 mg/L to 0.5 mg/L, and so the average DOC in test exposures was 0.4 mg/L. This value was used in the extrapolations of measured test values to what those values might have been if tested in standardized moderately hard water with 0.5 mg/L DOC (BLM normalization, Table4).
White sturgeon were less sensitive to cadmium exposure during the early life stages and tended to become more sensitive with the older life stages. This is consistent with what was documented in Wang et al. 15 during the first 4 d of the second phase of the chronic exposure, in which the sturgeon 4-d EC50 for cadmium at 27 dph was >11.0 µg Cd/L; the 4-d EC50 for 30 dph sturgeon was 102.7 µg Cd/L in the present study (Table1). This result could possibly be because of the size differences at each life stage. The most sensitive life stage for white sturgeon in the present study was at 72 dph, with a hardness-adjusted EC50 of 3.02 µg Cd/L (Table1), ranking in the species sensitivity distribution at the 19th percentile in a compiled cadmium toxicity database (Figure 3).
Zinc toxicity varied by life stage for white sturgeon. The reported EC50s in Table3 were normalized to a compiled BLM toxicity database for zinc 31 and tended to be more conservative, sometimes 37% more conservative, for white sturgeon. White sturgeon at 2 dph of development were most vulnerable to zinc toxicity, with a BLM-normalized EC50 of 209 µg/L (Table3). In Wang et al. 15, zinc toxicity at the start of the chronic exposures was consistent for white sturgeon at 2 dph (4-d EC50 > 369 µg Zn/L) and 27 dph (4-d EC50 > 395 µg Zn/L); however, the chronic exposure concentrations were quite lower than the concentrations used during the acute exposures.
Comparisons of effect and criteria concentrations
The median Upper Columbia River water quality characteristics for a given copper concentration indicates copper would be approximately 2.5-fold less toxic in the Upper Columbia River water compared with CERC laboratory water, using a BLM to compare the relative toxicities of copper 30. Zinc would be approximately 1.3-fold less toxic in the Upper Columbia River water compared with CERC laboratory water, using the BLM of DeForest and VanGenderen 31 to compare the relative toxicities of zinc. For cadmium, BLMs are less developed than for copper or zinc. A given concentration of cadmium was estimated to be approximately 1.5-fold more toxic in the Upper Columbia River than in the CERC laboratory water, using hardness-toxicity relations 29. Thus, absolute effect concentrations from the tests in the present study should not be extrapolated directly to Upper Columbia River conditions; instead, these effect concentrations for test water in the present study were compared with the BLM or hardness-dependent USEPA AWQC and to the Washington State WQS 50, calculated for test water conditions.
Effect concentrations for cadmium based on water hardness varied among life stage for white sturgeon (Table1). The most sensitive life stage for white sturgeon was at 72 dph, with an EC50 of 5.6 µg/L. This value is greater than the USEPA 51 FAV of 4.0 µg Cd/L, calculated for the test hardness of 100 mg/L. In contrast, 4 of the 7 rainbow trout life stages tested resulted in cadmium EC50s that were lower than the USEPA 51 FAV values calculated for the hardness of each test. The cadmium hardness adjusted EC50 for 72 dph white sturgeon (3.02 µg/L) was below the USEPA 52 AWQC for cadmium (FAV of 4.0 µg Cd/L) and below the Washington State WQS (7.4 µg/L) for acute toxicity of cadmium; however, rainbow trout at 18, 46, 60, and 95 dph all had cadmium EC50s below the AWQC and all life stages with the exception of 1 dph fell below the state of Washington State WQS for the acute toxicity of cadmium.
The Cu EC50s for white sturgeon at 2 dph and 30 dph were lower than the USEPA 30 BLM-based FAV concentrations calculated for test conditions, as was the EC50 for white sturgeon at 16 dph, although the EC50 for 16 dph white sturgeon was classified as a non-definitive effect concentration because control survival in that test was less than 90% (Table2). The EC50s for these developmental stages ranged from 2.7 µg Cu/L to 6.3 µg Cu/L compared, with the BLM-based FAV values of 7.0 µg Cu/L to 7.9 µg Cu/L for the test conditions. In contrast, the USEPA 53 hardness-based FAVs were much higher than test EC50 (FAVs approximately 36 µg Cu/L, compared with EC50s ranging from 2.7 µg Cu/L to 6.3 µg Cu/L). The rainbow trout EC50 at 95 dph was lower than the USEPA 53 hardness-based FAV that was used to define the Washington State WQS for acute Cu exposures. In turn, the rainbow trout copper EC50s at all life stages tested (Table2) were all greater than the USEPA 30 BLM-based FAV concentrations calculated for test conditions.
The Zn EC50s for the most sensitive life stage of white sturgeon was at 2 dph with an EC50 of 146.7 µg Zn/L (Table3). The USEPA 54 zinc FAV is higher, 204 µg Zn/L for the test water hardness of 100 mg/L. The rainbow trout EC50s at all life stages tested (Table3) were also greater than the USEPA 54 hardness-based FAV (204 µg Zn/L).
CONCLUSIONS
White sturgeon were more sensitive to copper than rainbow trout for 5 of the 7 life stages tested. White sturgeon at 2 dph, 16 dph, and 30 dph were highly sensitive to copper, whereas older life stages were less sensitive. For this reason, rainbow trout would not be a good surrogate for protectiveness of white sturgeon. Early life stage white sturgeon specifically at 2 dph were highly sensitive to zinc and the 2 dph life stage of white sturgeon was the only life stage that was more sensitive than rainbow trout at any life stage tested. White sturgeon tended to become more sensitive to cadmium in older life stages; however, when compared with rainbow trout, white sturgeon were considerably less sensitive. Immobilization and loss of equilibrium were affected by metal exposure, particularly for copper and zinc. The USEPA AWQC would be protective of rainbow trout from exposure to copper, and zinc; for cadmium, however, the AWQC and Washington State WQS would not necessarily be protective of early life stages of rainbow trout. For early life stage white sturgeon, the AWQC may not adequately be protective from exposure to copper or zinc but would be protective from cadmium exposures at all life stages. The Washington State WQS would also be protective of white sturgeon exposed to cadmium except for the 72 dph life stage. For copper and zinc, however, the Washington State WQS would not necessarily protect the early life stages of white sturgeon from acute exposure.
SUPPLEMENTAL DATA
Tables S1–S3. (33 KB XLSX).
Figures S1. (366 KB PDF).
Video S1. (40.8 MB WMV).
Video S2. (77.2 MB WMV).
Acknowledgments
We thank the staff in the Ecology Branch, Toxicology Branch, and Environmental Chemistry Branch of the US Geological Survey (USGS), Columbia Environmental Research Center, for technical assistance. We thank E. Van Genderen of the International Zinc Association, Durham, North Carolina, USA, for providing the biotic ligand model–based zinc data. Funding for the present study was provided in part by Teck Resources Limited through an agreement with the US Environmental Protection Agency (USEPA) Region 10 with funds provided by USEPA to USGS through the Department of Interior Central Hazmat Fund. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the United States Government.
Supporting Information
All Supplemental Data may be found in the online version of this article.
Supplementary Figure S1.
Supplementary Tables S1-S3.
Supplementary Video S1.
Supplementary Video S2.
REFERENCES
- Hildebrand L, McLeod C, McKenzie S. Status and management of white sturgeon in the Columbia River in British Columbia, Canada: An overview. J Appl Ichthyol. 1999;15:164–172. [Google Scholar]
- Howell MD, McLellan JG. 2009. Lake Roosevelt white sturgeon recovery project annual progress report January 2003–March 2004. DOE/BP-00022571-1. US Department of Energy, Portland, OR.
- Beamsderfer RCP, Farr RA. Alternatives for the protection and restoration of sturgeons and their habitat. Environ Biol Fish. 1997;48:407–417. [Google Scholar]
- Jager HI, Van Winkle W, Chandler JA, Lepla KB, Bates P, Counihan TD. A simulation study of factors controlling white sturgeon recruitment in the Snake River. In: Van Winkle W, Anders PJ, Secor DH, Dixon DA, editors. Biology, Management, and Protection of North American Sturgeon. Bethesda, MD: American Fisheries Society; 2001. pp. 127–150. Symposium 28. [Google Scholar]
- Smith CT, Nelson RJ, Pollard S, Rubidge E, McKay SJ, Rodzen J, May B, Koop B. Population genetic analysis of white sturgeon (Acipenser transmontanus) in the Fraser River. J Appl Ichthyol. 2002;18:307–312. [Google Scholar]
- Gadomski DM, Parsley MJ. Laboratory studies on the vulnerability of young white sturgeon to predation. North Am J Fish Manage. 2005;25:667–674. [Google Scholar]
- McAdam SO. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Can J Fish Aquat Sci. 2011;68:812–822. [Google Scholar]
- Upper Columbia White Sturgeon Recovery Initiative. 2002. Upper Columbia White Sturgeon Recovery Plan. [cited 2012 December]. Available from: http://uppercolumbiasturgeon.org.
- Kruse GO, Scarnecchia DL. Assessment of bioaccumulated metal and organochlorine compounds in relation to physiological biomarkers in Kootenai River white sturgeon. J Appl Ichthyol. 2002;18:430–438. [Google Scholar]
- Conte FS, Doroshov SI, Lutes PB, Strange EM. Hatchery Manual for the White Sturgeon Acipenser Transmontanus Richardson with Application to Other North American Acipenseridae. Oakland, CA, USA: University of California; 1988. [Google Scholar]
- Little EE, Calfee RD, Linder G. Toxicity of copper to early life stages of Kootenai River white sturgeon, Columbia River white sturgeon, and rainbow trout. Arch Environ Con Tox. 2012;63:400–408. doi: 10.1007/s00244-012-9782-3. [DOI] [PubMed] [Google Scholar]
- Vardy DW, Oellers J, Doering JA, Hollert H, Giesy JP, Hecker M. Sensitivity of early life stages of white sturgeon, rainbow trout, and fathead minnow to copper. Ecotoxicology. 2012;22:139–147. doi: 10.1007/s10646-012-1010-4. [DOI] [PubMed] [Google Scholar]
- Vardy DW, Santore R, Ryan A, Giesy JP, Hecker M. Acute toxicity of copper, lead, cadmium, and zinc to early life stages of white sturgeon (Acipenser transmontanus) in laboratory and Columbia River water. Environ Sci Pollut Res. 2014;21:8176–8187. doi: 10.1007/s11356-014-2754-6. [DOI] [PubMed] [Google Scholar]
- Dwyer FJ, Mayer FL, Sappington LC, Buckler DR, Bridges CM, Greer IE, Hardesty DK, Henke CE, Ingersoll CG, Kunz JL, Whites DW, Augspurger T, Mount DR, Hattala K, Neuderferv GN. Assessing contaminant sensitivity of endangered and threatened aquatic species: Part I. Acute toxicity of five chemicals. Arch Environ Con Tox. 2005;48:143–154. doi: 10.1007/s00244-003-3038-1. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingersoll C, Dorman R, Brumbaugh WG, Mebane CA, Kunz J, Hardesty D. Chronic sensitivity of white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) to cadmium, copper, or zinc in laboratory water-only exposures. In: Ingersoll CG, Mebane CA, editors. Acute and Chronic Sensitivity of White Sturgeon (Acipenser transmontanus) and Rainbow Trout (Oncorhynchus mykiss) to Cadmium, Copper, Lead, or Zinc in Laboratory Water-Only Exposure. Reston, Virginia: US Geological Survey; 2014. pp. 35–70. Scientific Investigations Report. [Google Scholar]
- Ingersoll CG, Mebane CA, editors. Acute and Chronic Sensitivity of White Sturgeon (Acipenser transmontanus) and Rainbow Trout (Oncorhynchus mykiss) to Cadmium, Copper, Lead, or Zinc in Laboratory Water-Only Exposure. Reston, Virginia: US Geological Survey; 2014. Scientific Investigations Report. [DOI] [PubMed] [Google Scholar]
- Paulson AJ, Cox SE. Release of elements to natural water from sediment of Lake Roosevelt, Washington, USA. Environ Toxicol Chem. 2007;26:2550–2559. doi: 10.1897/07-052.1. [DOI] [PubMed] [Google Scholar]
- Mount DI, Brungs WA. A simplified dosing apparatus for fish toxicological studies. Water Research. 1967;1:21–29. [Google Scholar]
- ASTM International. 2013. Standard guide for conducting early life-sage toxicity tests with fishes. E1241-05. In Annual Book of ASTM Standards, Vol 11.06. West Conshohocken, PA, USA.
- ASTM International. 2013. Standard guide for measurement of behavior during fish toxicity tests. E1711-95 (2008) In Annual Book of ASTM Standards, Vol 11.06. West Conshohocken, PA, USA.
- ASTM International. 2013. Standard guide for conducting acute toxicity tests on test materials with fishes, macroinvertebrates, and amphibians. E729-96 (2007) In Annual Book of ASTM Standards, Vol. 11.06. West Conshohocken, PA. USA.
- Eaton AD, Clesceri LS, Rice EW, Greenberg AE. Standard Methods for the Examination of Water and Wastewater. 21st ed. Washington, DC: American Public Health Association, Water Environment Federation, American Water Works Association; 2005. [Google Scholar]
- Brumbaugh WG, May TW, Besser JM, Allert AL, Schmitt CJ. 2007. Assessment of elemental concentrations in streams of the New Lead Belt in southeastern Missouri, 2002–2005. Scientific Investigations Report 2007-5057. US Geological Survey, Reston, VA.
- US Environmental Protection Agency. 1994. Method 200.7: Determination of metals and trace metals in water and wastes by inductively coupled plasma-atomic emission spectrometry, revision 4.4. Cincinnati, OH. [PubMed]
- US Environmental Protection Agency. 1983. Total organic carbon. Method 415.2: Total organic carbon. In Methods for Chemical Analysis of Water and Wastes. EPA 600/4-79/020. Washington, DC. [PubMed]
- Erickson RJ. 2012. Toxicity Relationship Analysis Program (TRAP), Ver 1.21. EPA 600/C-11/002. US Environmental Protection Agency, Washington, DC.
- Western EcoSystems. 1996. TOXSTAT, Ver 3.5. Cheyenne, WY, USA.
- US Environmental Protection Agency. 2002. Short-term methods for estimating the chronic toxicity of effluents and receiving water to freshwater organisms. EPA 821/R-02/013 (5th ed.). Washington, DC. [PubMed]
- Mebane CA. 2006. Cadmium risks to freshwater life: Derivation and validation of low-effect criteria values using laboratory and field studies, Ver 1.2. US Geological Survey Scientific Investigation Report.
- US Environmental Protection Agency. 2007. Aquatic life ambient freshwater quality criteria – Copper. EPA 822/R-07/001. Office of Water, Washington, DC. [PubMed]
- DeForest DK, Van Genderen EJ. Application of USEPA guidelines in a bioavailability-based assessment of ambient water quality criteria for zinc in freshwater. Environ Toxicol Chem. 2012;31:1264–1272. doi: 10.1002/etc.1810. [DOI] [PubMed] [Google Scholar]
- Stephan CE. Use of species sensitivity distributions in the derivation of water quality criteria for aquatic life by the US Environmental Protection Agency. In: Posthuma L, Suter GW II, Traas TP, editors. Species Sensitivity Distributions in Ecotoxicology. Boca Raton, FL, USA: CRC Press; 2002. pp. 211–220. [Google Scholar]
- US Geological Survey. Columbia MO: 2010. USGS-Columbia quality assurance project plan for an evaluation of the acute or chronic toxicity of individual chemicals of interest to white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) in water-only exposures. [Google Scholar]
- Scott GR, Sloman KA. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat Toxicol. 2004;68:369–392. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Rand GM, Wells PG, McCarty LS. Introduction to aquatic toxicology. In: Rand GM, editor. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment. 2nd ed. Washington, DC: Taylor and Francis; 1995. pp. 3–67. [Google Scholar]
- Hendriks AJ, Heikens A. The power of size. 2. Rate constants and equilibrium ratios for accumulation of inorganic substances related to species weight. Environ Toxicol Chem. 2001;20:1421–1437. [PubMed] [Google Scholar]
- Grosell M, Nielsen C, Bianchini A. Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Phys C. 2002;133:287–303. doi: 10.1016/s1532-0456(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Bills TL, Wenholz MD, LaPoint TW, Cobb GP, Forsythe BL., II 1996. pp. 125–130. Influence of age sensitivity on the acute toxicity of silver to fathead minnows at various quality parameters. Proceedings, 4th International Conference Transport, Fate and Effects of Silver in the Environment, Madison, WI, USA, August 25–28, 1996.
- Besser JM, Mebane CA, Mount DR, Ivey CD, Kunz JL, Greer EI, May TW, Ingersoll CG. Relative sensitivity of mottled sculpins (Cottus bairdi) and rainbow trout (Oncorhynchus mykiss) to toxicity of metals associated with mining activities. Environ Toxicol Chem. 2007;26:1657–1665. doi: 10.1897/06-571r.1. [DOI] [PubMed] [Google Scholar]
- Anderson PD, Weber LJ. Toxic response as a quantitative function of body size. Toxicol Appl Pharmacol. 1975;33:471–483. doi: 10.1016/0041-008x(75)90073-3. [DOI] [PubMed] [Google Scholar]
- Hedtke JL, Robinson-Wilson E, Weber LJ. Influence of body size and developmental stage of coho salmon (Oncorhynchus kisutch) on lethality of several toxicants. Fund Appl Toxicol. 1982;2:67–72. doi: 10.1016/s0272-0590(82)80116-4. [DOI] [PubMed] [Google Scholar]
- Mebane CA, Dillon FS, Hennessey DP. Acute toxicity of cadmium, lead, and zinc and their mixtures to stream resident fish and invertebrates. Environ Toxicol Chem. 2012;31:1334–1338. doi: 10.1002/etc.1820. [DOI] [PubMed] [Google Scholar]
- Fu C, Wilson JM, Rombough PJ, Brauner CJ. Ions first—Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc R Soc London B Bio. 2010;277:1553–1560. doi: 10.1098/rspb.2009.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert E, Williot P. Larval behavior and effect of the timing of initial feeding on growth and survival of Siberian sturgeon (Acipenser baeri) larvae under small scale hatchery production. Aquaculture. 1997;156:63–76. [Google Scholar]
- Meyer JS, Clearwater SJ, Doser TA, Rogaczewski MG, Hansen JA. Effects of Water Chemistry on the Bioavailability and Toxicity of Waterborne Cadmium, Copper, Nickel, Lead, and Zinc to Freshwater Organisms. Pensacola, FL, USA: SETAC Press; 2007. [Google Scholar]
- Meyer JS, Adams WJ, DeForest DK, Dwyer RL, Gensemer RW, Gorsuch JW, Johnston RK, Santore RC, Van Gendren E. Water chemistry matters in metal toxicity papers. Environ Toxicol Chem. 2012;31:689–690. doi: 10.1002/etc.1773. [DOI] [PubMed] [Google Scholar]
- Entrix and University of Saskatchewan. 2011. Acute water exposures of cadmium, copper, and zinc to early life-stages of white sturgeon (Acipenser transmontanus). Report to Teck American Incorporated. Saskatoon, SK, Canada.
- Ma H, Kim SD, Cha DK, Allen HE. Effect of kinetics of complexation by humic acid on toxicity of copper to Ceriodaphnia dubia. Environ Toxicol Chem. 1999;18:828–837. [Google Scholar]
- Santore RC, Di Toro DM, Paquin PR, Allen HE, Meyer JS. Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ Toxicol Chem. 2001;20:2397–2402. [PubMed] [Google Scholar]
- Washington State Department of Ecology. 2006. Water quality standards for surface waters of the State of Washington—Chapter 173-201A WAC, Amended November 20, 2006. Publication No. 06-10-091. Olympia, WA.
- US Environmental Protection Agency. 1984. Ambient water quality criteria for cadmium. EPA 440/5-84/032. Duluth, MN. [PubMed]
- US Environmental Protection Agency. 2001. 2001 update of ambient water quality criteria for cadmium. EPA 822/R-01/001. Washington, DC. [PubMed]
- US Environmental Protection Agency. 1985. Ambient water quality criteria for copper – 1984. EPA 440/5-84-031. Duluth, MN. [PubMed]
- US Environmental Protection Agency. 1996. Water quality criteria documents for the protection of aquatic life in ambient water: 1995 updates. EPA 820/B-96/001. Washington, DC. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1.
Supplementary Tables S1-S3.
Supplementary Video S1.
Supplementary Video S2.


