Abstract
The heparin sulfate proteoglycan Trol (Terribly Reduced Optic Lobes) is the D. melanogaster homolog of the vertebrate protein Perlecan. Trol is expressed as part of the extracellular matrix (ECM) found in the hematopoietic organ, called the lymph gland. In the normal lymph gland, the ECM forms thin basement membranes around individual or small groups of blood progenitors. The pattern of basement membranes, reported by Trol expression, is spatio-temporally correlated to hematopoiesis. The central, medullary zone which contain undifferentiated hematopoietic progenitors has many, closely spaced membranes. Fewer basement membranes are present in the outer, cortical zone, where differentiation of blood cells takes place. Loss of trol causes a dramatic change of the ECM into a three-dimensional, spongy mass that fills wide spaces scattered throughout the lymph gland. At the same time proliferation is reduced, leading to a significantly smaller lymph gland. Interestingly, differentiation of blood progenitors in trol mutants is precocious, resulting in the break-down of the usual zonation of the lymph gland which normally consists of an immature center (medullary zone) where cells remain undifferentiated, and an outer cortical zone, where differentiation sets in. We present evidence that the effect of Trol on blood cell differentiation is mediated by Hedgehog (Hh) signaling, which is known to be required to maintain an immature medullary zone. Overexpression of hh in the background of a trol mutation is able to rescue the premature differentiation phenotype. Our data provide novel insight into the role of the ECM component Perlecan during Drosophila hematopoiesis.
Keywords: Drosophila, hematopoiesis, lymph gland, extracellular matrix, hedgehog, Perlecan
Introduction
Animal cells secrete proteins that assemble into an extracellular matrix (ECM). The ECM plays important roles in determining the mechanical properties of cells and tissues, as well as in intercellular communication. In many cells, the ECM forms a layered structure called the basal lamina or the basement membrane. In epithelia, the basement membrane assembles over the basal cell surface; in other, mesenchymal tissues, such as muscle or cartilage, basement membranes surround cells on all sides (Durbeej, 2010). Many basement membranes are composed primarily of Laminin, type IV Collagen, Nidogen and heparin sulfate proteoglycans (HSPGs), which include Perlecan and Agrin (reviewed in Yurchenco et al., 2004; Laurila and Leivo, 1993). The heparin sulfate chains of these proteins are mainly responsible for the involvement of basement membranes in cell-cell signaling, because they bind (and thereby sequester or release in a controlled manner) ligands like Fibroblast Growth Factor (FGF), Platelet Derived Growth Factor (PDGF), Wnt, Hedgehog (Hh) and Transforming Growth Factor (TGFβ) (Lin, 2004; Lindner et al., 2007; Yurchenco et al., 2004).
Perlecan is a large macromolecule which consists of a core protein associated with three glycosaminoglycan side chains and that occurs in basement membranes around most cells. The core protein has five distinct domains. Domains I and V, located at the N and C terminal sides, respectively, both contain binding sites for heparin sulfate side chains (Whitelock et al., 2008). These two domains also interact with other ECM proteins, including Laminin and Collagen IV, as well cell adhesion molecules, such as integrins. Domain II is thought to interact with lipids (Fuki et al., 2000; Whitelock et al., 2008). Domains III and IV interact with other secreted signals and ECM proteins (Friedrich et al., 1999; Iozzo et al., 2005; Whitelock et al., 2008).
Given its widespread expression in basement membranes and its ability to bind to a multitude of known adhesion and signaling molecules, it is to be expected that Perlecan (along with other HSPGs) plays many different roles in tissue development and maintenance. Both in vitro and in vivo studies confirm this expectation. In vertebrates, Perlecan plays particularly important roles in the growth and morphogenesis of the skeleton and vascular system by modulating cell adhesion and/or the effect of the signaling molecules FGF, PDGF, and VEGF on chondrocytes, smooth muscle cells, and endothelial cells (Melrose et al., 2008; Segev et al., 2004; Whitelock et al., 2008). In C. elegans, Perlecan mediates the binding of muscle cells to epidermal cells via integrins (Rogalski et al., 2001). In Drosophila, the Perlecan homolog was originally identified as a mutation affecting postembryonic growth of the central nervous system. In larvae carrying this mutation, called trol, neuroblasts show a strongly decreased rate of proliferation (Datta, 1995). This phenotype was shown to be mediated by an effect of trol on FGF and Hh signaling (Caldwell et al., 1998; Lindner et al., 2007; Park et al., 2003). The effect of trol on proliferation is not confined to the CNS; experiments looking at hemocyte number in trol mutants have also shown a significant drop in circulating plasmatocyte numbers (Lindner et al., 2007).
Comparisons of the human Perlecan gene to trol have found 34% sequence identity in domain III, 24% identity in domain IV and 30% identity in domain V. No significant similarity was seen in domains I or II (Murdoch et al., 1992; Park et al., 2003). In Drosophila, Trol is expressed strongly in basement membranes of embryonic tissues, where it interacts with Laminin and Collagen (Urbano et al., 2009). It also appears around hemocytes, vascular cells, and the hematopoietic organ (“lymph gland”) of the late embryo. Given the reported effect of trol on blood cell number, we embarked on a more detailed analysis of the role of trol in Drosophila hematopoiesis.
The blood, or hemolymph, of Drosophila contains three major types of blood cells (hemocytes), called plasmatocytes, crystal cells and lamellocytes. Plasmatocytes act as macrophages during development, and together with crystal cells, play a role in immunity and response to injury (Crozatier and Meister, 2007; Martinez-Agosto et al., 2007). These two cell types comprise the hemocytes most commonly seen under non-immune challenged conditions. Lamellocytes are very rare under normal conditions. In cases of immune challenge, their numbers increase and they act to neutralize objects too large to be phagocytosed.
Hemocytes are produced during two phases of development. The first phase of hematopoiesis takes place in the head mesoderm of the early embryo; hemocytes produced during this phase populate the embryo and the circulating hemolymph of the larva. The second phase of hematopoiesis takes place in the lymph gland of the larva, a solid hematopoietic organ located alongside the dorsal vessel (“heart”). The lymph gland derives from a small population of hematopoietic blood progenitors that first appear in the trunk mesoderm of the embryo, consolidate into the lymph gland, and then proliferate during the larval stage. In the late larva, the lymph gland has grown into a series of several paired lobes flanking the dorsal vessel. Differentiation of hematopoietic progenitors into mature blood cells takes place in the periphery (cortex) of the large, anteriorly located primary lobe. A specialized subpopulation of hemocytes, called the posterior signaling center (PSC), signals to the medullary zone via the Hh pathway to maintain cells in an undifferentiated state (Mandal et al., 2007). Aside from Hh, the Wg signal (expressed in the medullary zone) and Adenosine deaminase growth factor A (Adgf-A), produced by differentiating cells in the cortical zone, antagonizes prohemocyte differentiation (and prolongs proliferation) in the medullary zone (Sinenko et al., 2009; Mondal et al., 2011; Grigorian and Hartenstein, 2012). Differentiated hemocytes are released from the lymph gland into circulation during early metamorphosis (Lanot et al., 2001; Grigorian et al., 2011). During this phase, the entire lymph gland dissociates; adult flies lack a solid hematopoietic organ. A lymph gland similar to that described for Drosophila has been documented for many insects (reviewed in Grigorian and Hartenstein, 2012). Similarities to the hematopoietic tissue of vertebrates are present; even though a prominent “stroma” (represented in the vertebrate bone marrow by the network of capillaries and reticular cells) is missing in invertebrates, cells described as “reticular cells”, surrounding prohemocytes and possibly acting as stem cells, have been described in several insect species (Hoffmann, 1970). In all insects investigated, profuse lamellae of extracellular matrix, formed by proteins that are found ubiquitously in basement membranes and other ECM assemblies of Drosophila and vertebrates (reviewed in Grigorian and Hartenstein, 2012), were observed.
In this paper, we show that Perlecan/Trol is expressed in basement membranes that both surround the surface of the lymph gland and form discrete chambers within the lymph gland interior. Loss of trol is associated with a dramatic change in the texture of the ECM: instead of forming thin membranes around blood cells, the matrix appears as a spongy mass filling large areas of the lymph gland. Concomitantly, proliferation of blood progenitors is reduced, and the lymph gland is significantly reduced in size and cell number. Finally, the altered composition of the extracellular matrix resulting from a loss of trol is associated with premature hemocyte differentiation. This phenotype can be rescued by the ectopic expression of Hh. We propose that trol is required for the organization of the lymph gland ECM, and the proper release and/or distribution of the Hh ligand throughout the lymph gland.
Material and Methods
Fly Lines
y trol 8 w/Bnsn (Park et al., 2001), y trol4 w/Bnsn (Park et al., 2001), y trolSD w/Bnsn (Datta and Kankel, 1992), ZCL1973X (Kelso et al., 2004), trol-GFP (Lindner et al., 2007; Medioni and Noselli, 2005; Kelso et al., 2004), domeless-Gal4 (Jung et al., 2005; Mandal et al., 2007; Ghiglione et al., 2002), Antennapedia-Gal4 (Mandal et al., 2007; Emerald and Cohen, 2004), UAS-trol Rnai (Vienna Drosophila RNAi Center), heatshock-hh (hh fused downstream of a Hsp70 promoter; Ingham et al., 1993; Park et al., 2003), Oregon R (Bloomington Stock Center).
Immunohistochemistry
α-Antennapedia (Mouse; 1:4; Developmental Hybridoma Bank), α- BrdU (Rat; 1:100; Abcam), α-Cleaved Caspase 3 (Rabbit; 1:100; Cell Signaling Technology, Inc.), α-Collagen IV (Mouse; 6G7; 1:25–1:50; Murray et al., 1995), α-GFP (Mouse; 1:400; Sigma), α-GFP (Rabbit; 1:2000; Molecular Probes), α-Laminin (Rabbit; 1:500; Fessler et al., 1987), α-Odd (Rabbit; 1:500; Ward and Skeath, 2000), α-P1 (Mouse; 1:10; Kurucz et al., 2007), α-Perlecan domain V (Rabbit; 1:1000; Friedrich et al., 2000), α-Peroxidasin (Mouse; 1:500; Nelson et al., 1994), α-ProPo (Rabbit; 1:1000; Muller et al., 1999). All secondary antibodies were acquired from Jackson Immunoresearch. Both Cy3 and FITC were used at a dilution of 1:200. Cy5 was used at a dilution of 1:100. Immunohistochemistry was carried out following standard protocols (Ashburner, 1989). Some preparations were counterstained with the nuclear marker Toto-3 iodide (Invitrogen; added at a concentration of 1:500 to the Vectashield in which the samples were mounted).
Mutant selection and Larval Fixation
Trol homozygous mutants were selected during 1st instar stage via yellow mouth hook color. Larvae were then allowed to mature till stated time point. All Larval lymph glands were dissected by either pulling out mouth hook with the lymph gland attached or pinning the larva dorsal side down and filleting it. Tissue fixed for 20 minutes in a 1:3 solution of 37% Formaldehyde: 1X PBS. Lymph glands then washed with 1X PBT (0.1%), 3 times for 15 minutes each at room temperature while rotating.
BrdU feeding, fixation and staining
56 hour larvae were placed on a fly food plate containing 1mg/ml of BrdU and left at 25°C for 30 minutes. Larvae were then shifted to regular fly food plates and left at 25°C until they were dissected at 73 hours. Tissue was then fixed in a 1:3 solution of 37% Formaldehyde: 1X PBS for 20–30 minutes. Staining was carried out as described previously (Grigorian et al., 2011).
Heatshock
WT, hs-hh, trol8 and hs-hh; trol8 fly lines were allowed to lay eggs over the weekend on food plates. Larvae were shifted to 37°C overnight (food plate was placed upside-down to better withstand drying). Larvae were then shifted to 29°C for two days before being selected, dissected, fixed as described above and labeled with antibodies. All larvae dissected were 72–132 hours post hatching (raised at 29°C, except during heat shock).
Electron microscopy
Larval lymph glands were dissected and fixed as described previously (Grigorian et al., 2011) Blocks were sectioned (60nm) and mounted on net grids (Ted Pella). Sections were treated with uranyl acetate and lead citrate. Samples were sectioned by the Microscopic Techniques Laboratory and examined using the JEOL 100CX transmission electron microscope at the Electron Microscope Laboratory of the UCLA Brain Research Institute.
Results
Trol is a component of the basement membranes that compartmentalize the lymph gland
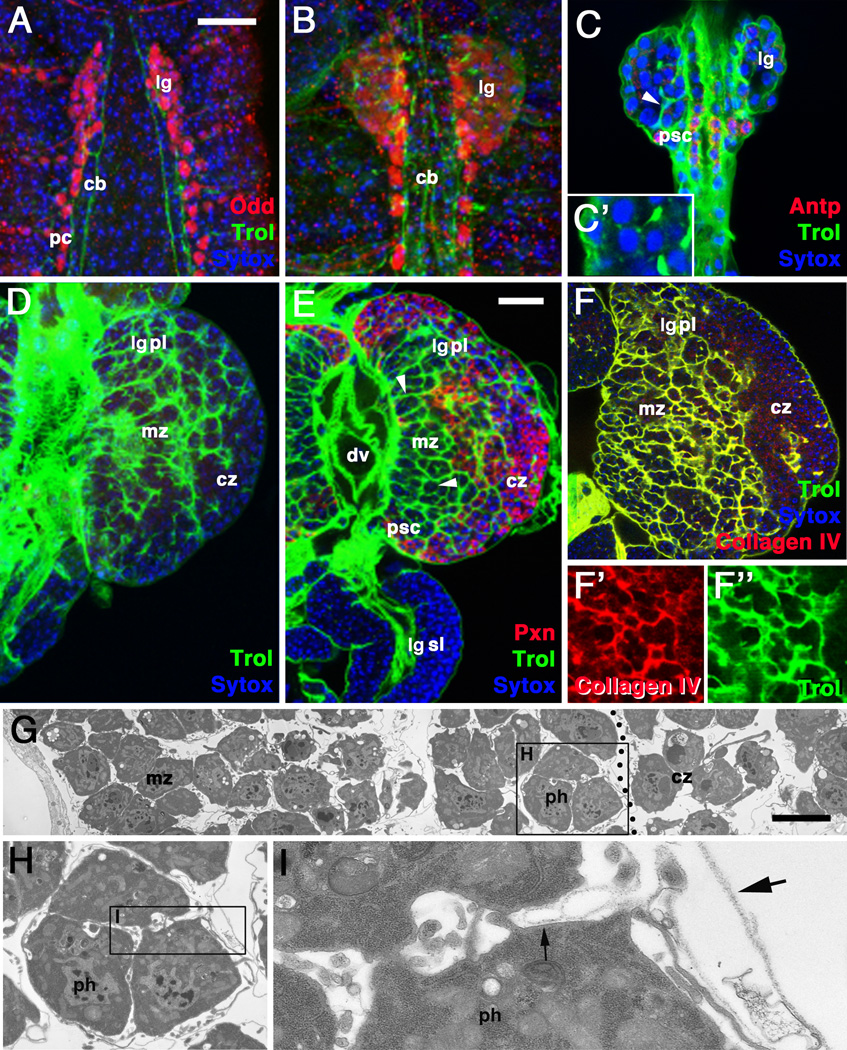
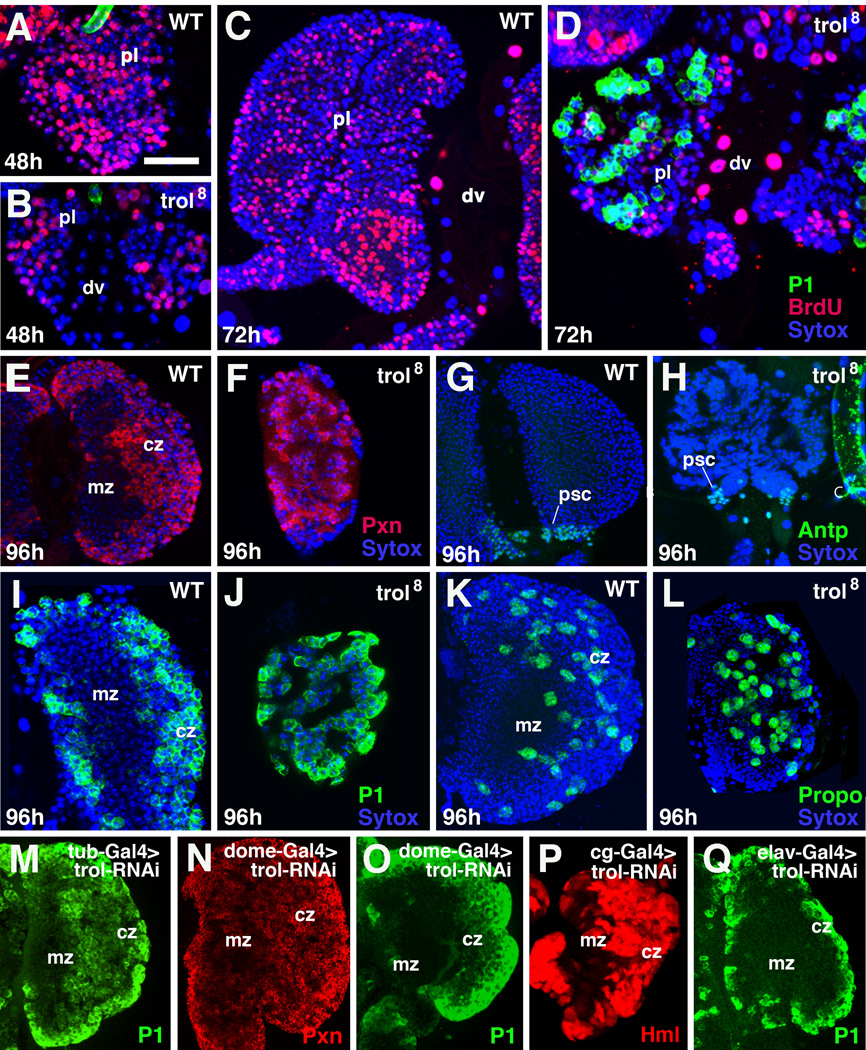
In order to assess the expression pattern of trol in the heart and blood system of Drosophila, we utilized the protein trap line ZCL1973X (Kelso et al., 2004), as well as an antibody against the Trol protein (Friedrich et al., 2000). During embryogenesis, Trol expression becomes visible around cardioblasts at stage 14 (Fig.1A). By stage 16, Trol expression also surrounds pericardial nephrocytes and the surface of the lymph gland, a paired cluster of approximately 30 hematopoietic progenitors (Fig.1B). Shortly after larval hatching, Trol covers each individual hematopoietic progenitor of the lymph gland. Expression is particularly strong around cells of the PSC, labeled with an antibody against Antennapedia (Antp) (Fig.1C).
Figure 1.
Expression of Perlecan/Trol in the lymph gland and dorsal vessel. A, B: Dorsal view of stage 14 (A) and stage 16 (B) embryos. Trol expression is visualized by GFP (green) driven by the ZCL1973X line. The lymph gland (lg) and pericardial cells (pc) are also labeled with anti-Odd antibody (red). Nuclei are labeled with Toto (blue). C: First instar larva (4–6h post hatching). Trol expression has increased and starts to surround individual hematopoietic progenitors (arrowhead). The Posterior Signaling Center (PSC) is labeled with anti-Antennapedia (Antp; red) antibody. Nuclei are labeled with Toto (blue). D: Second instar larva (56 hour post hatching). Trol expression increases and becomes more concentrated in the center of the lymph gland, the incipient medullary zone (mz). E: Third instar larva (96h post hatching). Trol expression is strong in the medullary zone and outlines chambers that include multiple cells (arrowhead; see also Fig.2). Expression is diminished in cortical zone (cz) where hemocytes, labeled here by anti-Peroxidasin (Pxn; red) differentiate. F–G: Co-expression of Trol (green) with Laminin A (F, F’) and Collagen IV (G, G’), recognized by their respective antibodies (red) in extracellular matrix of late larval lymph gland. H–I: Electron micrographs of third instar larval lymph gland. Basement membranes surround individual hematopoietic progenitors (ph; small arrow in J) and form irregular septa around groups of cells (large arrow in J. Other abbreviations: cb cardioblasts; dv dorsal vessel; lgpl primary lobe of lymph gland; lgsl secondary lobe of lymph gland.
Bars: 20µm (A–D; E–F); 5µm (G)
By the 2nd instar (56 hours), the lymph gland has expanded, and a separate central medullary zone (hematopoietic progenitors) has become morphologically distinct from an outer, more loosely packed cortical zone, in which some cells show expression of differentiation markers, such as Peroxidasin (Pxn) (Jung et al., 2005). At the same time, secondary and tertiary lobes appear posterior of the large primary lobes. Trol expression forms a solid layer around the surface of primary and secondary lobes. Within the lobes, Trol expression is seen strongly around clusters of cells; faint Trol signal appears around individual cells. In other words, the Trol pattern outlines a system of chambers that each contain small clusters of hematopoietic progenitors or differentiated hemocytes. These chambers are smaller in the medullary zone than in the cortical zone (Fig.1D, E), and are all but absent in the secondary/tertiary lobes (Fig.1E). Two other major components of basement membranes, laminin and collagen IV, are co-expressed with Trol in the lymph gland, as seen via antibody staining (Fig.1F–G’). It should be mentioned that, while it has been shown that hemocytes and their progenitors themselves express the genes encoding these ECM proteins (Kniebiehler et al., 1987; Lunstrum et al., 1988; Kusche-Gullberg et al., 1992), other organs, such as the fat body, could also contribute to the ECM of the lymph gland.
Electron microscopically, the structures containing Trol, Laminin and Collagen IV are revealed as typical basement membranes, forming 20–40nm thick, electron dense layers of granular material (Fig.1H–J). Clefts of variable diameter separate basement membranes and hemocytes. Thus, in many places, basement membranes adhere closely to the surface of the hemocyte (small arrow in Fig.1J). At other locations, basement membranes have separated from the hemocytes (large arrow in Fig.1J). Basement membranes of the lymph gland persist for several hours after hemocytes are released during the pupal stage (Grigorian et al., 2011), attesting to the fact that these membranes are extraordinarily stable structures.
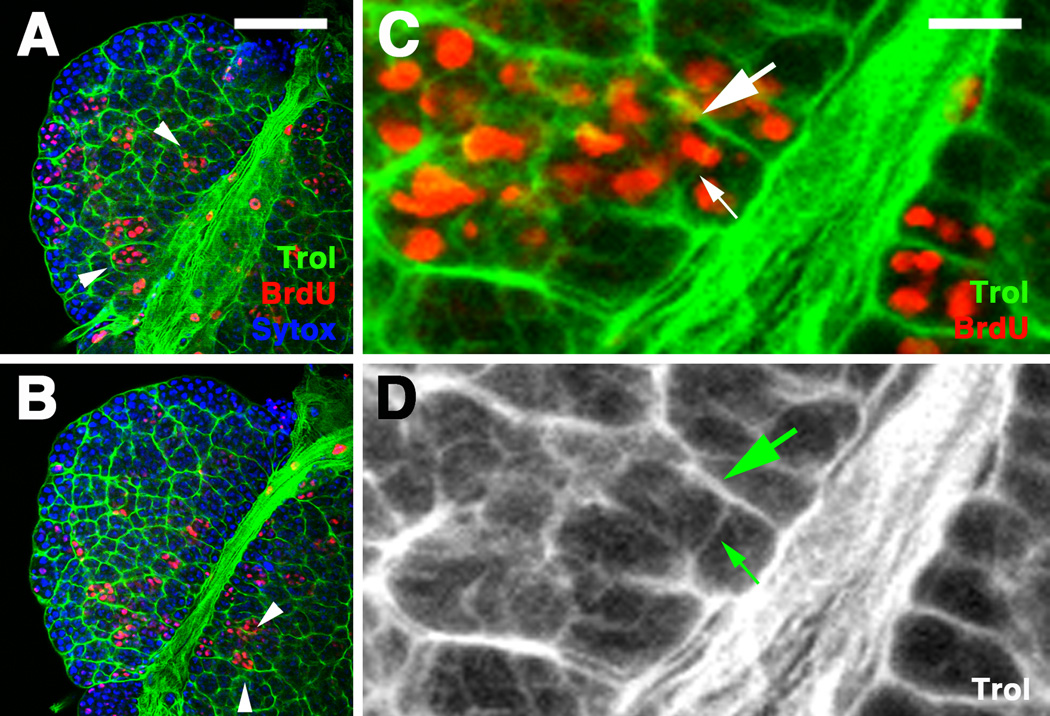
The chambered appearance of the basement membranes appears to be related to hemocyte proliferation. Thus, short pulses of BrdU fed to larvae, followed by a chase, resulted in 4–8 cell clones of labeled cells which frequently coincided with chambers (Fig.2). We propose a process where Trol (and other ECM proteins) is continuously secreted by a hematopoietic progenitor to form a basement membrane around the cell. As the hematopoietic progenitor divides, the newly formed membranes in between the daughter cells initially lack a coat of ECM, whereas this coat is already present on all other sides. In this manner, as proliferation continues, the membranes facing the “inside” of the clone are thinner (small arrow in Fig.2D) than those facing the outside (large arrow in Fig.2D)
Figure 2.
Pattern of basement membranes in lymph gland is related to hematopoietic progenitor proliferation. A, B: Two confocal section of a ZCL1973X, 72h larval lymph gland showing expression of trol (green), anti-BrdU (red), and TOTO (blue). Larva had been subjected to 1h pulse of BrdU at 48h. C, D: High magnification view of BrdU-positive cells (red in C) in relationship to trol-positive basement membranes (green in C; white in D). BrdU-positive hematopoietic progenitors form clusters of cells whose boundaries, in most cases, coincide with the septa formed by basement membranes (arrowheads in A, B; large arrows in C, D). Individual cells are surrounded by thin basement membranes (small arrows in C, D).
Bar: 40µm (A, B); 10µm (C, D)
Loss of trol causes a change in the ultrastructure of the lymph gland ECM
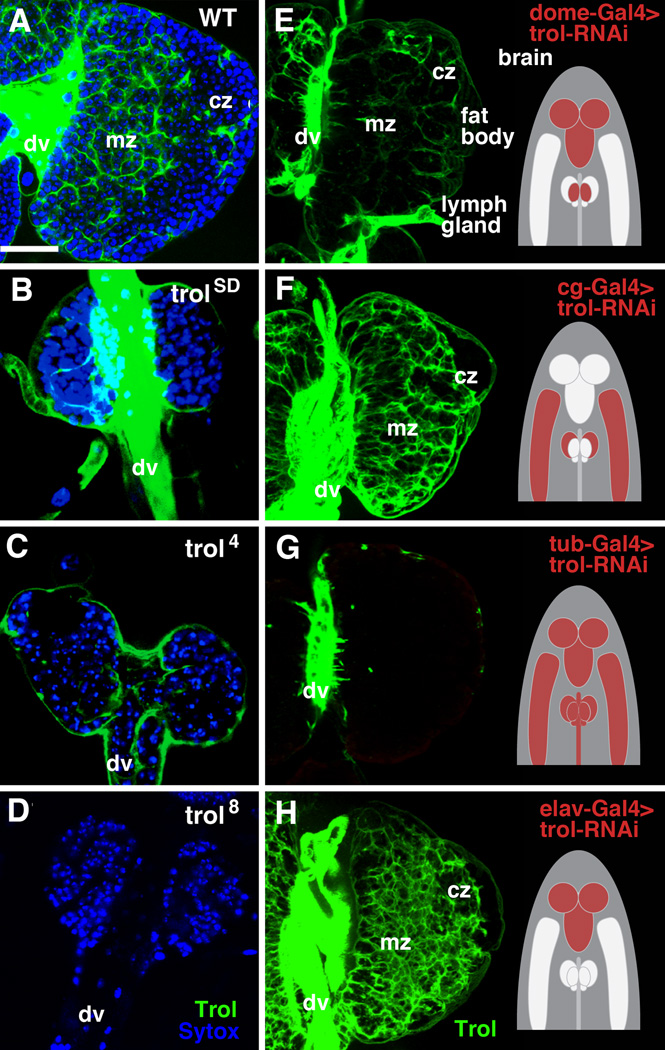
In order to assess the role of trol in hematopoiesis, we investigated three loss of function alleles, trolSD, trol4 and trol8 (Lindner et al., 2007). The trolsd and trol4 alleles are late larval lethals. However, some of the mutants of the trol8 allele are able to progress to pupal stages (Lindner et al., 2007). trol8 (the allele used in the following for phenotypic analysis) showed a complete loss of Trol protein expression (Fig.3B), whereas residual Trol signal, visible as a sheath encasing the lymph gland, remained in the other two alleles (Fig.3C, D). To learn more about the tissue-specific requirement of trol we used the UAS-Gal4 system to drive a trol-RNAi construct by dome-Gal4 (medullary zone, but also nervous system; Fig.3E), Antp-Gal4 (PSC; not shown); hml-Gal4 (cortical zone; not shown), cg-Gal4 (cortical zone, fat body; Fig.3F), tub-Gal4 (ubiquitous; Fig.3G), and elav-Gal4 (brain; Fig.3H). Ubiquitous and medullary zone-directed expression of trol-RNAi resulted in a severe reduction of anti-Trol antibody signal in the lymph gland; by contrast, trol-RNAi driven by cg-Gal4 and elav-Gal4 did not have a significant effect on Trol expression in the lymph gland.
Figure 3.
A-D: Expression of Trol (green) in 96h wild-type lymph gland (A) and three mutant trol alleles: trol 8 (B), trol 4 (C) and trol SD (D). Of the three mutants only trol8 shows a complete lack of anti-trol antibody staining. E–H: Expression of trol-RNAi by four different Gal4 driver lines. Pattern of expression of the drivers (E: dome-Gal4; F: cg-Gal4; G: tub-Gal4; H: elav-Gal4) is shown schematically at left of panels; Trol signal in lymph gland, detected by antibody, is showing at the right. Abbreviations: cz cortical zone; dv dorsal vessel; mz medullary zone; Bar: 20µm (A-H).
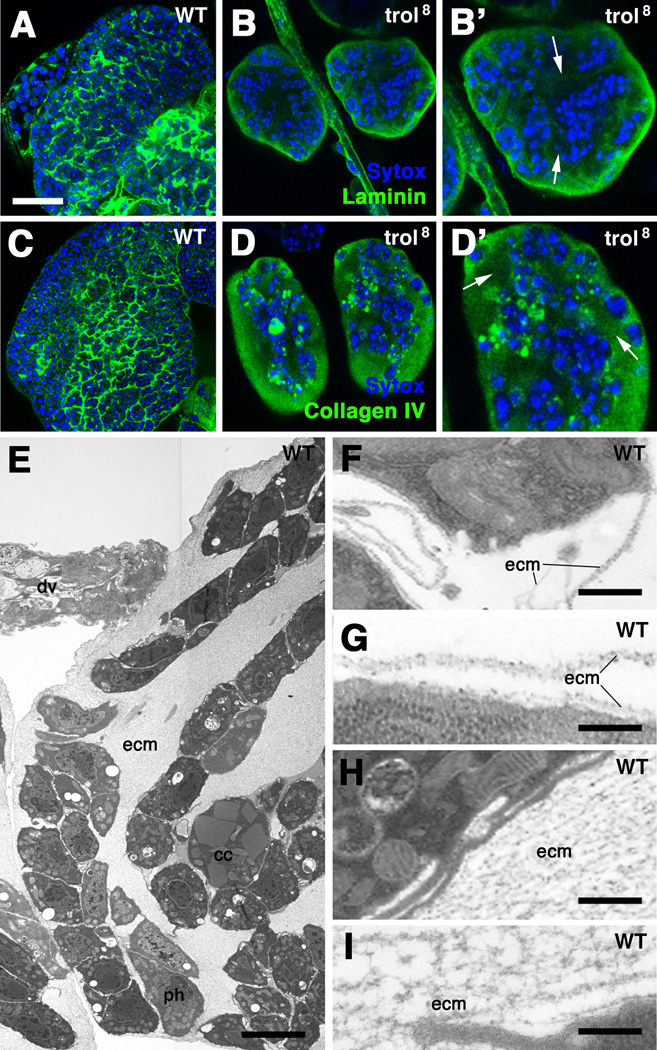
The most conspicuous phenotype of trol LOF alleles is a reduced lymph gland size and altered packing of prohemocytes. Light microscopically, the lymph gland of trol mutants showed large gaps (lacunae) devoid of cells (arrowheads in Fig.4B’,D’). A similar phenotype could be observed when expressing trol-RNAi ubiquitously by tub-Gal4 (not shown). Laminin and Collagen IV, normally concentrated in thin basement membranes surrounding (groups of) hemocytes (Fig.4A, C), occupied these lacunae in between strands of cells (Fig.4B, D). Electron microscopically, these lacunae were solidly filled with a fine-meshed, reticular material (Fig.4E). At the same time, thin basement membranes around individual cells, or the lymph gland as a whole, were absent. In electron density, the reticular ECM of trol mutant lymph glands resembled the basement membranes of wild type (compare panels 4F–I). The main difference is best characterized as a change in pattern: in wild type, the macromolecular components of the ECM assemble as a two-dimensional sheet (Fig.4F–G). In the absence of trol, these components are woven into a three-dimensional network of lamellae and fibers (Fig.4H–I).
Figure 4.
Loss of trol causes structural changes in extracellular matrix of lymph gland. A–D: confocal sections of 96h wild-type lymph gland (A, C) and trol8 mutant (B, D) labeled with anti-Laminin (A, B) and anti-Collagen IV antibodies (C, D). B’ and D’ show high magnifications of mutant lymph glands presented in B and D, respectively. Laminin and Collagen IV are distributed in thin layers (basement membranes) in wild type; in trol8 mutant, these proteins solidly fill wide extracellular spaces formed within the lymph gland (arrows in B’ and D’). E: Electron micrograph of trol8 mutant lymph gland. Wide clefts in between clusters of hematopoietic progenitors (ph) are filled with extracellular matrix (ecm). F, G: High magnification electron micrographs of extracellular matrix of wild-type lymph gland, forming thin, two-dimensional basement membranes around cells. H, I: In trol8 mutant, extracellular matrix is structured as a three-dimensional meshwork of fibers and membranes. Other abbreviations: cc crystal cell; dv dorsal vessel.
Bars: 20µm (A-D’); 5µm (E); 200nm (F, H); 100nm (G, I).
Loss of trol results in a reduction of proliferation and premature differentiation of hemocytes
The lymph glands of trol mutant larvae were smaller than their wild-type counterparts. Past studies have shown that cell cycle progression in the brain of trol mutants is arrested (Caldwell et al.,1998). We therefore carried out BrdU incorporation studies in order to assess whether cell proliferation was reduced in trol8 mutant lymph glands. We found that 48h and 72h trol8 lymph glands had lower levels of BrdU incorporation in comparison to their WT counterparts (Fig.5A–D). Since changes in size could also be due to cell death, we carried out an anti-cleaved Caspase staining in mutant lymph glands, however no noticeable cell death was seen (data not shown).
Figure 5.
Loss of trol leads to reduced proliferation and precocious differentiation of hematopoietic progenitors. A–D: One hour pulses of BrdU were applied to wild-type larvae (A, C) and trol8 mutant larvae (B, D) at time points indicated (48h after hatching: A, B; 72h: C, D). Lymph glands were labeled with anti-BrdU (red) and anti-P1 (green). BrdU-positive cells are strongly reduced in trol mutant at both time points. The plasmatocyte marker P1 is not yet expressed in 72h wild-type lymph glands (C), but labels clusters of cells in trol8 (D). E–L: Expression of hemocyte differentiation markers in lymph glands of 96h wild-type and trol8 mutant larvae. In 96h wild-type lymph glands, early hemocyte marker peroxidasin (Pxn, red in E), late plasmatocyte marker P1 (green in I) and crystal cell marker Propo (green in K) are restricted to the cortical zone (cz). These markers are evenly expressed throughout lymph gland in 96h trol8 mutant (F, J, L). Antp, a marker for the PSC (green in G, H) is expressed normally in trol8 mutant. M–Q: Driving a trol-RNAi construct with different driver lines [tub-Gal4 (M), dome-Gal4 (N, O), cg-Gal4 (P), elav-Gal4 (Q)] results precocious expression of differentiation markers (P1: green; Pxn or Hml: red). Bar:20µm (A-Q).
Significantly, the reduction in proliferation is accompanied by a premature differentiation of hemocytes. Late differentiation markers of plasmatocytes, like P1 which is expressed only in the late third instar (96–120h) of wild type larvae, appeared as early as 72h post hatching in trol mutants (Fig.5D). Furthermore, in mutant lymph glands, the zonation into a central medullary zone containing undifferentiated hematopoietic progenitors, surrounded by a cortical zone of differentiating hemocytes was abolished. Differentiation markers like P1(late plasmatocytes), Pxn (early plasmatocytes), or ProPo (crystal cells) were evenly distributed throughout the lymph gland (Fig.5E–F, I–L). Ubiquitous activation of trol-RNAi (tub-Gal4) had a similarly strong effect in regard to precocious differentiation of hemocytes (Fig.5M). A milder phenotype could be elicited by knock-down of trol via an RNAi construct driven by dome-Gal4 (medullary zone; Fig.5N, O) or cg-Gal4 (cortical zone plus fat body; Fig.5P). Knockdown of trol in the cortical zone alone or in the brain had no significant effect on hemocyte differentiation (Fig.5Q).
The loss of function and knock-down phenotype suggests that the block of differentiation that is normally exerted onto the medullary zone by signals from the PSC and the cortical zone is lifted. To assay for the presence of the PSC we used an antibody against Antp (Mandal et al., 2007). The expression of this marker remained unchanged (Fig.5G, H) in regard to level and cell number, indicating that the premature differentiation of the medullary zone is not the result of a lack of the PSC.
Loss of trol interferes with the Hh signaling pathway
Hh signaling plays an important role in determining hemocyte differentiation within the lymph gland. Hh signals originating from the PSC maintain cells of the medullary zone in an undifferentiated state (Mandal et al., 2007). Loss of this signal results in mass differentiation of the cells of the lymph gland. Trol is known to play a role in modulating molecular signals, including Hh. Since we had observed a phenotype where the cells of the trol8 mutant lymph glands were differentiated regardless of their location in a medullary or cortical zone, we decided to look into whether trol may be effecting Hh signaling.
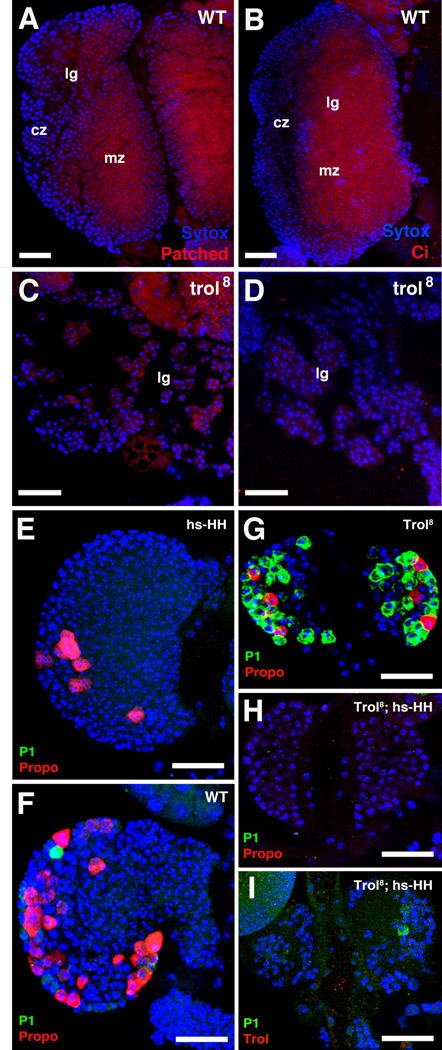
The expression level of the receptor patched (Ptc), a read-out of Hh-signaling activity, was reduced in the medullary zone (Fig.6A, C). The transcription factor Cubitus interruptus (Ci), which is activated by Hh signaling from the PSC, and is normally expressed in the medullary zone (Mandal et al., 2007), was also strongly reduced or absent in the trol8 mutant (Fig.6B, D). Both findings indicate that in the absence of trol, Hh signaling to the medullary zone is diminished. Genetic interaction between hh and trol further support this notion. We used the heat-shock inducible hs-hh construct (Ingham et al., 1993; Park et al., 2003) in the background of homozygous trol larvae to test the prediction that overexpression of hh is able to rescue the trol hemocyte differentiation phenotype in the lymph gland. Expression of the hs-hh construct alone in wild-type larvae caused a delay in hemocyte differentiation compared to WT (Fig. E, F). This was expected based on past studies that have shown that Hh acts to maintain lymph gland hematopoietic progenitors in an undifferentiated state (Mandal et al., 2007). Expressing hs-hh in the background of trol LOF animals rescued the global, premature differentiation of primary lobes characteristic for the trol mutant (compare Fig.6G and 6H, I). Lymph glands were still reduced in size and showed wide, cell-free gaps, but contained no differentiated cells in their central domain (“medullary zone”). P1 was almost entirely absent; Pxn was expressed in clusters of hemocytes restricted to the periphery (“cortical zone”) of the lymph gland (data not shown). These results suggest a model where the Hh signal, in order to act on cells of the medullary zone, requires the presence of the ECM protein Trol. Loss of Trol renders endogenous Hh ineffective; however, by applying Hh in excess by hs-Hh, its role as an inhibitor of differentiation can be partially restored.
Figure 6.
Genetic interaction between trol and Hh signaling pathway members. A–D: Genes activated by Hh signaling, patched (ptc; A) and cubitus interruptus (ci; B) are expressed in medullary zone (mz) of 96h wild-type lymph gland (Ptc and Ci proteins recognized by antibodies; red). Expression levels of these genes are strongly reduced in trol8 mutant (C, D). E-I: Precocious differentiation of hemocytes in trol can be rescued by ectopic hh expression. All panels show lymph glands of late third instar larvae which have been heat shocked (see Material and Methods). E–F: In wild-type and larvae expressing hs-hh in wild-type background, hemocyte differentiation is initiated in the cortical zone, as shown by expression of the crystal cell marker Propo (red). Expression of late plasmatocyte marker P1 (green) is just beginning. As expected, the hs-hh lymph gland displays reduced hemocyte differentiation in comparison to the WT. G: In a trol8 mutant that has been heat shocked, both Propo and P1 are expressed throughout the lymph gland. H,I: Expression of hs-hh in the background of trol8 (note absence of trol protein shown in I) prevents hemocyte differentiation. Bar: 20µm (A-E).
Other abbreviations: cz cortical zone; lg lymph gland. Bars: 20µm
Discussion
Perlecan/Trol is a secreted heparin sulfate proteoglycan (HSPG) that forms a widespread component of the ECM. Members of the HSPG family are comprised of a protein core to which heparin sulfate glycosaminoglycan chains are attached (Bernfield et al., 1999; Esko and Selleck, 2002; reviewed in Yan and Lin, 2009). A number of genes encoding glycosyltransferases and modification enzymes that include tout-velu (ttv), sister of ttv (sotv), brother of ttv (botv) and sulfateless (sfl) are conserved from vertebrates to Drosophila, and are important for the polymerization and modification of heparin sulfate chains during their biosynthesis (Esko and Selleck, 2002; Nybakken and Perrimon, 2002; Lin and Perrimon, 2003; reviewed in Yan and Lin, 2009). Loss of these enzymes has been shown to lead to defective morphogen signaling and gradient formation, showing the importance of heparin sulfate chains for the role of HSPGs in the ECM.
Our study of Drosophila Perlecan/Trol shows that this protein plays an important role in Drosophila hematopoiesis. Trol is expressed in the hematopoietic organ, the lymph gland, from embryonic stages onward. As shown in our previous work (Grigorian et al., 2011) and in this paper, the ECM of the lymph gland forms thin lamellae (“basement membranes”) surrounding individual hemocytes, as well as the organ as a whole. A similar organization of the ECM has also been noted in the hematopoietic organs of other insects (Hoffmann et al., 1979; Nardi et al., 2003; Grigorian and Hartenstein, 2012). For example, in Locusta migratoria, Hoffmann and colleagues (1979) describe“fine lamellae of basement membrane” around the hemocytes. The basal laminae within the Manduca hematopoietic organ also seem to form chambers around hemocytes;Nardi et al. (2003) describe groups of contiguous cells within the hematopoietic organ that appear to be tightly held together by basal laminae, thereby somewhat separating them from more loosely associated single cells that are also found in the organ. As hemocytes move from the hematopoietic organ into circulation, they locally break through this ECM (Grigorian et al., 2011). Similarly, in Manduca sexta, the basal lamina surrounding the hematopoietic organ shows gaps in the domain where hemocytes are released (Nardi et al., 2003).
Drosophila trol mutants exhibit a complex lymph gland phenotype. We speculate that this phenotype is caused by (1) the role of Trol as a structural component of the ECM, and (2) its role as a binding partner to extracellular signals, such as Hh. In regard to the first role we observed an anomalous ultrastructure of the lymph gland ECM, which forms a sponge-like, three dimensional mesh, as opposed to the two-dimensional lamellae seen in the wild type. Correspondingly, the distribution of Laminin and Collagen IV is also affected in trol mutants. Instead of forming the lamellae characteristicly seen in wild type, Collagen IV and Laminin are distributed diffusely throughout the wide lacunar spaces that riddle the trol-mutant lymph gland. This finding suggests that Trol exerts an organizing effects on other constituents of the ECM, such as Laminin and Collagen. Converse studies, looking at Trol protein in the background of deficiencies of other ECM components, have revealed that a mutation in laminin β1 chain causes a loss of both Collagen IV and Trol accumulation around the cardioblasts of D. melanogaster embryos (Urbano et al., 2009). Collagen IV mutants in D. melanogaster also lose Trol in the basement membranes of embryos and larval wing discs (Pastor-Pareja and Xu, 2011). A recent study (Pastor-Pareja and Xu, 2011) investigated the function of trol during wing disc development using trol-RNAi. This treatment did not cause the loss of Collagen IV or Laminin. Structurally, trol-RNAi reduced the thickness of the wing disc basement membrane. The mesh-like ECM phenotype that we witnessed in the trol8 mutant larval lymph gland was not observed in the wing disc. In the lymph gland, knocking down trol via dome-RNAi also causes only slightly premature expression of the hemocyte differentiation markers Pxn and P1, but does not have an apparent effect on general ECM structure (this study; Dragojlovick and Martinez-Agosto, 2012). On the other hand, driving the trol-RNAi construct ubiquitously (tub-Gal4) results in a much stronger phenotype, pointing to the importance of tissues outside the lymph gland in shaping the ECM surrounding hemocytes and their progenitors. Previous work had shown that the basement membranes surrounding a number of larval structures, notably the imaginal discs, are assembled from extracellular matrix produced in the fat body, rather than the epithelial cells of the discs themselves (). It is likely that the basement membrances surrounding hemocytes in the lymph gland may also be enriched by proteins diffusing from the fat body, via hemolymph, into the glands. The lack of any lymph gland phenotype when knocking down trol in the brain (elav-Gal4) demonstrates that general neurological abnormalities, which might have an indirect effect on growth and metabolism, did not account for the strong lymph phenotype in troll LOF alleles and RNAi knock-downs.
In vertebrates, Perlecan binds to Laminin and Collagen (Whitelock et al., 2008). It is known to play a role in Laminin organization in mouse embryonic stem cells (Henry et al., 2001). Perlecan is also involved in cartilage matrix assembly and has been seen to promote collagen fibril formation (Kvist et al., 2006). On the organismic level, mutations in vertebrate Perlecan also have a profound effect on blood and cardiovascular development. Morpholino-mediated knockdown of Perlecan in zebrafish showed abnormal development of the angiogenic intersegmental and dorsal longitudinal anastomotic vessels (Zoeller et al., 2008). Knock-out of Perlecan in mice causes a pleiotropic phenotype (Arikawa-Hirasawa et al., 1999; Costell et al., 1999; reviewed in Whitelock et al., 2008), reflecting the fact that Perlecan is expressed in many different tissues throughout development. In the cardiovascular system, Perlecan KO mice show massive blood leakage into the pericardial cavity (Sasse et al., 2008). Vascular defects include abnormal coronary vessels, as well as transposition of the great arteries (Hassel et al., 2002). Perlecan is also a component of the ECM in bone marrow (Klein et al., 1995), where it is expressed strongly between maturing hematopoietic cells, as well as in the basement membrane of endothelial cells. Perlecan is thought to interact closely with proliferating and differentiating hematopoietic cells. Interestingly, in vitro cell attachment assays indicate that Perlecan appears to reduce the adhesion of hematopoietic cells (Klein et al., 1995). This role as a repellant for hematopoietic cells may be important for the regulation of cell migration and egress into circulation.
Aside from the structural defects, loss of function in Perlecan or other glycoproteins causes defects that are attributable to disruptions in signaling mechanisms. These observations emphasize the (permissive) role of ECM components in shaping the pattern of release and the concentration gradients of secreted signals. We report here that the lymph glands are significantly smaller in size than their WT counterparts due to reduced cell divisions. This phenotype is reminiscent of the reduced proliferation in larval brain neuroblasts that gave the trol mutation (“terribly reduced optic lobe”) its name (Datta and Kankel, 1992). In the larval brain, trol promotes cell cycle progression of neuroblasts via the FGF and Hh signaling pathways (Park et al., 2003; Caldwell and Datta, 1998; Lindner et al., 2007). Datta and colleagues also noted a significant drop in the number of circulating larval hemocytes in the trol mutant (Lindner et al., 2007), and speculated that trol promotes Ras-MAPK via the Pvr (PDGF/VEGF) signaling pathway, which has been shown to play a role in hemocyte maintenance in the embryo (Brückner et al., 2004). It is conceivable that the reduced proliferation in the trol mutant larval lymph gland described in this paper is also mediated via an effect of Trol on PVR signaling.
A further characteristic phenotype of the trol mutation is precocious differentiation of the hematopoietic progenitors of the lymph gland. Whereas P1 expression is seen by 72 hour in the trol8 mutant, WT lymph glands do not show expression of this marker until 96 hours. In addition to its early onset, differentiation is not limited to the cortical zone of the lymph gland, as is seen in WT, but occurs in the medullary zone as well. In the lymph gland of D. melanogaster larvae, Hh is known to play a role in maintaining the cells of the medullary zone in an undifferentiated state (Mandal et al., 2007). Our findings indicate that the precocious and disordered differentiation observed in trol lymph glands is indeed due to a reduced activity in Hh signaling, prompting the hypothesis that hh, secreted from the PSC, requires trol and/or an intact ECM to act upon the medullary zone. Previous studies have shown that Perlecan can bind Hh, Wnt and FGF, thereby controlling the concentration of these molecules in the pericellular space and the amount of signal that is received by cells (reviewed in Iozzo, 2005; Whitelock et al., 2008). In other words, Perlecan and other glycoproteins of the ECM shape the gradient of Hh and other morphogens. In the wing disc, mutant clones of the glycosyltransferase ttv exhibit impaired Hh signaling. Hh appears to be unable to diffuse past these mutant cells (Bellaiche et al., 1998). Clones carrying a double mutation for the Drosophila HSPG glypicans division abnormally delayed (dally) and dally-like-protein (dlp) show a similar, albeit weaker phenotype to that seen for ttv (Han et al., 2004; reviewed in Yan and Lin, 2009). Furthermore, co-immunoprecipitation studies in third instar larvae of D. melanogaster demonstrate a direct interaction between trol and Hh (Park et al., 2003). Lipid-modification of Hh is needed for its interaction with HSPGs (Bellaiche et al., 1998; Callejo et al., 2006; Gallet et al., 2006; reviewed in Yan and Lin, 2009). Another way of picturing the involvement of trol in Hh signaling is to view the ECM as a physical conduit for the normal spreading of Hh. Even under normal circumstances, ECM acts as a migration and diffusion barrier (Korpos et al., 2010; Sabeh et al., 2004). The altered state of the ECM in trol mutant lymph glands may further inhibit proper Hh dispersion. We propose that the Drosophila larval lymph gland, with its simple structure and highly characteristic lamellar ECM architecture, represents a favorable system to approach genetically the role of ECM in cell-cell signaling pathways.
Acknowledgements
We are grateful to I. Ando, U. Banerjee, S. Baumgartner, L. Cooley, S. Datta, J. Fessler, L. Fessler, F. Kafatos, H. Muller, J. Skeath, the Bloomington Stock Center, the Vienna Drosophila Rnai Center and the Developmental Studies Hybridoma Bank for providing fly stocks and antibodies. We would also like to thank Marianne Cilluffo for technical help with the electron microscopy. This work was supported by the HFSP grant RGP0015/2008-C EM to V.H. and the Ruth L. Kirschstein National Research Service Award GM007185 to M.G.
References
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila. A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 214–217. [Google Scholar]
- Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Caldwell MC, Datta S. Expression of cyclin E or DP/E2F rescues the G1 arrest of trol mutant neuroblasts in the Drosophila larval central nervous system. Mech Dev. 1998;79:121–130. doi: 10.1016/s0925-4773(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Callejo A, Torroja C, Quijada L, Guerrero I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–483. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Datta S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development. 1995;121:1173–1182. doi: 10.1242/dev.121.4.1173. [DOI] [PubMed] [Google Scholar]
- Datta S, Kankel DR. l(1)trol and l(1)devl, loci affecting the development of the adult central nervous system in Drosophila melanogaster. Genetics. 1992;130:523–537. doi: 10.1093/genetics/130.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovick M, Martinez-Agosto J. Extracellular matrix-modulated FGF signaling in Drosophila blood progenitors regulates their differentiation via a ras/ETS/FOG pathway and TORC1 function. In Review. 2012 doi: 10.1016/j.ydbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- Emerald BS, Cohen SM. Spatial and temporal regulation of the homeotic selector gene Antennapedia is required for the establishment of leg identity in Drosophila. Dev Biol. 2004;267:462–472. doi: 10.1016/j.ydbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Campbell AG, Duncan KG, Fessler JH. Drosophila laminin: characterization and localization. J Cell Biol. 1987;105:2383–2391. doi: 10.1083/jcb.105.5.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MV, Gohring W, Morgelin M, Brancaccio A, David G, Timpl R. Structural basis of glycosaminoglycan modification and of heterotypic interactions of perlecan domain V. J Mol Biol. 1999;294:259–270. doi: 10.1006/jmbi.1999.3259. [DOI] [PubMed] [Google Scholar]
- Friedrich MV, Schneider M, Timpl R, Baumgartner S. Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur J Biochem. 2000;267:3149–3159. doi: 10.1046/j.1432-1327.2000.01337.x. [DOI] [PubMed] [Google Scholar]
- Fuki IV, Iozzo RV, Williams KJ. Perlecan heparan sulfate proteoglycan: a novel receptor that mediates a distinct pathway for ligand catabolism. J Biol Chem. 2000;275:25742–25750. doi: 10.1074/jbc.M909173199. [DOI] [PubMed] [Google Scholar]
- Gallet A, Ruel L, Staccini-Lavenant L, Therond PP. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–418. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]
- Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, Cerezo D, Noselli S. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development. 2002;129:5437–5447. doi: 10.1242/dev.00116. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol. 2011;221:121–131. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M, Hartenstein V. Hematopoiesis and Hematopietic organs in arthropods. Dev Genes Evol. 2012 doi: 10.1007/s00427-012-0428-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Hassell J, Yamada Y, Arikawa-Hirasawa E. Role of perlecan in skeletal development and diseases. Glycoconj J. 2002;19:263–267. doi: 10.1023/A:1025340215261. [DOI] [PubMed] [Google Scholar]
- Henry MD, Satz JS, Brakebusch C, Costell M, Gustafsson E, Fassler R, Campbell KP. Distinct roles for dystroglycan, beta1 integrin and perlecan in cell surface laminin organization. J Cell Sci. 2001;114:1137–1144. doi: 10.1242/jcs.114.6.1137. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Zachary D, Hoffmann D, Brehelin M. Postembryonic development and differentiation: hematopoietic tissues and their functions in some insects. In. In: Gupta A, editor. Insect Hemocytes. London: Cambridge University Press; 1979. pp. 29–82. [Google Scholar]
- Ingham PW. Localized hedgehog activity controls spatial limits of wingless transcription in the Drosophila embryo. Nature. 1993;366:560–562. doi: 10.1038/366560a0. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kelso RJ, Buszczak M, Quinones AT, Castiblanco C, Mazzalupo S, Cooley L. Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 2004;32:D418–D420. doi: 10.1093/nar/gkh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Conzelmann S, Beck S, Timpl R, Muller CA. Perlecan in human bone marrow: a growth-factor-presenting, but anti-adhesive, extracellular matrix component for hematopoietic cells. Matrix Biol. 1995;14:457–465. doi: 10.1016/0945-053x(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Korpos E, Wu C, Song J, Hallmann R, Sorokin L. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res. 2010;339:47–57. doi: 10.1007/s00441-009-0853-3. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Garrison K, MacKrell AJ, Fessler LI, Fessler JH. Laminin A chain: expression during Drosophila development and genomic sequence. EMBO J. 1992;11:4519–4527. doi: 10.1002/j.1460-2075.1992.tb05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist AJ, Johnson AE, Morgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszodi A, Fassler R, Sasaki T, Timpl R, et al. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Laurila P, Leivo I. Basement membrane and interstitial matrix components form separate matrices in heterokaryons of PYS-2 cells and fibroblasts. J Cell Sci. 1993;104(Pt 1):59–68. doi: 10.1242/jcs.104.1.59. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Developmental roles of heparan sulfate proteoglycans in Drosophila. Glycoconj J. 2002;19:363–368. doi: 10.1023/A:1025329323438. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Hillman PR, Barrett AL, Jackson MC, Perry TL, Park Y, Datta S. The Drosophila Perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev Biol. 2007;7:121. doi: 10.1186/1471-213X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunstrom GP, Bächinger HP, Fessler LI, Duncan KG, Nelson RE, Fessler JH. The Drosophila basement membrane procollagen IV.I. protein characterization and distribution. J Biol Chem. 1988;263:18318–18327. [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Medioni C, Noselli S. Dynamics of the basement membrane in invasive epithelial clusters in Drosophila. Development. 2005;132:3069–3077. doi: 10.1242/dev.01886. [DOI] [PubMed] [Google Scholar]
- Melrose J, Hayes AJ, Whitelock JM, Little CB. Perlecan, the "jack of all trades" proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays. 2008;30:457–469. doi: 10.1002/bies.20748. [DOI] [PubMed] [Google Scholar]
- Mondal, et al. 2011 [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- Murray MA, Fessler LI, Palka J. Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev Biol. 1995;168:150–165. doi: 10.1006/dbio.1995.1068. [DOI] [PubMed] [Google Scholar]
- Nardi JB, Pilas B, Ujhelyi E, Garsha K, Kanost MR. Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev Genes Evol. 2003;213:477–491. doi: 10.1007/s00427-003-0352-6. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybakken K, Perrimon N. Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim Biophys Acta. 2002;1573:280–291. doi: 10.1016/s0304-4165(02)00395-1. [DOI] [PubMed] [Google Scholar]
- Park Y, Fujioka M, Kobayashi M, Jaynes JB, Datta S. even skipped is required to produce a trans-acting signal for larval neuroblast proliferation that can be mimicked by ecdysone. Development. 2001;128:1899–1909. doi: 10.1242/dev.128.10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJ, McDermott S, Datta S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Xu T. Shaping Cells and Organs in Drosophila by Opposing Roles of Fat Body-Secreted Collagen IV and Perlecan. Dev Cell. 2011;21:245–256. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski TM, Mullen GP, Bush JA, Gilchrist EJ, Moerman DG. UNC-52/perlecan isoform diversity and function in Caenorhabditis elegans. Biochem Soc Trans. 2001;29:171–176. doi: 10.1042/0300-5127:0290171. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse P, Malan D, Fleischmann M, Roell W, Gustafsson E, Bostani T, Fan Y, Kolbe T, Breitbach M, Addicks K, et al. Perlecan is critical for heart stability. Cardiovasc Res. 2008;80:435–444. doi: 10.1093/cvr/cvn225. [DOI] [PubMed] [Google Scholar]
- Segev A, Nili N, Strauss BH. The role of perlecan in arterial injury and angiogenesis. Cardiovasc Res. 2004;63:603–610. doi: 10.1016/j.cardiores.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Sinenko [Google Scholar]
- Urbano JM, Torgler CN, Molnar C, Tepass U, Lopez-Varea A, Brown NH, de Celis JF, Martin-Bermudo MD. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127:4959–4969. doi: 10.1242/dev.127.22.4959. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Zoeller JJ, McQuillan A, Whitelock J, Ho SY, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]